1. Introduction
For a long time, the in vitro building of tissues/organs has been a goal in biomaterials, tissue engineering, and regenerative medicine [
1]. However, angiogenesis has always been a challenging issue that hinders organ construction and regeneration. Tissues thicker than 1 mm struggle to maintain normal vitality without vascularization, as the diffusion limit of nutrients/oxygen is approximately 100–200 μm [
2,
3].
So far, few studies have reported the successful construction of bioartificial blood vessels, as well as vascular networks, in vitro. For example, Bell et al. assembled a blood vessel using smooth muscle cells, endothelial cells, and collagen in 1986 [
4]. Yan et al. synthesized a polyurethane (PU)/heparin vascular graft for small-caliber vein repair in 2007 [
5]. Xu et al. used three-dimensional (3D)-printed PU for vascular network building in 2008 [
6]. Kirkton et al. designed a blood vessel that entered the clinical trial phase in 2019 [
7]. Nevertheless, when these blood vessels or vascular networks are used for in vivo implantation, they encounter a lot of bottleneck problems, such as an unmatched size and composition, a lack of endothelialized vascular networks, and the appearance of side reactions (e.g., thrombus and tissue necrosis). Consequently, there is an immediate requirement for a structural framework that can encompass the complete vascular hierarchy with the aim of facilitating organ repair and replacement.
Since 2005, 3D printing has been widely applied in the fields of tissue engineering, regenerative medicine, and organ manufacturing, providing a building method that enables the precise control of the 3D structures and cell arrangements in an object or construct [
8,
9,
10,
11]. Traditional 3D bioprinting technologies include extrusion-based 3D printing, inkjet-based 3D printing, and laser-assisted 3D printing [
11,
12]. Among them, extrusion-based 3D printing is currently the most widely used technology due to its ease of use, fast formation, and low cost [
13]. However, the resolution of squeeze-out printing is relatively low, and it is susceptible to external conditions. Recently, a special type of 3D printing, i.e., coaxial printing, has been explored. Compared to the single nozzle used in traditional extrusion-based 3D bioprinting, the nested coaxial nozzles provide more possibility and creativity [
14]. Two different kinds of living cells can be encapsulated in the outer and inner material barrels and 3D printed simultaneously, mimicking the arrangement of the cells in a natural blood vessel [
15].
An essential concern of 3D bioprinting pertains to the capability to consistently generate replicable 3D constructs using bioinks, a category of biomaterials that can encapsulate living cells and bioactive agents. In order to address this objective, hydrogels have gained significant popularity due to their biocompatible milieu and substantial water content. Hydrogels are 3D crosslinked networks of hydrophilic polymers, which are usually soft with a high water content, like the extracellular matrix (ECM) [
16]. Natural hydrogels, such as gelatin and alginate, have played key roles in cell activities, including proliferation, migration, and lineage-specific gene expression [
17,
18,
19]. The interconnected porous structures of the polymeric networks enable the transport of oxygen, nutrients, and metabolites for the encapsulated cells [
20].
Gelatin is a water-soluble protein derived from the partial hydrolysis of the natural polymer collagen with very special properties [
21]. Gelatin solution can form hydrogel via hydrogen bond formation in or among the polymeric molecules below 25 °C, which is a physical crosslinking process. This physical crosslinking is unstable and reversible. When the temperature reaches the phase transition temperature of 25 °C, the gel-state hydrogels return to sol-state solutions again. Considering this feature, gelatin hydrogels can be 3D printed layer-by-layer between 1 and 20 °C, but the poor thermostability of gelatin hydrogels has greatly limited their applications in physiological conditions, such as 37 °C [
22]. The chemical or biochemical crosslinking of gelatin molecules has often been used after 3D printing to enhance the structural stability of the 3D-printed constructs. The commonly used crosslinking agents include aldehydes (such as glutaraldehyde [
23] or glyceraldehyde [
24]), polyoxides [
25], 1-ethyl-3-(3-Dimethylaminopropyl)-carbodiimide [
26], transglutaminase [
27], and genipin [
28]. However, most of these crosslinking agents exhibit cytotoxicity, a high cost, and result in an excessive darkening of color post-crosslinking. In particular, a critical drawback arises when the gelatin molecules are crosslinked with aldehyde agents like glutaraldehyde and glyceraldehyde, as their degradation products can release toxic substances [
23].
In recent years, oxidized polysaccharides have been recognized as effective crosslinking reagents for amino-containing polymers. The oxidized polysaccharide crosslinking agent exhibits a favorable biocompatibility, with degradation products that are non-toxic [
29]. Furthermore, crosslinked gelatin molecules with modified polysaccharides are beneficial for simulating the main components (i.e., proteoglycan) of the ECM and increasing the stability of hydrogels [
30]. Polysaccharides acquire aldehyde groups via oxidation, leading to the formation of covalent crosslinks between the aldehyde groups and the free amino groups of lysine and hydroxylysine in gelatin molecules through the Schiff base reaction mechanism [
31,
32].
In this study, we utilized oxidized pullulan (OxP) to crosslink gelatin molecules, with the objective of extending their degradation rate. To achieve this, we created a dual crosslinked gelatin–alginate hydrogel by combining gelatin with sodium alginate solutions, aiming to mimic the ECM from various angles. 3D coaxial bioprinting technology was effectively created to produce a vascular network, followed by the successful establishment of a vascularized organ model around the vascular network.
