Moisture Sorption Isotherms of Polydextrose and Its Gelling Efficiency in Inhibiting the Retrogradation of Rice Starch
Abstract
1. Introduction
2. Results and Discussion
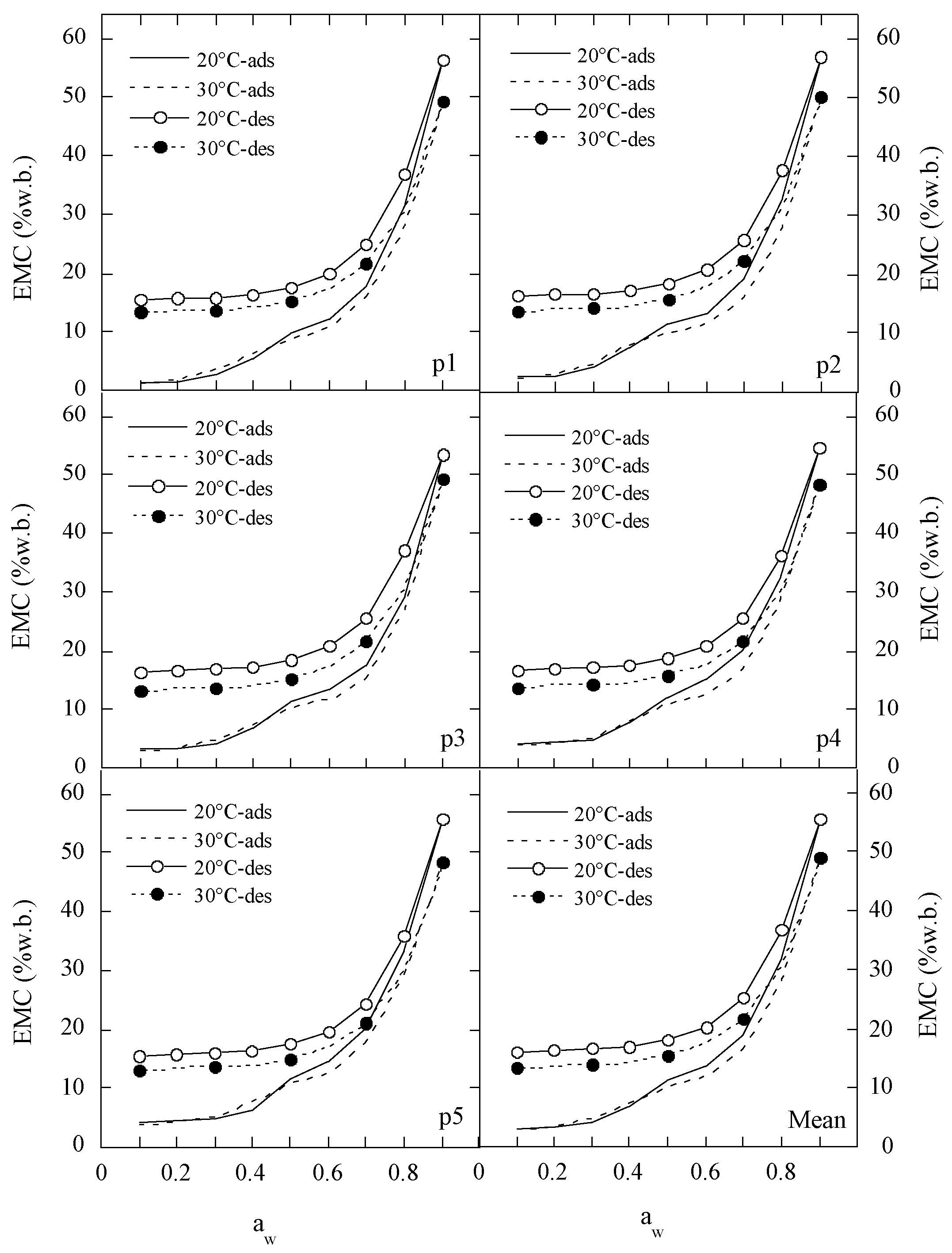
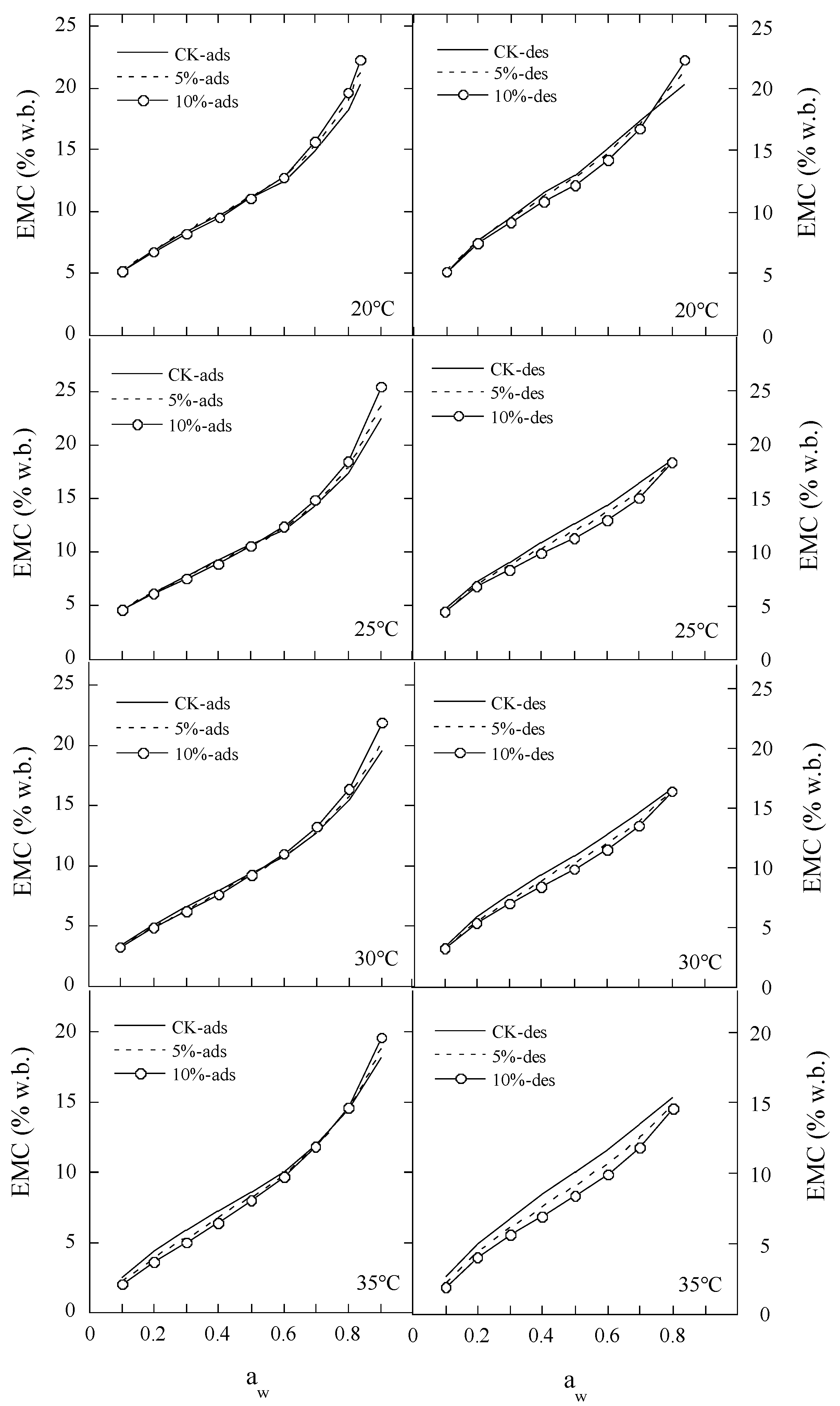
2.1. Experimental EMC/ERH Data for Polydextrose Samples
2.2. Fitting of Sorption Equations to Experimental Sorption Data
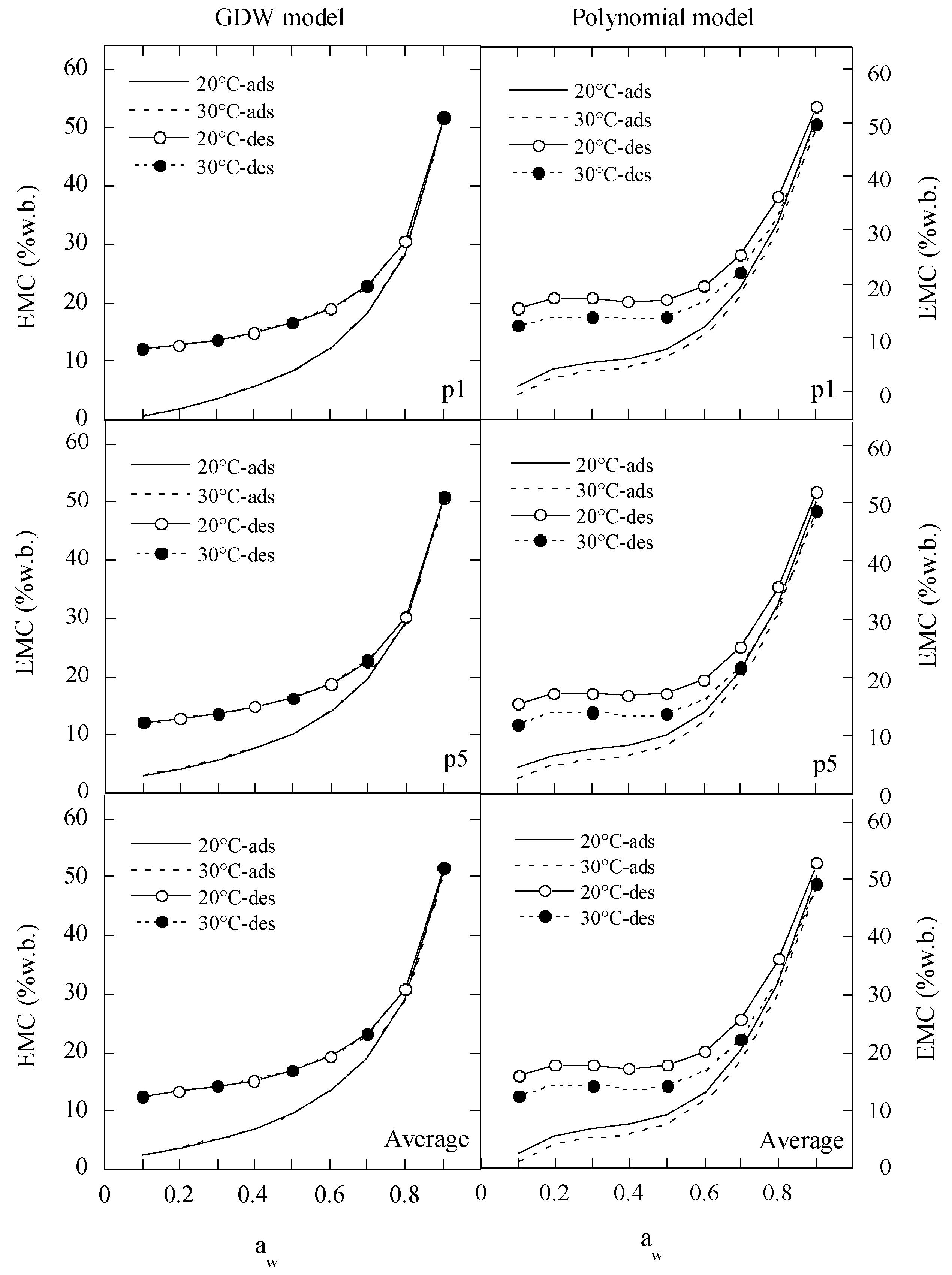
2.3. Prediction of Moisture Sorption Isotherms by the Best Fitting Equation
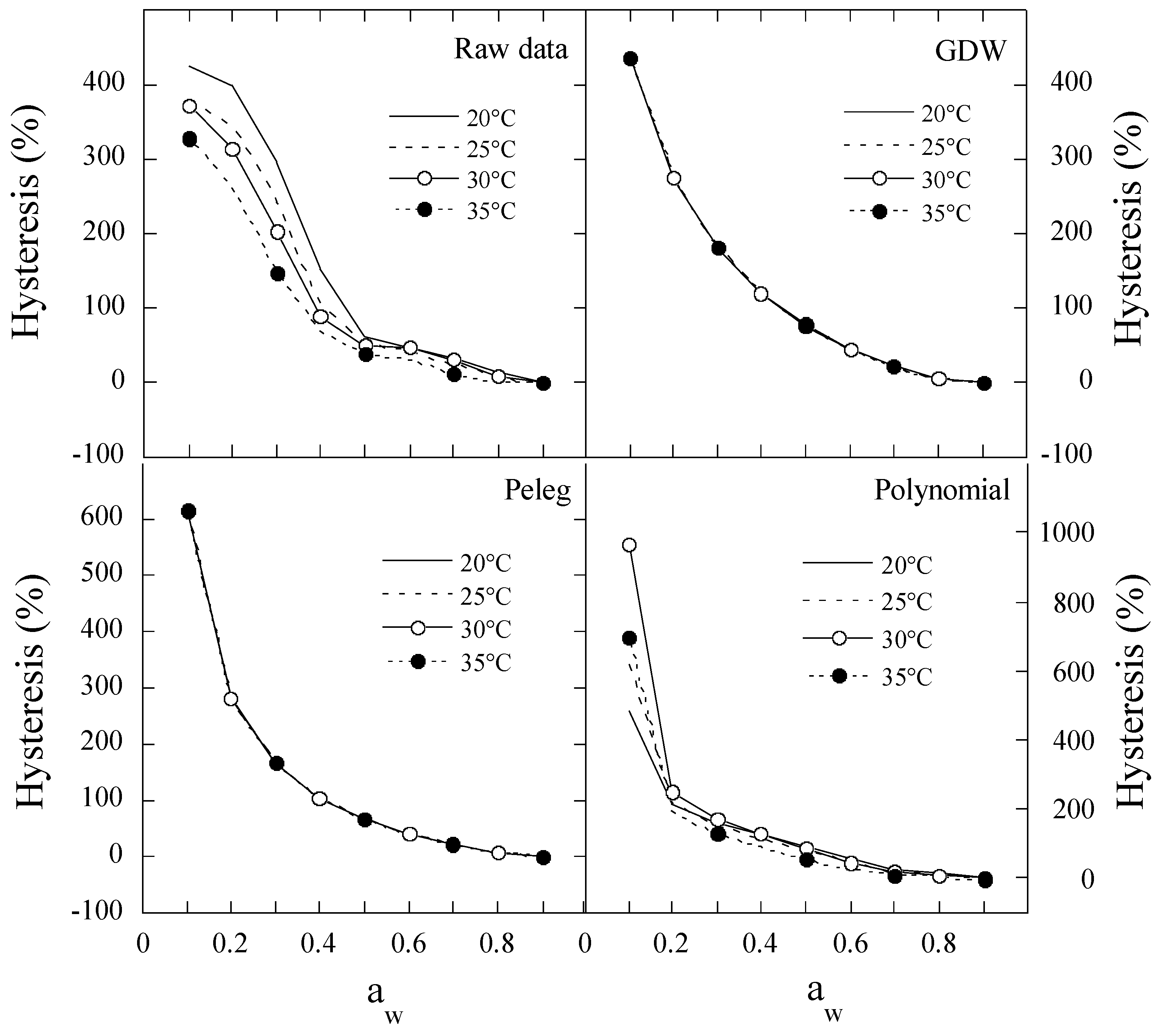
2.4. The Hysteresis Degree between Moisture Desorption and Adsorption of Polydextrose
