Development of Thermoresponsive Composite Hydrogel Loaded with Indocyanine Green and Camptothecin for Photochemotherapy of Skin Cancer After Surgery
Abstract
1. Introduction
2. Results and Discussion
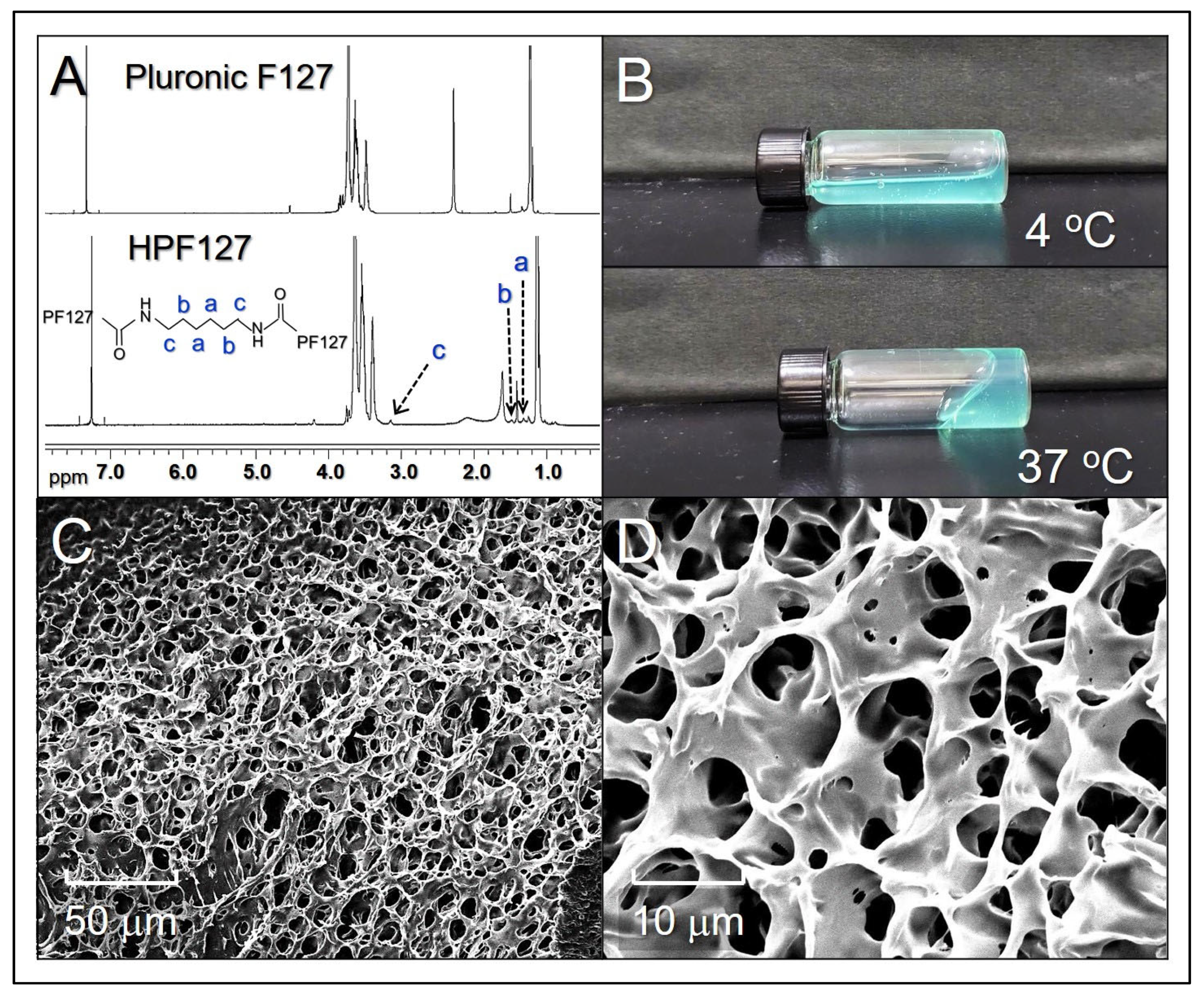
2.1. Characterization of ICHHPG
2.2. Thermal Stability of ICHHPG-Entrapped ICG
2.3. Effects of Hyperthermia on ICHHPG
2.4. Drug Release Kinetics of ICHHPG
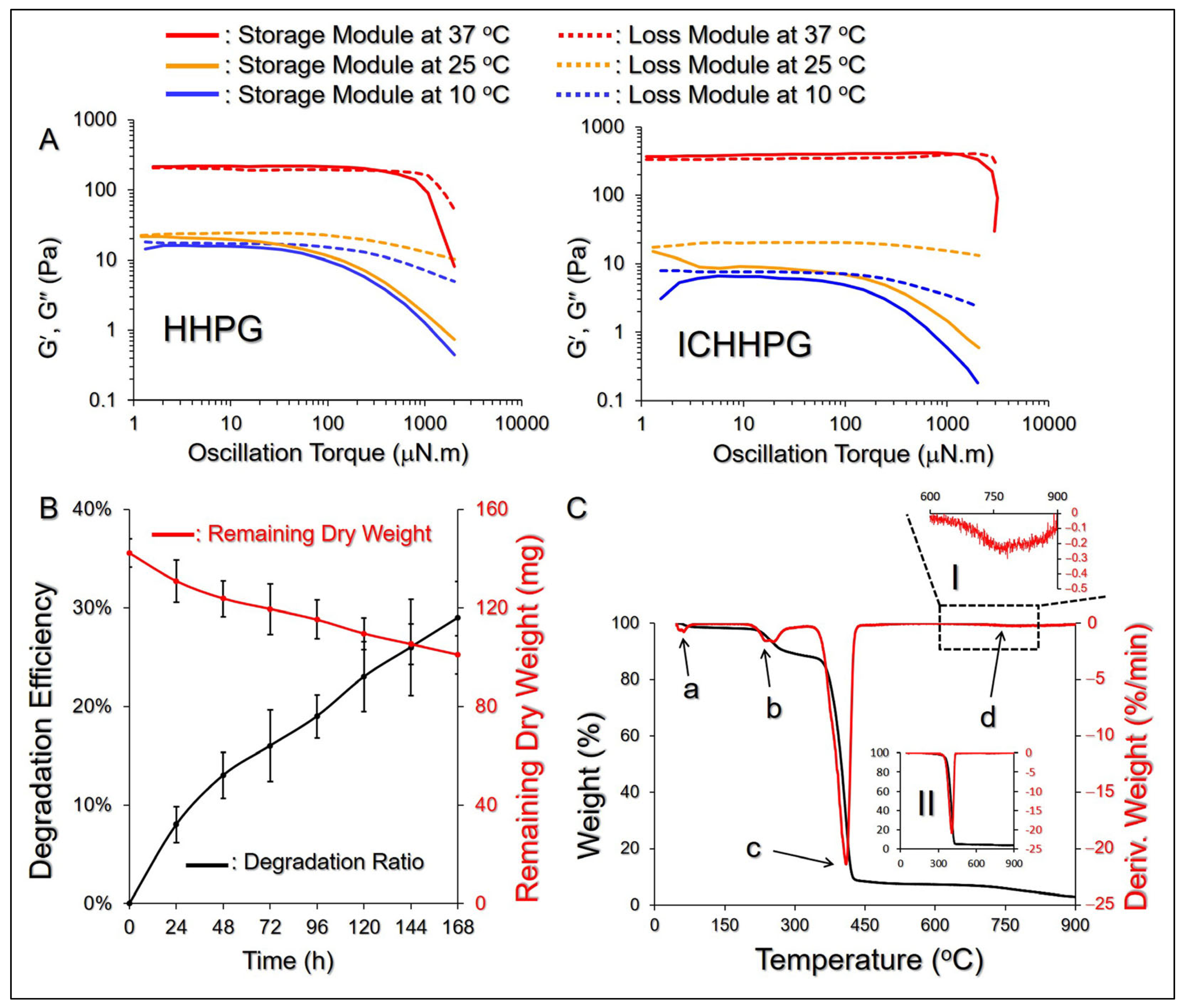
2.5. Rheology and Degradation of ICHHPG In Vitro
2.6. Thermal Properties of ICHHPG
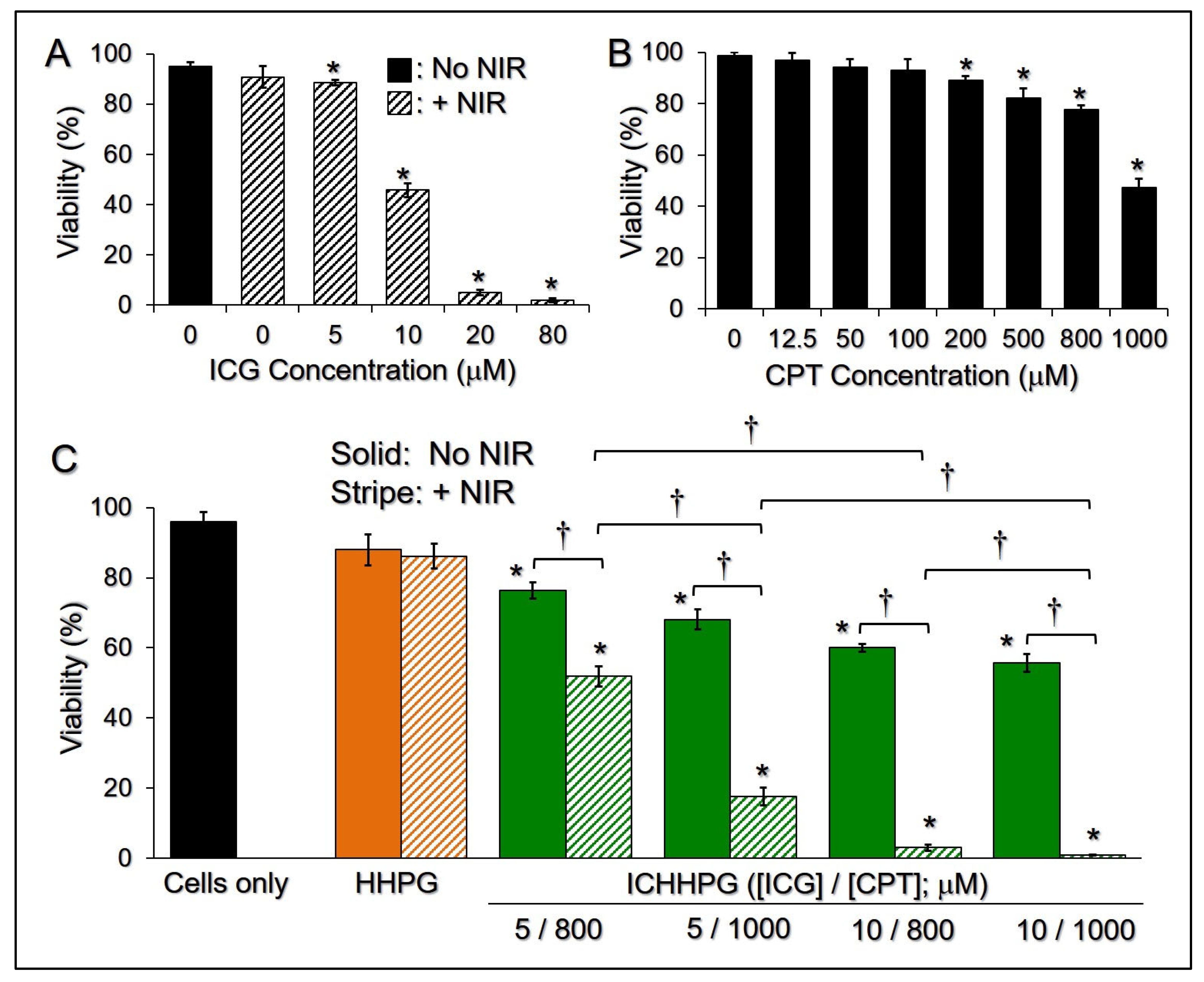
2.7. Cytotoxicity of ICHHPG In Vitro
3. Conclusions
4. Materials and Methods
4.1. Synthesis of HPF127 Copolymer
4.2. Fabrication and Characterization of ICHHPG
4.3. Evaluation of the Stability and Drug Release Kinetics of ICHHPG
4.4. Evaluation of ICHHPG-Induced Hyperthermia Effects
4.5. Evaluation of the Rheological and Thermal Properties of ICHHPG
4.6. Analysis of Degradation of ICHHPG In Vitro
4.7. Cell Culture
4.8. Assessment of Cytotoxicity of ICHHPG In Vitro
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Fidler, M.M.; Gupta, S.; Soerjomataram, I.; Ferlay, J.; Steliarova-Foucher, E.; Bray, F. Cancer incidence and mortality among young adults aged 20-39 years worldwide in 2012: A population-based study. Lancet Oncol. 2017, 18, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.K.; Simmons, J.L.; Parsons, P.G.; Boyle, G.M. Topical treatments for skin cancer. Adv. Drug Deliv. Rev. 2020, 153, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Quazi, S.J.; Aslam, N.; Saleem, H.; Rahman, J.; Khan, S. Surgical Margin of Excision in Basal Cell Carcinoma: A Systematic Review of Literature. Cureus 2020, 12, e9211. [Google Scholar] [CrossRef] [PubMed]