3. Discussion
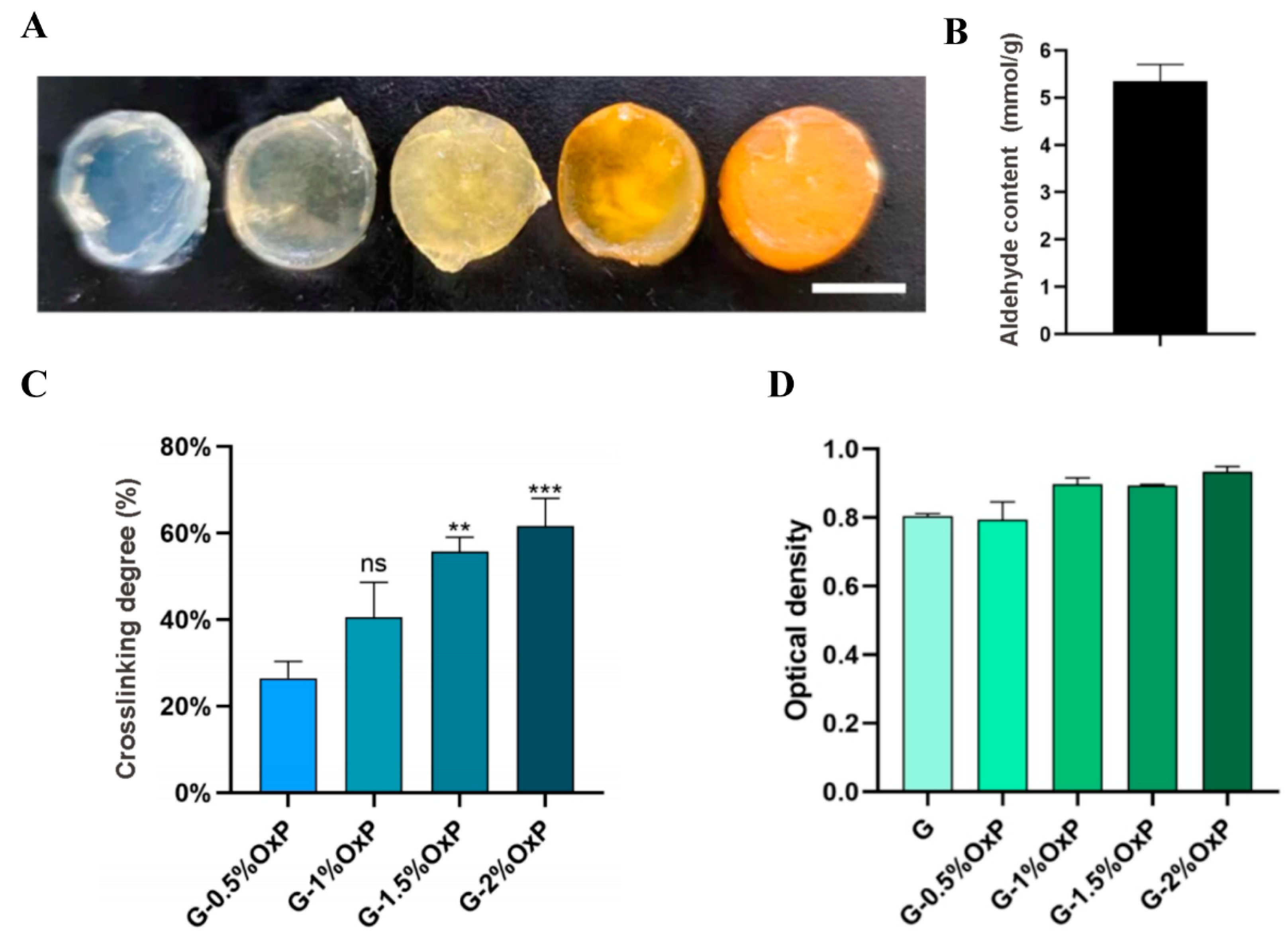
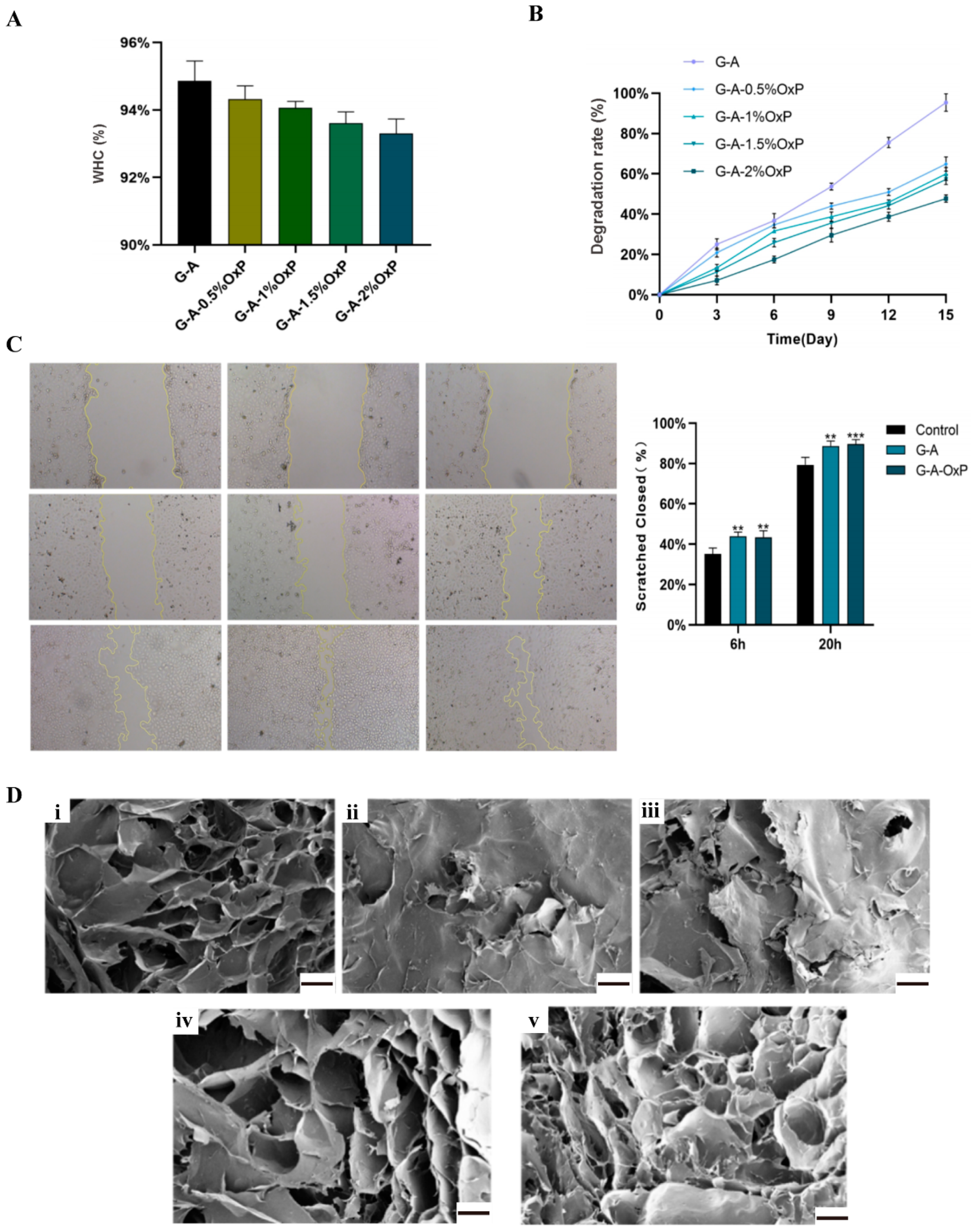
There are several indexes that are very important for hydrogel biomaterials. WHC refers to the capacity of a hydrogel to retain water in the presence of gravitational forces. This characteristic serves as a significant physical and chemical indicator for hydrogel materials. Simultaneously, an appropriate degradation rate of the polymers is also a crucial attribute for hydrogels, as it ensures the maintenance of a stable environment and the provision of adequate growth space for the enclosed cells. When the commonly utilized CaCl2 single-crosslinked gelatin–alginate hydrogel was subjected to a 37 °C culture after 3D printing, the gelatin molecules dissolved or degraded rapidly, resulting in the loss of certain cells and instability of the 3D constructs. However, by crosslinking the gelatin molecules with OxP, the structural stability can be significantly enhanced. An increase in the OxP concentration led to a relative decrease in the degradation rate. This phenomenon can be attributed to the fact that as the degree of OxP crosslinking in the gelatin molecules increased, the overall 3D structure became more compact or tight.
In order to solve the unstable problems of the physical crosslinked gelatin based hydrogels, biocompatible OxP was chosen as the chemical crosslinking agent. OxP was chosen due to its non-toxic nature, anti-bacterial properties, and minimal impact on color alteration, distinguishing it from other alternative crosslinking agents [
33,
34,
35]. A set of tests were carried out on the single- and double-crosslinked gelatin and gelatin–alginate hydrogels with varying concentrations of OxP. The results demonstrated that the hydrogels crosslinked with 2% (
w/
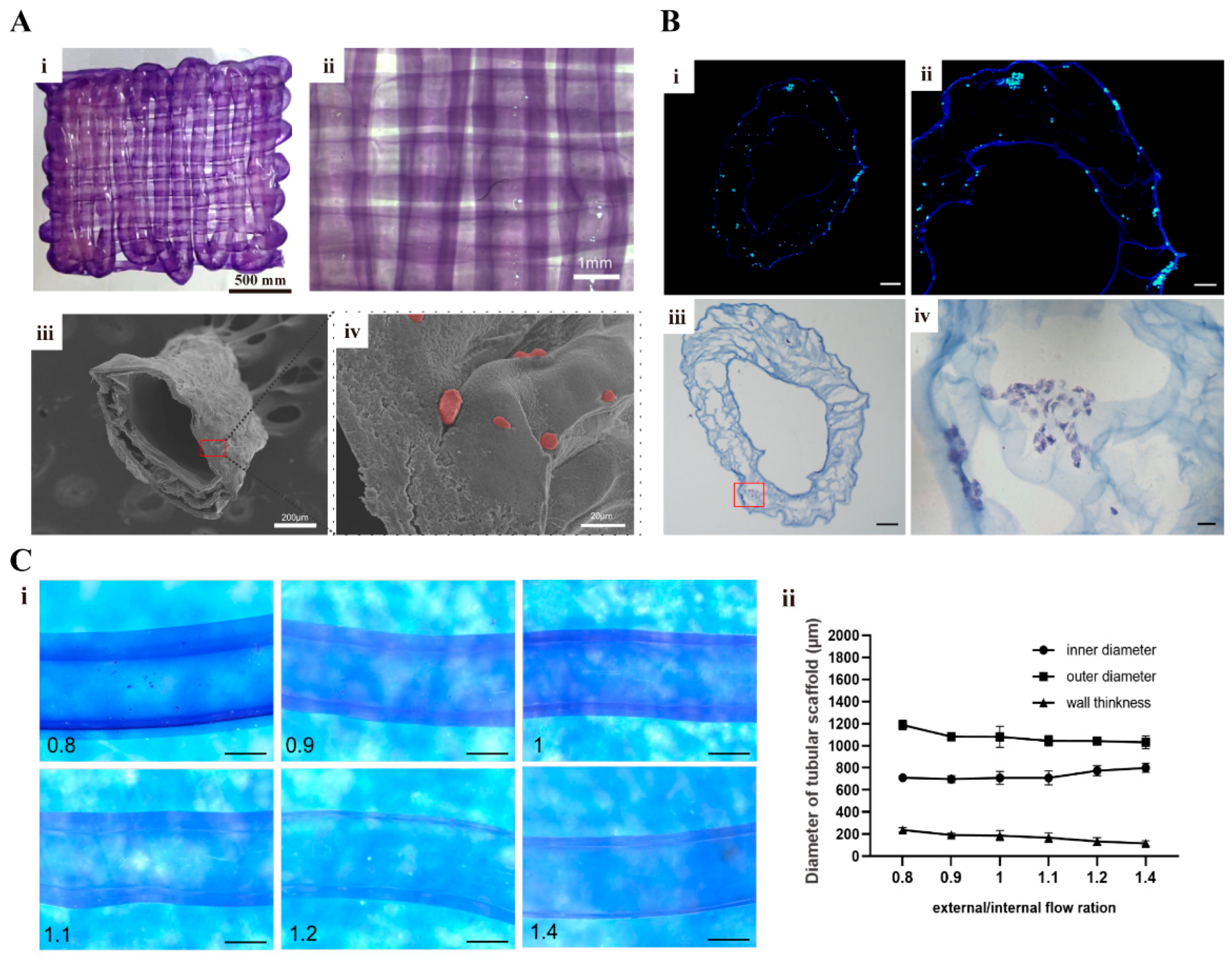