2.5. Change in the Phase State of Rice Starch with Polydextrose Addition
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Moisture Sorption Isotherms and Their Fitting
4.3. The Addition of Polydextrose in Rice Starch
4.4. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wüstenberg, T. General overview of food hydrocolloids. In Cellullose and Cellulose Derivatives in the Food Industry: Fundamentals and Application, 1st ed.; Wiley-VCH Verlag GmbH& Co. KGaA: Weinheim, Germany, 2015; pp. 1–68. [Google Scholar]
- Wong, K.Y.; Thoo, Y.Y.; Tan, C.P.; Siow, L.F. Moisture absorption behahavior and thermal properties of sucrose replacer mixture containing inulin and polydextrose. Appl. Food Res. 2022, 2, 100089. [Google Scholar] [CrossRef]
- Guan, Q.Q.; Shao, X.B.; Yang, T.T.; Dou, G.M.; Xu, T.C.; Gan, Z.B. A study on the preparation process of polydextrose. Agric. Tech. 2018, 38, 247–248. [Google Scholar]
- Guanyan Research Report. The Forecast of the Market Competition Pattern and Development Trend of China Polyglucose Industry on the Analysis Report of 2021 Year. Available online: https://market.chinabaogao.com/shipin/101455602R021.html (accessed on 14 October 2021).
