Molten Alkali-Assisted Formation of Silicate Gels and Its Application for Preparing Zeolites
Abstract
1. Introduction
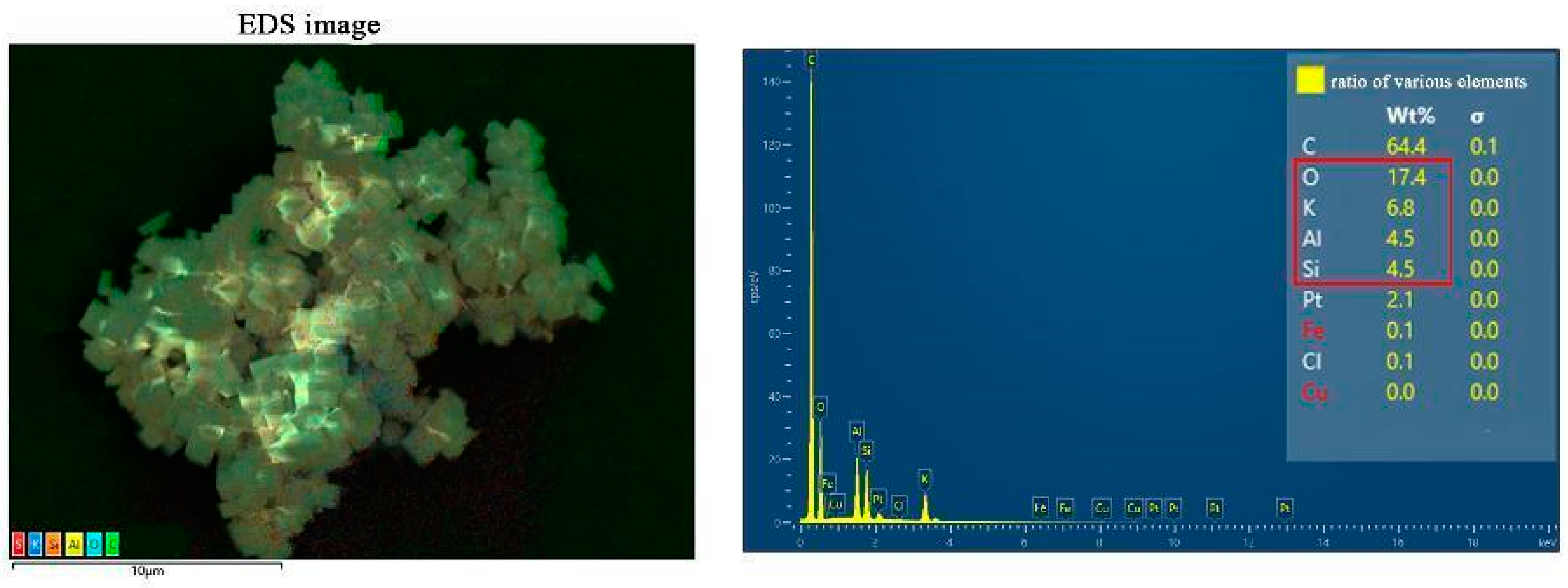
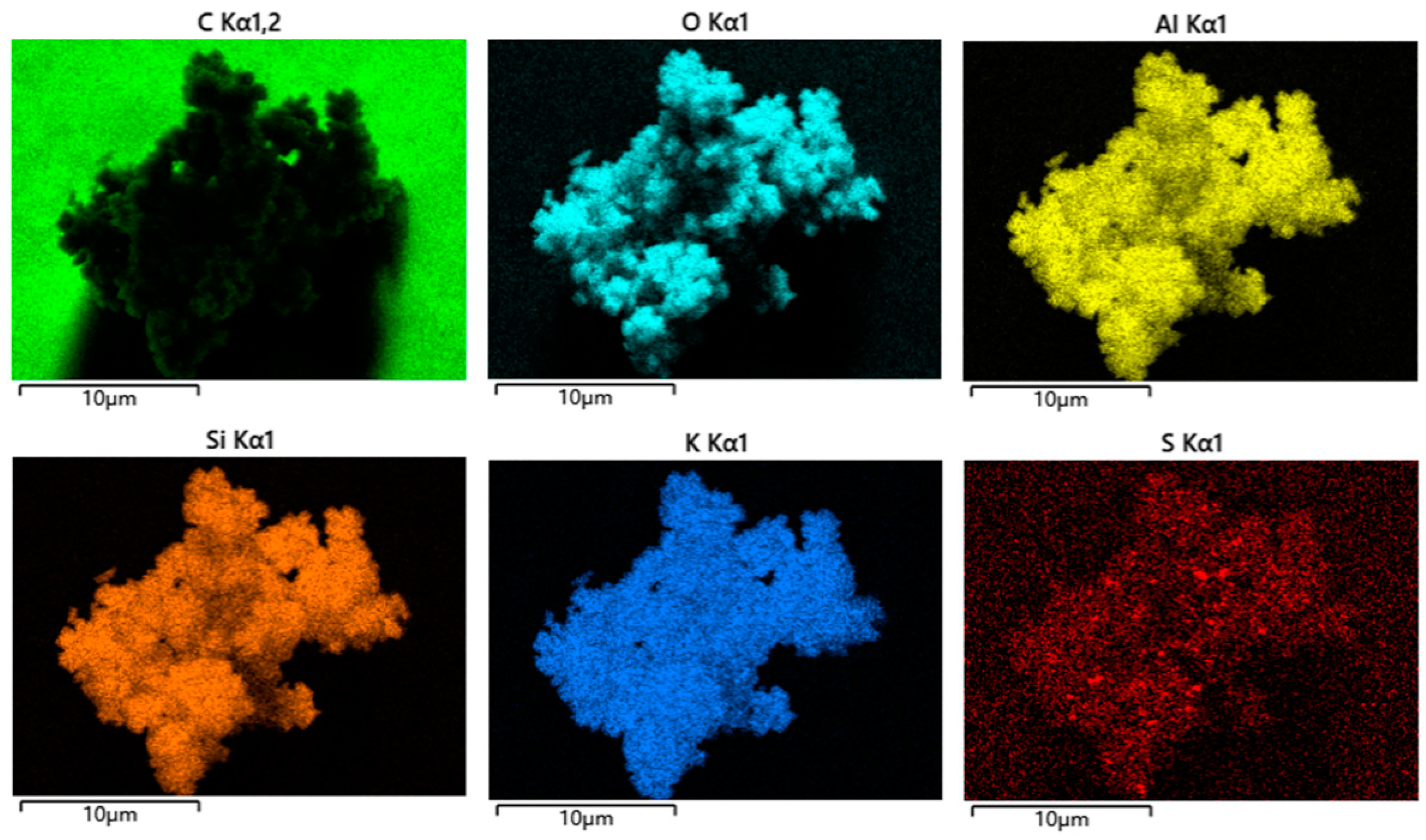
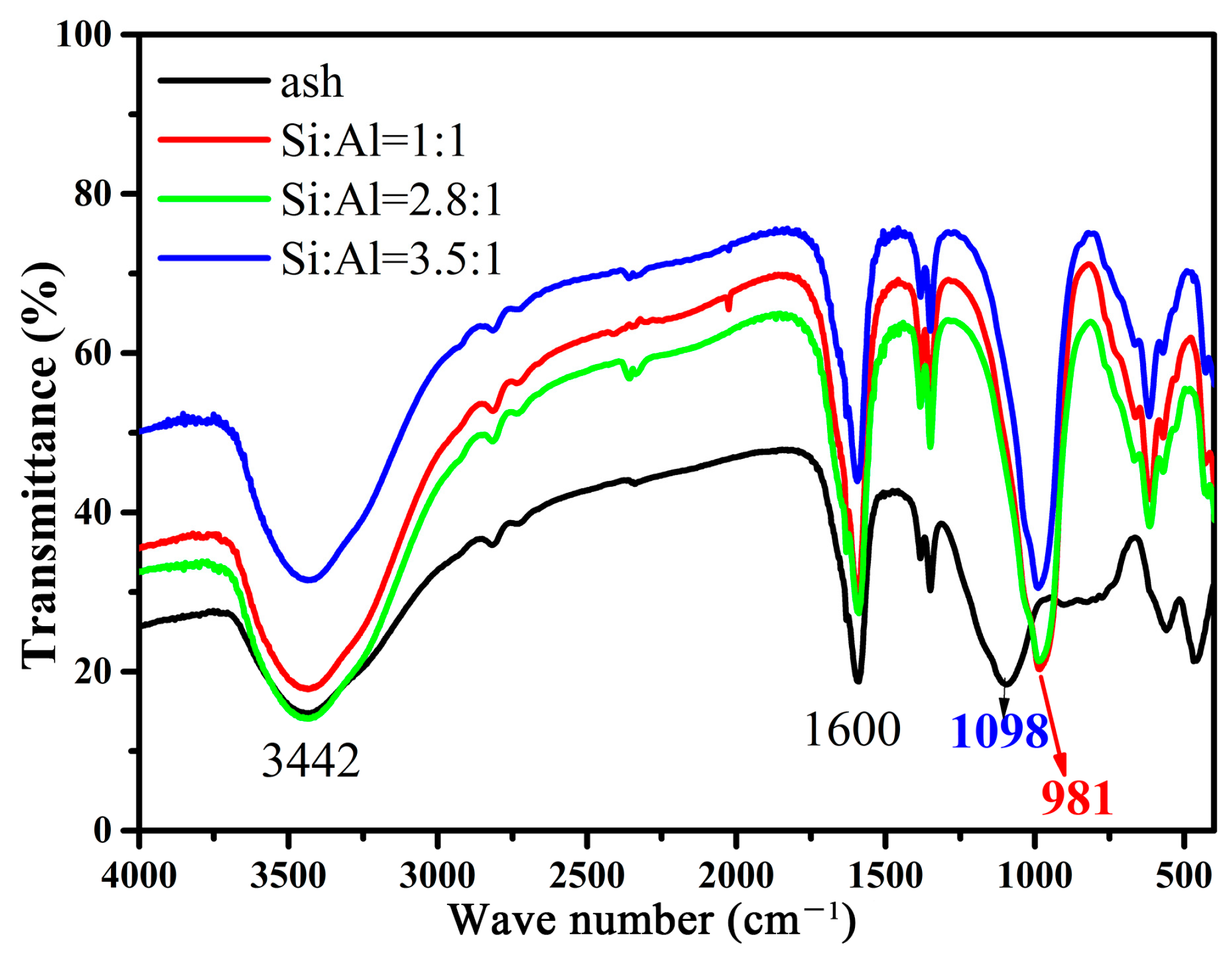
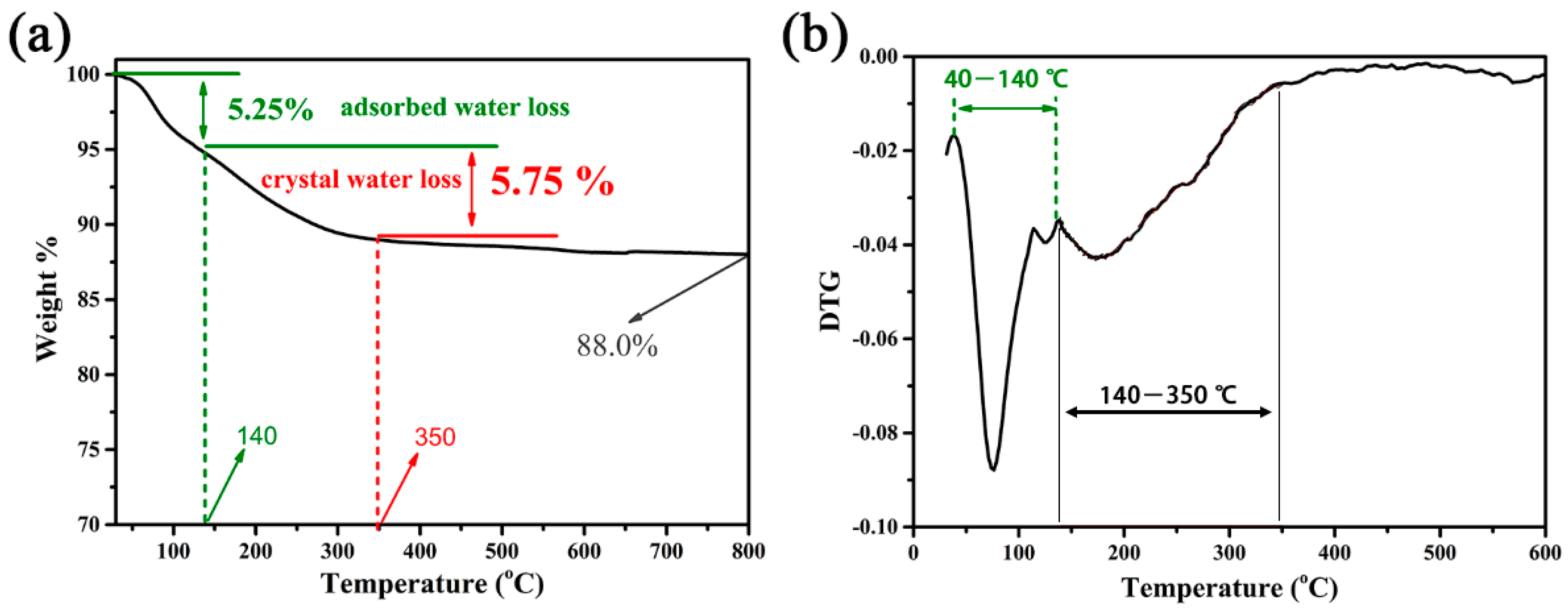
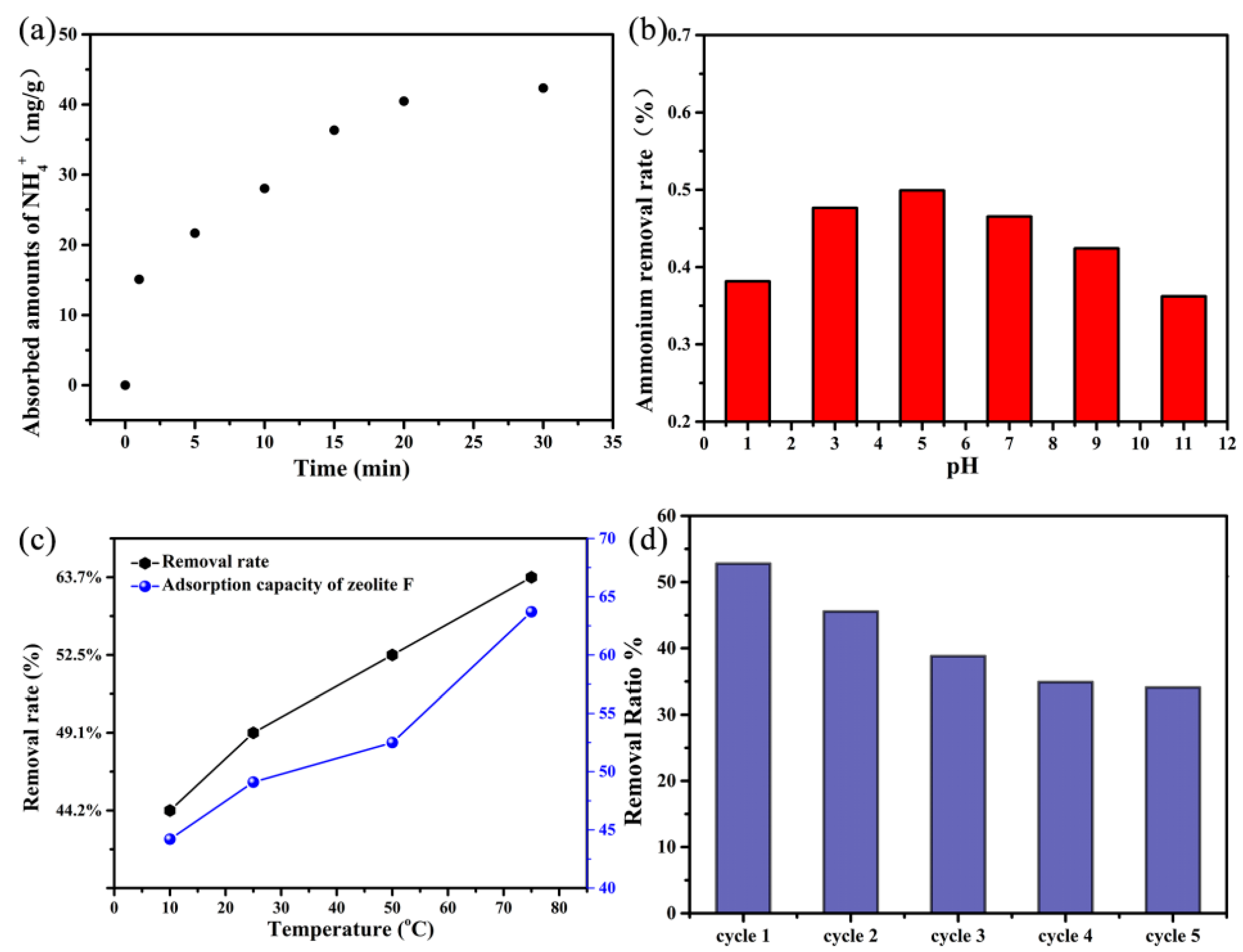
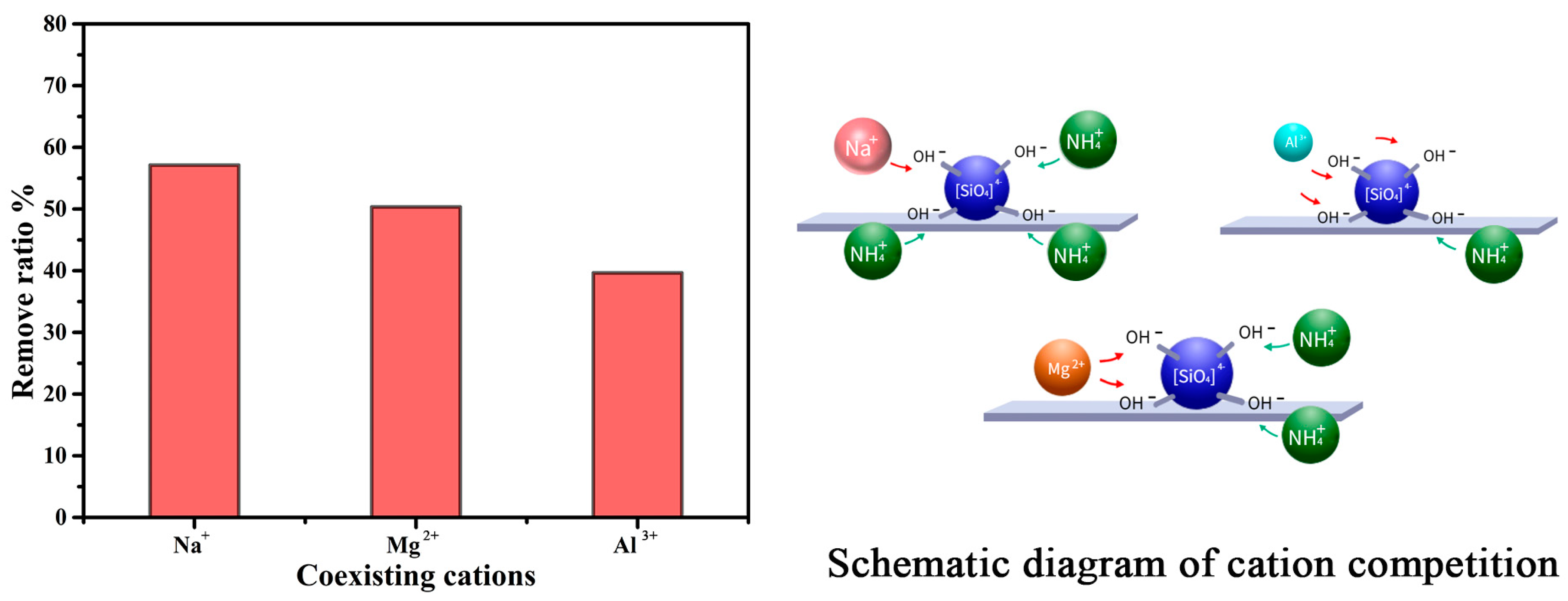
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Procedures of Zeolite Synthesis
4.2.1. The Formation of Silicate Gels
4.2.2. Hydrothermal Synthesis of Zeolite-F
4.2.3. Experiment of Ammonium Adsorption
4.3. Characterization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, R.; Zhu, H.; Zhou, J.; Luo, H.; Xue, K.; Yu, L.; Zhang, Y. Highly Water-Stable and Efficient Hydrogen-Producing Heterostructure Synthesized from Mn0.5Cd0.5S and a Zeolitic Imidazolate Framework ZIF-8 via Ligand and Cation Exchange. ACS Appl. Mater. Interfaces 2023, 15, 36477–36488. [Google Scholar] [CrossRef]
- He, J.; Deng, J.; Lan, T.; Liu, X.; Shen, Y.; Han, L.; Wang, J.; Zhang, D. Strong metal oxide-zeolite interactions during selective catalytic reduction of nitrogen oxides. J. Hazard. Mater. 2023, 465, 133164. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Franus, W.; Panek, R.; Sobczyk, M.; Rusiniak, P.; Szerement, J.; Jarosz, R.; Marcińska-Mazur, L.; Bajda, T.; Mierzwa-Hersztek, M. Zeolite Composite Materials from Fly Ash: An Assessment of Physicochemical and Adsorption Properties. Materials 2023, 16, 2142. [Google Scholar] [CrossRef]
- Tesana, S.; Kennedy, J.V.; Yip, A.C.K.; Golovko, V.B. In Situ Incorporation of Atomically Precise Au Nanoclusters within Zeolites for Ambient Temperature CO Oxidation. Nanomaterials 2023, 13, 3120. [Google Scholar] [CrossRef]
- Gu, J.; Liu, L.; Zhu, R.; Song, Q.; Yu, H.; Jiang, P.; Miao, C.; Du, Y.; Fu, R.; Wang, Y.; et al. Recycling Coal Fly Ash for Super-Thermal-Insulating Aerogel Fiber Preparation with Simultaneous Al2O3 Extraction. Molecules 2023, 28, 7978. [Google Scholar] [CrossRef]
- Shishkin, A.; Abramovskis, V.; Zalite, I.; Singh, A.K.; Mezinskis, G.; Popov, V.; Ozolins, J. Physical, Thermal, and Chemical Properties of Fly Ash Cenospheres Obtained from Different Sources. Materials 2023, 16, 2035. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Cecilia, J.A.; Ballesteros-Plata, D.; Barroso-Martín, I.; Núñez, P.; Infantes-Molina, A.; Rodríguez-Castellón, E. Microwave-Assisted Synthesis of Zeolite A from Metakaolinite for CO2 Adsorption. Int. J. Mol. Sci. 2023, 24, 14040. [Google Scholar] [CrossRef]