v) OxP exhibited a superior degradation persistence and structural stability compared to the physcial crosslinked gelatin hydrogels. When these hydrogels were 3D printed with a coaxial nozzle, a vascular network with an outer diameter of approximately 1 mm was effectively created, while tubular vascular networks with varying inner diameters and wall thicknesses were achieved by optimizing the extrusion parameters. These findings hold substantial implications for the advancement of complex tissue and organ engineering within the fields of biomaterials, tissue engineering, organ manufacturing, and regenerative medicine.
As stated above, vascularization plays a pivotal role in the successful construction of thick-volume organs for organ transplantation. However, it currently faces many challenges. A novel approach in bioprinting known as coaxial 3D bioprinting has emerged [
36]. This technique involves the simultaneous extrusion of two distinct bioink formulas in a core–shell configuration, enabling the direct formation of bionic vascular structures. The creation of a small-diameter tubular structure, averaging several hundred microns, can be achieved through regulating the inner and outer diameters of the coaxial nozzles or needles. Concurrently, the incorporation of endothelial cells within this structure offers a viable approach to facilitate channel endothelialization. In comparison to some other bioprinting technologies, such as single-nozzle inkjet-bioprinting, coaxial 3D printing has the capability to simultaneously print multiple functionalized biomaterials, thereby establishing a basis for the construction of intricate vascular tissues or organs [
37]. In contrast to photo-crosslinking printing, coaxial printing eliminates the need for extra crosslinking techniques involving ultraviolet light and potential cytotoxic agents [
38].
Alginate solution or hydrogel is the most used bioink in coaxial printing due to its rapid crosslinking properties with Ca
2+ [
39] The alginate bioink and crosslinking CaCl
