The Method of Direct and Reverse Phase Portraits as a Tool for Systematizing the Results of Studies of Phase Transitions in Solutions of Thermosensitive Polymers
Abstract
1. Introduction
1.1. Poly(ethylene glycol)—PEG
1.2. PEO/PPO-Based Systems, Poly(ethylene oxide)—b-poly(propylene oxide)-b-poly(ethylene oxide) (PEO-PPO-PEO) Also Known as Poloxamer, Pluronic, Kolliphor, and Synperonic
1.3. Poly(vinylcaprolactam)—PVCL
2. Results and Discussion
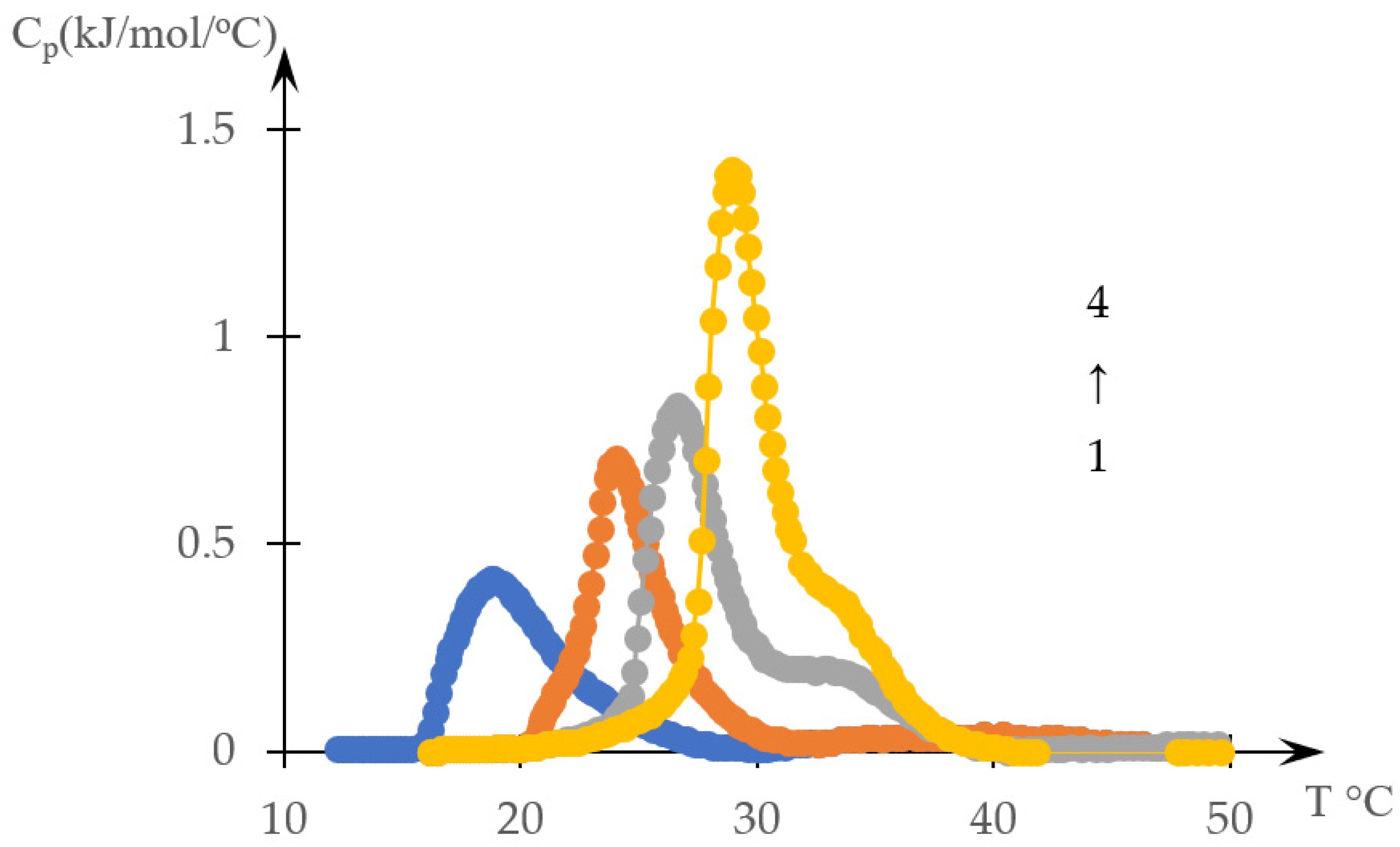
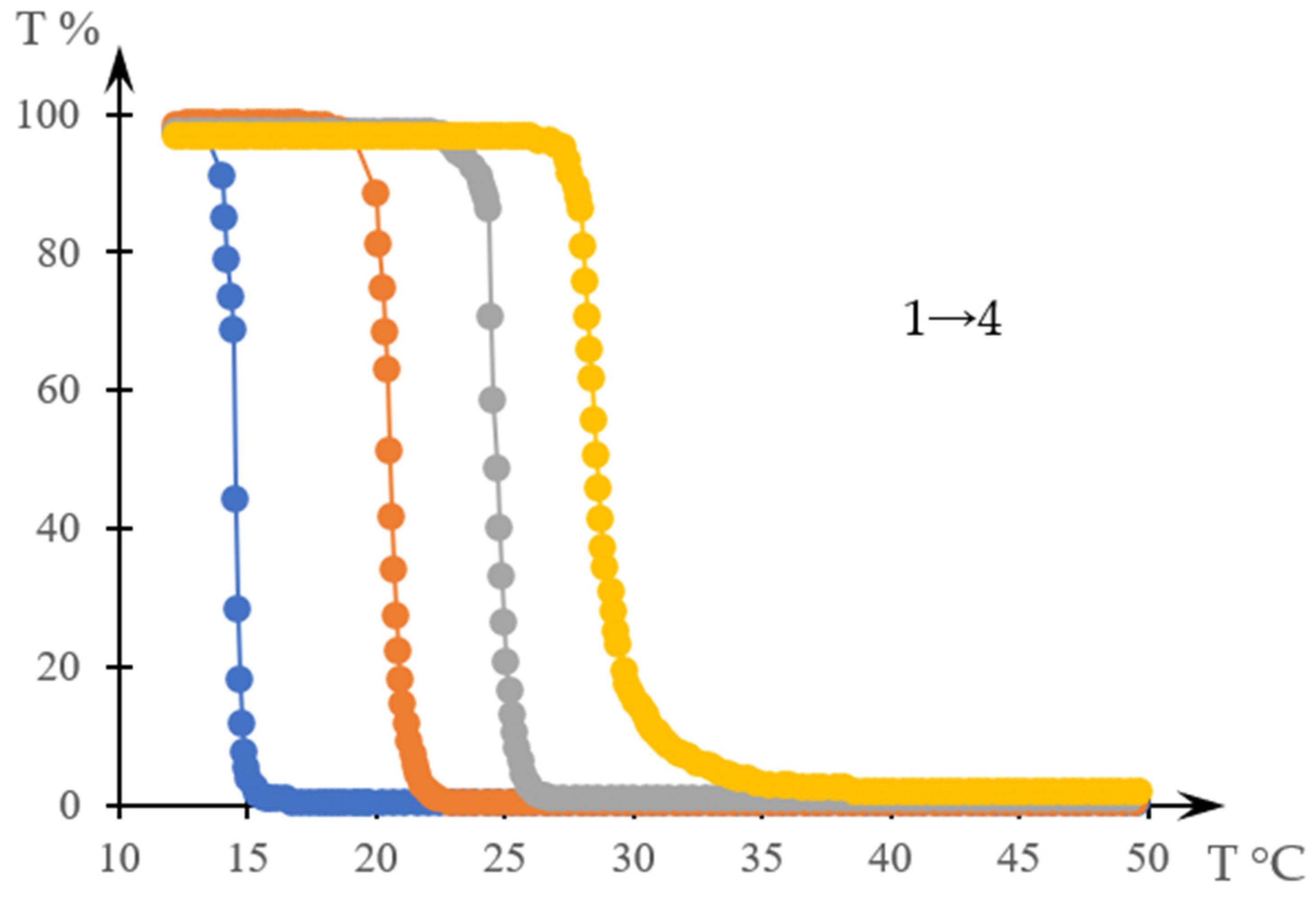
2.1. Results
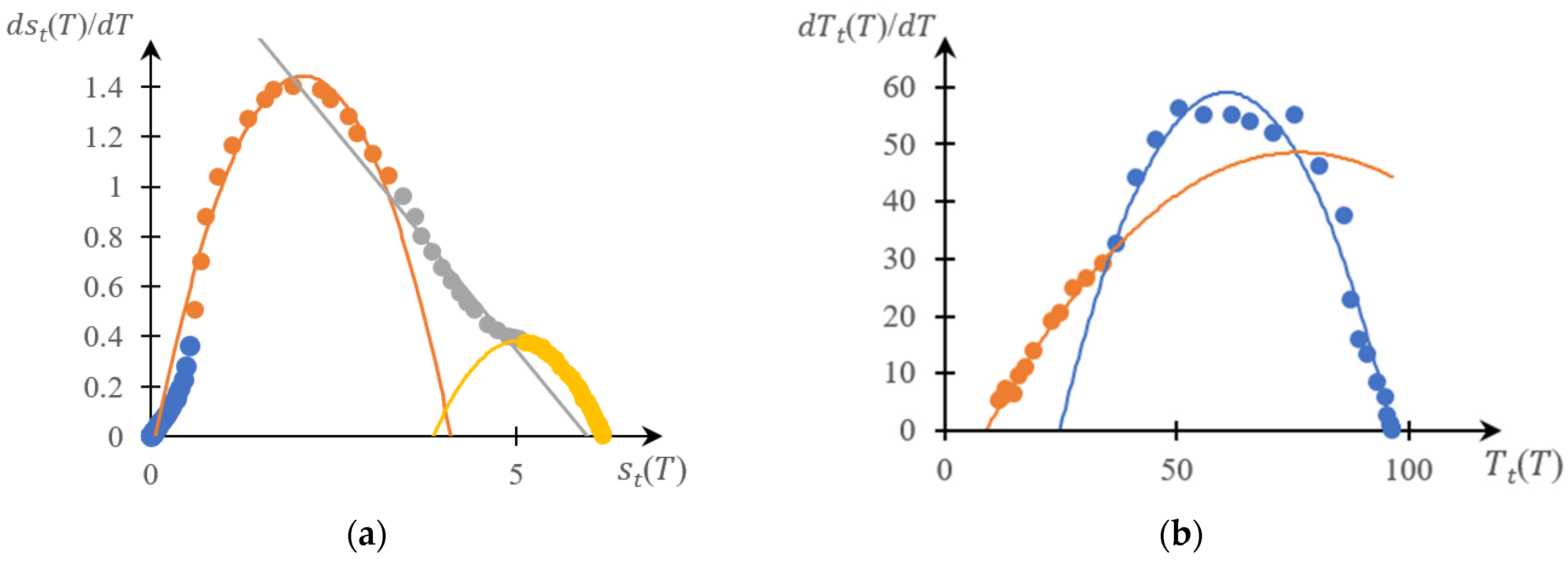
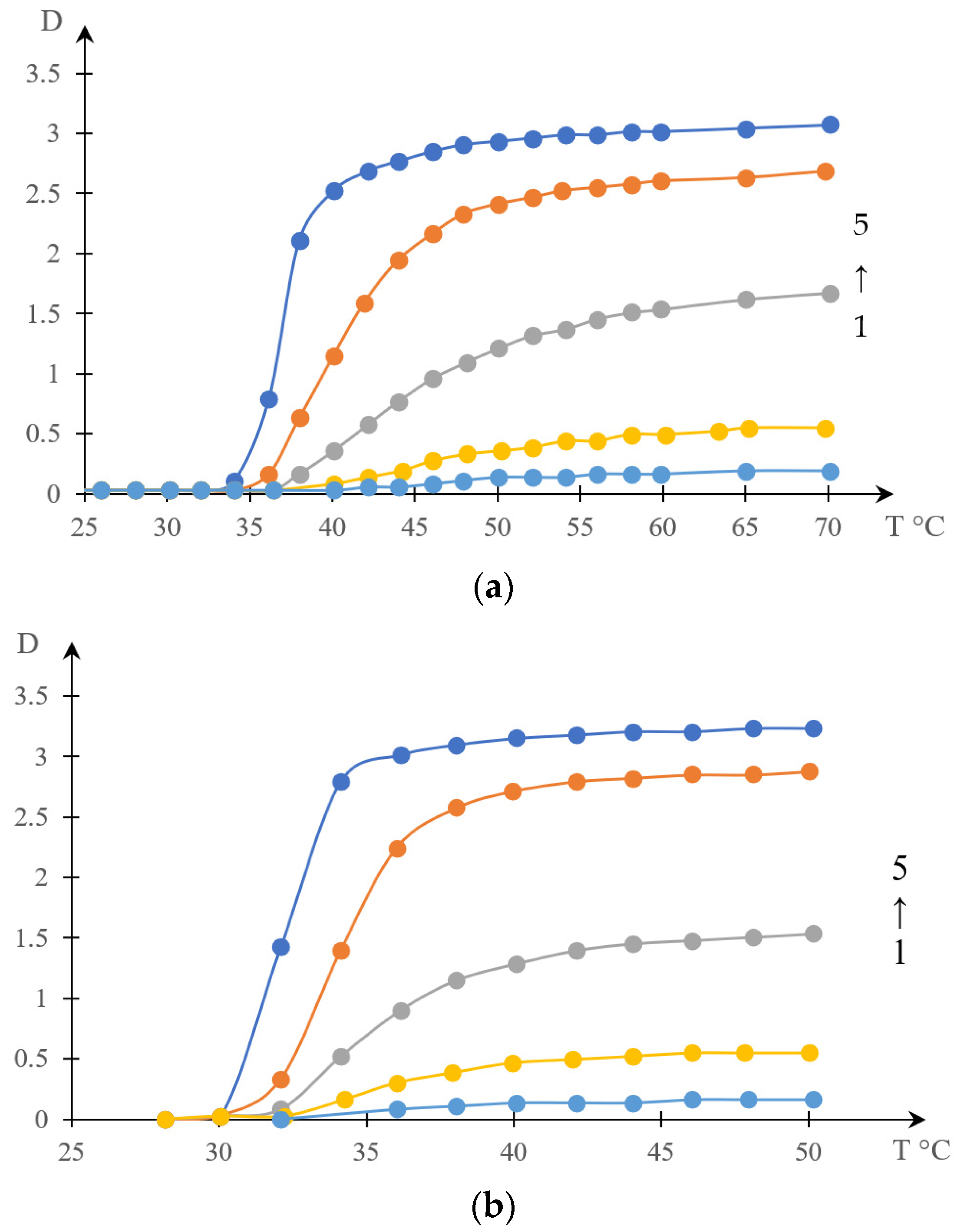
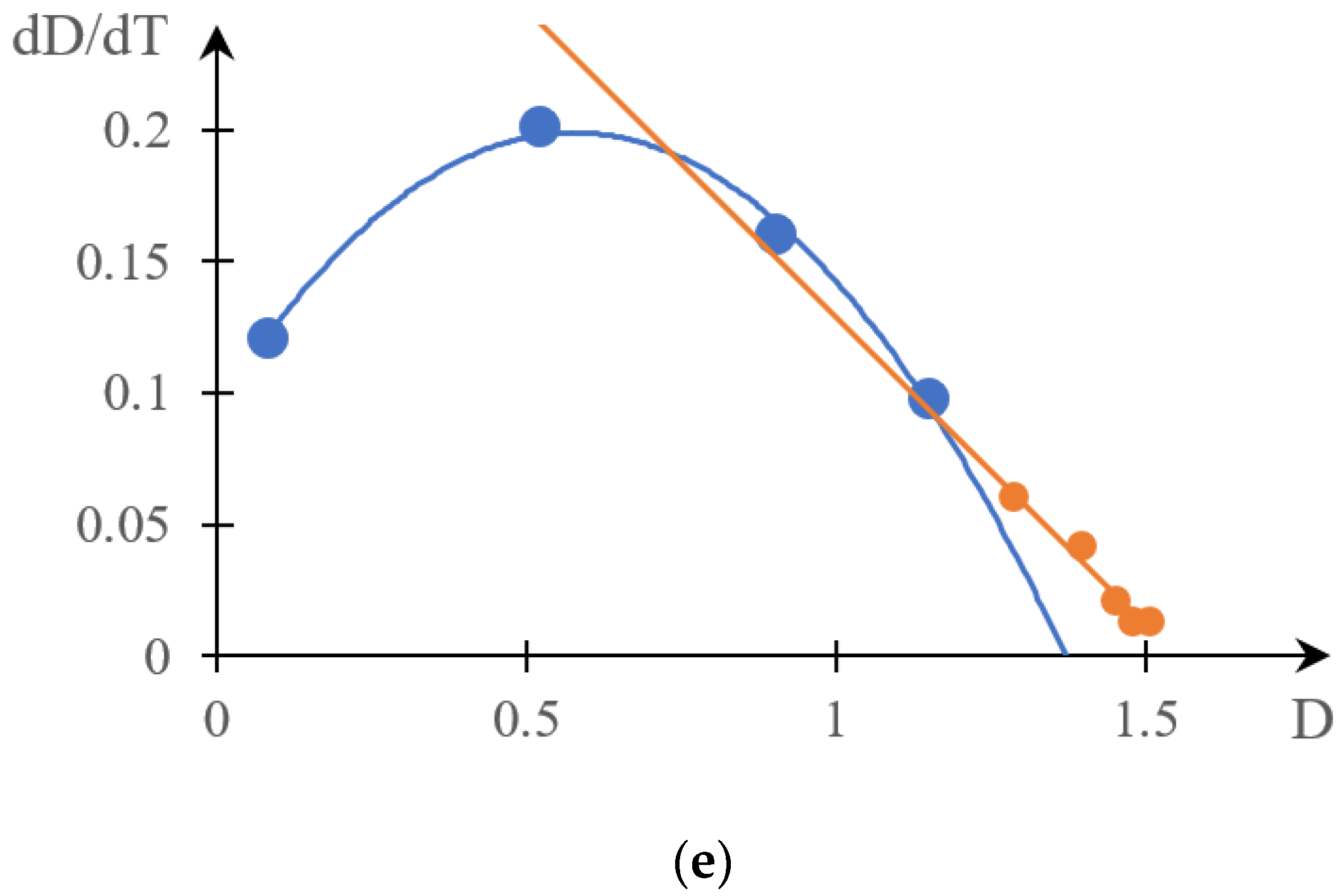
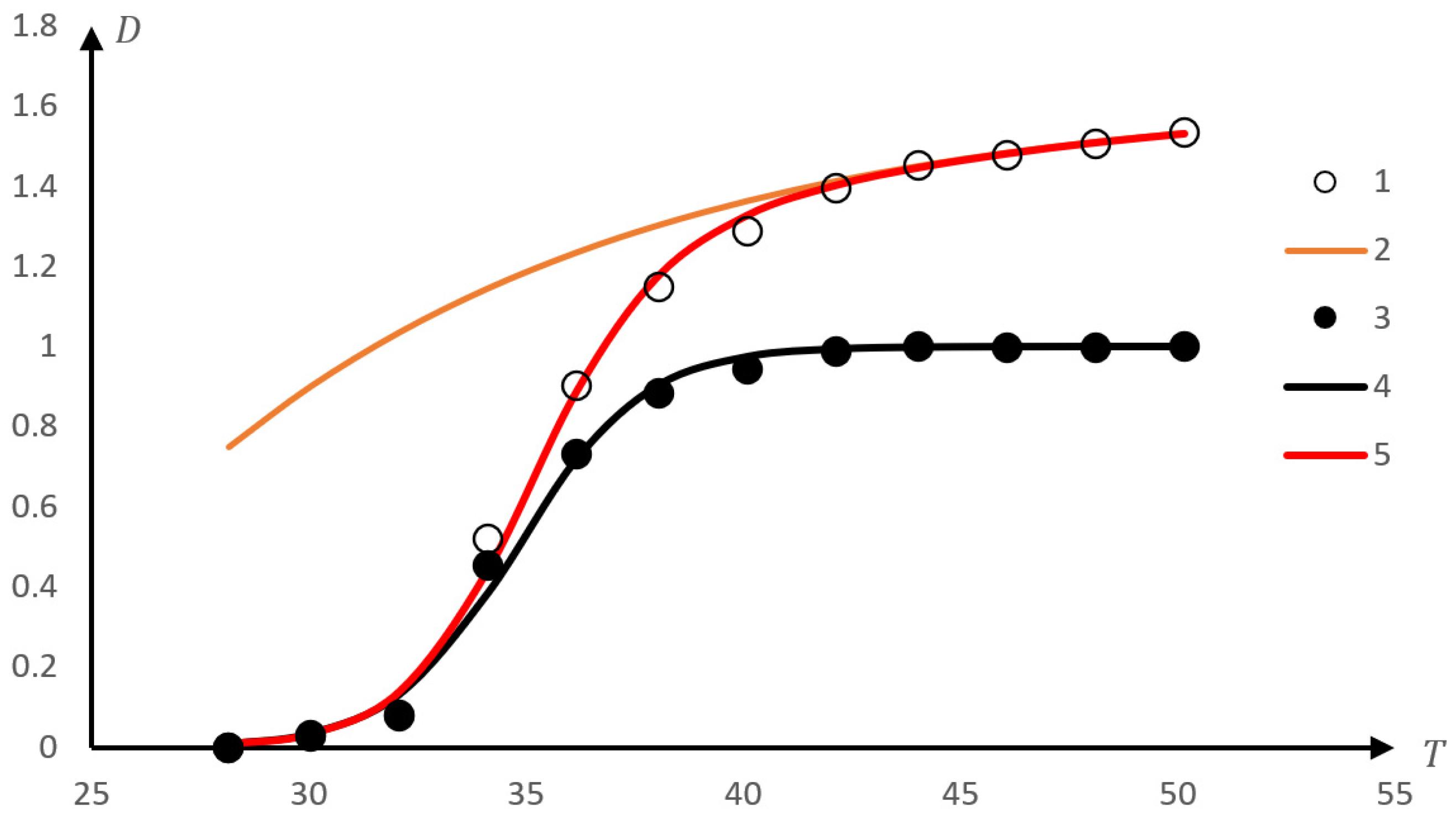
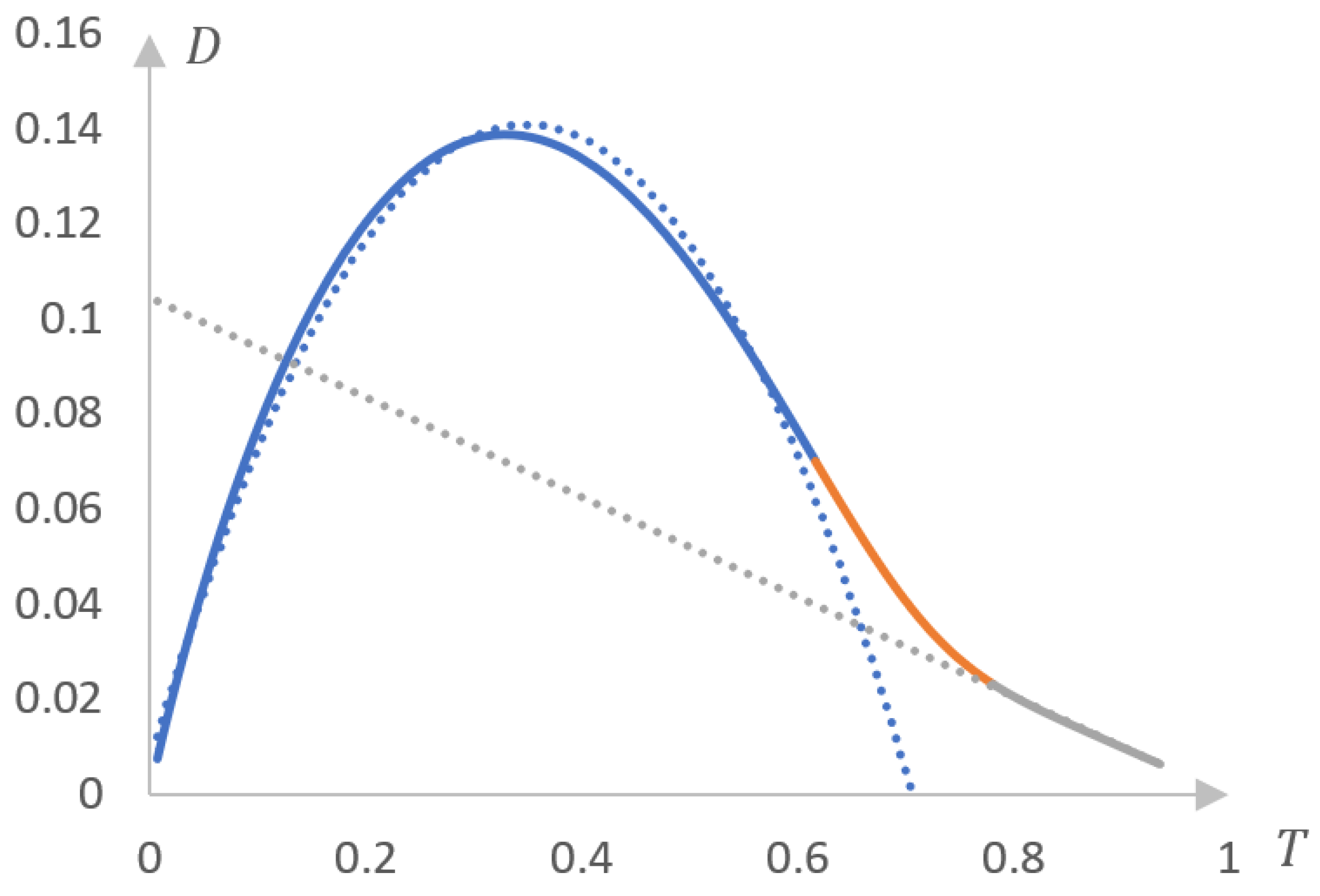
2.1.1. Rationale for Using the Inverse Phase Portrait Method to Study Phase Transitions in Solutions of Thermosensitive Polymers
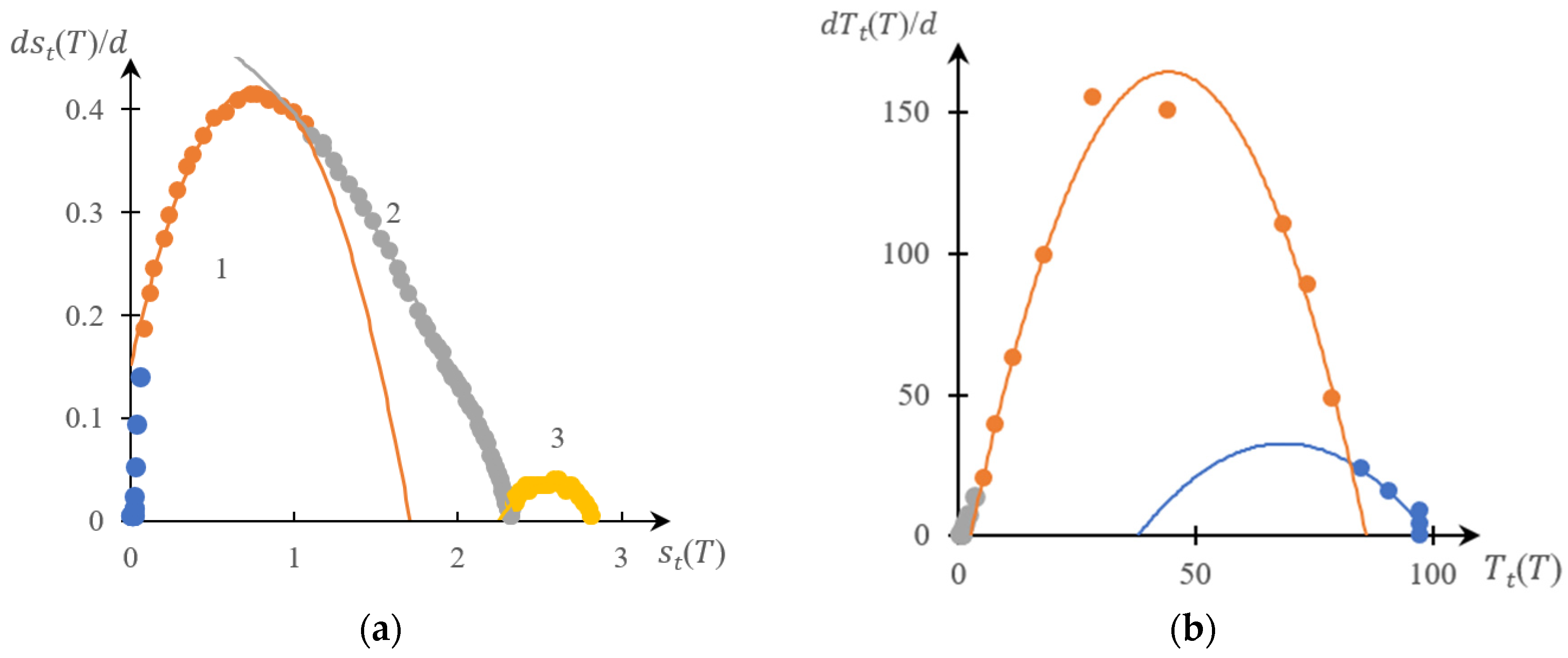
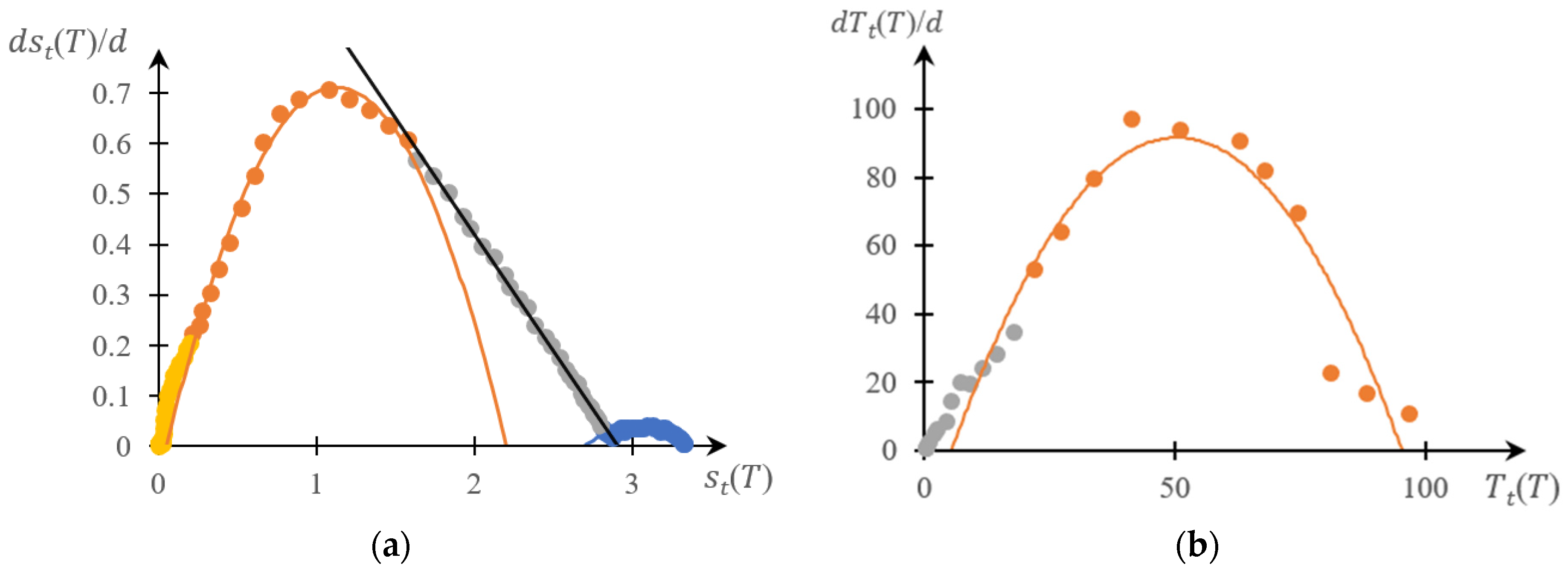
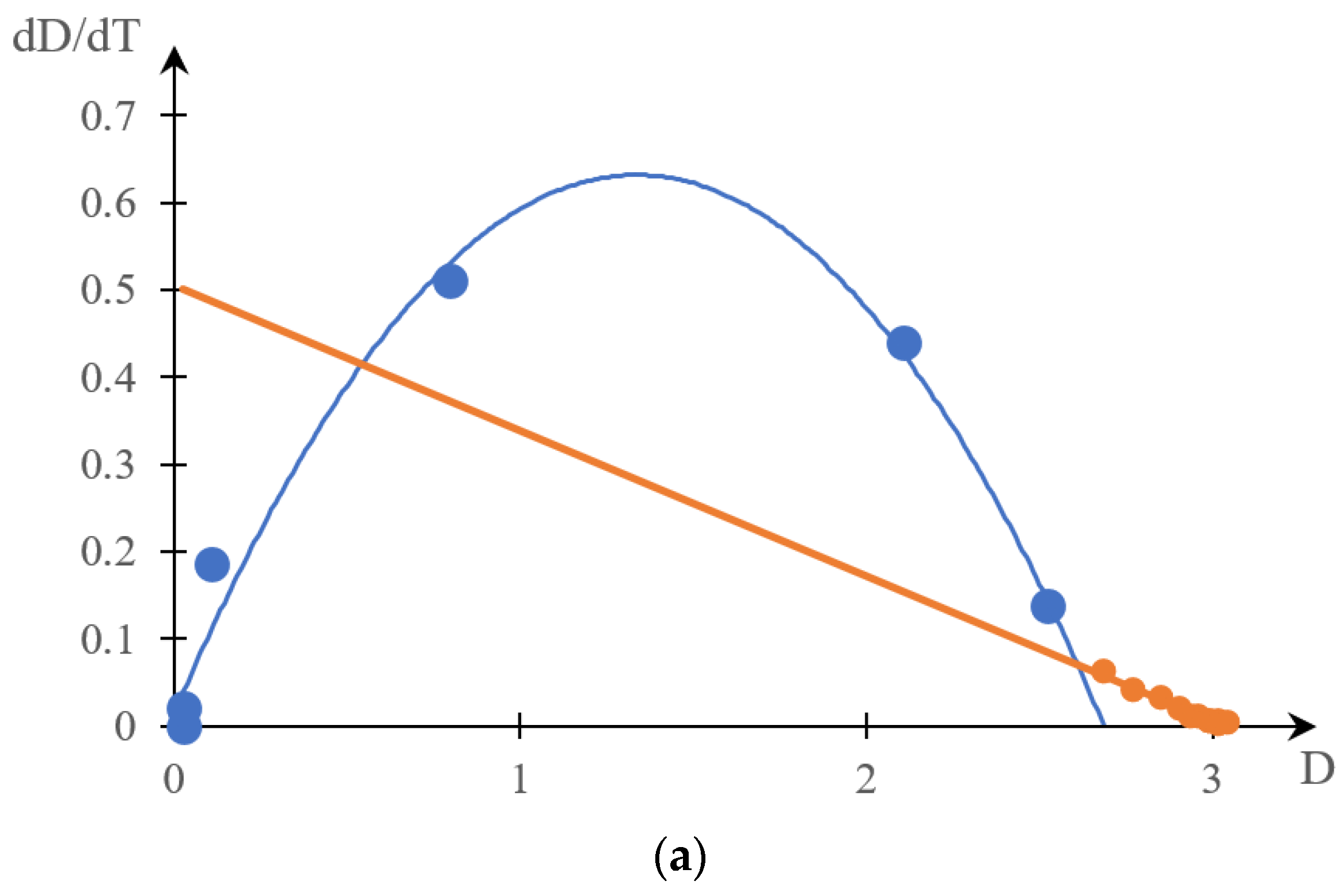
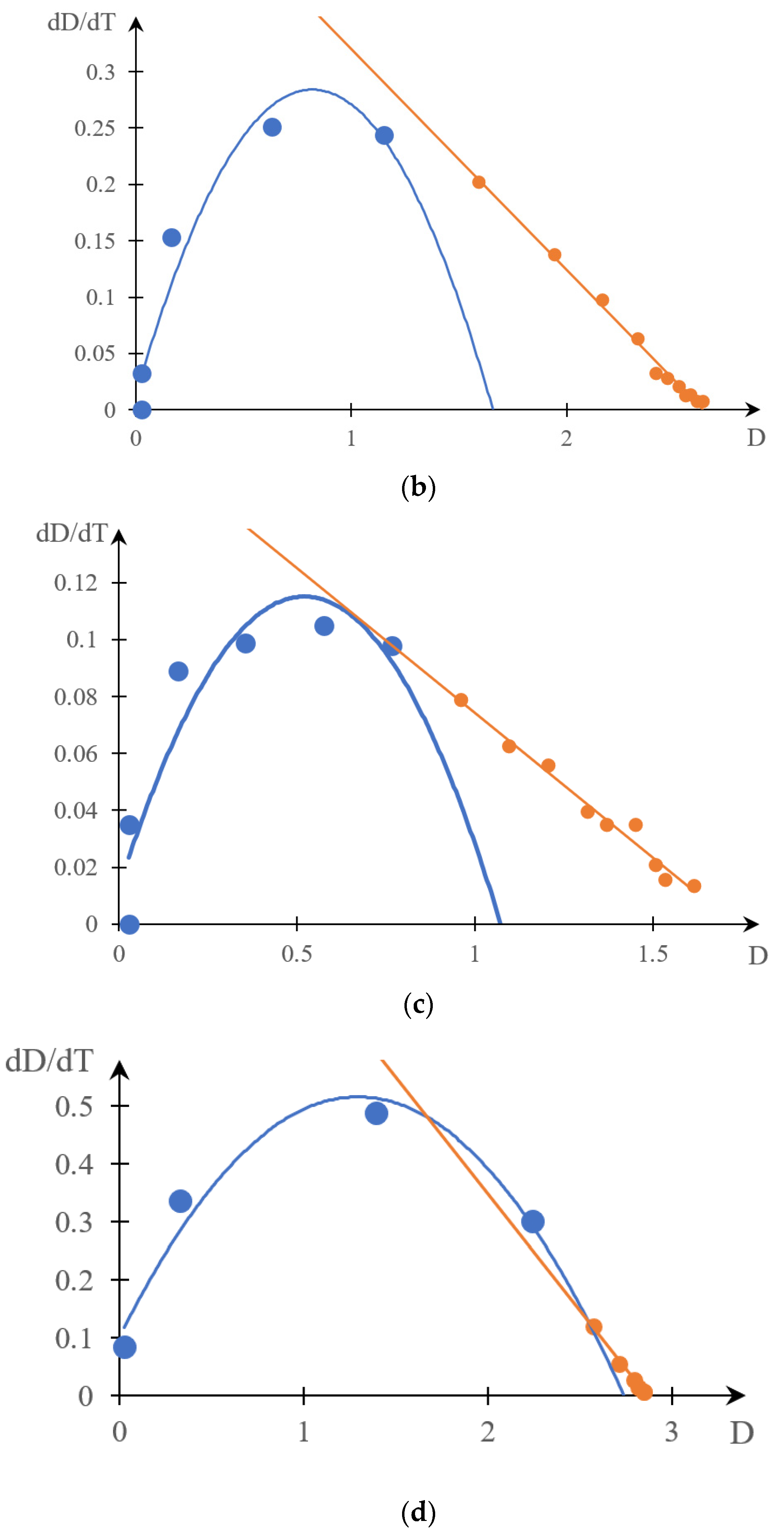
2.1.2. Comparison of Direct and Reverse Phase Portraits
2.2. Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.; Liu, L.; Zhang, S.; Liao, B.; Zhao, K.; Li, Y.; Xu, J.; Chen, L. Review of the Perspectives and Study of Thermo-Responsive Polymer Gels and Applications in Oil-Based Drilling Fluids. Gels 2023, 9, 969. [Google Scholar] [CrossRef]
- Gopinath, S.; Adarsh, N.N.; Radhakrishnan Nair, P.; Mathew, S. Recent Trends in thermo-responsive Elastomeric Shape Memory Polymer Nanocomposites. Polym. Compos. 2023, 44, 4433–4458. [Google Scholar] [CrossRef]
- Tian, S.; Geng, Y.; Huang, L.; Li, S.; Wang, Q.; Su, X. Fundamental and Perspectives of Thermo-Responsive Materials for Dehumidification and Water Harvesting. J. Clean. Prod. 2023, 412, 137446. [Google Scholar] [CrossRef]
- Buchholz, B.A.; Shi, W.; Barron, A.E. Microchannel DNA Sequencing Matrices with Switchable Viscosities. Electrophoresis 2002, 23, 1398. [Google Scholar] [CrossRef]
- Schmaljohann, D. Thermo- and PH-Responsive Polymers in Drug Delivery. Adv. Drug Deliv. Rev. 2006, 58, 1655–1670. [Google Scholar] [CrossRef] [PubMed]
- Ballauff, M.; Lu, Y. “Smart” Nanoparticles: Preparation, Characterization and Applications. Polymer 2007, 48, 1815–1823. [Google Scholar] [CrossRef]
- Rzaev, Z.M.O.; Dincer, S.; Piskin, E. Functional Copolymers of N-Isopropylacrylamide for Bioengineering Applications. Prog. Polym. Sci. 2007, 32, 534–595. [Google Scholar] [CrossRef]
- Bromberg, L. Temperature-Responsive Gels and Thermogelling Polymer Matrices for Protein and Peptide Delivery. Adv. Drug Deliv. Rev. 1998, 31, 197–221. [Google Scholar] [CrossRef]
- Zha, L.; Banik, B.; Alexis, F. Stimulus Responsive Nanogels for Drug Delivery. Soft Matter 2011, 7, 5908. [Google Scholar] [CrossRef]
- Suleimenov, I.E.; Mun, G.A.; Pak, I.T.; Kabdushev, S.B.; Kenessova, Z.A.; Kopishev, E.E. Redistribution of the Concentrations in Polyelectrolyte Hydrogels Contacts as the Basis of New Desalination Technologies. News Natl. Acad. Sci. Repub. Kazakhstan Ser. Geol. Tech. Sci. 2017, 423, 198–205. [Google Scholar]
- Budtova, T.; Suleimenov, I. Physical Principles of Using Polyelectrolyte Hydrogels for Purifying and Enrichment Technologies. J. Appl. Polym. Sci. 1995, 57, 1653–1658. [Google Scholar] [CrossRef]
- Suleimenov, I.; Egemberdieva, Z.; Bakirov, A.; Baipakbayeva, S.; Kopishev, E.; Mun, G. Efficiency Problem of Renewable Energetics Systems in the Context of «smart House» Concept. E3S Web Conf. 2020, 164, 13002. [Google Scholar] [CrossRef]
- Seidaliyeva, A.; Kabdushev, S.; Baipakbayeva, S.; Kopishev, E.; Suleimenov, I. The Diversification of Energy Sources in Kazakhstan as a Way to Dampen the Consequences of a Predicted Crisis. Polityka Energ. Energy Policy J. 2024, 27, 173–188. [Google Scholar] [CrossRef]
- Li, L.; Shan, H.; Yue, C.Y.; Lam, Y.C.; Tam, K.C.; Hu, X. Thermally Induced Association and Dissociation of Methylcellulose in Aqueous Solutions. Langmuir 2002, 18, 7291–7298. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Shao, Y.-H.; Lü, J. Preparation, Properties and Controlled Release Behaviors of PH-Induced Thermosensitive Amphiphilic Gels. Biomaterials 2006, 27, 4016–4024. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Hoffman, A.S.; Stayton, P.S. Poly(N-Isopropylacrylamide-co-Propylacrylic Acid) Copolymers That Respond Sharply to Temperature and PH. Biomacromolecules 2006, 7, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Pham, D.T.; Phewchan, P.; Navesit, K.; Chokamonsirikun, A.; Khemwong, T.; Tiyaboonchai, W. Development of Metronidazole-Loaded In Situ Thermosensitive Hydrogel for Periodontitis Treatment. Turk. J. Pharm. Sci. 2021, 18, 510–516. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, L.; Wang, Y.; Wang, Q.; Jing, D.; Liu, S. Nanomaterials Based on Thermosensitive Polymer in Biomedical Field. Front. Chem. 2022, 10, 946183. [Google Scholar] [CrossRef] [PubMed]
- Rohaľová, S.; Wolaschka, T.; Balážová, Ľ.; Paulovičová, K.; Tóthová, J.; Pavloková, S.; Stahorský, M.; Gajdziok, J. Formulation Optimization and Evaluation of Oromucosal in Situ Gel Loaded with Silver Nanoparticles Prepared by Green Biosynthesis. Eur. J. Pharm. Sci. 2024, 193, 106683. [Google Scholar] [CrossRef]
- Sun, L.; Lv, X.; Liu, N.; Qi, G.; Huang, Q. Spontaneous Coal Combustion Prevention Mechanisms of Thermosensitive Composite Hydrogel: An Experimental Study. Fuel 2023, 331, 125796. [Google Scholar] [CrossRef]
- Guo, S.; Yuan, S.; Geng, W.; Dong, Z. Preparation and Optimization of Thermosensitive Hydrogels for Inhibiting Coal Oxidation. Int. J. Energy Res. 2021, 45, 7783–7796. [Google Scholar] [CrossRef]
