Potential of Carbon Aerogels in Energy: Design, Characteristics, and Applications
Abstract
1. Introduction
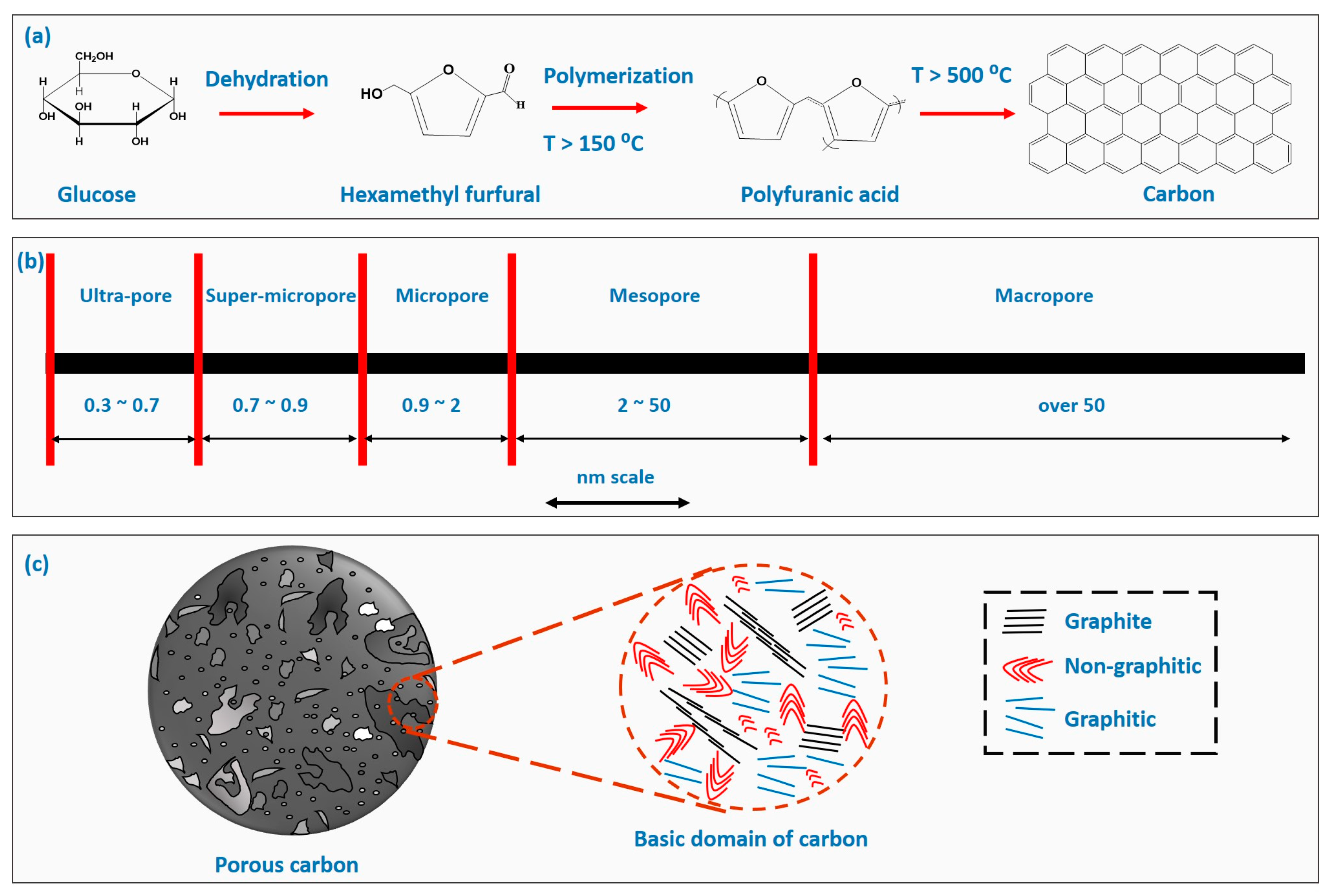
2. Fundamentals of Carbon
3. Carbon Aerogel Synthesis Strategy
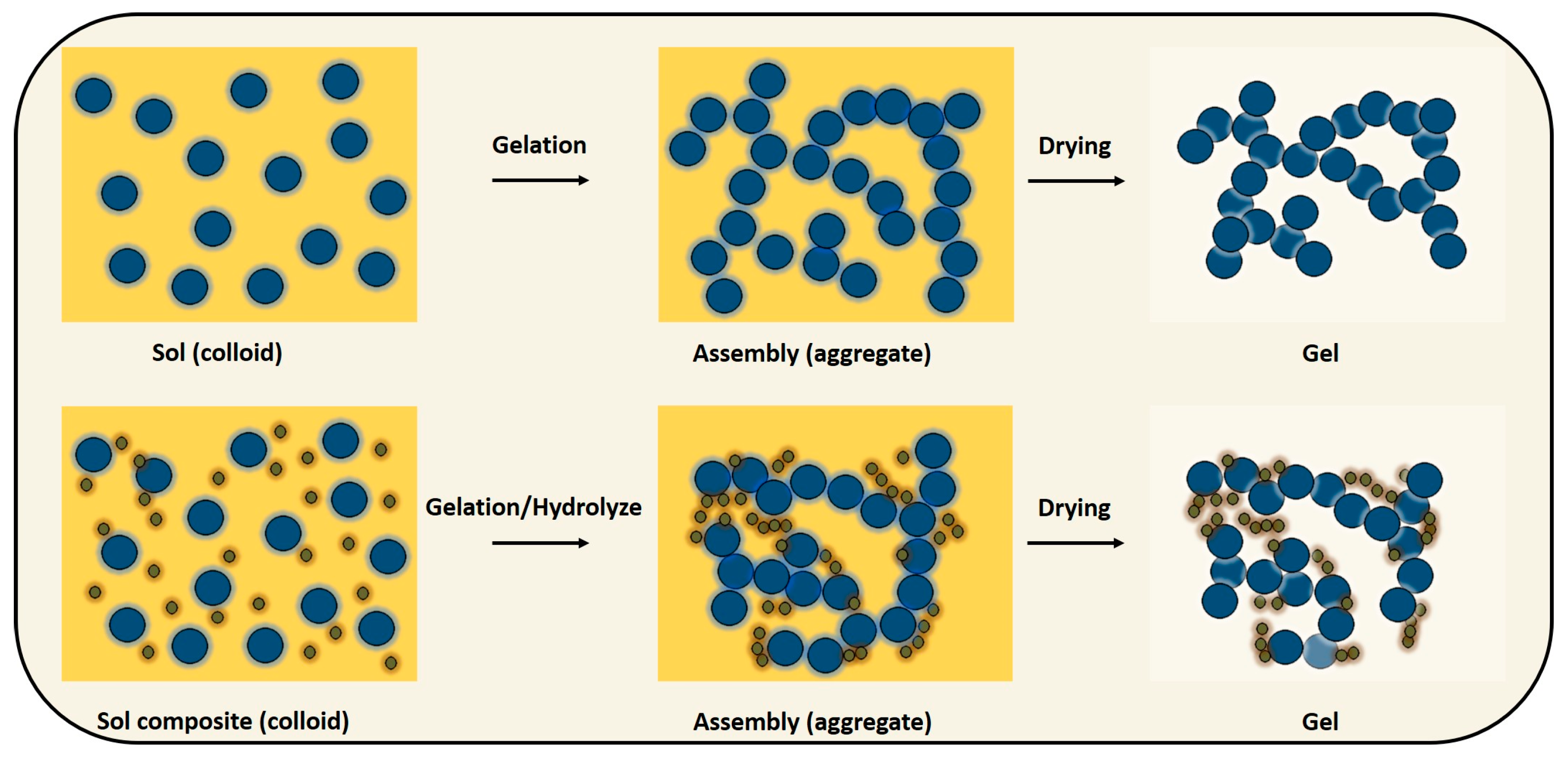
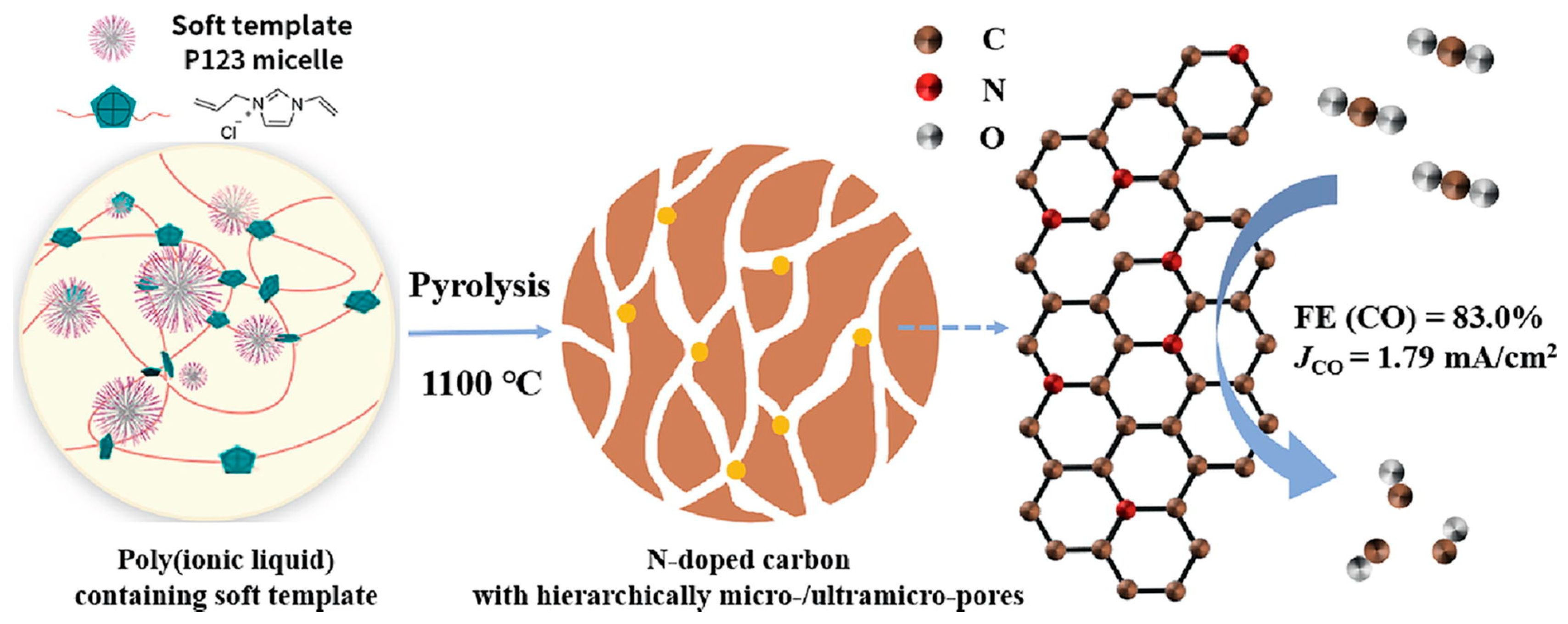
3.1. Gel Production
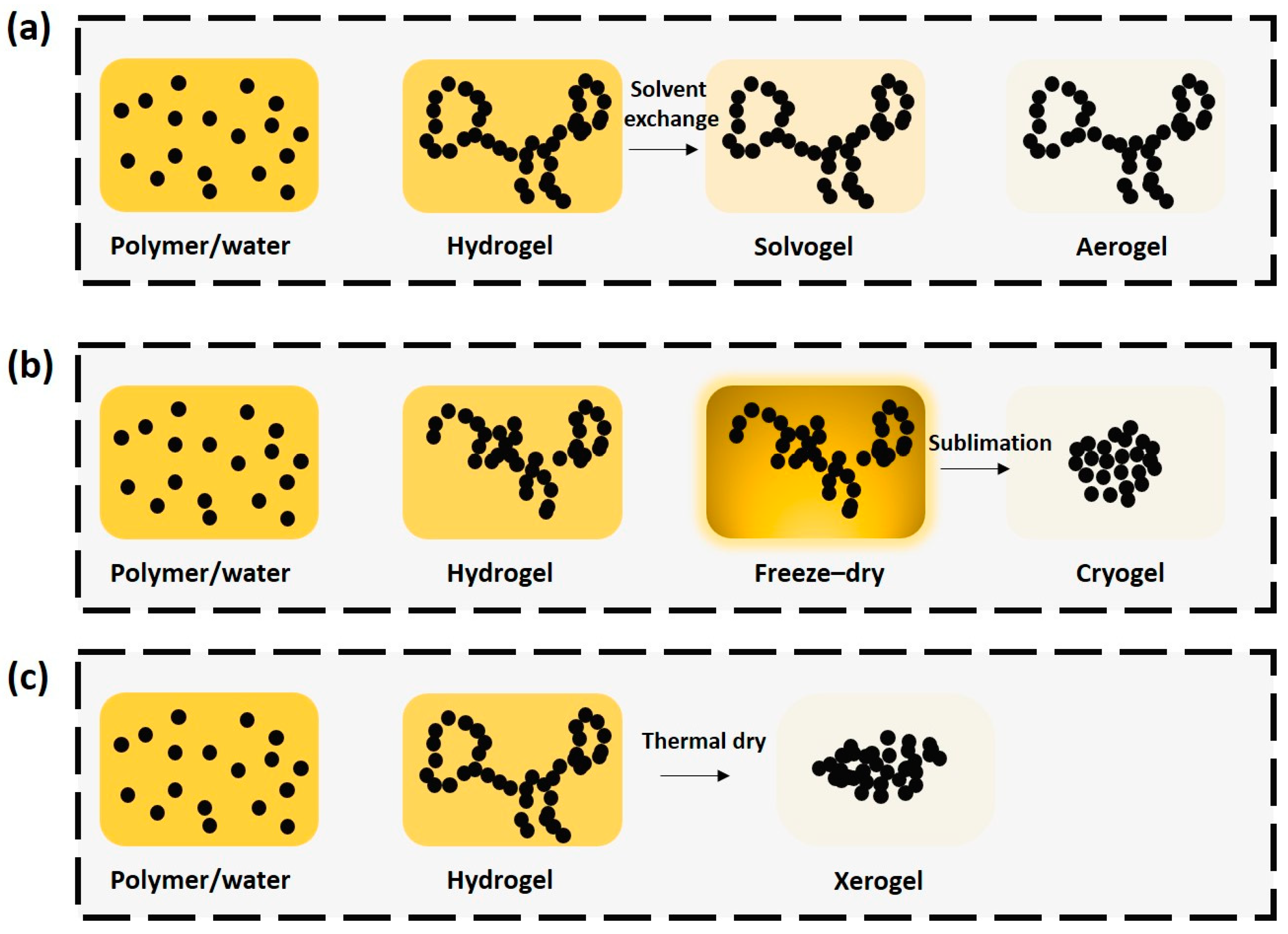
3.2. Drying of Gels
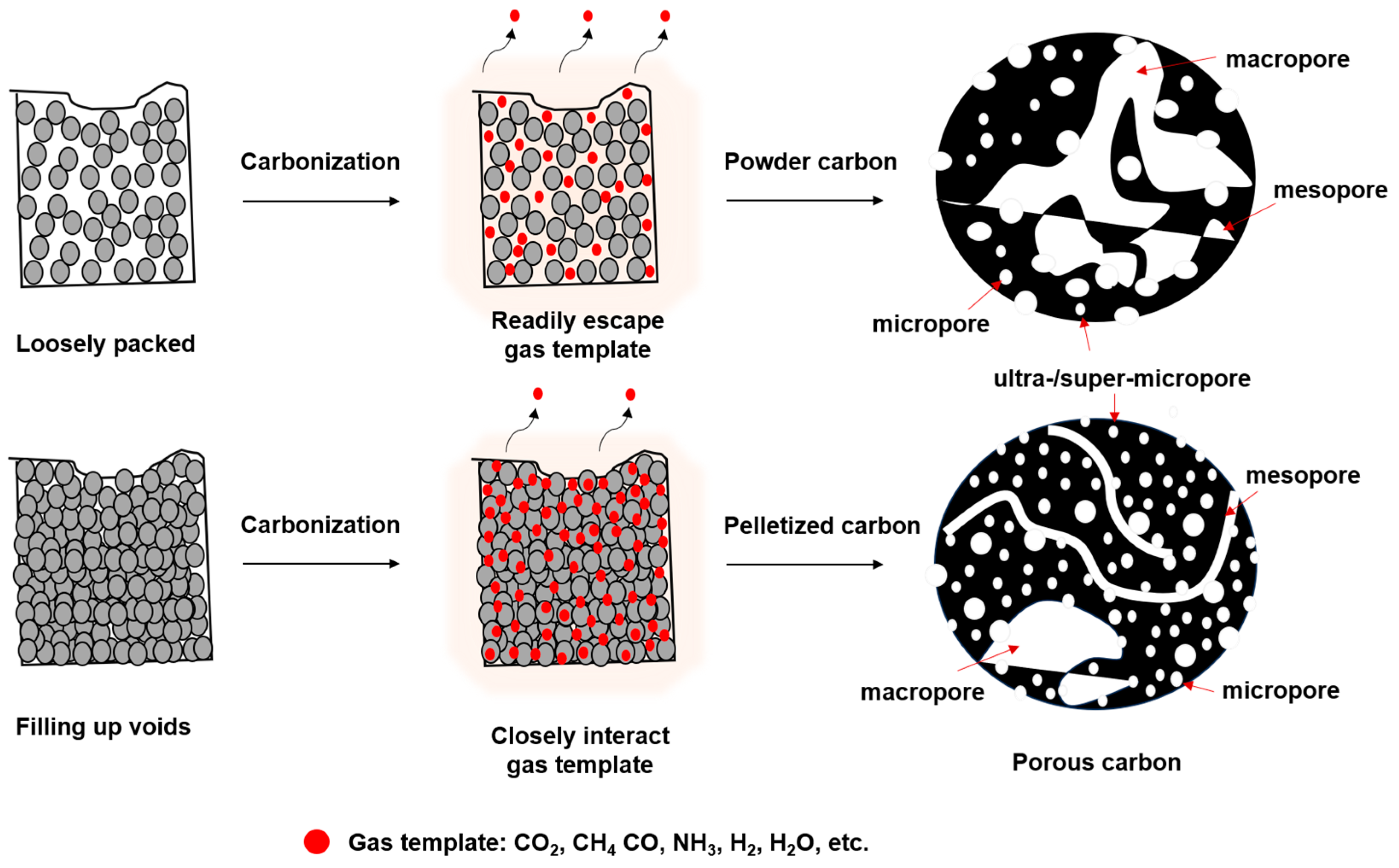
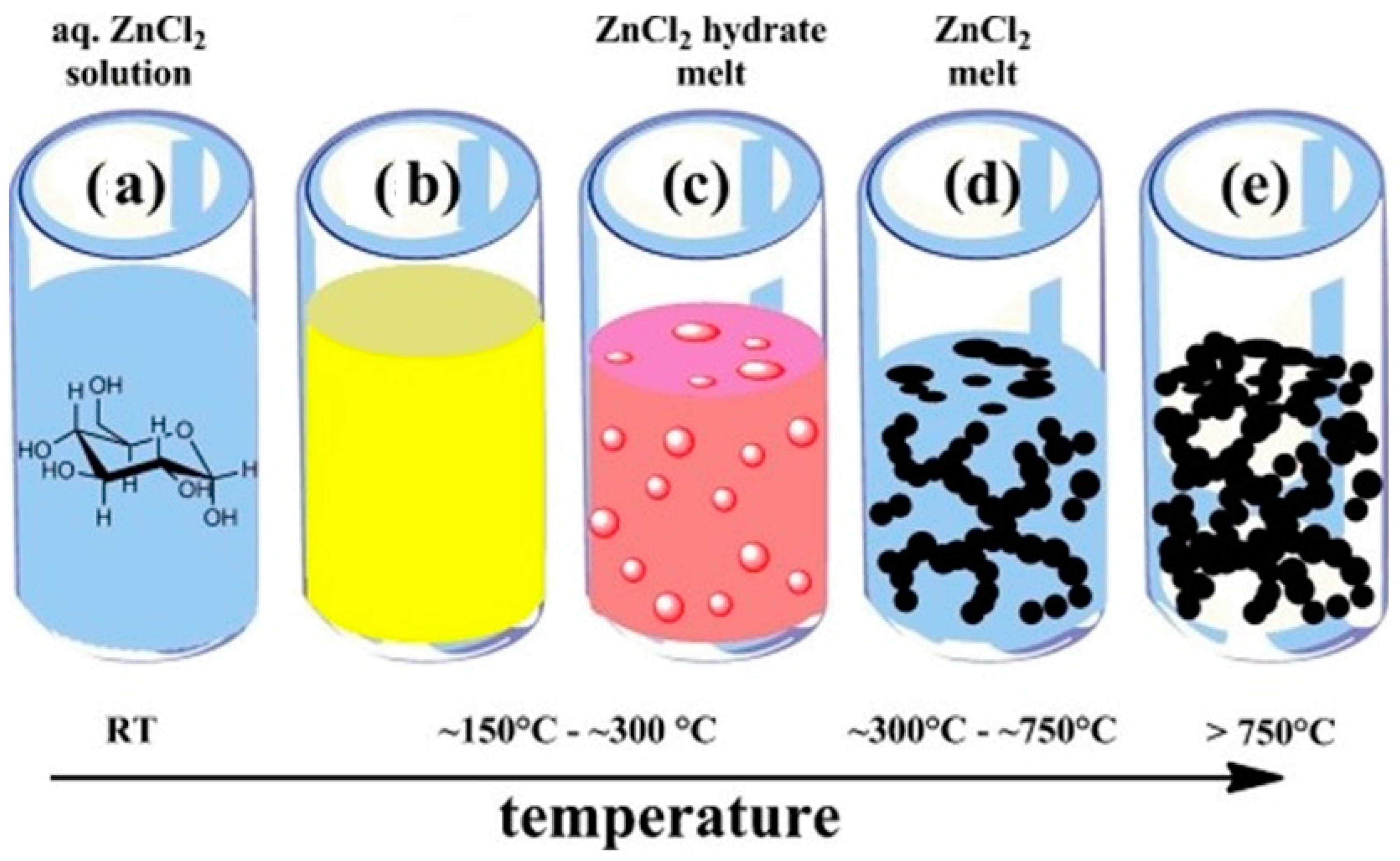
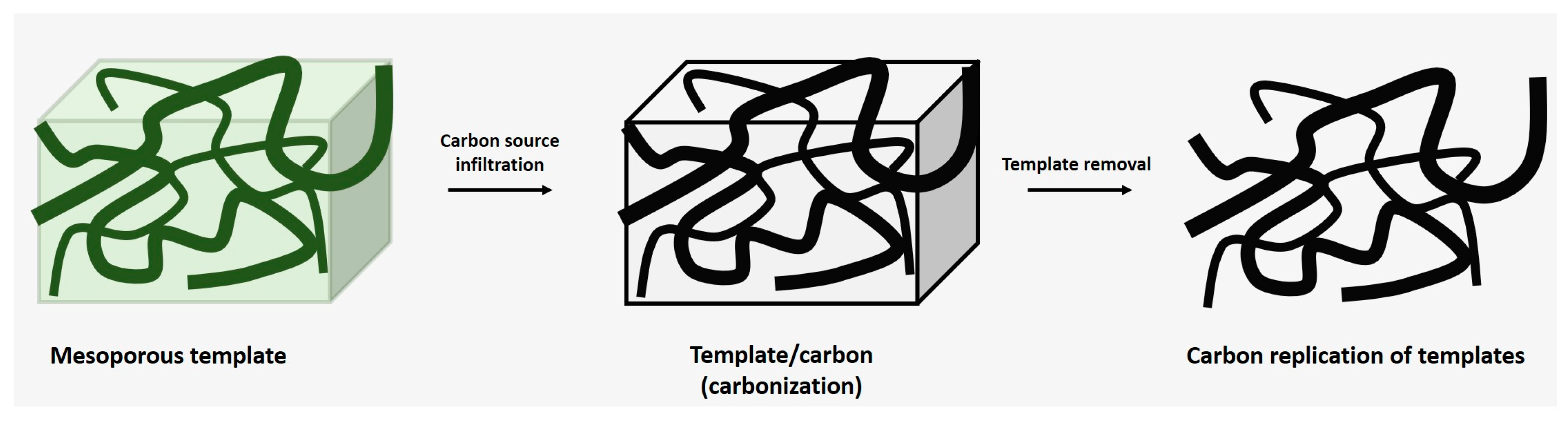
3.3. Carbonization of the Gel
3.4. Activation of the Carbon Gel
4. Carbon-Based Gel Materials for Energy Applications
4.1. Microbial Fuel Cell/Electrical Double-Layer Capacitance
4.2. Supercapacitors
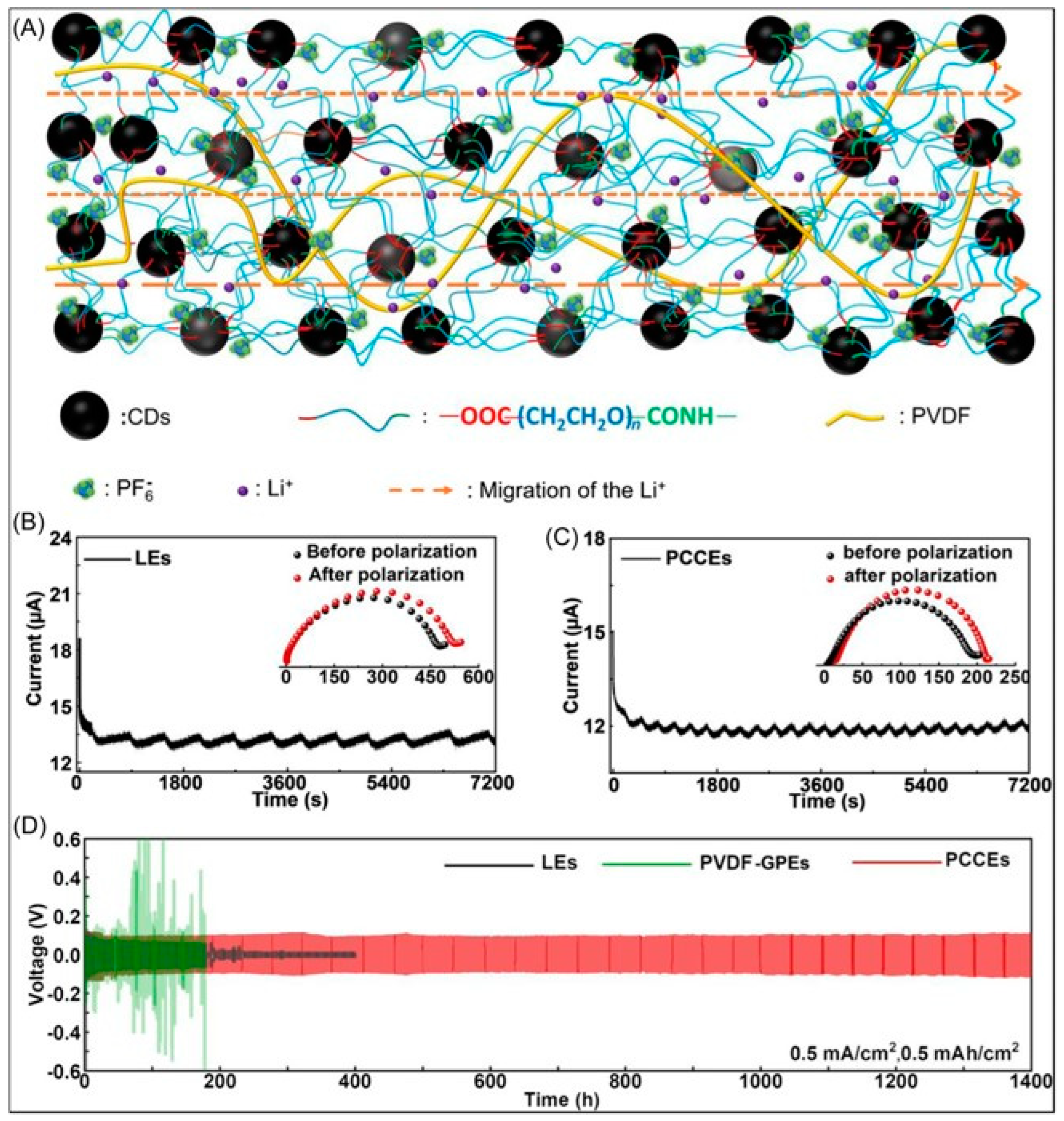
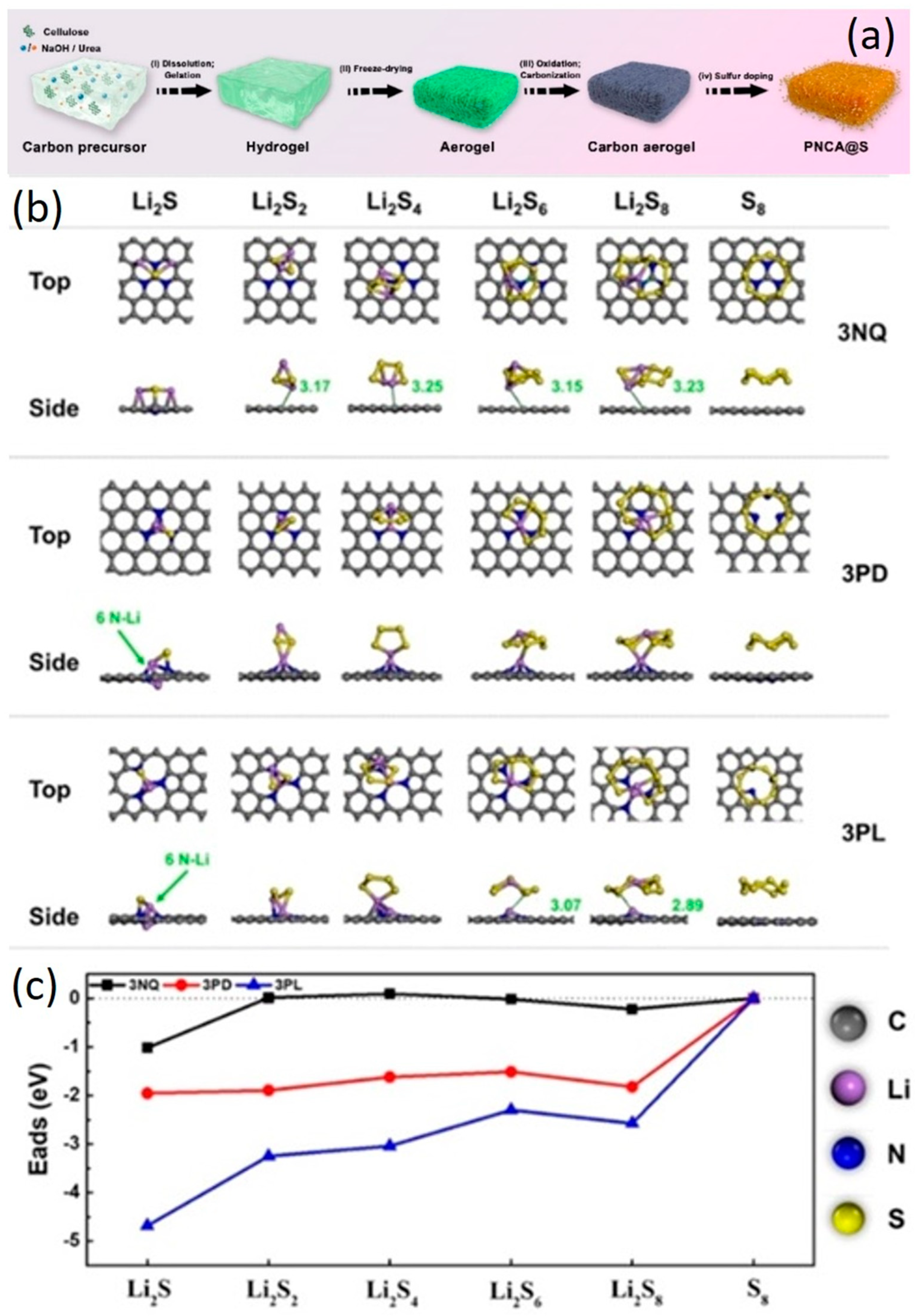
4.3. Lithium Ion/Lithium Sulfur Batteries
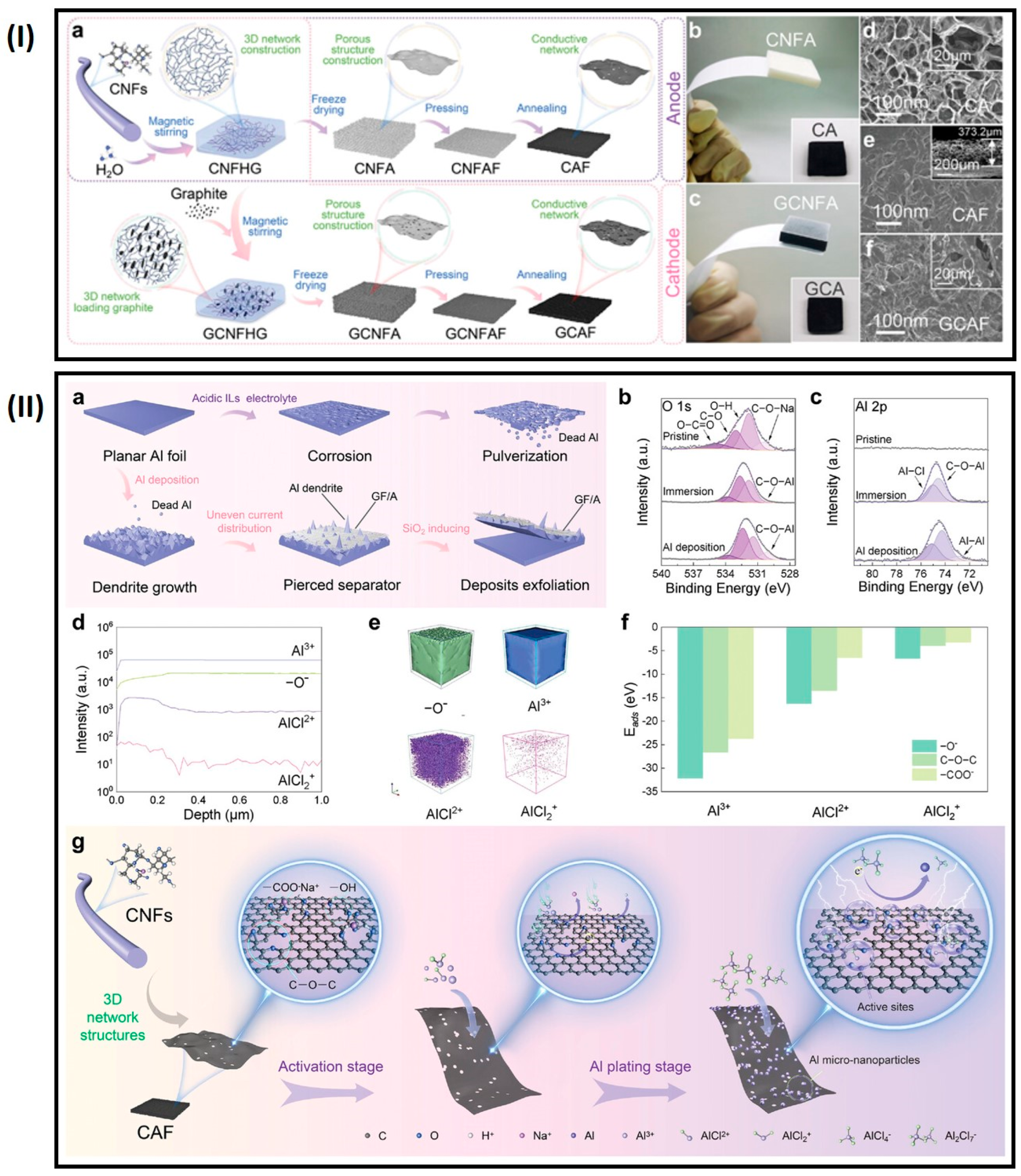
4.4. Al Batteries
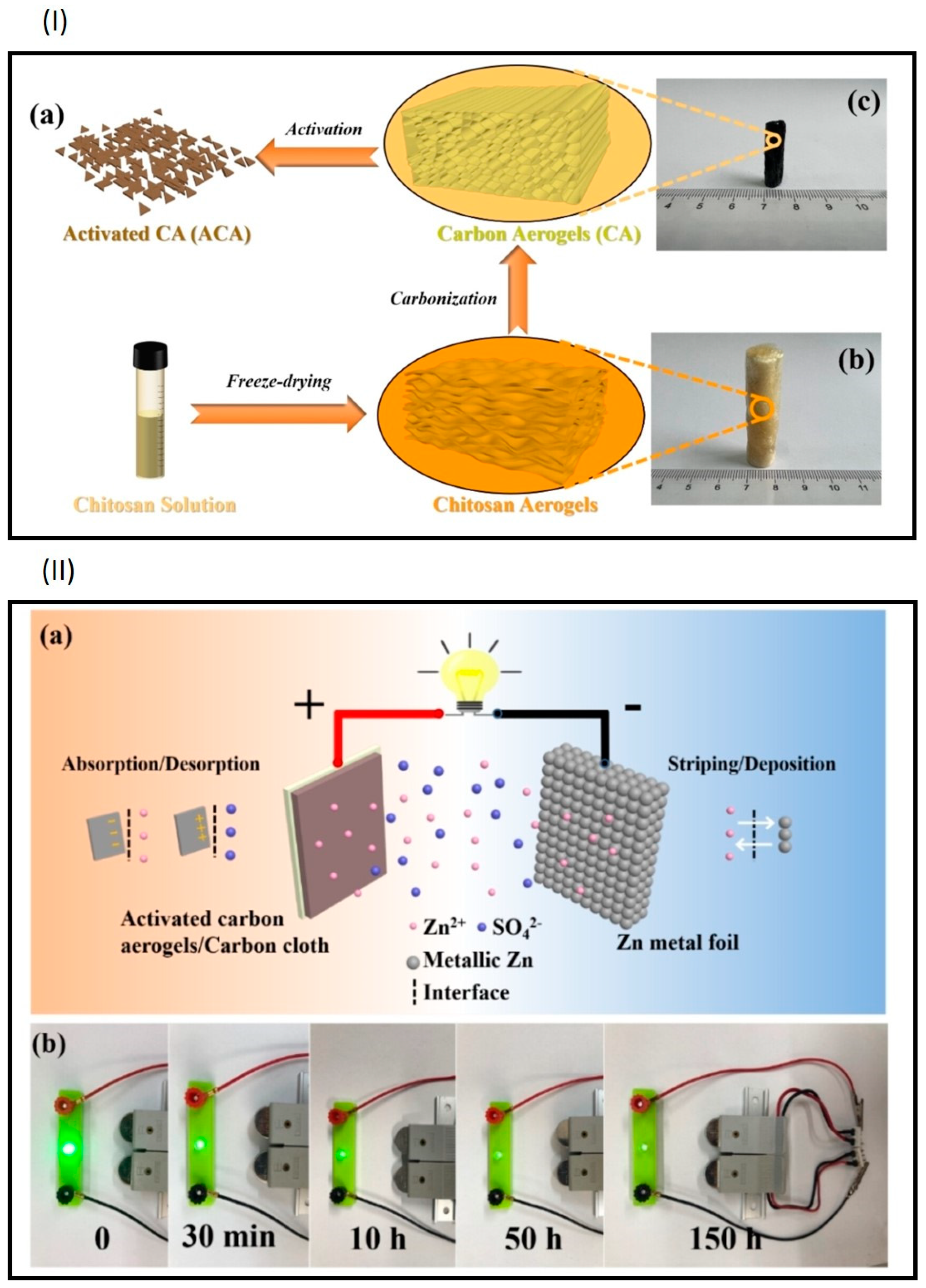
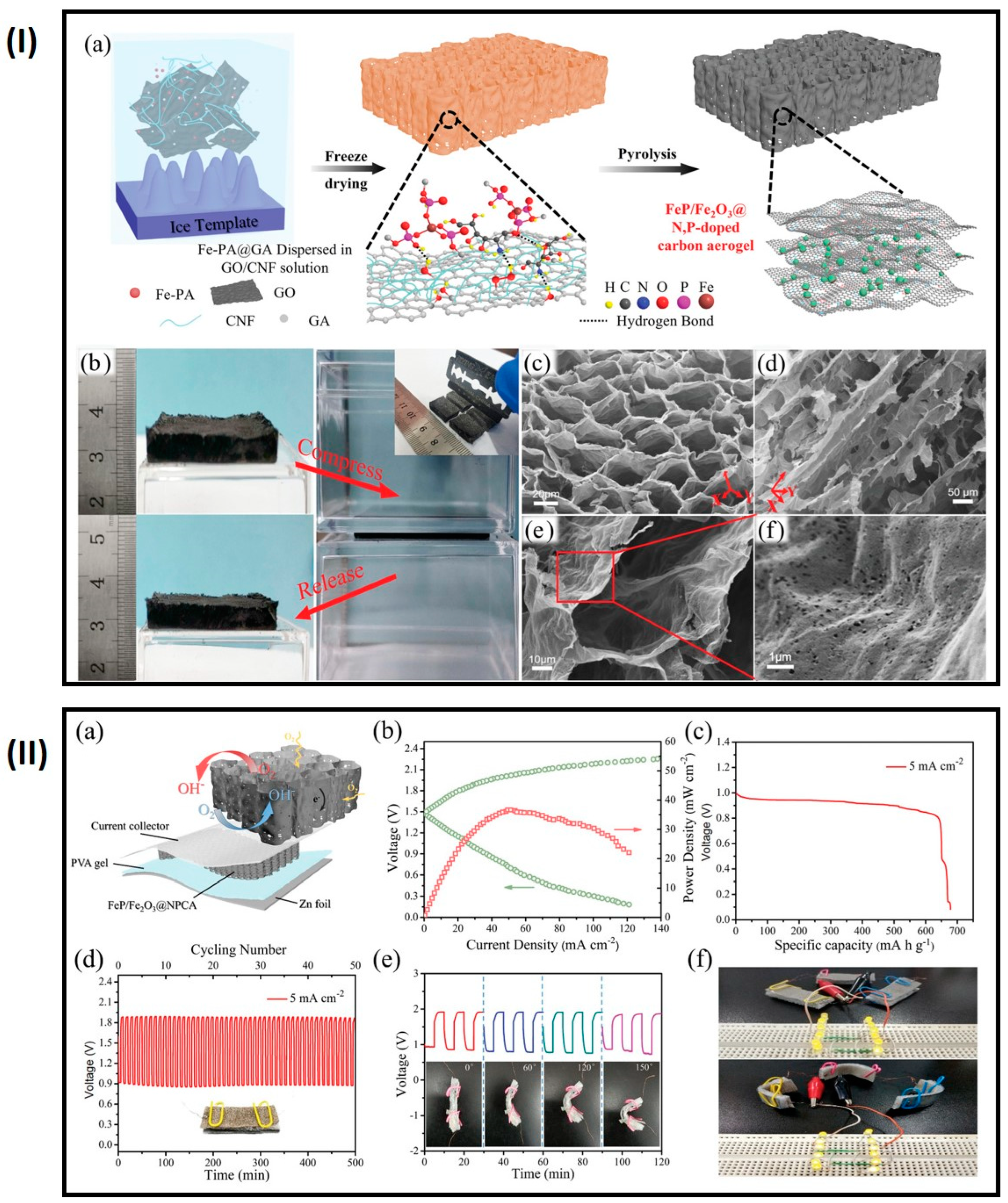
4.5. Zn–Air Batteries
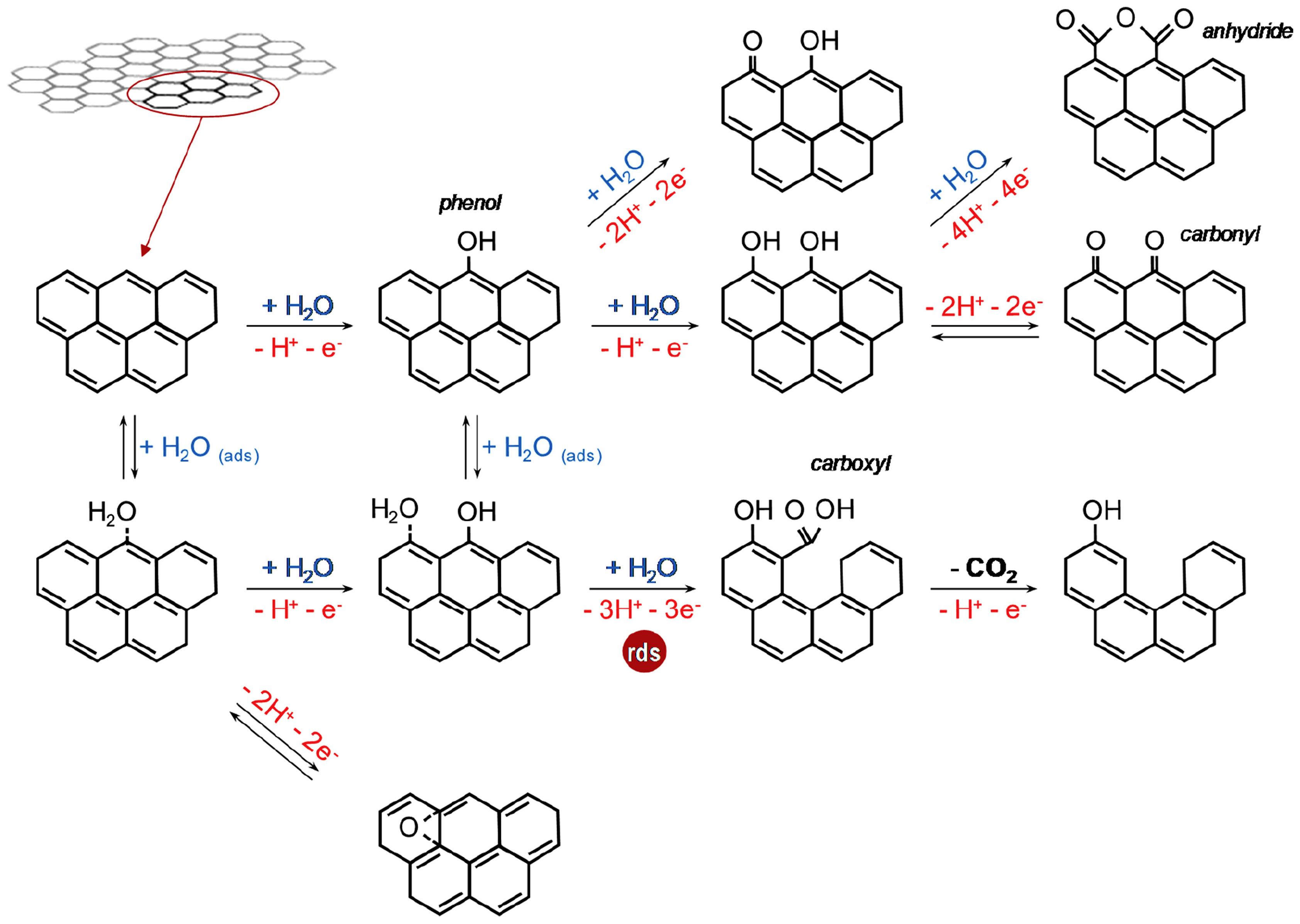
5. Stability of Carbon Materials
6. Sustainable Approach
7. Technology Readiness Levels (TRLs)
| Devices | Materials | Performances | Ref. |
|---|---|---|---|
| Li–S batteries | *C-aerogel | Capacities 1290 mAh g−1 (1st cycle) | [47] |
| Li–S | *C-aerogel | Capacity 1249 mAh g−1 (0.2 C) | [76] |
| *BMFC | *C-xerogel | Power density 895 mW m−3 | [60] |
| *BMFC | *C-felt | Power density 14 mW m−3 | [60] |
| *EDLC | Resorcinol–formaldehyde/KOH (activation) | Specific double-layer capacitance values 186.1 F g−1 at current density 0.1 A g−1 and 168.5 F g−1 at 1 A g−1 | [61] |
| Supercapacitor | Graphene-doped *C xerogel (GAG−/GAG+) | Energy density of 6.6 mWh cm−3, power density 165 W kg−1 | [67] |
| Li ion batteries | Polyethylene glycol–carbon dot composite electrolytes | Ionic conductivity 5.5 mS cm−1, ion transference number 0.71 | [73] |
| Li–S batteries | Glucose/ZnCl2 C gel | Initial capacity of 1290 mAh g−1, capacity 608 mAh g−1 (100 cycles) | [47] |
| Zn ion capacitor | Chitosan aerogel | Energy density 106.5 Wh Kg−1, power density 3108 W Kg−1 | [63] |
| Al batteries | *C aerogel film/graphite composite *C aerogel film | Discharge capacity 115.6 mAh g−1 after 2000 cycles | [89] |
| Zn–air batteries | *C aerogel (N, P doped)/FeP/Fe2O3 | Specific capacity 676 mAh g−1, energy density 517 Wh kg−1 at 5 mA cm−2, overpotential 402 mV at 10 mA cm−2, Tafel slope 86 mV dec−1 | [93] |
| *SSBs | N-, S-co-doped C aerogel | Reversible capacity 788 mAh g−1 at 0.1 C | [116] |
| Potassium supercapacitor | O, N, B–*C aerogel | Energy density51.8 Wh kg−1, power density 443 W kg−1 | [117] |
| OER | Fe, N–*C aerogel | Onset potential 0.9 V, half-potential 0.7 C (alkaline); onset potential 0.8 V, half-potential 0.5 C (acidic) | [118] |
8. Summary and Future Research Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bari, G.A.K.M.R.; Jeong, J.; Barai, H.R. Conductive Gels for Energy Storage, Conversion, and Generation: Materials Design Strategies, Properties, and Applications. Materials 2024, 17, 2268. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Xiao, Q.; Lv, H.; Li, B.; Wu, H.; Lu, Y.; Zhang, C. One-Step Synthesis of Microporous Carbon Monoliths Derived from Biomass with High Nitrogen Doping Content for Highly Selective CO2 Capture. Sci. Rep. 2016, 6, 30049. [Google Scholar] [CrossRef] [PubMed]
- Asrafali, S.P.; Periyasamy, T.; Bari, G.A.K.M.R.; Kim, S.C. Flexible Composite Hydrogels Based on Polybenzoxazine for Supercapacitor Applications. Gels 2024, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Kamran, U.; Rhee, K.Y.; Lee, S.Y.; Park, S.J. Solvent-Free Conversion of Cucumber Peels to N-Doped Microporous Carbons for Efficient CO2 Capture Performance. J. Clean. Prod. 2022, 369, 133367. [Google Scholar] [CrossRef]