- Bailey, R.L.; Gahche, J.J.; Lentino, C.V.; Dwyer, J.T.; Engel, J.S.; Thomas, P.R.; Betz, J.M.; Sempos, C.T.; Picciano, M.F. Dietary supplement use in the United States, 2003–2006. J. Nutr. 2011, 141, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Afoakwa, E.O.; Paterson, A.; Fowler, M. Factors influencing rheological and textural qualities in chocolate—A review. Trends Food Sci. Technol. 2007, 18, 290–298. [Google Scholar] [CrossRef]
- O’Brien-Nabor, L. Alternative Sweetneners, 4th ed.; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar] [CrossRef]
- Aviara, N.A. Moisture Sorption Phenomena in Biological Materials: Isotherm Model Fitting and Hysteresis Assessment Techniques, 1st ed.; Chapter 23, Festschrift in honour of Engr. Prof. Patrick Obi Ngoddy at 80 Promethaean Commentries with Seminal Paper Contributions; Iwe, M.O., Nkama, I., Eds.; University of Nigeria Nsukka Press Ltd.: Nsukka, Nigeria, 2021; pp. 382–402. [Google Scholar]
- Fan, K.; Chen, L.B.; Wei, X.H.; He, J.; Yan, P.W. Moisture adsorption isotherms and thermodynamic properties of Auricularia auricular. J. Food Process. Preserv. 2015, 39, 1534–1541. [Google Scholar] [CrossRef]
- Li, X.J.; Han, X.; Tao, L.S.; Jiang, P.; Qin, W. Sorption equilibrium moisture and isosteric heat of Chinese wheat bran products added to rice to increase its dietary fibre content. Grain Oil Sci. Technol. 2021, 4, 149–161. [Google Scholar] [CrossRef]
- Li, X. The Theory and Practice of Grain Moisture Content; China Light Industry Press: Beijing, China, 2022; pp. 1–100. [Google Scholar]
- Wang, S.J.; Li, C.L.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Chang, Q.; Zheng, B.D.; Zhang, Y.; Zeng, H.L. A comprehensive review of the factors influencing the formation of retrograded starch. Int. J. Biol. Macromol. 2021, 186, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Margaret, K. Polydextrose as Anti-Staling Agent. PCT/US98/02113, 5 February 1998. [Google Scholar]
- Lewicki, P.P. Water as the determinant of food engineering properties—A review. J. Food Eng. 2004, 61, 483–495. [Google Scholar] [CrossRef]
- Li, Q.E.; Schmidt, S.J. Use of ramping and equilibrium water-vapor sorption methods to determine the critical relative humidity at which the glassy to rubbery transition occurs in polydextrose. J. Food Sci. 2011, 76, E149–E153. [Google Scholar] [CrossRef] [PubMed]
- Vollenbroek, J.; Hebbink, G.A.; Ziffels, S.; Steckel, H. Determination of low levels of amorphous content in inhalation grade lactose by moisture sorption isotherms. Int. J. Pharm. 2010, 395, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Chen, J.; Luo, S.J.; Liu, C.M.; Liu, W. Effect of food additives on starch retrogradation-a review. Starch-Stärke 2014, 66, 1–10. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC; Association of Analytical Communities: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Synergy Software. Kaleidagraph for Windows Version 4.54 Software; Synergy: Reading, PA, USA, USA, 2021. [Google Scholar]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Water sorption isotherms of pea starch edible films and prediction models. Foods 2016, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Furmaniak, S.; Terzyk, P.; Gauden, P.A. The general mechanism of water sorption on foodstuffs-Important of the multitemperature fitting of data and the hierarchy of models. J. Food Eng. 2007, 82, 528–535. [Google Scholar] [CrossRef]