- Stabenfeldt, S.E.; García, A.J.; LaPlaca, M.C. Thermoreversible Laminin-functionalized Hydrogel for Neural Tissue Engineering. J. Biomed. Mater. Res. Part A 2006, 77A, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Tate, M. Biocompatibility of Methylcellulose-Based Constructs Designed for Intracerebral Gelation Following Experimental Traumatic Brain Injury. Biomaterials 2001, 22, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.P.; Budharaju, H.; Sethuraman, S.; Sundaramurthi, D. Carboxymethyl Cellulose-Agarose-Gelatin: A Thermoresponsive Triad Bioink Composition to Fabricate Volumetric Soft Tissue Constructs. SLAS Technol. 2023, 28, 183–198. [Google Scholar] [CrossRef]
- Li, X.; Chen, A.; Liu, Y.; Li, L. Preparation and Rectal Administration of Hydroxybutyl Chitosan/Graphene Oxide Composite Thermosensitive Hydrogel. React. Funct. Polym. 2023, 189, 105608. [Google Scholar] [CrossRef]
- Tohidi, H.; Maleki-Jirsaraei, N.; Simchi, A.; Mohandes, F.; Emami, Z.; Fassina, L.; Naro, F.; Conti, B.; Barbagallo, F. An Electroconductive, Thermosensitive, and Injectable Chitosan/Pluronic/Gold-Decorated Cellulose Nanofiber Hydrogel as an Efficient Carrier for Regeneration of Cardiac Tissue. Materials 2022, 15, 5122. [Google Scholar] [CrossRef]
- Mohamed, S.; Nasr, M.; Salama, A.; Refai, H. Novel Lipid–Polymer Hybrid Nanoparticles Incorporated in Thermosensitive in Situ Gel for Intranasal Delivery of Terbutaline Sulphate. J. Microencapsul. 2020, 37, 577–594. [Google Scholar] [CrossRef]
- Stanzione, A.; Polini, A.; La Pesa, V.; Romano, A.; Quattrini, A.; Gigli, G.; Moroni, L.; Gervaso, F. Development of Injectable Thermosensitive Chitosan-Based Hydrogels for Cell Encapsulation. Appl. Sci. 2020, 10, 6550. [Google Scholar] [CrossRef]
- Wasupalli, G.K.; Verma, D. Thermosensitive Injectable Hydrogel Based on Chitosan-Polygalacturonic Acid Polyelectrolyte Complexes for Bone Tissue Engineering. Carbohydr. Polym. 2022, 294, 119769. [Google Scholar] [CrossRef]
- Nawaz, A.; Ullah, S.; Alnuwaiser, M.A.; Rehman, F.U.; Selim, S.; Al Jaouni, S.K.; Farid, A. Formulation and Evaluation of Chitosan-Gelatin Thermosensitive Hydrogels Containing 5FU-Alginate Nanoparticles for Skin Delivery. Gels 2022, 8, 537. [Google Scholar] [CrossRef]
- Ziminska, M.; Wilson, J.J.; McErlean, E.; Dunne, N.; McCarthy, H.O. Synthesis and Evaluation of a Thermoresponsive Degradable Chitosan-Grafted PNIPAAm Hydrogel as a “Smart” Gene Delivery System. Materials 2020, 13, 2530. [Google Scholar] [CrossRef]
- Ku, J.; Seonwoo, H.; Park, S.; Jang, K.-J.; Lee, J.; Lee, M.; Lim, J.W.; Kim, J.; Chung, J.H. Cell-Laden Thermosensitive Chitosan Hydrogel Bioinks for 3D Bioprinting Applications. Appl. Sci. 2020, 10, 2455. [Google Scholar] [CrossRef]
- Chernykh, M.; Zavalny, D.; Sokolova, V.; Ponomarenko, S.; Prylutska, S.; Kuziv, Y.; Chumachenko, V.; Marynin, A.; Kutsevol, N.; Epple, M.; et al. A New Water-Soluble Thermosensitive Star-Like Copolymer as a Promising Carrier of the Chemotherapeutic Drug Doxorubicin. Materials 2021, 14, 3517. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, A.V.; Ravi, P.R.; Uppuluri, C.T.; Mahajan, R.R.; Katke, S.V.; Deshpande, V.S. Thermosensitive Nasal in Situ Gelling Systems of Rufinamide Formulated Using Modified Tamarind Seed Xyloglucan for Direct Nose-to-Brain Delivery: Design, Physical Characterization, and in Vivo Evaluation. J. Pharm. Investig. 2021, 51, 199–211. [Google Scholar] [CrossRef]
- Dalvi, A.; Ravi, P.R.; Uppuluri, C.T. Rufinamide-Loaded Chitosan Nanoparticles in Xyloglucan-Based Thermoresponsive In Situ Gel for Direct Nose to Brain Delivery. Front. Pharmacol. 2021, 12, 691936. [Google Scholar] [CrossRef] [PubMed]
- Dalvi, A.; Ravi, P.R.; Uppuluri, C.T. Design and Evaluation of Rufinamide Nanocrystals Loaded Thermoresponsive Nasal in Situ Gelling System for Improved Drug Distribution to Brain. Front. Pharmacol. 2022, 13, 943772. [Google Scholar] [CrossRef]
- Khan, M.S.; Ravi, P.R.; Mir, S.I.; Rawat, P.S. Optimization and in Vivo Evaluation of Triamcinolone Acetonide Loaded in Situ Gel Prepared Using Reacted Tamarind Seed Xyloglucan and Kappa-Carrageenan for Ocular Delivery. Int. J. Biol. Macromol. 2023, 233, 123533. [Google Scholar] [CrossRef] [PubMed]
- Kassab, H.; Alkufi, H.; Hussein, L. Use of Factorial Design in Formulation and Evaluation of Intrarectal in Situ Gel of Sumatriptan. J. Adv. Pharm. Technol. Res. 2023, 14, 119. [Google Scholar] [CrossRef]
- Talantikite, M.; Stimpson, T.C.; Gourlay, A.; Le-Gall, S.; Moreau, C.; Cranston, E.D.; Moran-Mirabal, J.M.; Cathala, B. Bioinspired Thermoresponsive Xyloglucan–Cellulose Nanocrystal Hydrogels. Biomacromolecules 2021, 22, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F. Thermosensitive Alginate-Gelatin-Nitrogen-Doped Carbon Dots Scaffolds as Potential Injectable Hydrogels for Cartilage Tissue Engineering Applications. RSC Adv. 2021, 11, 18423–18431. [Google Scholar] [CrossRef]
- Ghanbari, M.; Salavati-Niasari, M.; Mohandes, F.; Firouzi, Z. Modified Silicon Carbide NPs Reinforced Nanocomposite Hydrogels Based on Alginate-Gelatin by with High Mechanical Properties for Tissue Engineering. Arab. J. Chem. 2022, 15, 103520. [Google Scholar] [CrossRef]
- Ghanbari, M.; Sadjadinia, A.; Zahmatkesh, N.; Mohandes, F.; Dolatyar, B.; Zeynali, B.; Salavati-Niasari, M. Synthesis and Investigation of Physicochemical Properties of Alginate Dialdehyde/Gelatin/ZnO Nanocomposites as Injectable Hydrogels. Polym. Test. 2022, 110, 107562. [Google Scholar] [CrossRef]
- Gheysoori, P.; Paydayesh, A.; Jafari, M.; Peidayesh, H. Thermoresponsive Nanocomposite Hydrogels Based on Gelatin/Poly (N-Isopropylacrylamide) (PNIPAM) for Controlled Drug Delivery. Eur. Polym. J. 2023, 186, 111846. [Google Scholar] [CrossRef]