- Ren, X.; Li, H.; Chen, J.; Wei, L.; Modak, A.; Yang, H.; Yang, Q. N-Doped Porous Carbons with Exceptionally High CO2 Selectivity for CO2 Capture. Carbon 2017, 114, 473–481. [Google Scholar] [CrossRef]
- Khosrowshahi, M.S.; Abdol, M.A.; Mashhadimoslem, H.; Khakpour, E.; Emrooz, H.B.M.; Sadeghzadeh, S.; Ghaemi, A. The Role of Surface Chemistry on CO2 Adsorption in Biomass-Derived Porous Carbons by Experimental Results and Molecular Dynamics Simulations. Sci. Rep. 2022, 12, 8917. [Google Scholar] [CrossRef]
- Fernandes, D.M.; Peixoto, A.F.; Freire, C. Nitrogen-Doped Metal-Free Carbon Catalysts for (Electro)Chemical CO2 Conversion and Valorisation. Dalton Trans. 2019, 48, 13508–13528. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Guan, Z.; Liu, Y.; Cao, Y.; Zhu, Q.; Liu, H.; Wang, Z.; Zhang, P.; Xu, B. Extended “Adsorption–Insertion” Model: A New Insight into the Sodium Storage Mechanism of Hard Carbons. Adv. Energy Mater. 2019, 9, 1901351. [Google Scholar] [CrossRef]
- Kang, H.J.; Huh, Y.S.; Im, W.B.; Jun, Y.S. Molecular Cooperative Assembly-Mediated Synthesis of Ultra-High-Performance Hard Carbon Anodes for Dual-Carbon Sodium Hybrid Capacitors. ACS Nano 2019, 13, 11935–11946. [Google Scholar] [CrossRef]
- Zhao, L.F.; Hu, Z.; Lai, W.H.; Tao, Y.; Peng, J.; Miao, Z.C.; Wang, Y.X.; Chou, S.L.; Liu, H.K.; Dou, S.X. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Kang, H.J.; Rafiqul Bari, G.A.K.M.; Lee, T.G.; Khan, T.T.; Park, J.W.; Hwang, H.J.; Cho, S.Y.; Jun, Y.S. Microporous Carbon Nanoparticles for Lithium-Sulfur Batteries. Nanomaterials 2020, 10, 2012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Feng, J.; Jiang, Y.; Feng, J. Hierarchically Porous Carbon Aerogels with High Specific Surface Area Prepared from Ionic Liquids via Salt Templating Method. Carbon Lett. 2018, 28, 47–54. [Google Scholar] [CrossRef]
- Duy, P.H.A.; Tu, P.M.; Son, T.T.; Dat, N.M.; Nam, N.T.H.; Cong, C.Q.; Hai, N.D.; An, H.; Huyen, N.T.T.; Minh, P.P.D.; et al. A Facile Fabrication of Zinc Oxide-Doped Carbon Aerogel by Cellulose Extracted from Coconut Peat and Sodium Alginate for Energy Storage Application. J. Appl. Polym. Sci. 2023, 140, e53837. [Google Scholar] [CrossRef]
- Kiefer, R.; Elhi, F.; Puust, L.; Peikolainen, A.L.; Tamm, T. Dual Function Composite Fibers of Cellulose with Activated Carbon Aerogel and Carbide Derived Carbon. J. Appl. Polym. Sci. 2022, 139, 52297. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Ye, J.; Zhu, Y.; Cheng, S.; Ding, Y. Highly Interconnected Nanochannel and Silver, Nitrogen Co-Doped Carbon Aerogel via Metal Organic Knot–Modifying as Active Polysulfide Immobilizer for Lithium–Sulfur Battery. Energy Technol. 2021, 9, 2000736. [Google Scholar] [CrossRef]
- Cai, T.; Kuang, L.; Wang, C.; Jin, C.; Wang, Z.; Sun, Q. Cellulose as an Adhesive for the Synthesis of Carbon Aerogel with a 3D Hierarchical Network Structure for Capacitive Energy Storage. ChemElectroChem 2019, 6, 2586–2594. [Google Scholar] [CrossRef]
- Hua, Y.; Li, X.; Zhang, X.; Zhang, L.; Shu, Y.; Sheng, H.; Fang, H.; Wei, H.; Ding, Y. Active Anchoring Polysulfides of ZnS-Decorated Porous Carbon Aerogel for a High-Performance Lithium-Sulfur Battery. ChemElectroChem 2019, 6, 2570–2577. [Google Scholar] [CrossRef]
- Islam, M.; Ahmed, M.S.; Han, D.; Bari, G.A.R.; Nam, K.W. Electrochemical Storage Behavior of a High-Capacity Mg-Doped P2-Type Na2/3Fe1−yMnyO2 Cathode Material Synthesized by a Sol–Gel Method. Gels 2024, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Saurel, D.; Segalini, J.; Jauregui, M.; Pendashteh, A.; Daffos, B.; Simon, P.; Casas-Cabanas, M. A SAXS Outlook on Disordered Carbonaceous Materials for Electrochemical Energy Storage. Energy Storage Mater. 2019, 21, 162–173. [Google Scholar] [CrossRef]
