N-Oxalylglycine-Conjugated Hyaluronic Acid as a Macromolecular Prodrug for Therapeutic Angiogenesis
Abstract
1. Introduction
2. Results and Discussion
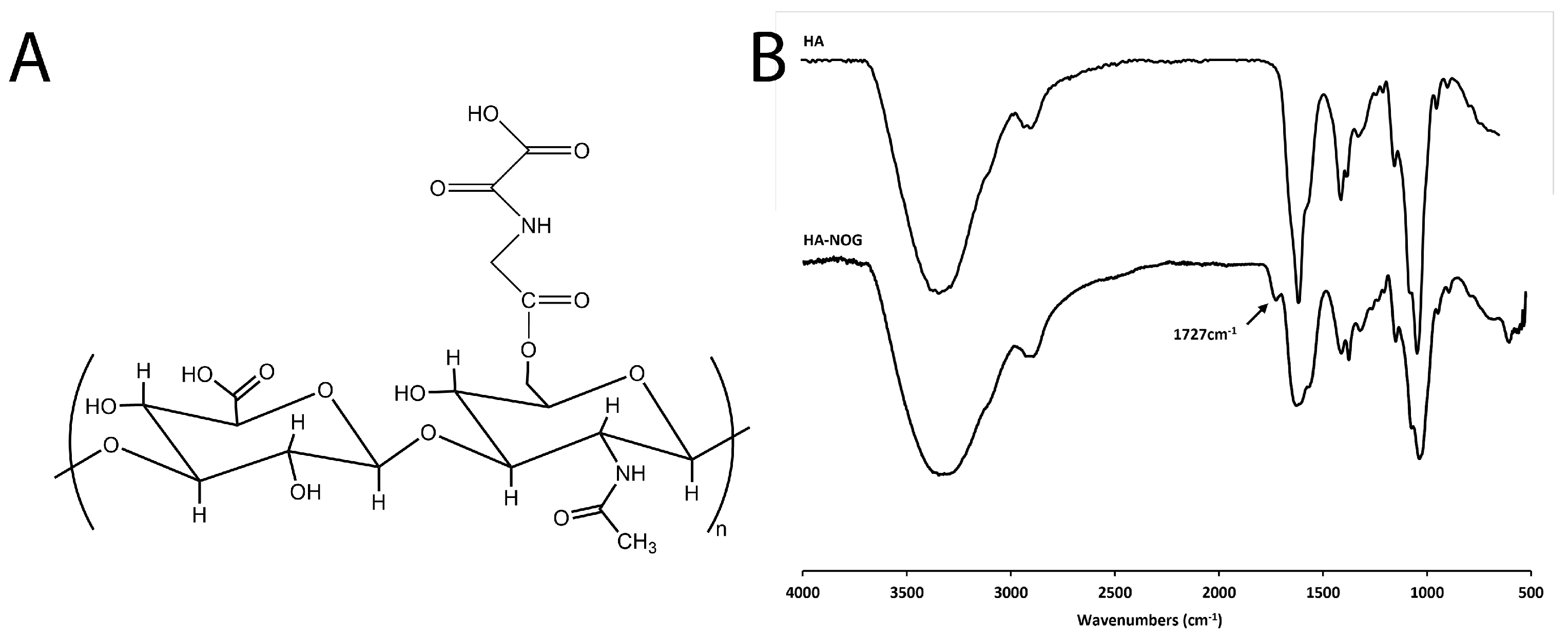
2.1. Synthesis and Characterization of HA-NOG
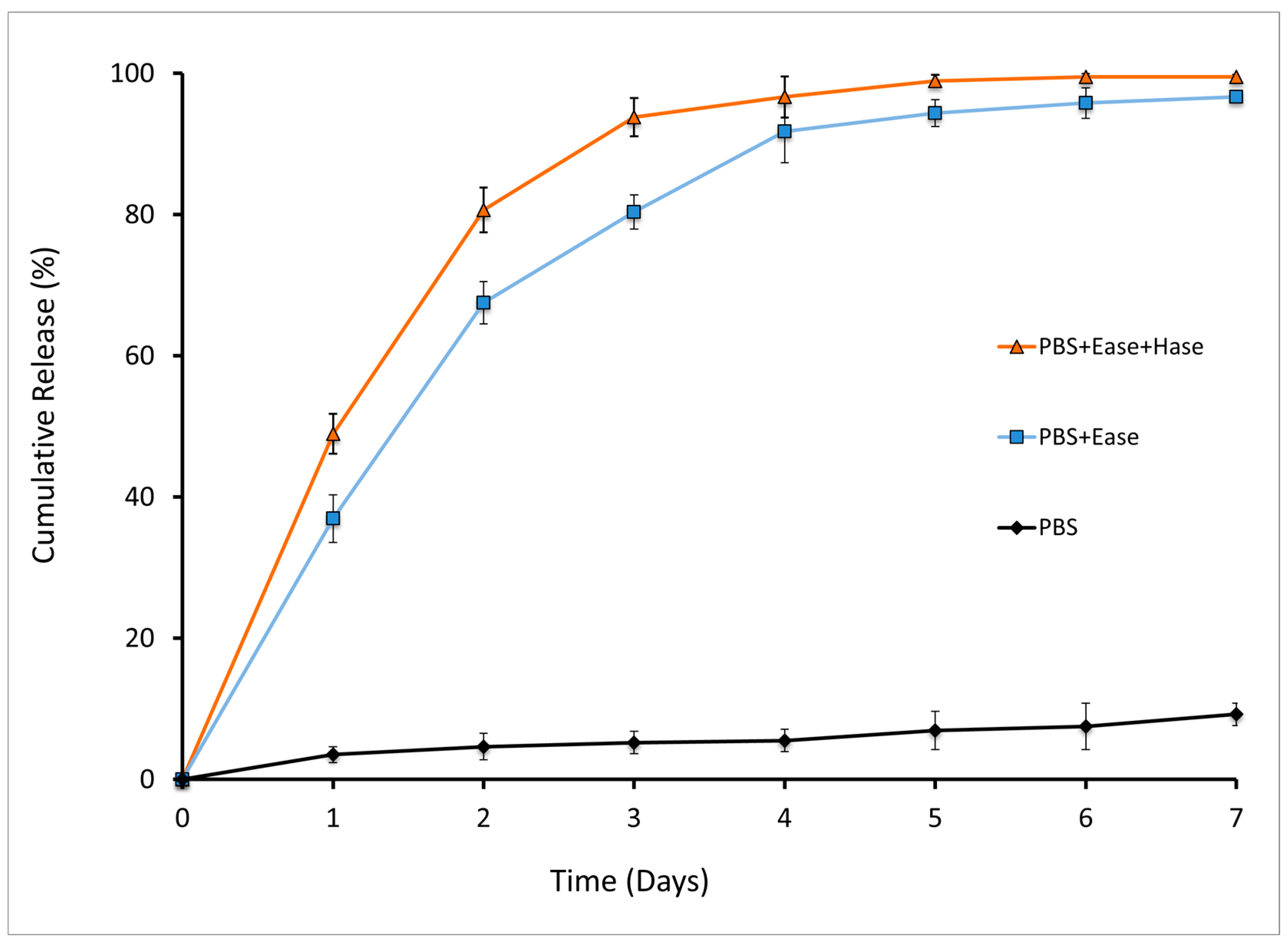
2.2. Enzymatic Release of NOG from HA-NOG Conjugate
2.3. Evaluation of Angiogenic Bioactivity
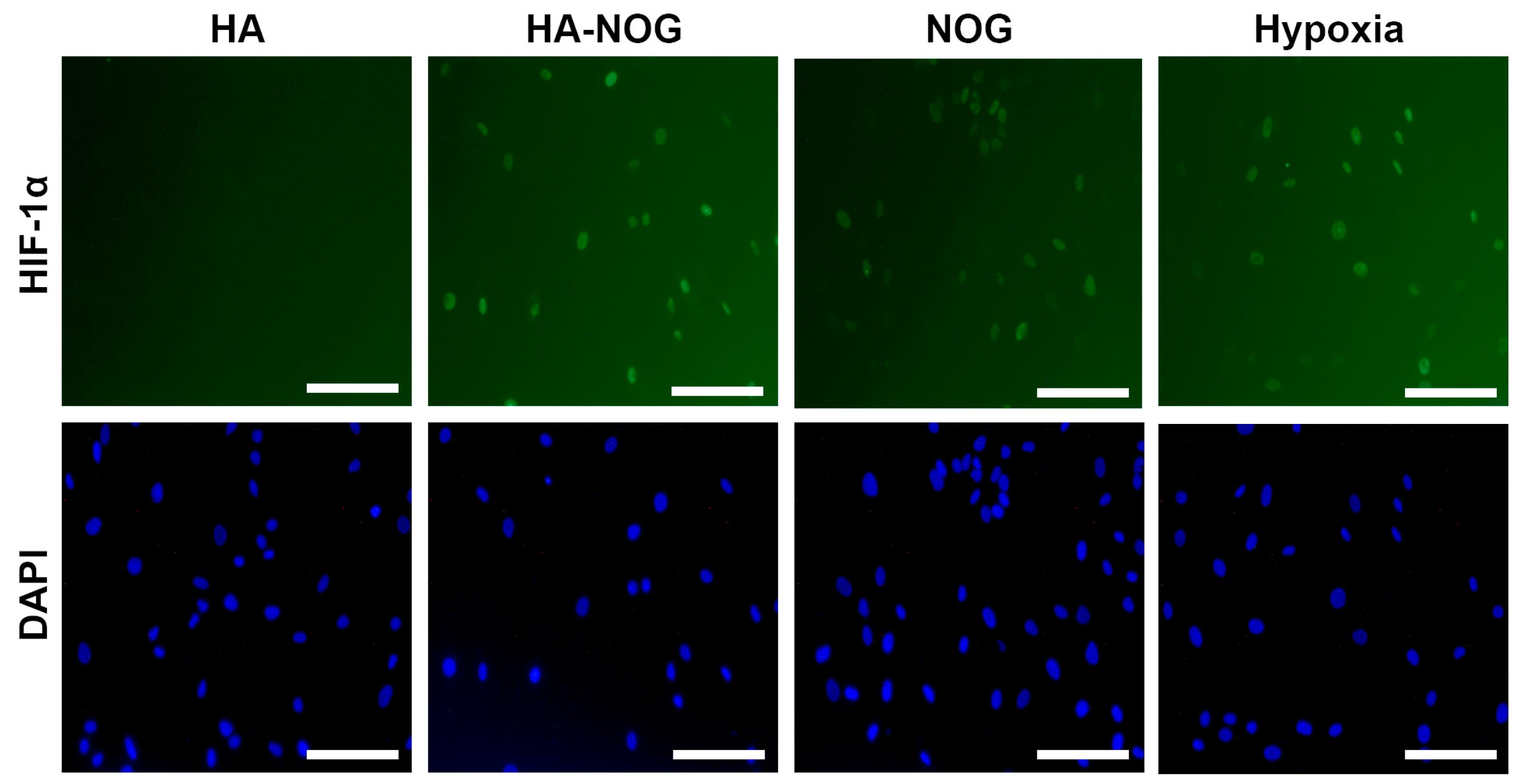
2.3.1. HIF-1α Stabilization
2.3.2. Expression of HIF-1 Target Genes
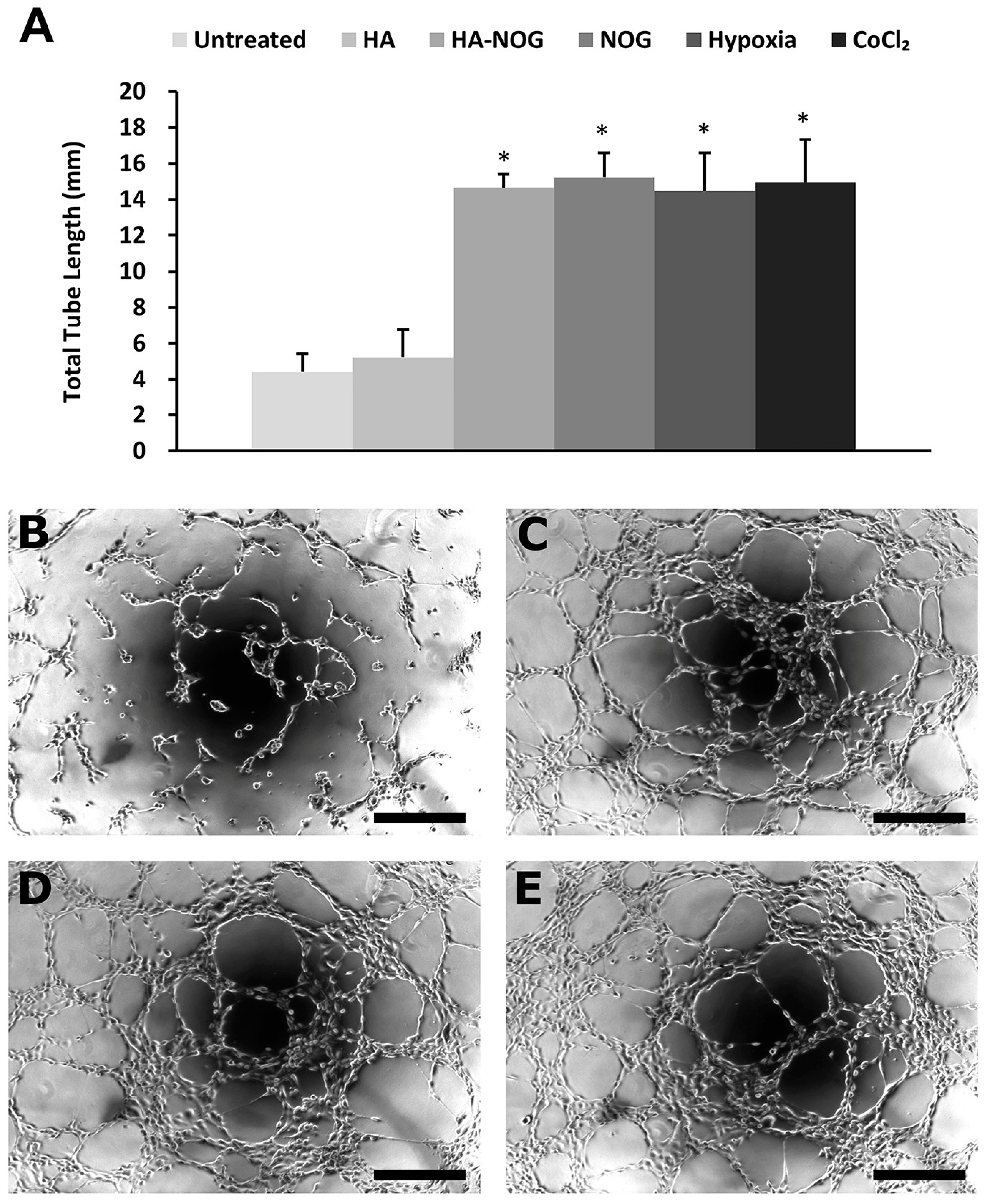
2.3.3. Endothelial Cell Tubulogenesis
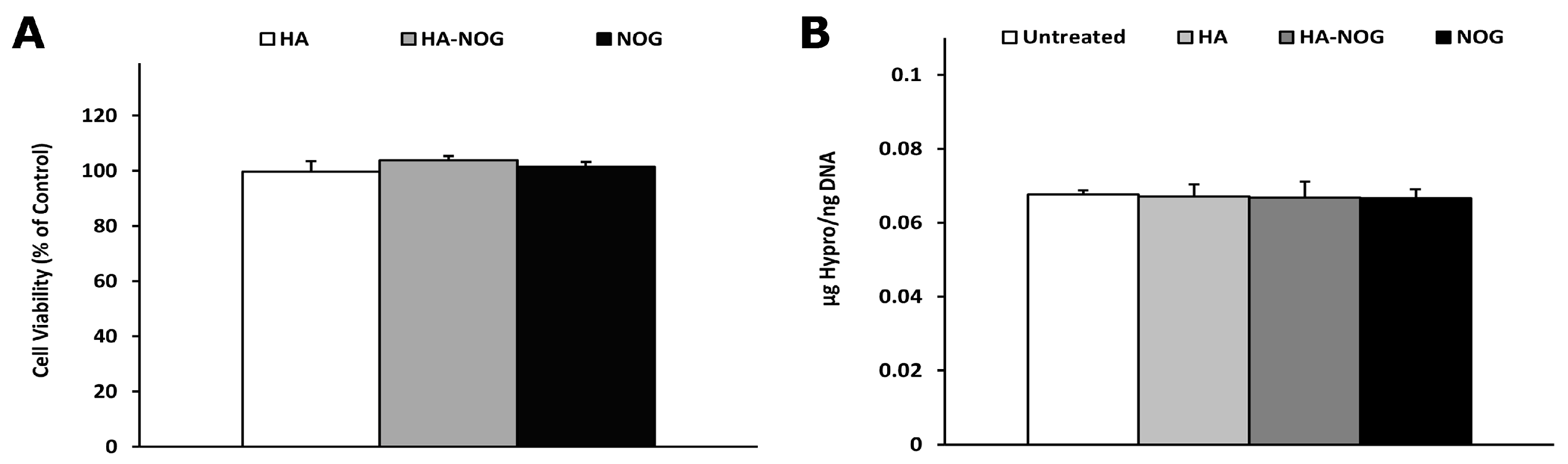
2.4. Cytotoxicity and Off-Target Effects
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Characterization
4.2.1. HA-TBA Synthesis
4.2.2. HA-NOG Conjugate
4.2.3. Enzymatic Release of NOG from HA-NOG Conjugate
4.3. Evaluation of Angiogenic Bioactivity
4.3.1. Cell Culture
4.3.2. HIF-1α Stabilization
4.3.3. Expression of HIF-1 Target Genes
4.3.4. Tubulogenesis Assay
4.4. Cytotoxicity of Off-Target Effects
4.4.1. Metabolic Activity Assay
4.4.2. DNA and Hydroxyproline Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Annex, B.H.; Cooke, J.P. New Directions in Therapeutic Angiogenesis and Arteriogenesis in Peripheral Arterial Disease. Circ. Res. 2021, 128, 1944–1957. [Google Scholar] [CrossRef]
- Gianni-Barrera, R.; Di Maggio, N.; Melly, L.; Burger, M.G.; Mujagic, E.; Gurke, L.; Schaefer, D.J.; Banfi, A. Therapeutic vascularization in regenerative medicine. Stem Cells Transl. Med. 2020, 9, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Chandel, N.S.; Simon, M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 2020, 21, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Ruan, W.; Bobrow, B.; Carmeliet, P.; Eltzschig, H.K. Targeting hypoxia-inducible factors: Therapeutic opportunities and challenges. Nat. Rev. Drug Discov. 2024, 23, 175–200. [Google Scholar] [CrossRef]
- Masson, N.; Willam, C.; Maxwell, P.H.; Pugh, C.W.; Ratcliffe, P.J. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001, 20, 5197–5206. [Google Scholar] [CrossRef]