- Mozafarian, D.; Jafari, M.; Paydayesh, A. Synthesis and Characterization of Gelatin/Poly N-Isopropylacryl Amide Nanocomposite Hydrogels by Response Surface Methodology. J. Macromol. Sci. Part B 2023, 62, 329–349. [Google Scholar] [CrossRef]
- Zhou, L.; Dai, C.; Fan, L.; Jiang, Y.; Liu, C.; Zhou, Z.; Guan, P.; Tian, Y.; Xing, J.; Li, X.; et al. Injectable Self-Healing Natural Biopolymer-Based Hydrogel Adhesive with Thermoresponsive Reversible Adhesion for Minimally Invasive Surgery. Adv. Funct. Mater. 2021, 31, 2007457. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Shao, C.; Nie, M.; Huang, Q.; Li, J.; Sun, L.; Zhao, Y. Photoresponsive Delivery Microcarriers for Tissue Defects Repair. Adv. Sci. 2019, 6, 1901280. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, W.; Li, W.; Xie, M.; Deng, C.; Sun, X.; Wang, C.; Liu, Y.; Shi, G.; Xu, Y.; et al. Fabrication of Thermoresponsive Hydrogel Scaffolds with Engineered Microscale Vasculatures. Adv. Funct. Mater. 2021, 31, 2102685. [Google Scholar] [CrossRef]
- Dabiri, S.M.H.; Samiei, E.; Shojaei, S.; Karperien, L.; Khun Jush, B.; Walsh, T.; Jahanshahi, M.; Hassanpour, S.; Hamdi, D.; Seyfoori, A.; et al. Multifunctional Thermoresponsive Microcarriers for High-Throughput Cell Culture and Enzyme-Free Cell Harvesting. Small 2021, 17, 2103192. [Google Scholar] [CrossRef] [PubMed]
- Perera, M.M.; Fischesser, D.M.; Molkentin, J.D.; Ayres, N. Stiffness of Thermoresponsive Gelatin-Based Dynamic Hydrogels Affects Fibroblast Activation. Polym. Chem. 2019, 10, 6360–6367. [Google Scholar] [CrossRef]
- Kamlungmak, S.; Rugmai, S.; Tinpun, K.; Nakpheng, T.; Srichana, T. Phase Behavior, in Vitro Drug Release, and Antibacterial Activity of Thermoresponsive poloxamer–Polyvinyl Alcohol Hydrogel-loaded Mupirocin Nanoparticles. J. Appl. Polym. Sci. 2020, 137, 49325. [Google Scholar] [CrossRef]
- Wong, L.W.; Pasbakhsh, P.; Cheng, W.T.; Goh, C.B.S.; Tan, J.B.L. One-Pot Synthesis of Injectable Self-Healing Thermoresponsive Halloysite Nanotube-Reinforced Nanocomposite Hydrogels for Tissue Engineering. Appl. Clay Sci. 2023, 232, 106812. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, X.; Xu, B.; Li, W. LCST and UCST-Type Thermoresponsive Behavior in Dendronized Gelatins. Polym. Chem. 2022, 13, 2813–2821. [Google Scholar] [CrossRef]
- Pang, Q.; Zhao, J.; Zhang, S.; Zhang, X. Near-Infrared Triggered on-Demand Local Anesthesia Using a Jammed Microgels System. J. Biomater. Sci. Polym. Ed. 2020, 31, 2252–2267. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Chen, C.; Mi, L.; Wang, J.-P.; Yang, J.; Wu, G.-P.; Xu, Z.-K.; Cubitt, R.; Mueller-Buschbaum, P. Thermoresponsive Diblock Copolymer Films with a Linear Shrinkage Behavior and Its Potential Application in Temperature Sensors. Langmuir 2020, 36, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Hu, N.; Mi, L.; Wang, J.-P.; Metwalli, E.; Biessmann, L.; Herold, C.; Yang, J.; Wu, G.-P.; Xu, Z.-K.; et al. Impact of Thermal History on the Kinetic Response of Thermoresponsive Poly(Diethylene Glycol Monomethyl Ether Methacrylate)-Block-Poly(Poly(Ethylene Glycol)Methyl Ether Methacrylate) Thin Films Investigated by In Situ Neutron Reflectivity. Langmuir 2020, 36, 6228–6237. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Chen, C.; Metwalli, E.; Biessmann, L.; Herold, C.; Fu, J.; Cubitt, R.; Zhong, Q.; Mueller-Buschbaum, P. Hydration and Thermal Response Kinetics of a Cross-Linked Thermoresponsive Copolymer Film on a Hydrophobic PAN Substrate Coating Probed by In Situ Neutron Reflectivity. Langmuir 2021, 37, 6819–6829. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Mi, L.; Metwalli, E.; Biessmann, L.; Herold, C.; Cubitt, R.; Zhong, Q.; Mueller-Buschbaum, P. Effect of Thermal Stimulus on Kinetic Rehydration of Thermoresponsive Poly(Diethylene Glycol Monomethyl Ether Methacrylate)-Block-Poly(Poly(Ethylene Glycol) Methyl Ether Methacrylate) Thin Films Probed by In Situ Neutron Reflectivity. Langmuir 2022, 38, 8094–8103. [Google Scholar] [CrossRef]
- Kasinski, A.; Zielinska-Pisklak, M.; Kowalczyk, S.; Plichta, A.; Zgadzaj, A.; Oledzka, E.; Sobczak, M. Synthesis and Characterization of New Biodegradable Injectable Thermosensitive Smart Hydrogels for 5-Fluorouracil Delivery. Int. J. Mol. Sci. 2021, 22, 8330. [Google Scholar] [CrossRef] [PubMed]
- Kasinski, A.; Zielinska-Pisklak, M.; Oledzka, E.; Nalecz-Jawecki, G.; Drobniewska, A.; Sobczak, M. Hydrogels Based on Poly(Ether-Ester)s as Highly Controlled 5-Fluorouracil Delivery Systems-Synthesis and Characterization. Materials 2021, 14, 98. [Google Scholar] [CrossRef]
- Kasinski, A.; Swierczek, A.; Zielinska-Pisklak, M.; Kowalczyk, S.; Plichta, A.; Zgadzaj, A.; Oledzka, E.; Sobczak, M. Dual-Stimuli-Sensitive Smart Hydrogels Containing Magnetic Nanoparticles as Antitumor Local Drug Delivery Systems-Synthesis and Characterization. Int. J. Mol. Sci. 2023, 24, 6906. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Miladinovic, Z.R.; Micic, M.; Suljovrujic, D.; Milicevic, D. The Influence of Monomer/Solvent Feed Ratio on POEGDMA Thermoresponsive Hydrogels: Radiation-Induced Synthesis, Swelling Properties and VPTT. Radiat. Phys. Chem. 2019, 158, 37–45. [Google Scholar] [CrossRef]
- Krstic, M.; Miladinovic, Z.R.; Barudzija, T.; Mladenovic, A.; Suljovrujic, E. Stimuli-Responsive Copolymeric Hydrogels Based on Oligo(Ethylene Glycol) Dimethacrylate for Biomedical Applications: An Optimisation Study of PH and Thermoresponsive Behaviour. React. Funct. Polym. 2022, 170, 105140. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Krstic, M.; Miladinovic, Z.R.; Petrovic, S.; Leskovac, A.; Stamboliev, G. Optimization of Thermoresponsive Hydrogels Based on Oligomers with Lower Critical Solution Temperature (LCST) Far below/above Physiological Temperatures for Biomedical Applications. React. Funct. Polym. 2023, 189, 105612. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M.; Morariu, S. Miscibility Study on Polymer Mixtures in Dilute Solution. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 559, 325–333. [Google Scholar] [CrossRef]