- Golda, N.; Hruza, G. Mohs Micrographic Surgery. Dermatol. Clin. 2023, 41, 39–47. [Google Scholar] [CrossRef]
- Carr, S.; Smith, C.; Wernberg, J. Epidemiology and risk factors of melanoma. Surg. Clin. 2020, 100, 1–12. [Google Scholar] [CrossRef]
- Wysong, A.; Higgins, S.; Blalock, T.W.; Ricci, D.; Nichols, R.; Smith, F.L.; Kossintseva, I. Defining skin cancer local recurrence. J. Am. Acad. Dermatol. 2019, 81, 581–599. [Google Scholar] [CrossRef]
- Holtkamp, L.H.J.; Lo, S.N.; Thompson, J.F.; Spillane, A.J.; Stretch, J.R.; Saw, R.P.M.; Shannon, K.F.; Nieweg, O.E.; Hong, A.M. Adjuvant radiotherapy after salvage surgery for melanoma recurrence in a node field following a previous lymph node dissection. J. Surg. Oncol. 2023, 128, 97–104. [Google Scholar] [CrossRef]
- Luke, J.J.; Schwartz, G.K. Chemotherapy in the management of advanced cutaneous malignant melanoma. Clin. Dermatol. 2013, 31, 290–297. [Google Scholar] [CrossRef]
- Bhave, P.; Pallan, L.; Long, G.V.; Menzies, A.M.; Atkinson, V.; Cohen, J.V.; Sullivan, R.J.; Chiarion-Sileni, V.; Nyakas, M.; Kahler, K.; et al. Melanoma recurrence patterns and management after adjuvant targeted therapy: A multicentre analysis. Br. J. Cancer 2021, 124, 574–580. [Google Scholar] [CrossRef]
- Jazirehi, A.R.; Lim, A.; Dinh, T. PD-1 inhibition and treatment of advanced melanoma-role of pembrolizumab. Am. J. Cancer Res. 2016, 6, 2117–2128. [Google Scholar] [PubMed]
- Worku, D.A.; Hewitt, V. The role and economics of immunotherapy in solid tumour management. J. Oncol. Pharm. Pract. 2020, 26, 2020–2024. [Google Scholar] [CrossRef] [PubMed]
- Kalal, B.S.; Upadhya, D.; Pai, V.R. Chemotherapy Resistance Mechanisms in Advanced Skin Cancer. Oncol. Rev. 2017, 11, 326. [Google Scholar] [CrossRef]