2 solution can be simultaneously extruded through the coaxial nozzle, resulting in immediate gelation within the dispensing head, followed by the direct formation of a hollow tubular structure. Consequently, when the double-crosslinked gelatin–alginate hydrogel was printed with the coaxial technique, the construction process of the endothelialized vascular networks was greatly facilitated with an enhanced structural stability. In forthcoming research, the manipulation of parameters such as the coaxial needle size, in conjunction with the integration of additional printing technologies, holds promise for the fabrication of full-size, multi-scale functionalized vascular networks. Through the development of intricate systems and the incorporation of various stromal cells, including but not limited to cancer cells, liver cells, and fibroblasts, the in vitro creation of vascularized tissues and organs that closely mimic their natural counterparts of the human body can be achieved.
There are several limitations that exist in this study. Primarily, our focus was on enhancing the biocompatibility, degradability, and printability of the gelatin based hydrogels through double-crosslinking modifications. However, due to the lack of an equivalent, we did not conduct any tests on their mechanical performances, such as tensile strength and torsional resistance. Future endeavors should prioritize the improvement of hydrogels’ mechanical strength and the reinforcement of vascular networks to withstand the filling pressure generated during blood flow. Moreover, the inherent vulnerability of the printed vascular networks, characterized by their thin wall thickness of approximately 200 μm, renders them susceptible to collapse and compromised structural integrity when subjected to prolonged in vitro cultures without supplementary reinforcement. It is hoped that the refinement of the coaxial printing structures can be achieved through incorporating additional biomaterials for the construction of a vascular smooth muscle layer, extending beyond the endothelial layer. Furthermore, the rheological properties of bioinks after OxP crosslinking are hard to define. Subsequent studies may need to integrate different printing technologies with novel bioinks to produce functionalized vascular networks with fully sized and multi-tissue characteristics.