- Mochida, I.; Yoon, S.H.; Qiao, W. Catalysts in Syntheses of Carbon and Carbon Precursors. J. Braz. Chem. Soc. 2006, 17, 1059–1073. [Google Scholar] [CrossRef]
- Molaiyan, P.; Dos Reis, G.S.; Karuppiah, D.; Subramaniyam, C.M.; García-Alvarado, F.; Lassi, U. Recent Progress in Biomass-Derived Carbon Materials for Li-Ion and Na-Ion Batteries—A Review. Batteries 2023, 9, 116. [Google Scholar] [CrossRef]
- Yang, I.; Jung, M.; Kim, M.S.; Choi, D.; Jung, J.C. Physical and Chemical Activation Mechanisms of Carbon Materials Based on the Microdomain Model. J. Mater. Chem. A Mater. 2021, 9, 9815–9825. [Google Scholar] [CrossRef]
- Canal-Rodríguez, M.; Ramírez-Montoya, L.A.; Villanueva, S.F.; Flores-López, S.L.; Angel Menéndez, J.; Arenillas, A.; Montes-Morán, M.A. Multiphase Graphitisation of Carbon Xerogels and Its Dependence on Their Pore Size. Carbon 2019, 152, 704–714. [Google Scholar] [CrossRef]
- Grunwald, T.; Wilhelm, D.P.; Dambon, O.; Bergs, T. Influence of Glassy Carbon Surface Finishing on Its Wear Behavior during Precision Glass Moulding of Fused Silica. Materials 2019, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Uskoković, V. A Historical Review of Glassy Carbon: Synthesis, Structure, Properties and Applications. Carbon Trends 2021, 5, 100116. [Google Scholar] [CrossRef]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and Properties of Resorcinol-Formaldehyde Organic and Carbon Gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nishimura, T.; Suzuki, T.; Tamon, H. Control of Mesoporosity of Carbon Gels Prepared by Sol-Gel Polycondensation and Freeze Drying. J. Non. Cryst. Solids 2001, 288, 46–55. [Google Scholar] [CrossRef]
- Salimian, S.; Zadhoush, A.; Kotek, N.R.; Ramakrishna, S. A Review on Aerogel: 3D Nanoporous Structured Fillers in Polymer-Based Nanocomposites. Polym. Compos. 2018, 39, 3383–3408. [Google Scholar] [CrossRef]
- Rafiqul Bari, G.A.K.M.; Kim, H. An Easy Fabricable Film for Organic Electronics Based on Phenoxy and Epoxy. Macromol. Chem. Phys. 2019, 220, 1900135. [Google Scholar] [CrossRef]
- Katiyar, S.; Mondal, K.; Sharma, A. One-Step Sol-Gel Synthesis of Hierarchically Porous, Flow-through Carbon/Silica Monoliths. RSC Adv. 2016, 6, 12298–12310. [Google Scholar] [CrossRef]
- Sungyool Bong, D.H. Supercritical, Freezing and Thermal Drying Process of Resorcinol-Formaldehyde Polymer Based Nano-Carbons and Their Highly Loaded PtRu Anode Electrocatalyst for DMFC. Electroanalysis 2019, 31, 1311–1315. [Google Scholar] [CrossRef]
- ElKhatat, A.M.; Al-Muhtaseb, S.A. Advances in Tailoring Resorcinol-Formaldehyde Organic and Carbon Gels. Adv. Mater. 2011, 23, 2887–2903. [Google Scholar] [CrossRef] [PubMed]
- Bari, G.A.K.M.R.; Jeong, J. Comprehensive Insights and Advancements in Gel Catalysts for Electrochemical Energy Conversion. Gels 2024, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyński, T.; Ślosarczyk, A.; Morawski, M. Synthesis of Silica Aerogel by Supercritical Drying Method. Procedia Eng. 2013, 57, 200–206. [Google Scholar] [CrossRef]
- Lee, T.G.; Kang, H.J.; Bari, G.A.K.M.R.; Park, J.W.; Seo, H.W.; An, B.H.; Hwang, H.J.; Jun, Y.S. Macroscopic Graphitic Carbon Nitride Monolith for Efficient Hydrogen Production by Photocatalytic Reforming of Glucose under Sunlight. Chemosphere 2021, 283, 131174. [Google Scholar] [CrossRef] [PubMed]
- Su, L.; Wang, C.Y.; Luo, Z.H.; Wu, J.K.; Zeng, M.; Xiao, Y.W.; Yi, Y. Reverse Microemulsion Synthesis of Mesopore Phloroglucinol-Resorcinol-Formaldehyde Carbon Aerogel Microsphere as Nano-Platinum Catalyst Support for ORR Lei. Chem. Sel. 2020, 5, 538–541. [Google Scholar] [CrossRef]