- Sachet, N.F.M.; Sajet, H.F.M.; Al-Owaidi, M.M.N.; Aljulihawi, S.R.M.; Al Idreis, M.K.J.; Al-Gharibawi, W.N.A.; AlKandali, H.A.I.; Kamel, D.J. Radiotherapy and Its Associated Side Effects in Breast, Head and neck, Liver, Thyroid, Non-melanoma skin Cancer and Recent Treatment Strategies. Curr. Clin. Med. Educ. 2024, 2, 106–115. [Google Scholar]
- Sánchez-Cid, P.; Jiménez-Rosado, M.; Romero, A.; Pérez-Puyana, V. Novel Trends in Hydrogel Development for Biomedical Applications: A Review. Polymers 2022, 14, 3023. [Google Scholar] [CrossRef]
- Khan, S.; Akhtar, N.; Minhas, M.U.; Badshah, S.F. pH/Thermo-Dual Responsive Tunable In Situ Cross-Linkable Depot Injectable Hydrogels Based on Poly(NIsopropylacrylamide)/Carboxymethyl Chitosan with Potential of Controlled Localized and Systemic Drug Delivery. AAPS PharmSciTech 2019, 20, 119. [Google Scholar] [CrossRef]
- He, J.; Zhang, Z.; Yang, Y.; Ren, F.; Li, J.; Zhu, S.; Ma, F.; Wu, R.; Lv, Y.; He, G.; et al. Injectable Self-Healing Adhesive pH-Responsive Hydrogels Accelerate Gastric Hemostasis and Wound Healing. Nano-Micro Lett. 2021, 13, 80. [Google Scholar] [CrossRef]
- PJ, R.J.; Oluwafemi, O.S.; Thomas, S.; Oyedeji, A.O. Recent advances in drug delivery nanocarriers incorporated in temperature-sensitive Pluronic F-127–A critical review. J. Drug Deliv. Sci. Technol. 2022, 72, 103390. [Google Scholar]
- Shamma, R.N.; Sayed, R.H.; Madry, H.; El Sayed, N.S.; Cucchiarini, M. Triblock Copolymer Bioinks in Hydrogel Three-Dimensional Printing for Regenerative Medicine: A Focus on Pluronic F127. Tissue Eng. Part B Rev. 2022, 28, 451–463. [Google Scholar] [CrossRef]
- Lippens, E.; Swennen, I.; Gironès, J.; Declercq, H.; Vertenten, G.; Vlaminck, L.; Gasthuys, F.; Schacht, E.; Cornelissen, R. Cell survival and proliferation after encapsulation in a chemically modified Pluronic(R) F127 hydrogel. J. Biomater. Appl. 2013, 27, 828–839. [Google Scholar] [CrossRef]