- Andrei, M.; Draghici, C.; Teodorescu, M. Thermoreversible Hydrogels from Hydrolytically Degradable Poly(N-isopropylacrylamide)-poly(ethylene glycol) Triblock copolymer and Gelatin. Univ. Politeh. Buchar. Sci. Bull. Ser. B-Chem. Mater. Sci. 2019, 81, 71–84. [Google Scholar]
- Stanescu, P.O.; Nachawati, R.; Abduraman, A.; Teodorescu, M. The Effect of Some Additives on the Thermal Response of Pluronic® L61 Poly(ethylene glycol)-poly(propylene glycol)-poly(ethylene glycol) Triblock Copolymer in Aqueous Solution. Univ. Politeh. Buchar. Sci. Bull. Ser. B-Chem. Mater. Sci. 2021, 83, 143–154. [Google Scholar]
- Stanescu, P.O.; Radu, I.C.; Draghici, C.; Teodorescu, M. Controlling the Thermal Response of Poly(N-Isopropylacrylamide)-Poly (Ethylene Glycol)-Poly(N-Isopropylacrylamide) Triblock Copolymers in Aqueous Solution by Means of Additives. React. Funct. Polym. 2020, 152, 104610. [Google Scholar] [CrossRef]
- Yan, X.; Fang, W.-W.; Xue, J.; Sun, T.-C.; Dong, L.; Zha, Z.; Qian, H.; Song, Y.-H.; Zhang, M.; Gong, X.; et al. Thermoresponsive in Situ Forming Hydrogel with Sol–Gel Irreversibility for Effective Methicillin-Resistant Staphylococcus Aureus Infected Wound Healing. ACS Nano 2019, 13, 10074–10084. [Google Scholar] [CrossRef]
- Song, X.; Zhang, Z.; Zhu, J.; Wen, Y.; Zhao, F.; Lei, L.; Phan-Thien, N.; Khoo, B.C.; Li, J. Thermoresponsive Hydrogel Induced by Dual Supramolecular Assemblies and Its Controlled Release Property for Enhanced Anticancer Drug Delivery. Biomacromolecules 2020, 21, 1516–1527. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, X.; Shi, K.; Yuan, L.; Yang, Y.; Liu, Q.; Ming, Y.; Yi, C.; Qian, Z. Injectable Thermosensitive Hydrogel Containing Erlotinib-Loaded Hollow Mesoporous Silica Nanoparticles as a Localized Drug Delivery System for NSCLC Therapy. Adv. Sci. 2020, 7, 2001442. [Google Scholar] [CrossRef]
- Cao, D.; Chen, X.; Cao, F.; Guo, W.; Tang, J.; Cai, C.; Cui, S.; Yang, X.; Yu, L.; Su, Y.; et al. An Intelligent Transdermal Formulation of ALA-Loaded Copolymer Thermogel with Spontaneous Asymmetry by Using Temperature-Induced Sol–Gel Transition and Gel–Sol (Suspension) Transition on Different Sides. Adv. Funct. Mater. 2021, 31, 2100349. [Google Scholar] [CrossRef]
- Kim, S.H.; Thambi, T.; Giang Phan, V.H.; Lee, D.S. Modularly Engineered Alginate Bioconjugate Hydrogel as Biocompatible Injectable Scaffold for in Situ Biomineralization. Carbohydr. Polym. 2020, 233, 115832. [Google Scholar] [CrossRef] [PubMed]
- Dutta, K.; Das, R.; Ling, J.; Monibas, R.M.; Carballo-Jane, E.; Kekec, A.; Feng, D.D.; Lin, S.; Mu, J.; Saklatvala, R.; et al. In Situ Forming Injectable Thermoresponsive Hydrogels for Controlled Delivery of Biomacromolecules. ACS Omega 2020, 5, 17531–17542. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-H.; Sun, M.-K.; Kung, Y.; Wang, Y.-C.; Chen, S.-L.; Shen, H.-H.; Chen, W.-S.; Young, T.-H. One Injection for One-Week Controlled Release: In Vitro and in Vivo Assessment of Ultrasound-Triggered Drug Release from Injectable Thermoresponsive Biocompatible Hydrogels. Ultrason. Sonochem. 2020, 62, 104875. [Google Scholar] [CrossRef] [PubMed]
- Kouvati, K.; Jaszczak, M.; Papagiannis, P.; Kadlubowski, S.; Wach, R.; Maras, P.; Dudek, M.; Kozicki, M. Leuco Crystal Violet-Pluronic F-127 3D Radiochromic Gel Dosimeter. Phys. Med. Biol. 2019, 64, 175017. [Google Scholar] [CrossRef] [PubMed]
- Jaszczak, M.; Maras, P.; Kozicki, M. Characterization of a New N-Vinylpyrrolidone-Containing Polymer Gel Dosimeter with Pluronic F-127 Gel Matrix. Radiat. Phys. Chem. 2020, 177, 109125. [Google Scholar] [CrossRef]
- Dudek, M.; Piotrowski, M.; Maras, P.; Jaszczak, M.; Kozicki, M. Anisotropic Diffusion of Fe Ions in Fricke-XO-Pluronic F-127 and Fricke-XO-Gelatine 3D Radiotherapy Dosimeters. Phys. Med. Biol. 2021, 66, 155005. [Google Scholar] [CrossRef] [PubMed]
- Jaszczak, M.; Sasiadek, E.; Kadlubowski, S.; Dudek, M.; Kozicki, M. Preliminary Study on a New 3D Radiochromic KI-Pluronic F-127 Gel Dosimeter for Radiotherapy. Radiat. Phys. Chem. 2021, 185, 109507. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Naglik, R.; Dudek, M.; Kadlubowski, S.; Wach, R. Preliminary Study on a 3D Lung Mimicking Dosimeter Based on Pluronic F-127 Matrix. Radiat. Phys. Chem. 2021, 185, 109479. [Google Scholar] [CrossRef]
- Piotrowski, M.; Maras, P.; Kadlubowski, S.; Kozicki, M. Study of the Optimal Composition and Storage Conditions of the Fricke-XO-Pluronic F-127 Radiochromic Dosimeter. Materials 2022, 15, 984. [Google Scholar] [CrossRef]
- Jaszczak, M.; Sasiadek-Andrzejczak, E.; Kozicki, M. Discolouring 3D Gel Dosimeter for UV Dose Distribution Measurements. Materials 2022, 15, 2546. [Google Scholar] [CrossRef]
- Piotrowski, M.; Maras, P.; Wach, R.; Kadlubowski, S.; Kozicki, M. Impact of Salt on Thermal Stability and Dose Response of the Fricke-XO-Pluronic F-127 3D Radiotherapy Dosimeter. Materials 2022, 15, 5223. [Google Scholar] [CrossRef]
- Kozicki, M.; Bartosiak, M.; Maras, P.; Wach, R.; Kadlubowski, S. First Combined, Double-Density LCV-Pluronic F-127 Radiochromic Dosimeter Mimicking Lungs and Muscles. Adv. Mater. Technol. 2023, 8, 2201023. [Google Scholar] [CrossRef]
- Sasiadek-Andrzejczak, E.; Jaszczak, M.; Kozicki, M. Capsule Dosimeters for Ultraviolet Radiation Measurements on Coral Reefs and in Seawater. Materials 2023, 16, 5734. [Google Scholar] [CrossRef]
