Synthesis of Ni-Doped Graphene Aerogels for Electrochemical Applications
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamba, P.; Singh, P.; Singh, P.; Singh, P.; Kumar, A.; Gupta, M.; Kumar, Y. Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance. J. Energy Storage 2022, 48, 103871. [Google Scholar] [CrossRef]
- Thakur, A.; Devi, P. Paper-based flexible devices for energy harvesting, conversion and storage applications: A review. Nano Energy 2022, 94, 106927. [Google Scholar] [CrossRef]
- Venkatesan, S.; Mitzel, J.; Wegner, K.; Costa, R.; Gazdzicki, P.; Friedrich, K.A. Nanomaterials and films for polymer electrolyte membrane fuel cells and solid oxide cells by flame spray pyrolysis. Renew. Sustain. Energy Rev. 2022, 158, 112080. [Google Scholar] [CrossRef]
- Yadlapalli, R.T.; Alla, R.R.; Kandipati, R.; Kotapati, A. Super capacitors for energy storage: Progress, applications and challenges. J. Energy Storage 2022, 49, 104194. [Google Scholar] [CrossRef]
- Jiang, B.; Cao, L.; Yuan, Q.; Ma, Z.; Huang, Z.; Lin, Z.; Zhang, P. Biomass Straw-Derived Porous Carbon Synthesized for Supercapacitor by Ball Milling. Materials 2022, 15, 924. [Google Scholar] [CrossRef] [PubMed]
- Sahu, R.K.; Gangil, S.; Bhargav, V.K.; Sahu, P.; Ghritalahre, B. Synthesizing biomass into nano carbon for use in high-performance supercapacitors—A brief critical review. J. Energy Storage 2023, 72, 108348. [Google Scholar] [CrossRef]
- Temesgen, T.; Bekele, E.T.; Gonfa, B.A.; Tufa, L.T.; Sabir, F.K.; Tadesse, S.; Dessie, Y. Advancements in biomass derived porous carbon materials and their surface influence effect on electrode electrochemical performance for sustainable supercapacitors: A review. J. Energy Storage 2023, 73, 109293. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Yao, F.; Wang, X.; Zheng, H.; Ye, G.; Cheng, H.; Wu, J.; Huang, H.; Ye, D. One-pot synthesis of N-doped petroleum coke-based microporous carbon for high-performance CO2 adsorption and supercapacitors. J. Environ. Sci. 2024, 139, 93–104. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Tailoring porosity in carbon materials for supercapacitor applications. Mater. Horiz. 2014, 1, 157–168. [Google Scholar] [CrossRef]
- Madheswaran, D.K.; Thangavelu, P.; Krishna, R.; Thangamuthu, M.; Joseph Chandran, A.; Colak, I. Carbon-based materials in proton exchange membrane fuel cells: A critical review on performance and application. Carbon Lett. 2023, 33, 1495–1518. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Lakshmy, S.; Santhosh, S.; Kalarikkal, N.; Chakraborty, B.; Rout, C.S. Recent Developments and Future Perspective on Electrochemical Glucose Sensors Based on 2D Materials. Biosensors 2022, 12, 467. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Tryk, D.A. The Role of Carbon Blacks as Catalyst Supports and Structural Elements in Polymer Electrolyte Fuel Cells. In Nanocarbons for Energy Conversion: Supramolecular Approaches; Nakashima, N., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 81–118. [Google Scholar]
- Roshandel, R.; Farhanieh, B.; Saievar-Iranizad, E. The effects of porosity distribution variation on PEM fuel cell performance. Renew. Energy 2005, 30, 1557–1572. [Google Scholar] [CrossRef]
- Walcarius, A. Mesoporous Materials-Based Electrochemical Sensors. Electroanalysis 2015, 27, 1303–1340. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Zhuge, W.; Huang, Q.; Xiang, G.; Peng, J. Conductive aluminum phthalocyanine-based porous organic polymer as an efficient electrocatalyst for nifedipine detection. Sens. Actuators B Chem. 2024, 404, 135191. [Google Scholar] [CrossRef]
- van Schalkwijk, W.; Scrosati, B. Advances in Lithium Ion Batteries Introduction. In Advances in Lithium-Ion Batteries; van Schalkwijk, W.A., Scrosati, B., Eds.; Springer: Boston, MA, USA, 2002; pp. 1–5. [Google Scholar]
- Canal-Rodríguez, M.; Arenillas, A.; Menéndez, J.A.; Beneroso, D.; Rey-Raap, N. Carbon xerogels graphitized by microwave heating as anode materials in lithium-ion batteries. Carbon 2018, 137, 384–394. [Google Scholar] [CrossRef]
- Ciszewski, M.; Koszorek, A.; Radko, T.; Szatkowski, P.; Janas, D. Review of the Selected Carbon-Based Materials for Symmetric Supercapacitor Application. J. Electron. Mater. 2019, 48, 717–744. [Google Scholar] [CrossRef]