- Zhou, Q.; Jiang, X.; Qiu, Q.; Zhao, Y.; Long, L. Synthesis of high-quality NaP1 zeolite from municipal solid waste incineration fly ash by microwave-assisted hydrothermal method and its adsorption capacity. Sci. Total Environ. 2023, 855, 158741. [Google Scholar] [CrossRef]
- Wang, B.; Wu, J.; Yuan, Z.-Y.; Li, N.; Xiang, S. Synthesis of MCM-22 zeolite by an ultrasonic-assisted aging procedure. Ultrason. Sonochem. 2008, 15, 334–338. [Google Scholar] [CrossRef]
- Panitchakarn, P.; Laosiripojana, N.; Viriya-Umpikul, N.; Pavasant, P. Synthesis of high-purity Na-A and Na-X zeolite from coal fly ash. J. Air Waste Manag. Assoc. 2014, 64, 586–596. [Google Scholar] [CrossRef]
- Koshlak, H. Synthesis of Zeolites from Coal Fly Ash Using Alkaline Fusion and Its Applications in Removing Heavy Metals. Materials 2023, 16, 4837. [Google Scholar] [CrossRef]
- Küçük, M.E.; Makarava, I.; Kinnarinen, T.; Häkkinen, A. Simultaneous adsorption of Cu(II), Zn(II), Cd(II) and Pb(II) from synthetic wastewater using NaP and LTA zeolites prepared from biomass fly ash. Heliyon 2023, 9, e20253. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, S.; Ding, B.; Jia, H.; Chen, P.; Du, T.; Wang, Y. Optimization of preparation of NaA zeolite from fly ash for CO2 capture. Environ. Sci. Pollut. Res. 2023, 30, 102803–102817. [Google Scholar] [CrossRef]
- Che, Q.; Yang, M.; Wang, X.; Yang, Q.; Chen, Y.; Chen, X.; Chen, W.; Hu, J.; Zeng, K.; Yang, H.; et al. Preparation of mesoporous ZSM-5 catalysts using green templates and their performance in biomass catalytic pyrolysis. Bioresour. Technol. 2019, 289, 121729. [Google Scholar] [CrossRef]
- Tarach, K.A.; Pyra, K.; Góra-Marek, K. Opening up ZSM-5 Hierarchical Zeolite’s Porosity through Sequential Treatments for Improved Low-Density Polyethylene Cracking. Molecules 2020, 25, 2878. [Google Scholar] [CrossRef]
- Visa, M.; Enesca, A. Opportunities for Recycling PV Glass and Coal Fly Ash into Zeolite Materials Used for Removal of Heavy Metals (Cd, Cu, Pb) from Wastewater. Materials 2022, 16, 239. [Google Scholar] [CrossRef]
- Haghjoo, S.; Lengauer, C.L.; Kazemian, H.; Roushani, M. Facile and innovative application of surfactant-modified-zeolite from Austrian fly ash for glyphosate removal from water solution. J. Environ. Manag. 2023, 346, 118976. [Google Scholar] [CrossRef]