- Epstein, A.C.; Gleadle, J.M.; McNeill, L.A.; Hewitson, K.S.; O’Rourke, J.; Mole, D.R.; Mukherji, M.; Metzen, E.; Wilson, M.I.; Dhanda, A.; et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 2001, 107, 43–54. [Google Scholar] [CrossRef]
- Ivan, M.; Kondo, K.; Yang, H.; Kim, W.; Valiando, J.; Ohh, M.; Salic, A.; Asara, J.M.; Lane, W.S.; Kaelin, W.G., Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 2001, 292, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Jaakkola, P.; Mole, D.R.; Tian, Y.M.; Wilson, M.I.; Gielbert, J.; Gaskell, S.J.; Kriegsheim, A.; Hebestreit, H.F.; Mukherji, M.; Schofield, C.J.; et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 2001, 292, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Lando, D.; Peet, D.J.; Whelan, D.A.; Gorman, J.J.; Whitelaw, M.L. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 2002, 295, 858–861. [Google Scholar] [CrossRef] [PubMed]
- McNeill, L.A.; Hewitson, K.S.; Claridge, T.D.; Seibel, J.F.; Horsfall, L.E.; Schofield, C.J. Hypoxia-inducible factor asparaginyl hydroxylase (FIH-1) catalyses hydroxylation at the beta-carbon of asparagine-803. Biochem. J. 2002, 367, 571–575. [Google Scholar] [CrossRef]
- Dengler, V.L.; Galbraith, M.; Espinosa, J.M. Transcriptional regulation by hypoxia inducible factors. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 1–15. [Google Scholar] [CrossRef]
- Omorphos, N.P.; Gao, C.; Tan, S.S.; Sangha, M.S. Understanding angiogenesis and the role of angiogenic growth factors in the vascularisation of engineered tissues. Mol. Biol. Rep. 2021, 48, 941–950. [Google Scholar] [CrossRef]
- Andrea, U.; Thomas, W.; Paolo, V.; Nunzia, D.M.; Matteo, P.; Lorenz, G.; Andrea, B.; Roberto, G.-B. Vascuar endothelial growth factor biology for regenerative angiogenesis. Swiss Med. Wkly. 2019, 149, w20011. [Google Scholar] [CrossRef]
- Rubanyi, G.M. Mechanistic, technical, and clinical perspectives in therapeutic stimulation of coronary collateral development by angiogenic growth factors. Mol. Ther. 2013, 21, 725–738. [Google Scholar] [CrossRef]