5. Materials and Methods
5.1. Synthesis of OxP and the OxP Crosslinked Gelatin Based Hydrogels
The synthesis process of the OxP referred to the report by Spatareanu and colleagues [
40]. Briefly, a dilute H
2SO
4 solution with a pH of 4.0, at a concentration of 100 mM, was prepared. In total, 2 g of pullulan was weighed and introduced into 70 mL of the diluted acid solution. The mixture was stirred in a dark environment for a duration of 30 min until complete dissolution occurred. Following this, 1.6 g of NaIO
4 was weighed and dissolved in 30 mL of the dilute acid solution, also under dark conditions. The resulting NaIO
4 solution was then added to the pullulan solution and stirred for 4 h.
In our previous study, a TG and CaCl
2 double-crosslinked gelatin–alginate hydrogel, consisting of 5% gelatin and 3% sodium alginate, was formed, with excellent physical, chemical, and biological properties such as water retention, degradation, and biocompatibility [
41]. In this study, our primary goal was to examine the influence of OxP on the gelatin based hydrogels. Consequently, we solely altered the ratio of OxP to gelatin within the OxP and CaCl
2 double-crosslinked gelatin–alginate hydrogels, while maintaining the concentrations of gelatin and sodium alginate at 5% and 3%, respectively, as indicated in
Table 1.
For measuring the crosslinking degree of the OxP crosslinked gelatin molecules, specific amounts of gelatin and OxP were weighed and dissolved in water. The resulting mixture was then subjected to a water bath at a temperature of 65 °C for a duration of 30 min. Once the gelatin was dissolved completely, the mixture was cooled to approximately 40 °C. Subsequently, the gelatin and OxP solutions were mixed thoroughly and allowed to react at a temperature of 37 °C for a period of 12 h.
The crosslinking degree of the gelatin molecules by OxP at various concentrations was assessed using the ninhydrin method, which is usually used to quantitatively measure free amino groups in samples. Simply, the OxP crosslinked hydrogel was mixed with 2 mL of deionized water and 1 mL of a 2% ninhydrin solution, heated in a water bath at 100 °C for 15 min, and then cooled. The quantification of free amino groups was carried out through spectrophotometry at a wavelength of 570 nm. The crosslinking degree was determined by employing the following calculation formula, as demonstrated by OD
d.
where OD
S is the mole fraction of the free amino groups in the OxP crosslinked gelatin hydrogel and OD
nc is the mole fraction of the free amino groups in the pure gelatin hydrogel.
5.2. Pullulan Oxidation Degree Measurement
The reaction between the amino group of hydroxylamine hydrochloride and the aldehyde group resulted in the formation of oxime and the release of HCl. Consequently, the concentration of aldehyde groups was determined by titrating the released HCl with NaOH, providing insight into the oxidation degree of the pullulan.
To determine the oxidation degree of the pullulan, 0.3 g of pullulan and OxP were dissolved in 15 mL of deionized water, respectively. This was followed by adding NaOH solution drop-by-drop while monitoring the pH with a meter until it reached 5.0. Then, 12 mL of a hydroxylamine hydrochloride solution with a concentration of 0.72 mol/L and a pH of 5.0 was introduced to the solution. A magnetic stirrer was used to stir the mixture using a water bath (40 °C) for a duration of 4 h. Upon the completion of the reaction, the sample was titrated with a 0.5 mol/L NaOH solution until the pH returned to 5.0. The aldehyde group content (AC) in the OxP molecules was determined according to the following formula.
Among them, Vc refers to the volume of the NaOH solution consumed in titrating the OxP solution. Vb refers to the volume of the NaOH solution consumed by the pullulan solution. CNaOH (mol/L) refers to the concentration of the NaOH solution and m (g) refers to the mass of pullulan.
5.3. Cytotoxicity Test of the OxP Crosslinked Gelatin Hydrogels
After the hydrogels were individually placed into a freeze-dryer and subjected to a 24 h drying period, six lots of 5 mg gels, each with varying concentrations of OxP, were weighed and sterilized for further use. The gels from each group were then immersed in 5 mL of DMEM/F12 medium containing 10% FBS and 1% penicillin–streptomycin and incubated at 37 °C for 24 h to obtain an extract. ASCs, separated as per our previous studies in passage 3, were subsequently inoculated into a 96-well plate, with each well containing 5000 cells [
11,
12,
41]. Six multiple wells were established for each group, and the cells were cultured for 24 h, denoted as OD
t. Next, the supernatant was aspirated, followed by the gradual addition of culture medium containing CCK-8 (CCK-8: DMEM/F12 culture medium = 1:9). The resulting mixture was then placed in a cell culture incubator and incubated for 2 h. Subsequently, a microplate reader was employed to measure the absorbance value at a wavelength of 450 nm. Cytotoxicity can be derived from the obtained optical density (OD) value according to the following formula.
where, OD
c and OD
bc refer to the optical densities of the control group (culture medium and cells) and blank control group (culture medium), respectively.
5.4. WHC and Degradation Rate of the Double-Crosslinked Hydrogels
The OxP and CaCl
2 double-crosslinked gelatin–alginate hydrogel was placed in individual wells of a 48-well plate, with each well containing 1 mL of the hydrogel. Subsequently, 0.5 mL of a 3% (
w/
v) CaCl
2 solution was added to each well. The plate was then placed in a 37 °C incubator for 30 min. After 12 h, the hydrogels were weighted. After the hydrogels were subjected to a freeze-drying for a period of 24 h, another weight measurement was carried out. The WHCs of the hydrogels were determined using the following formula:
where W
1 and W
2 refer to the wet and dry weights, respectively.