- Kancharla, S.; Zoyhofski, N.A.; Bufalini, L.; Chatelais, B.F.; Alexandridis, P. Association between Nonionic Amphiphilic Polymer and Ionic Surfactant in Aqueous Solutions: Effect of Polymer Hydrophobicity and Micellization. Polymers 2020, 12, 1831. [Google Scholar] [CrossRef]
- Kancharla, S.; Bedrov, D.; Tsianou, M.; Alexandridis, P. Structure and Composition of Mixed Micelles Formed by Nonionic Block Copolymers and Ionic Surfactants in Water Determined by Small-Angle Neutron Scattering with Contrast Variation. J. Colloid Interface Sci. 2022, 609, 456–468. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Z.; Alexandridis, P.; Tsianou, M. Polymeric Surfactant Micelle Structure Modulated by Ionic Liquids. J. Mol. Liq. 2022, 346, 118195. [Google Scholar] [CrossRef]
- Tsoutsoura, A.; He, Z.; Alexandridis, P. Phase Behavior and Structure of Poloxamer Block Copolymers in Protic and Aprotic Ionic Liquids. Molecules 2023, 28, 7434. [Google Scholar] [CrossRef]
- Tsoutsoura, A.; He, Z.; Alexandridis, P. Effects of Ionic Liquids on the Cylindrical Self-Assemblies Formed by Poly(Ethylene Oxide)-Poly(Propylene Oxide)-Poly(Ethylene Oxide) Block Copolymers in Water. Polymers 2024, 16, 349. [Google Scholar] [CrossRef]
- Kim, M.; Heinrich, F.; Haugstad, G.; Yu, G.; Yuan, G.; Satija, S.K.; Zhang, W.; Seo, H.S.; Metzger, J.M.; Azarin, S.M.; et al. Spatial Distribution of PEO-PPO-PEO Block Copolymer and PEO Homopolymer in Lipid Bilayers. Langmuir 2020, 36, 3393–3403. [Google Scholar] [CrossRef]
- Hassler, J.F.; Crabtree, A.; Liberman, L.; Bates, F.S.; Hackel, B.J.; Lodge, T.P. Effect of Bottlebrush Poloxamer Architecture on Binding to Liposomes. Biomacromolecules 2022, 24, 449–461. [Google Scholar] [CrossRef]
- Crabtree, A.A.; Bates, F.S.; Hackel, B.J. Concentration Threshold for Membrane Protection by PEO-PPO Block Copolymers with Variable Molecular Architectures. ACS Appl. Polym. Mater. 2022, 4, 3259–3269. [Google Scholar] [CrossRef]
- Hassler, J.F.; Lawson, M.; Arroyo, E.C.; Bates, F.S.; Hackel, B.J.; Lodge, T.P. Discovery of Kinetic Trapping of Poloxamers inside Liposomes via Thermal Treatment. Langmuir 2023, 39, 14263–14274. [Google Scholar] [CrossRef]
- Parekh, P.; Ohno, S.; Yusa, S.; Lv, C.; Du, B.; Ray, D.; Aswal, V.K.; Bahadur, P. Synthesis, Aggregation and Adsorption Behaviour of a Thermoresponsive Pentablock Copolymer. Polym. Int. 2020, 69, 1113–1121. [Google Scholar] [CrossRef]
- Pillai, S.A.; Kumar, V.; Kuperkar, K.; Ray, D.; Aswal, V.K.; Kumar, S. Micellization and Clouding Singularity amid the Mixture of EO-PO-Based Star-Block Copolymer and Cationic Surfactants in Aqueous Solution. Colloid Interface Sci. Commun. 2021, 42, 100414. [Google Scholar] [CrossRef]
- Machhi, H.K.; Ray, D.; Panjabi, S.H.; Aswal, V.K.; Soni, S.S. Effect of Redox Active Multivalent Metal Salts on Micellization of Amphiphilic Block Copolymer for Energy Storage Devices via SANS, DLS and NMR. J. Mol. Liq. 2021, 341, 116904. [Google Scholar] [CrossRef]
- Patel, D.; Bhojani, A.K.; Ray, D.; Singh, D.K.; Bhattacharjee, S.; Seth, D.; Aswal, V.K.; Kuperkar, K.; Bahadur, P. Glucose-Induced Self-Assembly and Phase Separation in Hydrophilic Triblock Copolymers and the Governing Mechanism. Phys. Chem. Chem. Phys. 2022, 24, 21141–21156. [Google Scholar] [CrossRef]
- Khamitova, T.O.; Burkeev, M.Z.; Havlicek, D. Synthesis and Study of the Properties of Polymer-Immobilized Silver and Nickel Nanoparticles. Bull. L.N. Gumilyov Eurasian Natl. Univ. Chem. Geogr. Ecol. Ser. 2022, 141, 7–18. [Google Scholar] [CrossRef]
- Lucia, A.; Toloza, A.C.; Fanucce, M.; Fernandez-Pena, L.; Ortega, F.; Rubio, R.G.; Coviella, C.; Guzman, E. Nanoemulsions Based on Thymol-Eugenol Mixtures: Characterization, Stability and Larvicidal Activity against Aedes aegypti. Bull. Insectology 2020, 73, 153–160. [Google Scholar]
- Laurano, R.; Abrami, M.; Grassi, M.; Ciardelli, G.; Boffito, M.; Chiono, V. Using Poloxamer(R) 407 as Building Block of Amphiphilic Poly(Ether Urethane)s: Effect of Its Molecular Weight Distribution on Thermo-Sensitive Hydrogel Performances in the Perspective of Their Biomedical Application. Front. Mater. 2020, 7, 594515. [Google Scholar] [CrossRef]
- Kolpek, A.; Kazankapova, M.; Perdekhanova, A. Purification of Wastewater Using Domestic Carbon Adsorbents and Conducting Physico-Chemical Analysis of Water Composition. Bull. L.N. Gumilyov Eurasian Natl. Univ. Chem. Geogr. Ecol. Ser. 2023, 145, 25–35. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Xue, P.; Liu, J.; Luo, L.; Zhuge, D.; Yao, Q.; Li, X.; Zhao, Y.; Xu, H. Thermo-Sensitive Hydrogel with Mussel-Inspired Adhesion Enhanced the Non-Fibrotic Repair Effect of EGF on Colonic Mucosa Barrier of TNBS-Induced Ulcerative Colitis Rats through Macrophage Polarizing. Chem. Eng. J. 2021, 416, 129221. [Google Scholar] [CrossRef]
- Rao, Y.; Li, R.; Liu, S.; Meng, L.; Wu, Q.; Yuan, Q.; Liang, H.; Qin, M. Enhanced Bioavailability and Biosafety of Cannabidiol Nanomicelles for Effective Anti-Inflammatory Therapy. Particuology 2022, 69, 1–9. [Google Scholar] [CrossRef]
- White, J.M.; Crabtree, A.A.; Bates, F.S.; Calabrese, M.A. Effect of Chain Architecture on the Structure, Dynamics, and Rheology of Thermoresponsive Poloxamer Hydrogels and Associated Blends. Macromolecules 2023, 56, 6834–6847. [Google Scholar] [CrossRef]
- Umapathi, R.; Kumar, K.; Venkatesu, P.; Deenadayalu, N. Quantifying the Influence of Ionic Liquid on the Phase Behaviour of a Biomedical Thermoresponsive Polymer: A Biophysical Experimental Approach. React. Funct. Polymers 2019, 143, 104327. [Google Scholar] [CrossRef]