- Martino, M.M.; Brkic, S.; Bovo, E.; Burger, M.; Schaefer, D.J.; Wolff, T.; Gurke, L.; Briquez, P.S.; Larsson, H.M.; Gianni-Barrera, R.; et al. Extracellular matrix and growth factor engineering for controlled angiogenesis in regenerative medicine. Front. Bioeng. Biotechnol. 2015, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Volkova, E.; Blatchley, M.R.; Gerecht, S. Hydrogel vehicles for sequential delivery of protein drugs to promote vascular regeneration. Adv. Drug Deliv. Rev. 2019, 149–150, 95–106. [Google Scholar] [CrossRef]
- Hadjipanayi, E.; Schilling, A.F. Hypoxia-based strategies for angiogenic induction: The dawn of a new era for ischemia therapy and tissue regeneration. Organogenesis 2013, 9, 261–272. [Google Scholar] [CrossRef]
- Lee, R.J.; Springer, M.L.; Blanco-Bose, W.E.; Shaw, R.; Ursell, P.C.; Blau, H.M. VEGF gene delivery to myocardium: Deleterious effects of unregulated expression. Circulation 2000, 102, 898–901. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; You, Q.; Zhang, X. Small-molecule modulators of the hypoxia-inducible factor pathway: Development and yherapeutic applications. J. Med. Chem. 2019, 62, 5725–5749. [Google Scholar] [CrossRef] [PubMed]
- Nagle, D.G.; Zhou, Y.D. Natural product-derived small molecule activators of hypoxia-inducible factor-1 (HIF-1). Curr. Pharm. Des. 2006, 12, 2673–2688. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.R.; McDonough, M.A.; King, O.N.; Kawamura, A.; Schofield, C.J. Inhibition of 2-oxoglutarate dependent oxygenases. Chem. Soc. Rev. 2011, 40, 4364–4397. [Google Scholar] [CrossRef]
- Donneys, A.; Weiss, D.M.; Deshpande, S.S.; Ahsan, S.; Tchanque-Fossuo, C.N.; Sarhaddi, D.; Levi, B.; Goldstein, S.A.; Buchman, S.R. Localized deferoxamine injection augments vascularity and improves bony union in pathologic fracture healing after radiotherapy. Bone 2013, 52, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Nie, C.; Si, Z.; Ma, Y. Deferoxamine enhances neovascularization and accelerates wound healing in diabetic rats via the accumulation of hypoxia-inducible factor-1alpha. Diabetes Res. Clin. Pract. 