- Boquet, R.; Chirife, J.; Iglesias, H.A. Equations for fitting water sorption isotherms of foods, part III: Evaluation of various three-parameter models. J. Food Technol. 1979, 14, 527–534. [Google Scholar] [CrossRef]
- Iglesias, H.A.; Chirife, J. An alternative to the Guggenheim Anderson and De boer model for mathematical description of moisture sorption isotherms of foods. Food Res. Int. 1995, 28, 317–321. [Google Scholar] [CrossRef]
- Cao, L.F.; Li, B.W.; Zhao, N.; Li, H.; Wang, Y.F.; Yu, X.; Huang, X. Moisture migration analysis of Chinese naked oat during different storage conditions by sorption isotherm model and low-field NMR. Food Sci. Nutr. 2020, 8, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Peleg, M. Assessment of a semi empirical four parameter general model for sigmoid moisture sorption isotherms. J. Food Process Eng. 1993, 16, 21–37. [Google Scholar] [CrossRef]
- SPSS Inc. SPSS for Windows, Release 18.0.1; SPSS Inc.: Chicago, IL, USA, 2017. [Google Scholar]
- Han, X.; Wang, X.; Li, X.; Jiang, P. Sorption equilibrium moisture and isosteric heat of Chinese wheat flours. Qual. Assur. Saf. Crops Foods 2017, 9, 435–449. [Google Scholar] [CrossRef]
- Tao, L.S.; Qin, W.; Wei, Z.; Li, X.J.; Zhang, H.Q. Effects of small-scale storage on the cooking property and fatty acid profile of sea rice paddy. Appl. Food Res. 2022, 2, 100175. [Google Scholar] [CrossRef]
| Equation | Sample | Equation Parameters | Statistical Parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | RSS | SE | R2 | MRE (%) | ||
| Ferro | p1 | 1.244 | 2.841 | 0.552 | 83.1454 | 2.5196 | 0.9901 | 11.3825 | |
| -Fontan | p2 | 1.119 | 4.403 | 0.758 | 92.1269 | 2.7917 | 0.9887 | 9.2898 | |
| p3 | 1.099 | 4.885 | 0.814 | 61.4220 | 1.8613 | 0.9917 | 8.8459 | ||
| p4 | 1.109 | 5.908 | 0.853 | 76.7037 | 2.3244 | 0.9894 | 8.3734 | ||
| p5 | 1.108 | 5.518 | 0.835 | 97.5545 | 2.9565 | 0.9867 | 9.8290 | ||
| GDW | p1 | 0.672 | −6.376 × 1016 | 0.934 | −9.809 | 87.7472 | 2.7421 | 0.9895 | 12.4027 |
| p2 | 2.241 | 3.603 × 1016 | 0.941 | −9.311 | 94.4429 | 2.9513 | 0.9885 | 8.9501 | |
| p3 | 2.633 | 3.076 × 1016 | 0.942 | −8.789 | 62.4652 | 1.9521 | 0.9916 | 8.3691 | |
| p4 | 3.185 | −4.221 × 1016 | 0.926 | −9.623 | 75.7441 | 2.3670 | 0.9896 | 7.7474 | |
| p5 | 3.097 | 5.885 × 1016 | 0.933 | −9.287 | 96.6885 | 3.0215 | 0.9869 | 8.6202 | |
| Boquet | p1 | 1.384 × 10−1 | −1.927 × 10−1 | −4.296 × 10−2 | 85.1817 | 2.5813 | 0.9898 | 11.3607 | |
| p2 | 6.955 × 10−2 | −9.27 × 10−3 | 5.449 × 10−2 | 99.8686 | 3.0263 | 0.9878 | 10.7312 | ||
| p3 | 5.971 × 10−2 | 2.001 × 10−2 | 7.405 × 10−2 | 71.0006 | 2.1515 | 0.9904 | 10.5478 | ||
| p4 | 4.544 × 10−2 | −9.275 × 10−3 | 5.449 × 10−2 | 91.1424 | 2.7619 | 0.9874 | 11.1783 | ||
| p5 | 4.983 × 10−2 | 3.389 × 10−2 | 7.733 × 10−2 | 116.6156 | 3.5338 | 0.9842 | 13.0292 | ||
| Lewicki | p1 | 38.691 | 0.399 | −0.252 | 91.4794 | 2.7721 | 0.9891 | 11.6048 | |
| p2 | 15.802 | 0.626 | 0.195 | 105.9026 | 3.2092 | 0.9871 | 11.1774 | ||
| p3 | 12.847 | 0.672 | 0.457 | 77.4541 | 2.3471 | 0.9896 | 11.5859 | ||
| p4 | 14.923 | 0.613 | 0.556 | 100.4813 | 3.0449 | 0.9861 | 12.3879 | ||
| p5 | 15.121 | 0.615 | 0.446 | 126.6168 | 3.8368 | 0.9828 | 14.0529 | ||
| Iglesias | p1 | 5.828 | 2.111 | 233.8069 | 6.8767 | 0.9719 | 39.7798 | ||
| p2 | 5.775 | 3.442 | 214.713 | 6.3151 | 0.9737 | 23.6655 | |||
| p3 | 5.502 | 3.686 | 167.7633 | 4.9321 | 0.9774 | 19.8521 | |||
| p4 | 5.424 | 4.706 | 226.4611 | 6.6606 | 0.9688 | 19.2150 | |||