- Liu, L.; Dai, X.; Kang, H.; Xu, Y.; Hao, W. Structural and Functional Properties of Hydrolyzed/Glycosylated Ovalbumin under Spray Drying and Microwave Freeze Drying. Food Sci. Human Wellness 2020, 9, 80–87. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, Drying Process and Medical Application of Polysaccharide-Based Aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Hwang, H.J.; Kang, H.J.; Rafiqul Bari, G.A.K.M.; Lee, T.G.; An, B.H.; Cho, S.Y.; Jun, Y.S. Hierarchical Porous, N-Containing Carbon Supports for High Loading Sulfur Cathodes. Nanomaterials 2021, 11, 408. [Google Scholar] [CrossRef]
- Bari, G.A.R.; Islam, M.; Jeong, J.H. Materials Design and Development of Photocatalytic NOx Removal Technology. Metals 2024, 14, 423. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Jeong, J.-H. Porous Carbon for CO2 Capture Technology: Unveiling Fundamentals and Innovations. Surfaces 2023, 6, 316–340. [Google Scholar] [CrossRef]
- Bari, G.A.K.M.R.; Kang, H.J.; Lee, T.G.; Hwang, H.J.; An, B.H.; Seo, H.W.; Ko, C.H.; Hong, W.H.; Jun, Y.S. Dual-Templating-Derived Porous Carbons for Low-Pressure CO2 Capture. Carbon Lett. 2023, 33, 811–822. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, J.; Xing, W.; Xue, Q.; Yan, Z.; Zhuo, S.; Qiao, S.Z. Critical Role of Small Micropores in High CO2 Uptake. Phys. Chem. Chem. Phys. 2013, 15, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Lee, T.G.; Bari, G.A.K.M.R.; Seo, H.W.; Park, J.W.; Hwang, H.J.; An, B.H.; Suzuki, N.; Fujishima, A.; Kim, J.H.; et al. Sulfuric Acid Treated G-CN as a Precursor to Generate High-Efficient G-CN for Hydrogen Evolution from Water under Visible Light Irradiation. Catalysts 2021, 11, 37. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Yao, F.; Wang, X.; Zheng, H.; Ye, G.; Cheng, H.; Wu, J.; Huang, H.; Ye, D. One-Pot Synthesis of N-Doped Petroleum Coke-Based Microporous Carbon for High-Performance CO2 Adsorption and Supercapacitors. J. Environ. Sci. 2024, 139, 93–104. [Google Scholar] [CrossRef]
- Jun, Y.S.; Lee, E.Z.; Wang, X.; Hong, W.H.; Stucky, G.D.; Thomas, A. From Melamine-Cyanuric Acid Supramolecular Aggregates to Carbon Nitride Hollow Spheres. Adv. Funct. Mater. 2013, 23, 3661–3667. [Google Scholar] [CrossRef]
- FlorianSchipper, A.V.; Jiawen Ren, R.D.; Fellinger, T.-P. Biomass-Derived Heteroatom-Doped Carbon Aerogels from ASalt Melt Sol–Gel Synthesis and Their Performance in Li–S Batteries. ChemSusChem 2015, 8, 3077–3083. [Google Scholar] [CrossRef]
- Xiao, F.; Bedane, A.H.; Mallula, S.; Sasi, P.C.; Alinezhad, A.; Soli, D.; Hagen, Z.M.; Mann, M.D. Production of Granular Activated Carbon by Thermal Air Oxidation of Biomass Charcoal/Biochar for Water Treatment in Rural Communities: A Mechanistic Investigation. Chem. Eng. J. Adv. 2020, 4, 100035. [Google Scholar] [CrossRef]
- Muthmann, J.; Bläker, C.; Pasel, C.; Luckas, M.; Schledorn, C.; Bathen, D. Characterization of Structural and Chemical Modifications during the Steam Activation of Activated Carbons. Microporous Mesoporous Mater. 2020, 309, 110549. [Google Scholar] [CrossRef]
- Yang, W.; Wu, D.; Fu, R. Porous Structure and Liquid-Phase Adsorption Properties of Activated Carbon Aerogels. J. Appl. Polym. Sci. 2007, 106, 2775–2779. [Google Scholar] [CrossRef]
- Rafiqul Bari, G.A.; Kim, H. High-Refractive-Index and High-Barrier-Capableepoxy-Phenoxy-Based Barrier Film for Organic Electronics. Polym. Adv. Technol. 2020, 31, 1752–1764. [Google Scholar] [CrossRef]
- Liu, X.; Giordano, C.; Antonietti, M. A Facile Molten-Salt Route to Graphene Synthesis. Small 2014, 10, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhao, B.; Zhu, L.; Shao, Z. Molten Salt Synthesis of Nitrogen-Doped Carbon with Hierarchical Pore Structures for Use as High-Performance Electrodes in Supercapacitors. Carbon 2015, 93, 48–58. [Google Scholar] [CrossRef]
- Itoi, H.; Nishihara, H.; Kogure, T.; Kyotani, T. Three-Dimensionally Arrayed and Mutually Connected 1.2-Nm Nanopores for High-Performance Electric Double Layer Capacitor. J. Am. Chem. Soc. 2011, 133, 1165–1167. [Google Scholar] [CrossRef]