2013, 101, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Duscher, D.; Neofytou, E.; Wong, V.W.; Maan, Z.N.; Rennert, R.C.; Inayathullah, M.; Januszyk, M.; Rodrigues, M.; Malkovskiy, A.V.; Whitmore, A.J.; et al. Transdermal deferoxamine prevents pressure-induced diabetic ulcers. Proc. Natl. Acad. Sci. USA 2015, 112, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Du, T.; Yang, X.; Zhang, W.; Wang, G.; Liu, X.; Li, T.; Jiang, Z. Desferoxamine protects against glucocorticoid-induced osteonecrosis of the femoral head via activating HIF-1alpha expression. J. Cell. Physiol. 2020, 235, 9864–9875. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.; Wu, X.; Xia, D.; Chen, Z.; Wang, D.; Liang, C.; Li, J. 3D nanofiber sponge with dimethyloxaloglycine-loaded Prussian blue analogue microspheres to promote wound healing. Biomed. Mater. 2023, 18, 035012. [Google Scholar] [CrossRef]
- Balmayor, E.R. Targeted delivery as key for the success of small osteoinductive molecules. Adv. Drug Deliv. Rev. 2015, 94, 13–27. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Ashe, K.M.; Henry, N.; Kan, H.M.; Lo, K.W. Delivery of small molecules for bone regenerative engineering: Preclinical studies and potential clinical applications. Drug Discov. Today 2014, 19, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Kidane, A.; Bhatt, P.P. Recent advances in small molecule drug delivery. Curr. Opin. Chem. Biol. 2005, 9, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Whittam, A.J.; Maan, Z.N.; Duscher, D.; Wong, V.W.; Barrera, J.A.; Januszyk, M.; Gurtner, G.C. Challenges and Opportunities in Drug Delivery for Wound Healing. Adv. Wound Care 2016, 5, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Fraser, I.P.; Yeo, Y.; Highley, C.B.; Bellas, E.; Kohane, D.S. Anti-inflammatory function of an in situ cross-linkable conjugate hydrogel of hyaluronic acid and dexamethasone. Biomaterials 2007, 28, 1778–1786. [Google Scholar] [CrossRef]