- Joseph, I.V.; Tosheva, L.; Miller, G.; Doyle, A.M. FAU-Type Zeolite Synthesis from Clays and Its Use for the Simultaneous Adsorption of Five Divalent Metals from Aqueous Solutions. Materials 2021, 14, 3738. [Google Scholar] [CrossRef]
- Panek, R.; Medykowska, M.; Wiśniewska, M.; Szewczuk-Karpisz, K.; Jędruchniewicz, K.; Franus, M. Simultaneous Removal of Pb2+ and Zn2+ Heavy Metals Using Fly Ash Na-X Zeolite and Its Carbon Na-X(C) Composite. Materials 2021, 14, 2832. [Google Scholar] [CrossRef]
- Osacký, M.; Binčík, T.; Hudcová, B.; Vítková, M.; Pálková, H.; Hudec, P.; Bačík, P.; Czímerová, A. Low-cost zeolite-based sorbents prepared from industrial perlite by-product material for Zn2+ and Ni2+ removal from aqueous solutions: Synthesis, properties and sorption efficiency. Heliyon 2022, 8, e12029. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, R.; Liu, Q.; Cao, Q.; Guo, R. Synthesis of zeolite A from fly ash and its application in the slow release of urea. Waste Manag. 2023, 158, 47–55. [Google Scholar] [CrossRef]