- Zhu, S.; Zhao, N.; Li, J.; Deng, X.; Sha, J.; He, C. Hard-Template Synthesis of Three-Dimensional Interconnected Carbon Networks: Rational Design, Hybridization and Energy-Related Applications. Nano Today 2019, 29, 100796. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Z.; Chi, J.; Lei, E.; Liu, Y.; Yin, Y.; Yang, Z.; Ma, C.; Li, W.; Luo, S.; et al. Soft-Template Hydrothermal Synthesis of N and B Co-Doped Walnut-Shaped Porous Carbon Spheres with Hydrophilic Surfaces for Supercapacitors. Appl. Surf. Sci. 2023, 638, 158016. [Google Scholar] [CrossRef]
- Sun, M.; Bian, Z.; Cui, W.; Zhao, X.; Dong, S.; Ke, X.; Zhou, Y.; Wang, J. Pyrolyzing Soft Template-Containing Poly(Ionic Liquid) into Hierarchical N-Doped Porous Carbon for Electroreduction of Carbon Dioxide. Chin. J. Chem. Eng. 2022, 43, 192–201. [Google Scholar] [CrossRef]
- Pushkar, P.; Prakash, O.; Mungray, A.A.; Kumar, K.S.; Chongdar, S.; Mungray, A.K. Evaluation of the Effect of Position and Configuration of Electrodes in Benthic Microbial Fuel Cell. Fuel Cells 2018, 18, 509–517. [Google Scholar] [CrossRef]
- Han, T.H.; Sawant, S.Y.; Hwang, S.J.; Cho, M.H. Three-Dimensional, Highly Porous N-Doped Carbon Foam as Microorganism Propitious, Efficient Anode for High Performance Microbial Fuel Cell. RSC Adv. 2016, 6, 25799–25807. [Google Scholar] [CrossRef]
- Pushkar, P.; Mungray, A.K. Synthesis of 3-Dimensional Resorcinol-Urea-Formaldehyde Carbon Xerogel Electrode and Its Application in Benthic Microbial Fuel Cell. Electrochim. Acta 2019, 317, 281–288. [Google Scholar] [CrossRef]
- Chavhan, M.P.; Ganguly, S. Activated Xerogel Nanoporous-Materials for Energy Storage Applications. Mater. Today Proc. 2018, 5, 9754–9759. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y.; Dunn, B. Where Do Batteries End and Supercapacitors Begin? Science (1979) 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, L.; Jia, D.; Yue, L.; Zhang, H.; Liu, J. Hierarchical Porous Doped Carbon Plates Derived from Chitosan Aerogel as Cathode for High Performance Zn-Ion Hybrid Capacitor. ChemElectroChem 2023, 10, e202200972. [Google Scholar] [CrossRef]
- Cho, Y.; Pak, S.; Lee, Y.G.; Hwang, J.S.; Giraud, P.; An, G.H.; Cha, S.N. Hybrid Smart Fiber with Spontaneous Self-Charging Mechanism for Sustainable Wearable Electronics. Adv. Funct. Mater. 2020, 30, 1908479. [Google Scholar] [CrossRef]
- Zhang, X.; Pei, Z.; Wang, C.; Yuan, Z.; Wei, L.; Pan, Y.; Mahmood, A.; Shao, Q.; Chen, Y. Flexible Zinc-Ion Hybrid Fiber Capacitors with Ultrahigh Energy Density and Long Cycling Life for Wearable Electronics. Small 2019, 15, 1903817. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Amal, R.; Wang, D.W. An Aqueous Metal-Ion Capacitor with Oxidized Carbon Nanotubes and Metallic Zinc Electrodes. Front. Energy Res. 2016, 4, 34. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Flores-López, S.L.; dos Santos-Gómez, L.; Brigandì, A.; Thomas, M.; Stoyanova, A.E.; Lufrano, F.; Arenillas, A. Graphene Doped Carbon-Gels and MnO2 for Next Generation of Solid-State Asymmetric Supercapacitors. ChemElectroChem 2023, 10, e202300161. [Google Scholar] [CrossRef]
- Ren, W.; Ding, C.; Fu, X.; Huang, Y. Advanced Gel Polymer Electrolytes for Safe and Durable Lithium Metal Batteries: Challenges, Strategies, and Perspectives. Energy Storage Mater. 2021, 34, 515–535. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium Battery Chemistries Enabled by Solid-State Electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Yang, P.; Gao, X.; Tian, X.; Shu, C.; Yi, Y.; Liu, P.; Wang, T.; Qu, L.; Tian, B.; Li, M.; et al. Upgrading Traditional Organic Electrolytes toward Future Lithium Metal Batteries: A Hierarchical Nano-SiO2-Supported Gel Polymer Electrolyte. ACS Energy Lett. 2020, 5, 1681–1688. [Google Scholar] [CrossRef]