- Narang, P.; de Oliveira, T.E.; Venkatesu, P.; Netz, P.A. The Role of Osmolytes in the Temperature-Triggered Conformational Transition of Poly(N-Vinylcaprolactam): An Experimental and Computational Study. Phys. Chem. Chem. Phys. 2020, 22, 5301–5313. [Google Scholar] [CrossRef]
- Yadav, R.; Kumar, K.; Venkatesu, P. Monitoring Phase Transition Behavior of Poly(N-Vinylcaprolactam) via Nanostructure-Based Functionalized Carbon Nanotubes. J. Mol. Liq. 2020, 318, 114062. [Google Scholar] [CrossRef]
- Kumar, K.; Umapathi, R.; Ramesh, K.; Hwang, S.-K.; Lim, K.T.; Huh, Y.S.; Venkatesu, P. Biological Stimuli-Induced Phase Transition of a Synthesized Block Copolymer: Preferential Interactions between PNIPAM-b-PNVCL and Heme Proteins. Langmuir 2021, 37, 1682–1696. [Google Scholar] [CrossRef]
- Kumar, K.; Umapathi, R.; Bisht, M.; Ghoreishian, S.M.; Huh, Y.S.; Venkatesu, P. Tweaking Behavior of Hydrogen Bond Donor in Choline Chloride-Based Deep Eutectic Solvents for Regulating the Phase Transition of Poly(N-Vinylcaprolactam): A Sustainable Medium for an Early Hydrophobic Collapse. ACS Sustain. Chem. Eng. 2021, 9, 14335–14344. [Google Scholar] [CrossRef]
- Yadav, R.; Kahlon, N.K.; Kumar, S.; Devunuri, N.; Venkatesu, P. Biophysical Study on the Phase Transition Behaviour of Biocompatible Thermoresponsive Polymer Influenced by Tryptophan-Based Amino Acid Ionic Liquids. Polymer 2021, 228, 123871. [Google Scholar] [CrossRef]
- Umapathi, R.; Kumar, K.; Ghoreishian, S.M.; Rani, G.M.; Park, S.Y.; Huh, Y.S.; Venkatesu, P. Tunnelling the Structural Insights between Poly(N-Isopropylacrylamide) and Imidazolium Sulfate Ionic Liquids. J. Mol. Liq. 2022, 360, 119404. [Google Scholar] [CrossRef]
- Umapathi, R.; Kumar, K.; Ghoreishian, S.M.; Rani, G.M.; Park, S.Y.; Huh, Y.S.; Venkatesu, P. Effect of Imidazolium Nitrate Ionic Liquids on Conformational Changes of Poly(N-Vinylcaprolactam). ACS Omega 2022, 7, 39742–39749. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Kumar, S.; Kumar, K.; Venkatesu, P. Gold Nanospheres/Nanorods as Highly Promising Candidates for the Hydrophilic/Hydrophobic Balance of Poly(N-Vinylcaprolactam): A Thoughtful Design of Nanocomposites. New J. Chem. 2022, 46, 12381–12393. [Google Scholar] [CrossRef]
- Rawat, P.; Mor, S.; Yadav, R.; Narang, P.; Bisht, M.; Venkatesu, P. The Hydrophobic Collapse of Thermoresponsive Polymer Poly(N-Vinyl Caprolactam): A New Class of Biocompatible Solvents. New J. Chem. 2024, 48, 1771–1780. [Google Scholar] [CrossRef]
- Saha, P.; Kather, M.; Banerjee, S.L.; Singha, N.K.; Pich, A. Aqueous Solution Behavior of Thermoresponsive Polyzwitterionic Microgels Based on Poly(N-Vinylcaprolactam) Synthesized via RAFT Precipitation Polymerization. Eur. Polym. J. 2019, 118, 195–204. [Google Scholar] [CrossRef]
- Tan, K.H.; Demco, D.E.; Fechete, R.; Pich, A. Functional Selenium Modified Microgels: Temperature-Induced Phase Transitions and Network Morphology. Soft Matter 2019, 15, 3227–3240. [Google Scholar] [CrossRef] [PubMed]
- Djeljadini, S.; Lohaus, T.; Gausmann, M.; Rauer, S.; Kather, M.; Krause, B.; Pich, A.; Moeller, M.; Wessling, M. Trypsin-Free Cultivation of 3D Mini-Tissues in an Adaptive Membrane Bioreactor. Adv. Biosyst. 2020, 4, e2000081. [Google Scholar] [CrossRef]
- Banerjee, S.L.; Saha, P.; Ganguly, R.; Bhattacharya, K.; Kalita, U.; Pich, A.; Singha, N.K. A Dual Thermoresponsive and Antifouling Zwitterionic Microgel with PH Triggered Fluorescent “on-off” Core. J. Colloid Interface Sci. 2021, 589, 110–126. [Google Scholar] [CrossRef]
- Saha, P.; Palanisamy, A.R.; Santi, M.; Ganguly, R.; Mondal, S.; Singha, N.K.; Pich, A. Thermoresponsive Zwitterionic Poly(Phosphobetaine) Microgels: Effect of Macro-RAFT Chain Length and Cross-Linker Molecular Weight on Their Antifouling Properties. Polym. Adv. Technol. 2021, 32, 2710–2726. [Google Scholar] [CrossRef]
- Ganguly, R.; Saha, P.; Kringe, L.A.; Pich, A.; Singha, N.K.K. Thermoresponsive Microgels with High Loading of Zwitterions Exhibiting Superior Performance: A Macromonomer Approach. Macromol. Chem. Phys. 2023, 224, 2200349. [Google Scholar] [CrossRef]
- Muralter, F.; Perrotta, A.; Werzer, O.; Coclite, A.M. Interlink between Tunable Material Properties and Thermoresponsiveness of Cross-Linked Poly(N-Vinylcaprolactam) Thin Films Deposited by Initiated Chemical Vapor Deposition. Macromolecules 2019, 52, 6817–6824. [Google Scholar] [CrossRef] [PubMed]
- Muralter, F.; Coclite, A.M.; Werzer, O. Wrinkling of an Enteric Coating Induced by Vapor-Deposited Stimuli-Responsive Hydrogel Thin Films. J. Phys. Chem. C 2019, 123, 24165–24171. [Google Scholar] [CrossRef]
- Muralter, F.; Greco, F.; Coclite, A.M. Applicability of Vapor-Deposited Thermoresponsive Hydrogel Thin Films in Ultrafast Humidity Sensors/Actuators. ACS Appl. Polym. Mater. 2020, 2, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, A.; Kindlhofer, P.; Greco, F.; Coclite, A.M. Multiresponsive Soft Actuators Based on a Thermoresponsive Hydrogel and Embedded Laser-Induced Graphene. ACS Appl. Polym. Mater. 2021, 3, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Sumbarda, Y.D.; Dominguez-Gonzalez, C.; Zizumbo-Lopez, A.; Licea-Claverie, A. Thermoresponsive Nanocomposite Hydrogels with Improved Properties Based on Poly(N-Vinylcaprolactam). Mater. Today Commun. 2020, 24, 101041. [Google Scholar] [CrossRef]
- Gonzalez-Ayon, M.A.; Licea-Claverie, A.; Adriana Sanudo-Barajas, J. Different Strategies for the Preparation of Galactose-Functionalized Thermo-Responsive Nanogels with Potential as Smart Drug Delivery Systems. Polymers 2020, 12, 2150. [Google Scholar] [CrossRef]
- Gonzalez-Ayon, M.A.; Licea-Rodriguez, J.; Mendez, E.R.; Licea-Claverie, A. NVCL-Based Galacto-Functionalized and Thermosensitive Nanogels with GNRDs for Chemo/Photothermal-Therapy. Pharmaceutics 2022, 14, 560. [Google Scholar] [CrossRef]