- Wang, G.; Chen, C.; Li, J.; Yang, F.; Wang, L.; Lin, X.; Wu, H.; Zhang, J. A clean method for gallium recovery and the coproduction of silica-potassium compound fertilizer and zeolite F from brown corundum fly ash. J. Hazard. Mater. 2024, 461, 132625. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, J.; Miao, J.; Yue, L.; Cheng, M.; Li, Y.; Jing, Z. Self-regulated immobilization behavior of multiple heavy metals via zeolitization towards a novel hydrothermal technology for soil remediation. Environ. Res. 2023, 216 Pt 3, 114726. [Google Scholar] [CrossRef]
- Fan, W.; Morozumi, K.; Kimura, R.; Yokoi, T.; Okubo, T. Synthesis of nanometer-sized sodalite without adding organic additives. Langmuir 2008, 24, 6952–6958. [Google Scholar] [CrossRef]
- Liu, H. Conversion of Harmful Fly Ash Residue to Zeolites: Innovative Processes Focusing on Maximum Activation, Extraction, and Utilization of Aluminosilicate. ACS Omega 2022, 7, 20347–20356. [Google Scholar] [CrossRef]
- Hardin, J.L.; Oyler, N.A.; Steinle, E.D.; Meints, G.A. Spectroscopic analysis of interactions between alkylated silanes and alumina nanoporous membranes. J. Colloid Interface Sci. 2010, 342, 614–619. [Google Scholar] [CrossRef]