- Peng, S.; Lin, J.Y.; Cheng, M.H.; Wu, C.W.; Chu, I.M. A cell-compatible PEO-PPO-PEO (Pluronic®)-based hydrogel stabilized through secondary structures. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 69, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Lane, D. Designer combination therapy for cancer. Nat. Biotechnol. 2006, 24, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T.; et al. Emerging Combination Strategies with Phototherapy in Cancer Nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “Smart” Materials-Based Near-Infrared Light-Responsive Drug Delivery Systems for Cancer Treatment: A Review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Fontana, F.; Tapeinos, C.; Shahbazi, M.A.; Han, H.; Santos, H.A. Nanoparticles-based phototherapy systems for cancer treatment: Current status and clinical potential. Bioact. Mater. 2022, 23, 471–507. [Google Scholar] [CrossRef]
- Lee, Y.H.; Pham, U.N.T. Engineered indocyanine green and PD-L1 inhibitors co-loaded perfluorochemical double-layered nanodroplets offer effective photoimmunotherapy against colorectal cancer. Chem. Eng. J. 2023, 460, 141819. [Google Scholar] [CrossRef]
- Jiang, Z.; Li, J.; Chen, S.; Guo, Q.; Jing, Z.; Huang, B.; Pan, Y.; Wang, L.; Hu, Y. Zoledronate and SPIO dual-targeting nanoparticles loaded with ICG for photothermal therapy of breast cancer tibial metastasis. Sci. Rep. 2020, 10, 13675. [Google Scholar] [CrossRef]