For the degradation rate (DR) measurement, the hydrogels with varying proportions were firstly immersed in PBS solution and incubated at 37 °C for 12 h before being frozen and dried for an additional 12 h, following which, their weights were measured as W1. The hydrogels were then re-immersed in PBS solution for consecutive intervals of 3, 6, 9, 12, and 15 d. Following each immersion period, the freeze-drying process was repeated, and the resulting dry weight was recorded as W2. The DR of the single- or double-crosslinked hydrogels was determined using the following calculation:
5.5. Micropore Structure Characterization
The inner micropore structure of the hydrogels after dehydration was characterized using scanning electron microscopy (SEM). Briefly, after the samples were subjected to 30 s of immersion in liquid nitrogen, they were precisely cut into sections using a surgical blade. The sections underwent gold sputter coating and were examined under SEM for the purpose of microstructural analysis.
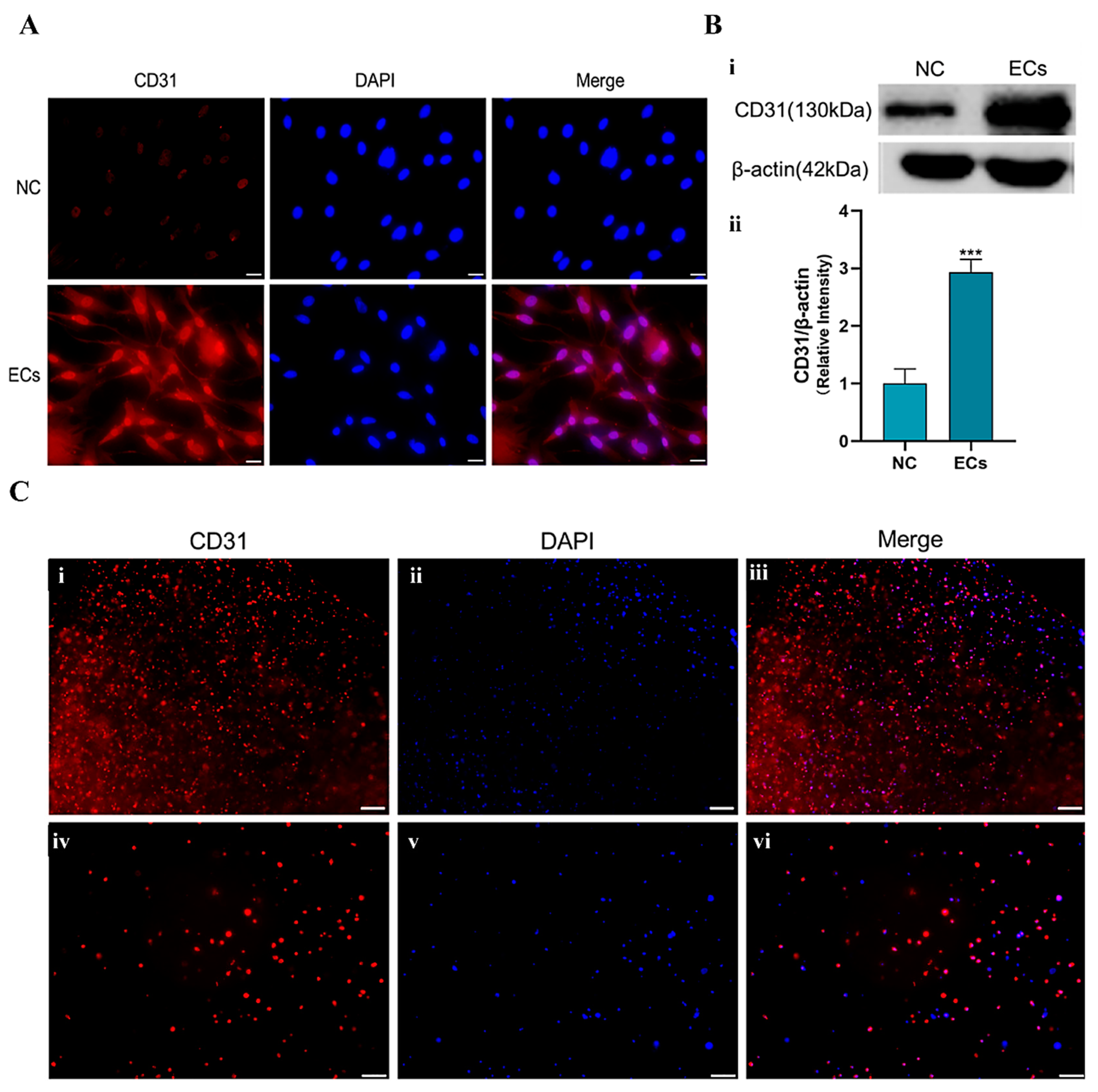
5.6. Induction of the ASCs into Endothelial Cells
According to our previous reports, bFGF and VEGF were used to stimulate the differentiation of the ASCs into endothelial cells [
42,
43]. Here, the cell induction solution (or medium) was prepared according to
Table 2.
Induction steps: (1) weighting 1000 ng of VEGF and 200 ng of b-FGF, respectively, and adding them to 20 mL of DMEM/F-12 culture medium (containing 2% FBS and 1% penicillin–streptomycin) to obtain a cell induction medium; and (2) taking the passage 3 generation ASCs for the experiments. When the ASCs grew to about 85% confluence, they were cultured with the cell induction medium. The induction medium was changed every 48 h for a total of 7 d of induction.
5.7. Identification of the Endothelial-Induced ASCs
Immunofluorescence and Western blot techniques were used to detect the expression of endothelial cell marker CD31.