- Marquez-Castro, J.E.; Licea-Claverie, A.; Licea-Rodriguez, J.; Quiroga-Sanchez, L.P.; Mendez, E.R. Surface Grafted Gold Nanorods (GNRDs) Using Thermosensitive Copolymers with Various Transition Temperatures: Nanomaterials with Potential Application for Photothermal Therapy. Eur. Polym. J. 2023, 197, 112341. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Sala, R.L.; de Jesus, L.A.O.; Cruz, S.A.; Camargo, E.R. Analyzing the Effects of Silica Nanospheres on the Sol-Gel Transition Profile of Thermosensitive Hydrogels. Langmuir 2021, 37, 7373–7379. [Google Scholar] [CrossRef]
- Sala, R.L.; Venancio, T.; Camargo, E.R. Probing the Structural Dynamics of the Coil-Globule Transition of Thermosensitive Nanocomposite Hydrogels. Langmuir 2021, 37, 1531–1541. [Google Scholar] [CrossRef]
- Assis, J.F.; Gabriel, A.M.; Goncalves, L.F.; Machado, M.R.F.; Morgado, D.L.; Sala, R.L.; Cristovan, F.H.; Oliveira, M.P.; Arantes, T.M.; Camargo, E.R. Thermosensitive and Biocompatible Nanocomposites of Poly(N-Vinylcaprolactam) and Hydroxyapatite with Potential Use for Bone Tissue Repair. Bionanoscience 2022, 12, 766–773. [Google Scholar] [CrossRef]
- Ribeiro, L.S.; Sala, R.L.; Robeldo, T.A.; Borra, R.C.; Camargo, E.R. Injectable Thermosensitive Nanocomposites Based on Poly(N-Vinylcaprolactam) and Silica Particles for Localized Release of Hydrophilic and Hydrophobic Drugs. Langmuir 2023, 39, 2380–2388. [Google Scholar] [CrossRef] [PubMed]
- Siirila, J.; Karesoja, M.; Pulkkinen, P.; Malho, J.-M.; Tenhu, H. Soft Poly(N-Vinylcaprolactam) Nanogels Surface-Decorated with AuNPs. Response to Temperature, Light, and RF-Field. Eur. Polym. J. 2019, 115, 59–69. [Google Scholar] [CrossRef]
- Fernandez-Quiroz, D.; Loya-Duarte, J.; Silva-Campa, E.; Arguelles-Monal, W.; Sarabia-Sainz, A.-I.; Lucero-Acuna, A.; del Castillo-Castro, T.; San Roman, J.; Lizardi-Mendoza, J.; Burgara-Estrella, A.J.; et al. Temperature Stimuli-Responsive Nanoparticles from Chitosan-Graft-Poly(N-Vinylcaprolactam) as a Drug Delivery System. J. Appl. Polym. Sci. 2019, 136, 47831. [Google Scholar] [CrossRef]
- Peng, J.; Tang, D.; Jia, S.; Zhang, Y.; Sun, Z.; Yang, X.; Zou, H.; Lv, H. In Situ Thermal Synthesis of Molybdenum Oxide Nanocrystals in Thermoresponsive Microgels. Colloids Surfaces A-Physicochem. Eng. Asp. 2019, 563, 130–140. [Google Scholar] [CrossRef]
- Yin, F.; Nguyen, H.H.; Coutelier, O.; Destarac, M.; Lauth-de Viguerie, N.; Marty, J.-D. Effect of Copolymer Composition of Controlled (N-Vinylcaprolactam/ N-Vinylpyrrolidone) Statistical Copolymers on Formation, Stabilization, Thermoresponsiveness and Catalytic Properties of Gold Nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 630, 127611. [Google Scholar] [CrossRef]
- Roh, Y.H.; Eom, J.Y.; Choi, D.G.; Moon, J.Y.; Shim, M.S.; Bong, K.W. Gold Nanorods-Encapsulated Thermosensitive Drug Carriers for NIR Light-Responsive Anticancer Therapy. J. Ind. Eng. Chem. 2021, 98, 211–216. [Google Scholar] [CrossRef]
- Ren, H.; Qiu, X.P.; Shi, Y.; Yang, P.; Winnik, F.M.; Winnik, F.M. The Two Phase Transitions of Hydrophobically End-Capped Poly(N-Isopropylacrylamide)s in Water. Macromolecules 2020, 53, 5105–5115. [Google Scholar] [CrossRef] [PubMed]
- Nakan, U.; Rahmetullaeva, R.K.; Mun, G.A.; Shaihutdinov, E.M.; Yeligbaeva, G.Z.; Negim, E.S.M. Linear Copolymer of N-Isopropylacrylamide and 2-Hydroxyethylacrylate: Synthesis, Characterization and Monomer Reactivity Ratios. Orient. J. Chem. 2016, 32, 2347–2354. [Google Scholar] [CrossRef]
- Ermukhambetova, B.B.; Suleimenov, I.E.; Alikulov, A.Z.; Moldakhan, I.; Baipakbaeva, S.T.; Mun, G.A. On the Question of the Method for Determining the Critical PH Value during the Formation of Complexes between Nonionic Polymers and Polyacid in Aqueous Solutions. Polym. Sci. Ser. A 2021, 63, 8–14. [Google Scholar] [CrossRef]
- Mun, G.A.; Moldakhan, I.; Serikbay, A.M.; Kaldybekov, D.; Suleimenov, I.E.; Park, K. Hydrophilic Interpolymer Associates—The Key to Solving the Problem of Pre-Biological Evolution. Int. J. Biol. Chem. 2020, 13, 4–13. [Google Scholar] [CrossRef]
- Suleimenov, I.; Shaltykova, D.; Mun, G.; Obukhova, P.; Panchenko, S.; Isembergenov, N. Influence of Hydrophilic Interpolymer Associates on the Formation of Interpolymer Complexes. Adv. Mater. Res. 2013, 749, 60–64. [Google Scholar] [CrossRef]
- Suleimenov, I.; Shaltykova, D.; Sedlakova, Z.; Mun, G.; Semenyakin, N.; Kaldybekov, D.; Obukhova, P. Hydrophilic Interpolymer Associates as a Satellite Product of Reactions of Formation of Interpolymer Complexes. Appl. Mech. Mater. 2013, 467, 58–63. [Google Scholar] [CrossRef]
- Kabdushev, S.; Mun, G.; Suleimenov, I.; Alikulov, A.; Shaikhutdinov, R.; Kopishev, E. Formation of Hydrophobic–Hydrophilic Associates in the N-Vinylpyrrolidone and Vinyl Propyl Ether Copolymer Aqueous Solutions. Polymers 2023, 15, 3578. [Google Scholar] [CrossRef]
- Shaikhutdinov, R.; Mun, G.; Kopishev, E.; Bakirov, A.; Kabdushev, S.; Baipakbaeva, S.; Suleimenov, I. Effect of the Formation of Hydrophilic and Hydrophobic–Hydrophilic Associates on the Behavior of Copolymers of N-Vinylpyrrolidone with Methyl Acrylate in Aqueous Solutions. Polymers 2024, 16, 584. [Google Scholar] [CrossRef]
- Suleimenov, I.; Güven, O.; Mun, G.; Beissegul, A.; Panchenko, S.; Ivlev, R. The Formation of Interpolymer Complexes and Hydrophilic Associates of Poly(Acrylic Acid) and Non-Ionic Copolymers Based on 2-Hydroxyethylacrylate in Aqueous Solutions. Polym. Int. 2013, 62, 1310–1315. [Google Scholar] [CrossRef]
- Nakan, U.; Rakhmetullaeva, R.; Mun, G.A.; Yeligbaeva, G.Z.; Amitova, A.A.; Tolkyn, B.; Shaihutdinov, E.M.; Nursultanov, M.E.; Negim, E.S.M. Synthesis of Water Soluble Copolymers and Their Interpolymer Complexes with Poly(Acrylic Acid). J. Chem. Technol. Metall. 2018, 53, 83–87. [Google Scholar]