- Ellerbrock, R.; Stein, M.; Schaller, J. Comparing amorphous silica, short-range-ordered silicates and silicic acid species by FTIR. Sci. Rep. 2022, 12, 11708. [Google Scholar] [CrossRef]
- Fu, H.; Li, Y.; Yu, Z.; Shen, J.; Li, J.; Zhang, M.; Ding, T.; Xu, L.; Lee, S.S. Ammonium removal using a calcined natural zeolite modified with sodium nitrate. J. Hazard. Mater. 2020, 393, 122481. [Google Scholar] [CrossRef]
- Zhou, C.; An, Y.; Zhang, W.; Yang, D.; Tang, J.; Ye, J.; Zhou, Z. Inhibitory effects of Ca2+ on ammonium exchange by zeolite in the long-term exchange and NaClO-NaCl regeneration process. Chemosphere 2021, 263, 128216. [Google Scholar] [CrossRef]
- He, X.; Chen, W.; Sun, F.; Jiang, Z.; Li, B.; Li, X.-Y.; Lin, L. Enhanced NH4+ Removal and Recovery from Wastewater Using Na-Zeolite-based Flow-Electrode Capacitive Deionization: Insight from Ion Transport Flux. Environ. Sci. Technol. 2023, 57, 8828–8838. [Google Scholar] [CrossRef]



| Materials | Melt Point/°C | Solubility/ | Source |
|---|---|---|---|
| NaOH | 318 (low) | strong corrosive | Aladdin |
| KOH | 361 (low) | strong corrosive | Aladdin |
| Na2SiO3 | 1089 (high) | water soluble | Aladdin |
| NaAlO2 | 1650 (high) | water soluble | Aladdin |
| K2SiO3 | 976 (high) | water soluble | Aladdin |