| p5 | 5.464 | 4.432 | 230.5623 | 6.7812 | 0.9687 | 17.1287 | |||
| MGAB | p1 | 11.146 | 0.924 | 19.784 | 56.4852 | 1.7117 | 0.9932 | 15.3594 | |
| p2 | 8.848 | 0.945 | 39.663 | 79.2495 | 2.4015 | 0.9903 | 15.0906 | ||
| p3 | 7.585 | 0.957 | 59.231 | 62.9130 | 1.9064 | 0.9915 | 13.1556 | ||
| p4 | 8.481 | 0.938 | 61.969 | 73.5209 | 2.2279 | 0.9899 | 13.4585 | ||
| p5 | 9.211 | 0.931 | 44.778 | 88.1195 | 2.6703 | 0.9881 | 16.5624 | ||
| Peleg | p1 | 20.311 | 72.472 | 1.416 | 7.227 | 76.1193 | 2.3787 | 0.9909 | 12.7732 |
| p2 | 18.333 | 77.065 | 1.027 | 7.213 | 82.8187 | 2.5881 | 0.9899 | 11.5972 | |
| p3 | 17.251 | 74.145 | 0.947 | 7.166 | 56.1971 | 1.7562 | 0.9924 | 11.2992 | |
| p4 | 15.861 | 68.392 | 0.7668 | 6.221 | 76.9654 | 2.4052 | 0.9894 | 10.7701 | |
| p5 | 13.249 | 70.388 | 0.6474 | 5.875 | 92.1769 | 2.8805 | 0.9875 | 10.6562 | |
| Equation | Sample | Equation | Parameters | Statistical | Parameters | ||||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | d | RSS | SE | R2 | MRE (%) | ||
| Ferro- | p1 | 0.939 | 729.174 | 2.474 | 271.7619 | 6.2349 | 0.9454 | 11.7105 | |
| Fontan | p2 | 0.936 | 1185.896 | 2.599 | 269.6140 | 8.1701 | 0.9466 | 11.2267 | |
| p3 | 0.938 | 1130.646 | 2.601 | 264.5025 | 8.0152 | 0.9432 | 11.8144 | ||
| p4 | 0.935 | 1500.841 | 2.699 | 290.6119 | 8.8064 | 0.9365 | 12.3165 | ||
| p5 | 0.936 | 917.159 | 2.559 | 271.8086 | 8.2366 | 0.9428 | 11.8704 | ||
| GDW | p1 | 12.538 | −6.452 × 1017 | 0.985 | −5.126 | 184.7613 | 5.7738 | 0.9628 | 9.1302 |
| p2 | 13.377 | −7.379 × 1017 | 0.995 | −5.161 | 178.9945 | 5.5936 | 0.9645 | 8.7242 | |
| p3 | 13.095 | −4.461 × 1017 | 0.981 | −5.111 | 178.6485 | 5.5827 | 0.9617 | 9.4301 | |
| p4 | 13.346 | −1.765 × 1018 | 0.986 | −4.849 | 205.6944 | 6.4279 | 0.9551 | 10.2768 | |
| p5 | 12.661 | 3.323 × 1018 | 0.987 | −4.914 | 188.0357 | 5.8761 | 0.9604 | 9.6024 | |
| Boquet | p1 | −5.186 × 10-3 | 0.135 | 0.122 | 187.7547 | 5.6895 | 0.9623 | 9.6116 | |
| p2 | −4.957 × 10-3 | 0.128 | 0.115 | 189.0566 | 5.7289 | 0.9625 | 9.3613 | ||
| p3 | −4.957 × 10-3 | 0.129 | 0.116 | 185.2875 | 5.6148 | 0.9602 | 9.8677 | ||
| p4 | −5.073 × 10-3 | 0.129 | 0.116 | 222.9486 | 6.7561 | 0.9513 | 11.1029 | ||
| p5 | −5.287 × 10-3 | 0.136 | 0.122 | 196.6704 | 5.9597 | 0.9586 | 10.2154 | ||
| Lewicki | p1 | 10.293 | 0.694 | 866.000 | 214.2943 | 6.4938 | 0.9569 | 10.4041 | |
| p2 | 11.002 | 0.674 | 866.000 | 213.6649 | 6.4747 | 0.9576 | 10.0556 | ||
| p3 | 10.844 | 0.664 | 866.000 | 208.2569 | 6.3108 | 0.9553 | 10.4865 | ||
| p4 | 10.925 | 0.658 | 866.000 | 241.9401 | 7.3315 | 0.9471 | 11.5011 | ||
| p5 | 10.323 | 0.685 | 866.000 | 220.6965 | 6.6877 | 0.9536 | 10.8351 | ||
| Iglesias | p1 | 4.474 | 11.995 | 191.4131 | 5.6298 | 0.9615 | 8.8098 | ||
| p2 | 4.509 | 12.834 | 185.6057 | 5.4589 | 0.9632 | 8.4282 | |||
| p3 | 4.319 | 12.644 | 188.1148 | 5.5328 | 0.9596 | 9.1332 | |||
| p4 | 4.272 | 12.752 | 210.9327 | 6.2039 | 0.9539 | 9.9994 | |||
| p5 | 4.368 | 12.046 | 192.7602 | 5.6694 | 0.9594 | 9.3108 | |||
| MGAB | p1 | 8.841 | 0.915 | 9.553 × 109 | 290.0764 | 8.7902 | 0.9417 | 12.8749 | |
| p2 | 9.407 | 0.906 | 1.067 × 1010 | 306.6459 | 9.2923 | 0.9392 | 12.7336 | ||
| p3 | 9.269 | 0.901 | 8.621 × 109 | 293.5406 | 8.8952 | 0.9369 | 13.0781 | ||
| p4 | 9.311 | 0.899 | 1.146 × 1010 | 339.8126 | 10.2974 | 0.9257 | 13.7825 | ||
| p5 | 8.841 | 0.911 | 9.154 × 109 | 303.4783 | 9.1963 | 0.9362 | 13.1996 | ||
| Peleg | p1 | 14.803 | 69.557 | 4.795 × 10−2 | 6.175 | 142.7679 | 4.4615 | 0.9713 | 7.1544 |