- Luo, Y.; Ziebell, M.R.; Prestwich, G.D. A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules 2000, 1, 208–218. [Google Scholar] [CrossRef]
- Prestwich, G.D.; Kuo, J.W. Chemically-modified HA for therapy and regenerative medicine. Curr. Pharm. Biotechnol. 2008, 9, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sahu, K.; Singh, S.P.; Jain, B. Wound healing activity of curcumin conjugated to hyaluronic acid: In vitro and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1009–1017. [Google Scholar] [CrossRef]
- Bae, S.; Lee, H.J.; Lee, J.S.; Webb, K. Cell-Mediated Dexamethasone Release from Semi-IPNs Stimulates Osteogenic Differentiation of Encapsulated Mesenchymal Stem Cells. Biomacromolecules 2015, 16, 2757–2765. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.U.; Bae, S.; Macks, C.; Whitaker, J.; Lynn, M.; Webb, K.; Lee, J.S. Hydrogel-mediated local delivery of dexamethasone reduces neuroinflammation after traumatic brain injury. Biomed. Mater. 2021, 16, 035002. [Google Scholar] [CrossRef] [PubMed]
- Macks, C.; Jeong, D.; Bae, S.; Webb, K.; Lee, J.S. Dexamethasone-loaded hydrogels improve motor and cognitive functions in a rat Mild traumatic brain injury model. Int. J. Mol. Sci. 2022, 23, 11153. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.; Elliott, B.; Liao, Z.; Johnson, Z.; Ma, F.; Bailey, Z.S.; Gilsdorf, J.; Scultetus, A.; Shear, D.; Webb, K.; et al. PEG hydrogel containing dexamethasone-conjugated hyaluronic acid reduces secondary injury and improves motor function in a rat moderate TBI model. Exp. Neurol. 2023, 369, 114533. [Google Scholar] [CrossRef] [PubMed]
- Gilli, R.; Kacurakova, M.; Mathlouthi, M.; Navarini, L.; Paoletti, S. FTIR studies of sodium hyaluronate and its oligomers in the amorphous solid phase and in aqueous solution. Carbohydr. Res. 1994, 263, 315–326. [Google Scholar] [CrossRef]