- Caméan, I.; Lobato, B.; Rey-Raap, N.; dos Santos-Gómez, L.; Flores-López, S.; Arenillas, A.; García, A.B. Optimizing the Performance of a Graphitized Carbon Xerogel as Cathode for Sodium Dual-Ion Batteries. ChemElectroChem 2023, 10, e202201069. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, M.; Zheng, L.; Guan, D.; Huang, X.; Xu, J.; Yu, J. Porous Materials Applied in Nonaqueous Li–O2 Batteries: Status and Perspectives. Adv. Mater. 2020, 32, 2002559. [Google Scholar] [CrossRef]
- Sultanov, F.; Mentbayeva, A.; Kalybekkyzy, S.; Zhaisanova, A.; Myung, S.-T.; Bakenov, Z. Advances of graphene-based aerogels and their modifications in lithium-sulfur batteries. Carbon 2023, 201, 679–702. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, Z.; Xu, B.; Yue, S.; Luo, Y.; Zhan, W.; Liu, B. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J. Mater. Chem. 2011, 21, 6494–6497. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Campbell, P.G.; Baumann, T.F.; Worsley, M.A. Carbon aerogel evolution: Allotrope, graphene-inspired, and 3D-printed aerogels. J. Mater. Res. 2017, 32, 4166–4185. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Angel Menéndez, J.; Arenillas, A. RF xerogels with tailored porosity over the entire nanoscale. Microporous Mesoporous Mater. 2014, 195, 266–275. [Google Scholar] [CrossRef]
- Alonso-Buenaposada, I.D.; Rey-Raap, N.; Calvo, E.G.; Menendez, J.A.; Arenillas, A. Acid-based resorcinol-formaldehyde xerogels synthesized by microwave heating. J. Sol-Gel Sci. Technol. 2017, 84, 60–69. [Google Scholar] [CrossRef]
- Veselov, G.B.; Vedyagin, A.A. Resorcinol–Formaldehyde-Derived Carbon Xerogels: Preparation, Functionalization, and Application Aspects. Materials 2023, 16, 6566. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Xie, L.; Sun, G.; Kong, Q.; Su, F.; Cao, Y.; Wei, J.; Ahmad, A.; Guo, X.; Chen, C.-M. Resorcinol-formaldehyde based carbon aerogel: Preparation, structure and applications in energy storage devices. Microporous Mesoporous Mater. 2019, 279, 293–315. [Google Scholar] [CrossRef]
- Canal-Rodríguez, M.; Arenillas, A.; Rey-Raap, N.; Ramos-Fernández, G.; Martín-Gullón, I.; Menéndez, J.A. Graphene-doped carbon xerogel combining high electrical conductivity and surface area for optimized aqueous supercapacitors. Carbon 2017, 118, 291–298. [Google Scholar] [CrossRef]
- Morawa Eblagon, K.; Rey-Raap, N.; Figueiredo, J.L.; RPereira, M.F. Relationships between texture, surface chemistry and performance of N-doped carbon xerogels in the oxygen reduction reaction. Appl. Surf. Sci. 2021, 548, 149242. [Google Scholar] [CrossRef]
- Ding, M.; Li, C. Recent Advances in Simple Preparation of 3D Graphene Aerogels Based on 2D Graphene Materials. Front. Chem. 2022, 10, 815463. [Google Scholar] [CrossRef]
- dos Santos-Gómez, L.; García, J.R.; Montes-Morán, M.A.; Menéndez, J.A.; García-Granda, S.; Arenillas, A. Ultralight-Weight Graphene Aerogels with Extremely High Electrical Conductivity. Small 2021, 17, 2103407. [Google Scholar] [CrossRef]
- Duan, S.; Li, A.; Wang, Y.; Chen, X.; Liu, B.; Liang, B.; Dai, C.; Yan, L.; Guo, J. Fabrication and performance of a 3D porous graphene aerogel-supported Ni–ZnS composite photocatalyst. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132948. [Google Scholar] [CrossRef]
- Lim, M.B.; Hu, M.; Manandhar, S.; Sakshaug, A.; Strong, A.; Riley, L.; Pauzauskie, P.J. Ultrafast sol–gel synthesis of graphene aerogel materials. Carbon 2015, 95, 616–624. [Google Scholar] [CrossRef]
- Worsley, M.A.; Pauzauskie, P.J.; Olson, T.Y.; Biener, J.; Satcher, J.H., Jr.; Baumann, T.F. Synthesis of Graphene Aerogel with High Electrical Conductivity. J. Am. Chem. Soc. 2010, 132, 14067–14069. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, J.; Sang, X.; Liu, C.; Luo, T.; Peng, L.; Han, B.; Tan, X.; Ma, X.; Wang, D.; et al. Cellular graphene aerogel combines ultralow weight and high mechanical strength: A highly efficient reactor for catalytic hydrogenation. Sci. Rep. 2016, 6, 25830. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yan, L. In situ self-assembly of mild chemical reduction graphene for three-dimensional architectures. Nanoscale 2011, 3, 3132–3137. [Google Scholar] [CrossRef]