- Lin, D.; Liu, W.; Liu, Y.; Lee, H.R.; Hsu, P.C.; Liu, K.; Cui, Y. High Ionic Conductivity of Composite Solid Polymer Electrolyte via in Situ Synthesis of Monodispersed SiO2 Nanospheres in Poly(Ethylene Oxide). Nano Lett. 2016, 16, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Mangal, R.; Agrawal, A.; Archer, L.A. A Highly Reversible Room-Temperature Lithium Metal Battery Based on Crosslinked Hairy Nanoparticles. Nat. Commun. 2015, 6, 10101. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.H.; Wei, J.S.; Song, T.B.; Ni, J.W.; Wang, F.; Xiong, H.M. Carbon Dots Crosslinked Gel Polymer Electrolytes for Dendrite-Free and Long-Cycle Lithium Metal Batteries. SmartMat 2022, 3, 323–336. [Google Scholar] [CrossRef]
- Li, X.Y.; Feng, S.; Zhao, M.; Zhao, C.X.; Chen, X.; Li, B.Q.; Huang, J.Q.; Zhang, Q. Surface Gelation on Disulfide Electrocatalysts in Lithium–Sulfur Batteries. Angew. Chem. Int. Ed. 2022, 61, e202114671. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Tian, H.; Su, D.; Zhang, Q.; Wang, G. Advances in Lithium–Sulfur Batteries: From Academic Research to Commercial Viability. Adv. Mater. 2021, 33, 2003666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lu, C.; Bi, Z.; Xu, X.; Ren, X.; Li, X.; Lu, K.; Yuan, S. Preparation of Highly Pyrrolic-Nitrogen-Doped Carbon Aerogels for Lithium-Sulfur Batteries. ChemElectroChem 2021, 8, 895–902. [Google Scholar] [CrossRef]
- Wang, D.Y.; Wei, C.Y.; Lin, M.C.; Pan, C.J.; Chou, H.L.; Chen, H.A.; Gong, M.; Wu, Y.; Yuan, C.; Angell, M.; et al. Advanced Rechargeable Aluminium Ion Battery with a High-Quality Natural Graphite Cathode. Nat. Commun. 2017, 8, 14283. [Google Scholar] [CrossRef]
- Das, S.K. Graphene:ACathodeMaterialof ChoiceforAluminum-IonBatteries. Angew. Chem. Int. Ed. 2018, 57, 16606–16617. [Google Scholar] [CrossRef]
- Yang, H.; Li, H.; Li, J.; Sun, Z.; He, K.; Cheng, H.M.; Li, F. The Rechargeable Aluminum Battery: Opportunities and Challenges. Angew. Chem. Int. Ed. 2019, 58, 11978–11996. [Google Scholar] [CrossRef]
- Zhang, X.; Jiao, S. Modified Al Negative Electrode for Stable High-Capacity Al—Te Batteries. Int. J. Miner. Metall. Mater. 2022, 29, 896–904. [Google Scholar] [CrossRef]
- Tu, J.; Song, W.L.; Lei, H.; Yu, Z.; Chen, L.L.; Wang, M.; Jiao, S. Nonaqueous Rechargeable Aluminum Batteries: Progresses, Challenges, and Perspectives. Chem. Rev. 2021, 121, 4903–4961. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Fu, C.; Meng, P.; Ren, J.; Wang, J.; Bu, J.; Dong, A.; Zhang, J.; Xiao, W.; Sun, B. Challenges and Strategies of Low-Cost Aluminum Anodes for High-Performance Al-Based Batteries. Adv. Mater. 2022, 34, 2102026. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Song, W.L.; Liu, Y.; Wang, W.; Wang, M.; Ge, J.; Jiao, H.; Jiao, S. Stable Quasi-Solid-State Aluminum Batteries. Adv. Mater. 2022, 34, 2104557. [Google Scholar] [CrossRef] [PubMed]
- Faegh, E.; Ng, B.; Hayman, D.; Mustain, W.E. Practical Assessment of the Performance of Aluminium Battery Technologies. Nat. Energy 2021, 6, 21–29. [Google Scholar] [CrossRef]
- Ng, K.L.; Amrithraj, B.; Azimi, G. Nonaqueous Rechargeable Aluminum Batteries. Joule 2022, 6, 134–170. [Google Scholar] [CrossRef]
- Lei, H.; Tu, J.; Yu, Z.; Jiao, S. Exfoliation Mechanism of Graphite Cathode in Ionic Liquids. ACS Appl. Mater. Interfaces 2017, 9, 36702–36707. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, Z.; Tu, J.; Wang, J.; Tian, D.; Liu, Y.; Jiao, S. A Novel Aluminum-Ion Battery: Al/AlCl3-[EMIm]Cl/Ni3S2@Graphene. Adv. Energy Mater. 2016, 6, 1600137. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, W.; Lei, H.; Wang, M.; Jiao, S. Alternate Storage of Opposite Charges in Multisites for High-Energy-Density Al–MOF Batteries. Adv. Mater. 2022, 34, 2110109. [Google Scholar] [CrossRef]