- Pan, N.C.; Pereira, H.C.B.; da Silva, M.L.C.; Vasconcelos, A.F.D.; Celligoi, M. Improvement production of hyaluronic ccid by Streptococcus zooepidemicus in sugarcane molasses. Appl. Biochem. Biotechnol. 2017, 182, 276–293. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Miller, R.J.; Dhal, P.K. Hyaluronic acid-based drug conjugates: State-of-the-art and perspectives. J. Biomed. Nanotechnol. 2014, 10, 4–16. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Park, T.G. Hyaluronic acid-paclitaxel conjugate micelles: Synthesis, characterization, and antitumor activity. Bioconjug. Chem. 2008, 19, 1319–1325. [Google Scholar] [CrossRef]
- Gianolio, D.A.; Philbrook, M.; Avila, L.Z.; Young, L.E.; Plate, L.; Santos, M.R.; Bernasconi, R.; Liu, H.; Ahn, S.; Sun, W.; et al. Hyaluronan-tethered opioid depots: Synthetic strategies and release kinetics in vitro and in vivo. Bioconjug. Chem. 2008, 19, 1767–1774. [Google Scholar] [CrossRef]
- Camacho, K.M.; Kumar, S.; Menegatti, S.; Vogus, D.R.; Anselmo, A.C.; Mitragotri, S. Synergistic antitumor activity of camptothecin-doxorubicin combinations and their conjugates with hyaluronic acid. J. Control. Release 2015, 210, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Yeoh, K.K.; Chan, M.C.; Thalhammer, A.; Demetriades, M.; Chowdhury, R.; Tian, Y.M.; Stolze, I.; McNeill, L.A.; Lee, M.K.; Woon, E.C.Y.; et al. Dual-action inhibitors of HIF prolyl hydroxylases that induce binding of a second iron ion. Org. Biomol. Chem. 2013, 11, 732–745. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Takahashi, M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J. Biol. Chem. 2007, 282, 5597–5607. [Google Scholar] [CrossRef]
- Pugh, C.W.; Ratcliffe, P.J. Regulation of angiogenesis by hypoxia: Role of the HIF system. Nat. Med. 2003, 9, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, L.; Yang, J.; Wang, Z.; Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol. Med. Rep. 2018, 18, 3547–3554. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chen, S.; Song, W.Q.; Gao, Y.S.; Guan, J.J.; Wang, Y.; Sun, Y.; Zhang, C.Q. Dimethyloxaloylglycine improves angiogenic activity of bone marrow stromal cells in the tissue-engineered bone. Int. J. Biol. Sci. 2014, 10, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Guan, J.; Qi, X.; Ding, H.; Yuan, H.; Xie, Z.; Chen, C.; Li, X.; Zhang, C.; Huang, Y. Dimethyloxaloylglycine Promotes the Angiogenic Activity of Mesenchymal Stem Cells Derived from iPSCs via Activation of the PI3K/Akt Pathway for Bone Regeneration. Int. J. Biol. Sci. 2016, 12, 639–652. [Google Scholar] [CrossRef]
- Lawley, T.J.; Kubota, Y. Induction of morphologic differentiation of endothelial cells in culture. J. Investig. Dermatol. 1989, 93, 59S–61S. [Google Scholar] [CrossRef]
- Staton, C.A.; Reed, M.W.; Brown, N.J. A critical analysis of current in vitro and in vivo angiogenesis assays. Int. J. Exp. Pathol. 2009, 90, 195–221. [Google Scholar] [CrossRef] [PubMed]
- Dery, M.A.; Michaud, M.D.; Richard, D.E. Hypoxia-inducible factor 1: Regulation by hypoxic and non-hypoxic activators. Int. J. Biochem. Cell Biol. 2005, 37, 535–540. [Google Scholar] [CrossRef]
- Malkov, M.I.; Lee, C.T.; Taylor, C.T. Regulation of the Hypoxia-Inducible Factor (HIF) by Pro-Inflammatory Cytokines. Cells 2021, 10, 2340. [Google Scholar] [CrossRef]
- Cunliffe, C.J.; Franklin, T.J.; Hales, N.J.; Hill, G.B. Novel inhibitors of prolyl 4-hydroxylase. 3. Inhibition by the substrate analogue N-oxaloglycine and its derivatives. J. Med. Chem. 1992, 35, 2652–2658. [Google Scholar] [CrossRef] [PubMed]
- Bentovim, L.; Amarilio, R.; Zelzer, E. HIF1alpha is a central regulator of collagen hydroxylation and secretion under hypoxia during bone development. Development 2012, 139, 4473–4483. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.; Li, W.; Hitchcock, R.W.; Smeal, R.M.; Gray, S.D.; Tresco, P.A. Comparison of human fibroblast ECM-related gene expression on elastic three-dimensional substrates relative to two-dimensional films of the same material. Biomaterials 2003, 24, 4681–4690. [Google Scholar] [CrossRef]