Immunofluorescence steps: after 7 d of induction, the cells were collected, fixed with paraformaldehyde, and blocked with goat serum for 30 min. Primary antibody (ABclonal, Wuhan, China, CD31Rabbit, 1:500) was added to the samples before they were incubated at 4 °C overnight. Then, a secondary antibody (APEXBIO, Goat Anti-rabbit IgG/AlexaFluro555, 1:200) was added dropwise before the samples were incubated at 37 °C in the dark for 1 h. After the samples were stained with DAPI, they were placed under a fluorescence microscope for observation.
Western blot steps: after 7 d of induction, the cells were collected and placed in a 105 °C metal bath for 5 min to obtain the protein-containing samples. The samples were analyzed via an 8% SDS-PAGE electrophoresis gel, beginning at a voltage of 80 V and completing at 120 V. After 2 h of transfer at 100 V, the protein strips were blocked with 5% skimmed milk powder for 1 h. The primary antibodies CD31 (ABclonal, CD31 Rabbit, 1:1000) and β-actin (ABclonal, 1:1000) were added to the samples before they were placed in a shaker at 4 °C overnight. A corresponding secondary antibody (1:20,000) was then employed before the samples were incubated at room temperature for 1.5 h, washed with TBST, stained with luminescent liquid, and inspected using a fluorescence imager.
5.8. Cell Migration Test
HUVECs were chosen to assess the migratory capability of the cells cultured with the double-crosslinked hydrogel extraction media. The cells, extracts of G-A and G-A-2% OxP hydrogels, and a control (i.e., standard culture medium) were prepared accordingly. Once the cells, cultured in a 6-well plate, reached approximately 90% confluence, they were gently scraped using the tip of a sterile 200 μL pipette to make uniform scratches. The culture medium was then replaced with the extraction media supplemented with 2% FBS. Subsequently, images were captured using an inverted microscope at 0, 6, and 24 h after scratching, and these images were analyzed using Image J software (version 1.8.0). The closure area of the scratches was determined by measuring the distance of cell migration, and the resulting data are expressed as a relative percentage of the initial scratch size.
5.9. Printability and Biocompatibility of the Hydrogels
After a mixture with the final concentration of 5% (w/v), gelatin—3% (w/v), and sodium alginate—2% (w/v) OxP was prepared, it was transferred into the 3D printing barrel. The printing parameters were set up, including a layer height of 0.15 mm, contour filling once, a grid filling degree of 1.5, a printing speed of 3 mm/s, a printing angle ranging from 0 to 90°, a platform temperature of 4 °C, and manual regulated printing air pressure. A computer-aided design (CAD) model, such as a hollow cylinder, cuboid, five-pointed star, and kidney, was established using Solidworks. The 3D printing was carried out according to the predetermined CAD models, followed by crosslinking the 3D objects with a 3% (w/v) CaCl2 solution.
The biocompatibility of the cells in the 3D-printed gelatin–alginate hydrogels was characterized through AO/PI staining. Briefly, the gelatin–alginate-OxP solution (i.e., 5%, gelatin—3%, alginate—2% OxP) was firstly sterilized at 75 °C for 1 h, before the temperature was decreased to 37 °C and ASCs were added with a density of 5 × 105 cells/mL. After 3D printing, the samples were put into a 6-well plate, followed by the addition of 5 mL of DMEM/F-12 complete culture medium to the wells and culturing in a 37 °C, 5% CO2 incubator. After 1, 3, and 14 d of culture, the samples were washed thrice with PBS buffer and stained with 1 mL of AO/PI dye solution for 15 min. Following this, the samples were washed again with PBS solution before being examined with an inverted fluorescence microscope.
5.10. Coaxially 3D Printing Vascular Networks
A micro syringe pump was used to install the coaxial 3D printing instrument. The cell density of the ASCs and HUVECs in the gelatin–alginate–OxP solution (i.e., 5% gelatin—3% alginate—2% OxP) was 5 × 105 cells/mL.
There were several parameters that directly influenced the wall thickness and porosity of the 3D-printed vascular networks. One of the parameters was the extrusion velocities of the inner and outer shaft syringe pumps. The optimized parameters for the coaxial 3D printing nozzle are shown in
Table 3. This means that the inner shaft used 3% CaCl
2 solution with an extrusion speed of 500 μL/min, meanwhile the outer shaft used the gelatin–alginate–OxP solution as the bioink with an extrusion rate of 400–700 μL/min. Post-processing, the 3D objects were cut into sections and checked under a stereomicroscope. The obtained images were then analyzed using ImageJ to calculate the inner diameter, outer diameter, and wall thickness of the vascular networks. Statistical analysis was performed using Prism 8.0.2 software.
5.11. Histological Characterization of the Cell-Containing Vascular Networks
After 3D printing, the tubular vascular networks were cultured for a duration of three days, followed by freeze-drying and subsequent staining with DAPI, as stated above. The stained samples were observed using Olympus Fluorescence microscopy.
For HE staining, the cut samples were immersed in PBS buffer for a duration of 10 min after rewarming. Following this step, the samples were exposed to a hematoxylin staining solution for a period of 4 min and rinsed with tap water three times. A differentiation solution was used for a duration of 3 s, followed by another three rinses with tap water. After the blue solution was restored for a duration of 40 s and subsequently soaked in tap water for 30 s, eosin staining was performed for a duration of 1 min, followed by a 20 s soak in tap water. The samples were finally watched under an upright microscope after being dehydrated and sealed with neutral gum.