| Fly ash | / | water insoluble | SiO2 48%, Al2O3 33% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Yang, Y.; Zhang, L.; Li, M.; Wang, Y.; Chen, Y.; Ling, R.; Yan, J.; Chen, Y.; Hu, J.; et al. Molten Alkali-Assisted Formation of Silicate Gels and Its Application for Preparing Zeolites. Gels 2024, 10, 392. https://doi.org/10.3390/gels10060392
Ye J, Yang Y, Zhang L, Li M, Wang Y, Chen Y, Ling R, Yan J, Chen Y, Hu J, et al. Molten Alkali-Assisted Formation of Silicate Gels and Its Application for Preparing Zeolites. Gels. 2024; 10(6):392. https://doi.org/10.3390/gels10060392
Chicago/Turabian StyleYe, Juan, Yanchun Yang, Li Zhang, Man Li, Yiling Wang, Yuxuan Chen, Ruhui Ling, Jiefeng Yan, Yan Chen, Jinxing Hu, and et al. 2024. "Molten Alkali-Assisted Formation of Silicate Gels and Its Application for Preparing Zeolites" Gels 10, no. 6: 392. https://doi.org/10.3390/gels10060392
APA StyleYe, J., Yang, Y., Zhang, L., Li, M., Wang, Y., Chen, Y., Ling, R., Yan, J., Chen, Y., Hu, J., & Fang, Z. (2024). Molten Alkali-Assisted Formation of Silicate Gels and Its Application for Preparing Zeolites. Gels, 10(6), 392. https://doi.org/10.3390/gels10060392