- Lv, A.; Wang, M.; Shi, H.; Lu, S.; Zhang, J.; Jiao, S. A Carbon Aerogel Lightweight Al Battery for Fast Storage of Fluctuating Energy. Adv. Mater. 2023, 35, 2303943. [Google Scholar] [CrossRef]
- Wang, Y.; Waterhouse, G.I.N.; Shang, L.; Zhang, T. Electrocatalytic Oxygen Reduction to Hydrogen Peroxide: From Homogeneous to Heterogeneous Electrocatalysis. Adv. Energy Mater. 2021, 11, 2003323. [Google Scholar] [CrossRef]
- Sumboja, A.; Lübke, M.; Wang, Y.; An, T.; Zong, Y.; Liu, Z. All-Solid-State, Foldable, and Rechargeable Zn-Air Batteries Based on Manganese Oxide Grown on Graphene-Coated Carbon Cloth Air Cathode. Adv. Energy Mater. 2017, 7, 1700927. [Google Scholar] [CrossRef]
- Chen, S.; Chen, S.; Zhang, J. Thermal Sugar Bubbling Preparation of N-Doped PorousCarbon for High-Performance Solid-State Zn-Air Batteries. Batter. Supercaps 2019, 2, 373–379. [Google Scholar] [CrossRef]
- Wu, K.; Zhang, L.; Yuan, Y.; Zhong, L.; Chen, Z.; Chi, X.; Lu, H.; Chen, Z.; Zou, R.; Li, T.; et al. An Iron-Decorated Carbon Aerogel for Rechargeable Flow and Flexible Zn–Air Batteries. Adv. Mater. 2020, 32, 2002292. [Google Scholar] [CrossRef] [PubMed]
- Tong, M.; Sun, F.; Xing, G.; Tian, C.; Wang, L.; Fu, H. Potential Dominates Structural Recombination of Single Atom Mn Sites for Promoting Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2023, 62, e202314933. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Ma, F.; Yang, H.; Wu, X.; Wang, R.; Jia, D.; Wang, Z.; Lu, N.; Ran, F.; Peng, S. Mn, N Co-Doped Co Nanoparticles/Porous Carbon as Air Cathode for Highly Efficient Rechargeable Zn-Air Batteries. Nano Res. 2022, 15, 1942–1948. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, C.; Meng, T.; Pu, Z.; Jin, H.; He, D.; Zhang, J.; Mu, S. Iron Oxide and Phosphide Encapsulated within N,P-Doped Microporous Carbon Nanofibers as Advanced Tri-Functional Electrocatalyst toward Oxygen Reduction/Evolution and Hydrogen Evolution Reactions and Zinc-Air Batteries. J. Power Sources 2019, 413, 367–375. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A.; Niu, J. Doping Strategy, Properties and Application of Heteroatom-Doped Ordered Mesoporous Carbon. RSC Adv. 2021, 11, 5361–5383. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, N.; Zámbó, D.; Sardella, F.; Kißling, P.A.; Schlosser, A.; Graf, R.T.; Pluta, D.; Deiana, C.; Bigall, N.C. Pd-Doped Cellulose Carbon Aerogels for Energy Storage Applications. Adv. Mater. Interfaces 2021, 8, 2100310. [Google Scholar] [CrossRef]
- Xiao, B.; Rojo, T.; Li, X. Hard Carbon as Sodium-Ion Battery Anodes: Progress and Challenges. ChemSusChem 2019, 12, 133–144. [Google Scholar] [CrossRef]
- Kinoshita, K. Carbon: Electrochemical and Physicochemical Properties; Kinoshita, K., Ed.; Wiley: New York, NY, USA, 1988. [Google Scholar]
- Artyushkova, K.; Pylypenko, S.; Dowlapalli, M.; Atanassov, P. Structure-to-Property Relationships in Fuel Cell Catalyst Supports: Correlation of Surface Chemistry and Morphology with Oxidation Resistance of Carbon Blacks. J. Power Sources 2012, 214, 303–313. [Google Scholar] [CrossRef]
- Antonucci, P.L.; Romeo, F.; Minutoli, M.; Alderucci, E.; Giordano, N. Electrochemical Corrosion Behavior of Carbon Black in Phosphoric Acid. Carbon 1988, 26, 197–203. [Google Scholar] [CrossRef]
- Berenguer, R.; Ruiz-Rosas, R.; Gallardo, A.; Cazorla-Amorós, D.; Morallón, E.; Nishihara, H.; Kyotani, T.; Rodríguez-Mirasol, J.; Cordero, T. Enhanced Electro-Oxidation Resistance of Carbon Electrodes Induced by Phosphorus Surface Groups. Carbon 2015, 95, 681–689. [Google Scholar] [CrossRef]
- Jang, S.E.; Kim, H. Effect of Water Electrolysis Catalysts on Carbon Corrosion in Polymer Electrolyte Membrane Fuel Cells. J. Am. Chem. Soc. 2010, 132, 14700–14701. [Google Scholar] [CrossRef] [PubMed]