5.12. Construction of the Vascularized Tissues
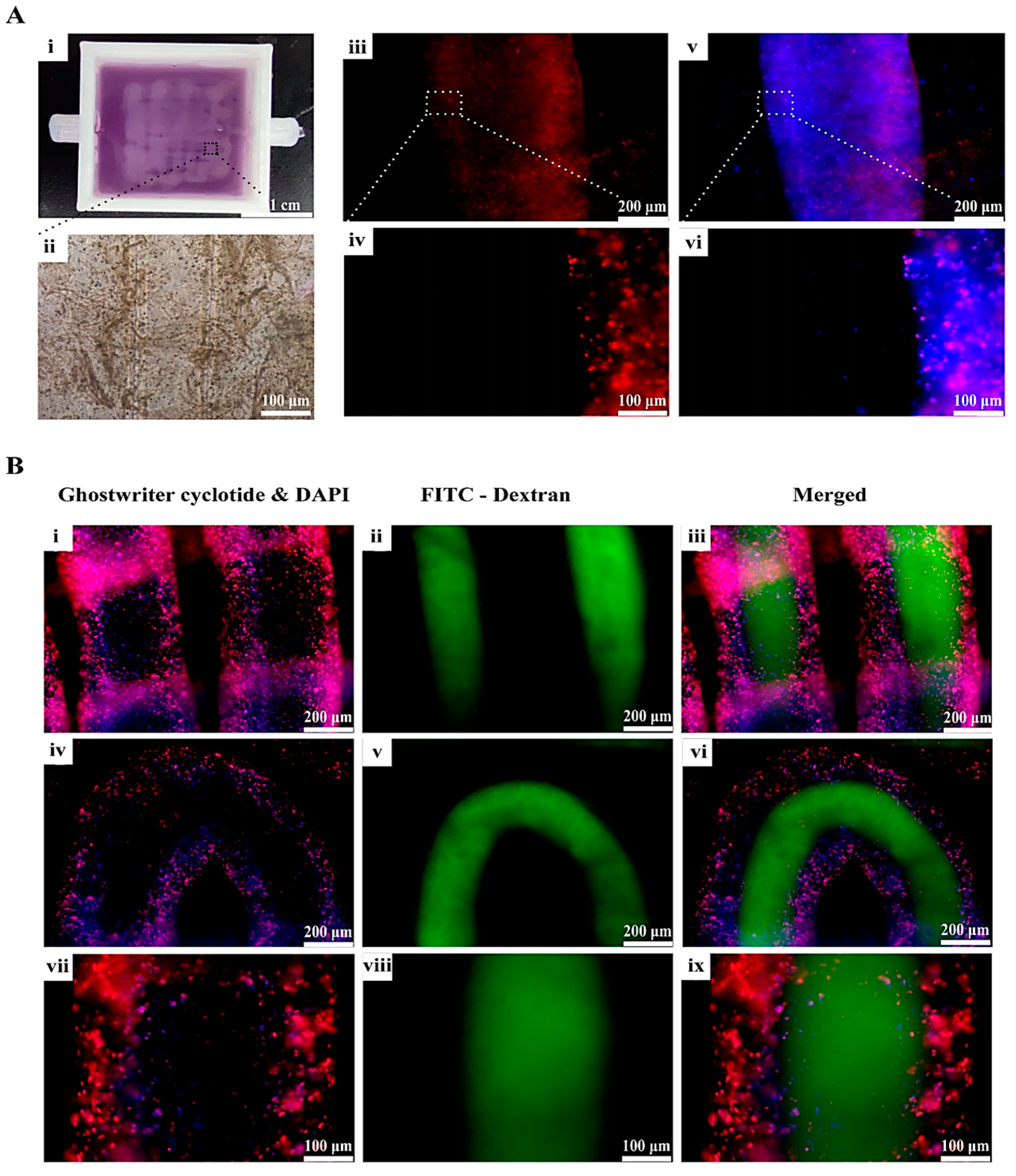
The design of the vascularized tissues with go-through channels was accomplished using SolidWorks software (SolidWorks 2021), followed by the construction of the physical CAD model utilizing extrusion-based 3D printing technologies. Upon the completion of the 3D printing process, the 3D-printed constructs were crosslinked using a 3% (w/v) CaCl2 solution before they were cultured and examined.
5.13. Phalloidin Staining and Vascular Connectivity Testing
It has been reported that fluorescently labeled phalloidin exhibits a specific affinity towards F-actins, which led to the fluorescence labelling of the cytoskeleton and visualization of the cellular growth dynamics within the 3D constructs [
44].
AbFluor 594-labeled Phalloidin was employed for the purpose of staining the coaxially printed vascular networks, thereby facilitating enhanced observation of the growth status of the HUVECs within the vascular networks. The procedure entailed, after the 3D-printed constructs were put into a 6-well plate and stained with phalloidin, fixing the samples with 4% paraformaldehyde and washing them three times with PBS buffer supplemented with 0.1% TritonX-100. AbFluor594-labeled Phalloidin (Abbkine) was then added in the dark and incubated at room temperature for 1 h. A DAPI dye solution was then added dropwise and incubated for a period of 5 min. After removing the DAPI solution, the samples were washed again, with each wash lasting 5 min.
To assess the connectivity of the vascular network, a 1 mL syringe was employed to administer a gradual injection of a 25 μg/mL FITC-conjugated 40 kDa dextran solution into a specific segment of the vascular network. Upon the solution’s emergence from the opposite end, a hamostatic clip was utilized to secure the two sections. Subsequently, PBS was employed for further rinsing to eliminate any residual fluorescent solution at the external end. Finally, the samples were observed using an inverted fluorescence microscope.