| p2 | 15.649 | 70.007 | 4.615 × 10−2 | 6.162 | 137.3941 | 4.2936 | 0.9728 | 7.3192 | |
| p3 | 15.185 | 66.077 | 4.065 × 10−2 | 6.013 | 136.8964 | 4.2781 | 0.9706 | 8.1769 | |
| p4 | 15.506 | 66.721 | 4.609 × 10−2 | 6.226 | 169.0143 | 5.2817 | 0.9631 | 8.7802 | |
| p5 | 14.831 | 68.398 | 4.708 × 10−2 | 6.238 | 149.8277 | 4.6821 | 0.9685 | 8.4051 |
| Type | Sample | Equation | Parameters | Statistical | Parameters | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | R2 | MRE (%) | ||
| Ads | p1 | 6.485 | −0.958 | 2.071 × 10−2 | −1.180 × 10−4 | 84.302 | −229.706 | 228.285 | 0.9866 | 33.7978 |
| p2 | −10.394 | 1.192 | −6.541 × 10−2 | 9.960 × 10−4 | 92.916 | −249.788 | 240.707 | 0.9848 | 22.7372 | |
| p3 | −71.023 | 7.841 | −2.974 × 10−1 | 3.640 × 10−3 | 83.347 | −227.405 | 223.018 | 0.9858 | 19.7183 | |
| p4 | −9.024 | 1.397 | −7.000 × 10−2 | 1.010 × 10−3 | 66.456 | −183.069 | 191.313 | 0.9867 | 14.8199 | |
| p5 | 27.979 | −2.822 | 8.911 × 10−2 | −9.540 × 10−4 | 65.568 | −186.908 | 196.687 | 0.9862 | 14.9129 | |
| Aver-ads | −13.251 | 1.539 | −7.190 × 10−2 | 9.990 × 10−4 | 79.082 | −217.042 | 217.289 | 0.9865 | 19.0038 | |
| Des | p1 | 99.812 | −9.624 | 3.376 × 10−1 | −3.990 × 10−3 | 69.7483 | −221.873 | 218.486 | 0.9907 | 4.5319 |
| p2 | 65.482 | −5.537 | 1.832 × 10−1 | −2.090 × 10−3 | 70.029 | −222.948 | 219.752 | 0.9918 | 4.2282 | |
| p3 | 62.348 | −5.044 | 1.608 × 10−1 | −1.780 × 10−3 | 64.764 | −207.252 | 206.103 | 0.9941 | 3.8077 | |
| p4 | 104.235 | −9.905 | 3.454 × 10−1 | −4.080 × 10−3 | 67.713 | −214.584 | 210.458 | 0.9927 | 3.9853 | |
| p5 | 59.269 | −5.022 | 1.688 × 10−1 | −1.980 × 10−3 | 69.376 | −220.301 | 215.993 | 0.9907 | 4.4116 | |
| Aver-des | 77.473 | −6.946 | 2.360 × 10−1 | −2.750 × 10−3 | 68.274 | −217.403 | 214.324 | 0.9925 | 4.1292 | |
| Mean | 32.109 | −2.703 | 8.220 × 10−2 | −8.760 × 10−4 | 73.678 | −217.223 | 215.807 | 0.9897 | 7.7274 |
| Types | Equation | Statistical | Parameters | Order | ||
|---|---|---|---|---|---|---|
| RSS | SE | R2 | MRE (%) | |||
| Adsorption | Ferro-Fontan | 82.1905 ± 14.1148 | 2.4907 ± 0.4278 | 0.9893 ± 0.0018 | 9.5441 ± 1.1601 | 2 |
| GDW | 83.4176 ± 14.2680 | 2.6068 ± 0.4458 | 0.9892 ± 0.0017 | 9.2179 ± 1.8340 | 1 | |
| Boquet | 92.7618 ± 16.9791 | 2.8110 ± 0.5145 | 0.9879 ± 0.0024 | 11.3694 ± 0.9841 | 5 | |
| Lewicki | 100.3868 ± 18.1913 | 3.0420 ± 0.5512 | 0.9869 ± 0.0027 | 12.1618 ± 1.1444 | 6 | |
| Iglesias | 214.6613 ± 27.1940 | 6.3131 ± 0.8007 | 0.9721 ± 0.0036 | 23.9282 ± 9.1707 | 8 | |
| MGAB | 72.0576 ± 12.6291 | 2.1836 ± 0.3827 | 0.9906 ± 0.0019 | 14.7253 ± 1.4124 | 4 | |
| Peleg | 76.8555 ± 13.2063 | 2.4017 ± 0.4127 | 0.9900 ± 0.0018 | 11.4192 ± 0.8490 | 3 | |
| Polynomial | 107.8395 ± 10.6417 | 3.7186 ± 0.3669 | 0.9860 ± 0.0008 | 21.1972 ± 7.8022 | 7 | |
| Desorption | Ferro-Fontan | 273.6598 ± 9.9326 | 7.8926 ± 0.9738 | 0.9429 ± 0.0039 | 11.7877 ± 0.3898 | 7 |
| GDW | 187.2269 ± 11.0583 | 5.8508 ± 0.3456 | 0.9609 ± 0.0036 | 9.4327 ± 0.5777 | 4 | |
| Boquet | 196.3436 ± 15.4681 | 5.9498 ± 0.4688 | 0.9589 ± 0.0046 | 10.0318 ± 0.6771 | 5 | |
| Lewicki | 219.7705 ± 13.1546 | 6.6597 ± 0.3986 | 0.9541 ± 0.0042 | 10.6565 ± 0.5475 | 6 | |
| Iglesias | 193.7653 ± 9.9971 | 5.6989 ± 0.2940 | 0.9595 ± 0.0035 | 9.1363 ± 0.5881 | 3 | |
| MGAB | 306.7108 ± 19.7252 | 9.2943 ± 0.5977 | 0.9359 ± 0.0061 | 13.1337 ± 0.4048 | 8 | |
| Peleg | 147.1801 ± 13.2734 | 4.5994 ± 0.4148 | 0.9693 ± 0.0038 | 7.9672 ± 0.7031 | 2 | |