- Ma, R.; Alifu, N.; Du, Z.; Chen, S.; Heng, Y.; Wang, J.; Zhu, L.; Ma, C.; Zhang, X. Indocyanine Green-Based Theranostic Nanoplatform for NIR Fluorescence Image-Guided Chemo/Photothermal Therapy of Cervical Cancer. Int. J. Nanomed. 2021, 16, 4847–4861. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation Kinetics of Indocyanine Green in Aqueous Solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef]
- Mindt, S.; Karampinis, I.; John, M.; Neumaier, M.; Nowak, K. Stability and Degradation of Indocyanine Green in Plasma, Aqueous Solution and Whole Blood. Photochem. Photobiol. Sci. 2018, 17, 1189–1196. [Google Scholar] [CrossRef]
- Veloso, A.; Biewen, B.; Paulsen, M.T.; Berg, N.; Carmo de Andrade Lima, L.; Prasad, J.; Bedi, K.; Magnuson, B.; Wilson, T.E.; Ljungman, M. Genome-wide transcriptional effects of the anti-cancer agent camptothecin. PLoS ONE 2013, 8, e78190. [Google Scholar] [CrossRef]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Ramsey, J.D.; Samadi, A.; Atoufi, Z.; Yazdi, M.K.; Ganjali, M.R.; Amirabad, L.M.; Zangene, E.; Farokhi, M.; Formela, K.; et al. Poloxamer: A versatile tri-block copolymer for biomedical applications. Acta Biomater. 2020, 110, 37–67. [Google Scholar] [CrossRef] [PubMed]
- Russo, G.; Delpiano, G.R.; Carucci, C.; Grosso, M.; Dessì, C.; Söderman, O.; Lindman, B.; Monduzzi, M.; Salis, A. Tuning Pluronic F127 phase transitions by adding physiological amounts of salts: A rheology, SAXS, and NMR investigation. Eur. Polym. J. 2024, 204, 112714. [Google Scholar] [CrossRef]
- Kim, M.R.; Park, T.G. Temperature-responsive and degradable hyaluronic acid/Pluronic composite hydrogels for controlled release of human growth hormone. J. Control. Release 2002, 80, 69–77. [Google Scholar] [CrossRef]
- De Paula, G.F.; Netto, G.I.; Mattoso, L.H.C. Physical and Chemical Characterization of Poly(hexamethylene biguanide) Hydrochloride. Polymers 2011, 3, 928–941. [Google Scholar] [CrossRef]