- Toktabayeva, A.; Rakhmetullayeva, R.; Abutalip, M.; Mamutova, A.; Batyrbayeva, A.; Mun, G. The New Hybrid Copolymers Based on N-Isopropylacrylamide. J. Chem. Technol. Metall. 2019, 54, 475–482. [Google Scholar]
- Toktabayeva, A.K.; Alikulov, A.Z.; Rakhmetullayeva, R.K.; Mun, G.A.; Tumabayeva, A.M.; Urkimbayeva, P.I.; Yu, V.K.; Seilkhanov, T.M. Synthesis and Study of Physico-Chemical Characteristics of Novel Cationic (Co)Polymers Based on n-Isopropylacrylamide and N-(2-Vinyloxyethyl)-n-(2-Cyanoethyl) Amine. J. Chem. Technol. Metall. 2019, 54, 467–474. [Google Scholar]
- Rakhmetullayeva, R.K.; Azhkeyeva, A.N.; Yeligbayeva, G.Z.; Shaikhutdynov, Y.M.; Mun, G.A.; Abutalip, M. New Thermo-Sensitive Hydrogel Based on Copolymer of 2-Hydroxyethyl Acrylate and Ethyl Acrylate. Eurasian Chem. J. 2017, 19, 47–55. [Google Scholar] [CrossRef]
- Mun, G.A.; Moldakhan, I.; Kabdushev, S.B.; Yermukhambetova, B.B.; Shaikhutdinov, R.; Yeligbayeva, G.Z. To the Methodology of Phase Transition Temperature Determination in Aqueous Solutions of Thermo-Sensitive Polymers. Eurasian Chem. J. 2020, 22, 129. [Google Scholar] [CrossRef]
- Lou, G.; Shi, H. Face Image Recognition Based on Convolutional Neural Network. China Commun. 2020, 17, 117–124. [Google Scholar] [CrossRef]
- Chen, H.; Geng, L.; Zhao, H.; Zhao, C.; Liu, A. Image Recognition Algorithm Based on Artificial Intelligence. Neural Comput. Appl. 2022, 34, 6661–6672. [Google Scholar] [CrossRef]
- Godyak, V.A.; Alexandrovich, B.M. Comparative Analyses of Plasma Probe Diagnostics Techniques. J. Appl. Phys. 2015, 118, 233302. [Google Scholar] [CrossRef]
- Demidov, V.I.; Ratynskaia, S.V.; Rypdal, K. Electric Probes for Plasmas: The Link between Theory and Instrument. Rev. Sci. Instrum. 2002, 73, 3409–3439. [Google Scholar] [CrossRef]
- Suleimenov, I.E.; Guven, O.; Mun, G.A.; Uzun, C.; Gabrielyan, O.A.; Kabdushev, S.B.; Agibaeva, L.; Nurtazin, A. Hysteresis Effects During the Phase Transition in Solutions of Temperature Sensitive Polymers. Eurasian Chem. J. 2017, 19, 41. [Google Scholar] [CrossRef]
- Suleimenov, I.; Kadyrzhan, K.; Kabdushev, S.; Bakirov, A.; Kopishev, E. New Equipment for Aromatherapy and Related Mobile App: A Tool to Support Small Peasant Farms in Kazakhstan in Crisis. In Smart Innovation, Systems and Technologies; Shamtsyan, M., Pasetti, M., Beskopylny, A., Eds.; Springer Nature Singapore Pte Ltd.: Berlin/Heidelberg, Germany, 2022; pp. 347–355. [Google Scholar]
- Suleimenov, I.E.; Kabdushev, S.B.; Kadyrzhan, K.; Shaltikova, D.B.; Moldakhan, I. New Technologies for Measuring Viscosity: Using Mobile Applications. In Proceedings of the 2020 6th International Conference on Computer and Technology Applications, Antalya, Turkey, 14–16 April 2020; pp. 129–133. [Google Scholar] [CrossRef]
- Suleimenov, I.E.; Mun, G.A.; Kabdushev, S.B.; Alikulov, A.; Shaltykova, D.B.; Moldakhan, I. The Design of Viscometer with Smartphone Controlling. Indones. J. Electr. Eng. Comput. Sci. 2022, 27, 366. [Google Scholar] [CrossRef]
- Suleimenov, I.E.; Budtova, T.V.; Adil’bekov, S.A.; Pereladov, I.Y.; Bekturov, E.A. Application of the Method of Phase Portraits to the Analysis of the Kinetics of Redistribution of Metal Ion Concentrations in the Polyelectrolyte Hydrogel-Multicomponent Solution System. Polym. Sci. Ser. A 2004, 46, 797–805. [Google Scholar]
- Ivanchina, E.D.; Chuzlov, V.A.; Ivanchin, N.R.; Borissov, A.; Seitenov, G.Z.; Dusova, R.M. Mathematical Modeling of the Process Catalytic Isomerization of Light Naphtha. Pet. Coal 2019, 61, 413–417. [Google Scholar]
- Safarov, R.; Berdenov, Z.; Urlibay, R.; Nossenko, Y.; Shomanova, Z.; Bexeitova, Z.; Kulak, A.; Varga, I.; Balog, A.; Domjánné, R.N.; et al. Spatial Distribution of Elements, Environmental Effects, and Economic Potential of Waste from the Aksu Ferroalloy Plant [Kazakhstan]. PLoS ONE 2023, 18, e0283251. [Google Scholar] [CrossRef]
- Safarov, R.Z.; Kargin, J.B.; Aibuldinov, Y.K.; Zhandildenova, A.K.; Makhmutov, B.B.; Sviderskiy, A.K.; Vatin, N.I. Structure and Content Analysis of Raw Materials for Production of Trimanganese Tetraoxide Pigment. Crystals 2021, 11, 1460. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakirov, A.; Kopishev, E.; Kadyrzhan, K.; Donbaeva, E.; Zhaxybayeva, A.; Duisembiyev, M.; Suyundikova, F.; Suleimenov, I. The Method of Direct and Reverse Phase Portraits as a Tool for Systematizing the Results of Studies of Phase Transitions in Solutions of Thermosensitive Polymers. Gels 2024, 10, 395. https://doi.org/10.3390/gels10060395
Bakirov A, Kopishev E, Kadyrzhan K, Donbaeva E, Zhaxybayeva A, Duisembiyev M, Suyundikova F, Suleimenov I. The Method of Direct and Reverse Phase Portraits as a Tool for Systematizing the Results of Studies of Phase Transitions in Solutions of Thermosensitive Polymers. Gels. 2024; 10(6):395. https://doi.org/10.3390/gels10060395
Chicago/Turabian StyleBakirov, Akhat, Eldar Kopishev, Kaisarali Kadyrzhan, Elvira Donbaeva, Aigerim Zhaxybayeva, Marat Duisembiyev, Faiziya Suyundikova, and Ibragim Suleimenov. 2024. "The Method of Direct and Reverse Phase Portraits as a Tool for Systematizing the Results of Studies of Phase Transitions in Solutions of Thermosensitive Polymers" Gels 10, no. 6: 395. https://doi.org/10.3390/gels10060395
APA StyleBakirov, A., Kopishev, E., Kadyrzhan, K., Donbaeva, E., Zhaxybayeva, A., Duisembiyev, M., Suyundikova, F., & Suleimenov, I. (2024). The Method of Direct and Reverse Phase Portraits as a Tool for Systematizing the Results of Studies of Phase Transitions in Solutions of Thermosensitive Polymers. Gels, 10(6), 395. https://doi.org/10.3390/gels10060395