| Polynomial | 38.5874 ± 8.0125 | 1.3306 ± 0.2763 | 0.9920 ± 0.0014 | 4.1929 ± 0.2981 | 1 |
| Equation | Types | Equation | Parameters | Statistical | Parameters | ||
|---|---|---|---|---|---|---|---|
| a | b | c | d | R2 | MRE (%) | ||
| Ferro-Fontan | Ads | 1.125 | 4.501 | 0.763 | 0.9905 | 8.6309 | |
| Des | 0.937 | 1052.959 | 2.584 | 0.9438 | 11.7343 | ||
| Mean | 0.982 | 38.377 | 1.544 | 0.9779 | 9.4624 | ||
| GDW | Ads | 1.376 | 1.090 × 1012 | 0.936 | 6.764 | 0.9902 | 8.3919 |
| Des | 11.997 | 9.200 × 1011 | 0.985 | 0.421 | 0.9618 | 9.3906 | |
| Mean | 6.749 | 8.550 × 107 | 0.961 | 1.036 | 0.9836 | 7.1238 | |
| Boquet | Ads | 6.820 × 10−2 | −6.640 × 10−3 | 5.520 × 10−2 | 0.9891 | 11.0751 | |
| Des | −5.100 × 10−3 | 0.132 | 0.118 | 0.9601 | 9.9857 | ||
| Mean | −1.300 × 10−3 | 0.151 | 0.144 | 0.9838 | 6.6885 | ||
| Peleg | Ads | 16.837 | 73.032 | 0.944 | 6.766 | 0.9911 | 10.9682 |
| Des | 15.166 | 68.252 | 4.423 × 10−2 | 6.148 | 0.9704 | 7.9234 | |
| Mean | 13.241 | 71.532 | 0.2304 | 6.026 | 0.9861 | 7.0115 |
| Sample | ΔH (J/g) | To (°C) | Tp (°C) | Tc (°C) | Peak Width (°C) | Peak Height (0.01 mW/mg) |
|---|---|---|---|---|---|---|
| CK | 9.63 ± 0.03 a | 64.00 ± 0.10 a | 70.63 ± 0.31 ab | 78.50 ± 0.44 a | 7.70 ± 0.20 a | 16.37 ± 0.53 a |
| 3%PD | 9.26 ± 0.29 b | 64.00 ± 0.20 a | 70.47 ± 0.15 b | 77.30 ± 0.17 b | 7.57 ± 0.12 a | 16.2 ± 0.43 a |
| 5%PD | 8.91 ± 0.26 bc | 64.07 ± 0.23 a | 70.37 ± 0.15 b | 77.20 ± 0.46 b | 7.53 ± 0.21 a | 15.6 ± 0.95 a |
| 7%PD | 8.82 ± 0.09 c | 64.15 ± 0.21 a | 70.90 ± 0.14 a | 77.55 ± 0.35 b | 7.50 ± 0.28 ab | 15.3 ± 0.55 a |
| 10%PD | 8.81 ± 0.39 bc | 64.10 ± 0.20 a | 71.07 ± 0.32 a | 77.47 ± 0.35 b | 7.23 ± 0.06 b | 15.8 ± 0.44 a |
| No | Producing Plant | Region | Moisture Content (% Wet Basis) |
|---|---|---|---|
| p1 | Renliang Biotechnology Co., LTD. | Shanghai, China | 1.00 |
| p2 | Taili Jie Biotechnology Co., LTD. | Mengzhou, Henan province, China | 2.60 |
| p3 | Baoling Bao Biotechnology Co., LTD. | Yucheng, Shandong province, China | 3.31 |
| p4 | BailongChuangyuan Biotechnology Co., LTD. | Yucheng, Shandong province, China | 4.42 |
| p5 | XingguangShouchuang Biotechnology Co., LTD. | Dezhou, Shandong province, China | 4.66 |
| Equation Name | Formula | Reference |
|---|---|---|
| Ferro-Fontan | Saberi, et al. [21] | |
| GDW | Furmaniak, et al. [22] | |
| Boquet | Boquet, et al. [23] | |
| Lewicki | Saberi, et al. [21] | |
| Iglesias–Chirife | Iglesias and Chirife [24] | |
| MGAB | Cao, et al. [25] | |
| Peleg | Peleg [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, X.; Song, H.; Li, X. Moisture Sorption Isotherms of Polydextrose and Its Gelling Efficiency in Inhibiting the Retrogradation of Rice Starch. Gels 2024, 10, 529. https://doi.org/10.3390/gels10080529
Liu C, Li X, Song H, Li X. Moisture Sorption Isotherms of Polydextrose and Its Gelling Efficiency in Inhibiting the Retrogradation of Rice Starch. Gels. 2024; 10(8):529. https://doi.org/10.3390/gels10080529
Chicago/Turabian StyleLiu, Chang, Xiaoyu Li, Hongdong Song, and Xingjun Li. 2024. "Moisture Sorption Isotherms of Polydextrose and Its Gelling Efficiency in Inhibiting the Retrogradation of Rice Starch" Gels 10, no. 8: 529. https://doi.org/10.3390/gels10080529
APA StyleLiu, C., Li, X., Song, H., & Li, X. (2024). Moisture Sorption Isotherms of Polydextrose and Its Gelling Efficiency in Inhibiting the Retrogradation of Rice Starch. Gels, 10(8), 529. https://doi.org/10.3390/gels10080529