- Lopez, K.M.; Ravula, S.; Pérez, R.L.; Ayala, C.E.; Losso, J.N.; Janes, M.E.; Warner, I.M. Hyaluronic Acid-Cellulose Composites as Patches for Minimizing Bacterial Infections. ACS Omega 2020, 5, 4125–4132. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Dinh, V.T.; Dang, L.H.; Nguyen, D.N.; Giang, B.L.; Nguyen, C.T.; Nguyen, T.B.T.; Thu, L.V.; Tran, N.Q. Dual Interactions of Amphiphilic Gelatin Copolymer and Nanocurcumin Improving the Delivery Efficiency of the Nanogels. Polymers 2019, 11, 814. [Google Scholar] [CrossRef]
- Mordon, S.; Devoisselle, J.M.; Soulie-Begu, S.; Desmettre, T. Indocyanine green: Physicochemical factors affecting its fluorescence in vivo. Microvasc. Res. 1998, 55, 146–152. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-H.; Chung, C.-L. Development of Thermoresponsive Composite Hydrogel Loaded with Indocyanine Green and Camptothecin for Photochemotherapy of Skin Cancer After Surgery. Gels 2025, 11, 71. https://doi.org/10.3390/gels11010071
Lee Y-H, Chung C-L. Development of Thermoresponsive Composite Hydrogel Loaded with Indocyanine Green and Camptothecin for Photochemotherapy of Skin Cancer After Surgery. Gels. 2025; 11(1):71. https://doi.org/10.3390/gels11010071
Chicago/Turabian StyleLee, Yu-Hsiang, and Chieh-Lin Chung. 2025. "Development of Thermoresponsive Composite Hydrogel Loaded with Indocyanine Green and Camptothecin for Photochemotherapy of Skin Cancer After Surgery" Gels 11, no. 1: 71. https://doi.org/10.3390/gels11010071
APA StyleLee, Y.-H., & Chung, C.-L. (2025). Development of Thermoresponsive Composite Hydrogel Loaded with Indocyanine Green and Camptothecin for Photochemotherapy of Skin Cancer After Surgery. Gels, 11(1), 71. https://doi.org/10.3390/gels11010071