- Alegre, C.; Sebastián, D.; Lázaro, M.J. Carbon Xerogels Electrochemical Oxidation and Correlation with Their Physico-Chemical Properties. Carbon 2019, 144, 382–394. [Google Scholar] [CrossRef]
- Radovic, L.R.; Mora-Vilches, C.V.; Salgado-Casanova, A.J.A.; Buljan, A. Graphene Functionalization: Mechanism of Carboxyl Group Formation. Carbon 2018, 130, 340–349. [Google Scholar] [CrossRef]
- Cherstiouk, O.V.; Simonov, A.N.; Moseva, N.S.; Cherepanova, S.V.; Simonov, P.A.; Zaikovskii, V.I.; Savinova, E.R. Microstructure Effects on the Electrochemical Corrosion of Carbon Materials and Carbon-Supported Pt Catalysts. Electrochim. Acta 2010, 55, 8453–8460. [Google Scholar] [CrossRef]
- Hsieh, T.H.; Huang, Y.S.; Shen, M.Y. Mechanical Properties and Toughness of Carbon Aerogel/Epoxy Polymer Composites. J. Mater. Sci. 2015, 50, 3258–3266. [Google Scholar] [CrossRef]
- Li, S.; Hou, D.; Cui, Y.; Jia, S.; Lan, G.; Sun, W.; Li, G.; Li, X.; Feng, W. Highly Ordered Carbon Aerogels: Synthesis, Structures, Properties and Applications. Carbon 2024, 218, 118669. [Google Scholar] [CrossRef]
- Kang, S.; Qiao, S.; Cao, Y.; Hu, Z.; Yu, J.; Wang, Y. Compression Strain-Dependent Tubular Carbon Nanofibers/Graphene Aerogel Absorber with Ultrabroad Absorption Band. Chem. Eng. J. 2022, 433, 133619. [Google Scholar] [CrossRef]
- Wu, R.; Ye, Q.; Wu, K.; Wang, L.; Dai, H. Highly Efficient CO2 Adsorption of Corn Kernel-Derived Porous Carbon with Abundant Oxygen Functional Groups. J. CO2 Util. 2021, 51, 101620. [Google Scholar] [CrossRef]
- Jagiello, J.; Chojnacka, A.; Pourhosseini, S.E.M.; Wang, Z.; Beguin, F. A Dual Shape Pore Model to Analyze the Gas Adsorption Data of Hierarchical Micro-Mesoporous Carbons. Carbon 2021, 178, 113–124. [Google Scholar] [CrossRef]
- Hotová, G.; Slovák, V.; Soares, O.S.G.P.; Figueiredo, J.L.; Pereira, M.F.R. Oxygen Surface Groups Analysis of Carbonaceous Samples Pyrolysed at Low Temperature. Carbon 2018, 134, 255–263. [Google Scholar] [CrossRef]
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. J. Carbon Res. 2021, 7, 39. [Google Scholar] [CrossRef]
- Chen, M.; Qin, X.; Zeng, G. Biodegradation of Carbon Nanotubes, Graphene, and Their Derivatives. Trends Biotechnol. 2017, 35, 836–846. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, W.; Zou, R.; Huang, Y.; Lai, H.; Chen, Z.; Peng, X. Cellulose Nanofiber-derived Carbon Aerogel for Advanced Room-temperature Sodium–Sulfur Batteries. Carbon Energy 2023, 5, e203. [Google Scholar] [CrossRef]
- Xie, K.; Liu, X.; Li, H.; Fang, L.; Xia, K.; Yang, D.; Zou, Y.; Zhang, X. Heteroatom Tuning in Agarose Derived Carbon Aerogel for Enhanced Potassium Ion Multiple Energy Storage. Carbon Energy 2024, 6, e427. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Wu, N.; Wang, B.; He, W.; Lei, Y.; Wang, Y. N-Doped 3D Carbon Aerogel with Trace Fe as an Efficient Catalyst for the Oxygen Reduction Reaction. ChemElectroChem 2017, 4, 514–520. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bari, G.A.K.M.R.; Jeong, J.-H. Potential of Carbon Aerogels in Energy: Design, Characteristics, and Applications. Gels 2024, 10, 389. https://doi.org/10.3390/gels10060389
Bari GAKMR, Jeong J-H. Potential of Carbon Aerogels in Energy: Design, Characteristics, and Applications. Gels. 2024; 10(6):389. https://doi.org/10.3390/gels10060389
Chicago/Turabian StyleBari, Gazi A. K. M. Rafiqul, and Jae-Ho Jeong. 2024. "Potential of Carbon Aerogels in Energy: Design, Characteristics, and Applications" Gels 10, no. 6: 389. https://doi.org/10.3390/gels10060389
APA StyleBari, G. A. K. M. R., & Jeong, J.-H. (2024). Potential of Carbon Aerogels in Energy: Design, Characteristics, and Applications. Gels, 10(6), 389. https://doi.org/10.3390/gels10060389