- West, D.C.; Sattar, A.; Kumar, S. A simplified in situ solubilization procedure for the determination of DNA and cell number in tissue cultured mammalian cells. Anal. Biochem. 1985, 147, 289–295. [Google Scholar] [CrossRef]
- Webb, K.; Hitchcock, R.W.; Smeal, R.M.; Li, W.; Gray, S.D.; Tresco, P.A. Cyclic strain increases fibroblast proliferation, matrix accumulation, and elastic modulus of fibroblast-seeded polyurethane constructs. J. Biomech. 2006, 39, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Cissell, D.D.; Link, J.M.; Hu, J.C.; Athanasiou, K.A. A modified hydroxyproline assay based on hydrochloric acid in Ehrlich’s solution accurately measures tissue collagen content. Tissue Eng. Part C Methods 2017, 23, 243–250. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | GeneBank No. | Product Size (bp) |
|---|---|---|---|---|
| VEGF | CCTTGCCTTGCTGCTCTACC | ACCAGGGTCTCGATTGGATG | NM_001171630 | 144 |
| PHD2 | TGTTATCCGGGCAATGGAAC | AAACTGGGCTTTGCCTTCTG | NM_022051 | 156 |
| GLUT1 | ACTCTTCAGCCAGGGTCCAC | CGTAGGGACCACACAGTTGC | NM_006516 | 119 |
| β2MG | TGTGCTCGCGCTACTCTCTC | CGGATGGATGAAACCCAGAC | NM_004048 | 137 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeMaria, A.H.; Lee, J.S.; Webb, K. N-Oxalylglycine-Conjugated Hyaluronic Acid as a Macromolecular Prodrug for Therapeutic Angiogenesis. Gels 2025, 11, 27. https://doi.org/10.3390/gels11010027
DeMaria AH, Lee JS, Webb K. N-Oxalylglycine-Conjugated Hyaluronic Acid as a Macromolecular Prodrug for Therapeutic Angiogenesis. Gels. 2025; 11(1):27. https://doi.org/10.3390/gels11010027
Chicago/Turabian StyleDeMaria, Andrew H., Jeoung Soo Lee, and Ken Webb. 2025. "N-Oxalylglycine-Conjugated Hyaluronic Acid as a Macromolecular Prodrug for Therapeutic Angiogenesis" Gels 11, no. 1: 27. https://doi.org/10.3390/gels11010027
APA StyleDeMaria, A. H., Lee, J. S., & Webb, K. (2025). N-Oxalylglycine-Conjugated Hyaluronic Acid as a Macromolecular Prodrug for Therapeutic Angiogenesis. Gels, 11(1), 27. https://doi.org/10.3390/gels11010027