- Zhang, L.; Song, G.; Song, Y.; Li, J.; Li, Z.; Yang, X.; Li, Z.; Li, X.; Jia, Z. Ultralight 3D cross-linked reinforced graphene@Fe3O4 composite aerogels for electromagnetic wave absorption. Mater. Res. Bull. 2024, 173, 112696. [Google Scholar] [CrossRef]
- Deng, Z.; Gao, C.; Feng, S.; Zhang, H.; Liu, Y.; Zhu, Y.; Wang, J.; Xiang, X.; Xie, H. Highly Compressible, Light-Weight and robust Nitrogen-Doped graphene composite aerogel for sensitive pressure sensors. Chem. Eng. J. 2023, 471, 144790. [Google Scholar] [CrossRef]
- Cao, L.; Wang, C.; Huang, Y. Structure optimization of graphene aerogel-based composites and applications in batteries and supercapacitors. Chem. Eng. J. 2023, 454, 140094. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, R.; Wang, Y.; Cao, Y.; Shen, Y.; Huang, Y.; Chen, Y. Recent advances in graphene aerogels as absorption-dominated electromagnetic interference shielding materials. Carbon 2023, 205, 112–137. [Google Scholar] [CrossRef]
- Bibi, A.; Chen, C.-Y.; Huang, K.-N.; Sathishkumar, N.; Chen, H.-T.; Lin, Y.-F.; Yeh, J.-M.; Santiago, K.S. Comparative study on the H2S gas-sensing properties of graphene aerogels synthesized through hydrothermal and chemical reduction. J. Taiwan Inst. Chem. Eng. 2024, 154, 105155. [Google Scholar] [CrossRef]
- Liang, W.; Wang, Y.; Gao, F.; Hou, S.; Wu, Q.; Yang, H.; Jin, F.; Bai, G.; Wang, Y.; Li, Z.; et al. Ni–Co Prussian blue analogue/graphene aerogel: A green synthesis approach for high-performance electromagnetic wave absorption and radar stealth applications. J. Mater. Chem. C 2023, 11, 14371–14381. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; Deng, W.; Kong, S. Preparation and Adsorption Properties of Graphene-Modified, Pitch-Based Carbon Foam Composites. Polymers 2022, 14, 4455. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yan, Q.; Wu, M.; Zhao, X. Carbon aerogel based materials for secondary batteries. Sustain. Mater. Technol. 2021, 30, e00342. [Google Scholar] [CrossRef]
- González, M.; Baselga, J.; Pozuelo, J. Modulating the electromagnetic shielding mechanisms by thermal treatment of high porosity graphene aerogels. Carbon 2019, 147, 27–34. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Wang, A.-J.; Wu, X.-L.; Chen, J.-R.; Feng, J.-J. Green-assembly of three-dimensional porous graphene hydrogels for efficient removal of organic dyes. J. Colloid Interface Sci. 2016, 484, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Zhang, F.; Pei, L.; Cui, Z.; Shen, J.; Ye, M. Ni, Co and Mn doped SnS2-graphene aerogels for supercapacitors. J. Alloys Compd. 2018, 767, 583–591. [Google Scholar] [CrossRef]
- Osińska, M.; Krawczyk, P.; Łuczak, T.; Rozmanowski, T. Nitrogen, nickel and graphene oxide doped carbon xerogel as an active electrode of an electrochemical capacitor. J. Sol-Gel Sci. Technol. 2023, 106, 827–836. [Google Scholar] [CrossRef]
- Chi, C.; Xu, H.; Zhang, K.; Wang, Y.; Zhang, S.; Liu, X.; Liu, X.; Zhao, J.; Li, Y. 3D hierarchical porous graphene aerogels for highly improved adsorption and recycled capacity. Mater. Sci. Eng. B 2015, 194, 62–67. [Google Scholar] [CrossRef]
- Wei, G.; Miao, Y.-E.; Zhang, C.; Yang, Z.; Liu, Z.; Tjiu, W.W.; Liu, T. Ni-doped graphene/carbon cryogels and their applications as versatile sorbents for water purification. ACS Appl. Mater. Interfaces 2013, 5, 7584–7591. [Google Scholar] [CrossRef]
- Oliva, P.; Leonardi, J.; Laurent, J.; Delmas, C.; Braconnier, J.; Figlarz, M.; Fievet, F.; Guibert, A. Review of the structure and the electrochemistry of nickel hydroxides and oxy-hydroxides. J. Power Sources 1982, 8, 229–255. [Google Scholar] [CrossRef]
- Abdelkader-Fernández, V.K.; Fernandes, D.M.; Balula, S.S.; Cunha-Silva, L.; Pérez-Mendoza, M.J.; Freire, C. Unveiling the structural transformations of the PW11Co@ZIF-67 nanocomposite induced by thermal treatment. Dalton Trans. 2022, 51, 17844–17857. [Google Scholar] [CrossRef] [PubMed]
- Morais, R.G.; Rey-Raap, N.; Figueiredo, J.L.; Pereira, M.F.R. Optimization of cobalt on CNT towards the oxygen evolution reaction and its synergy with iron(II) phthalocyanine as bifunctional oxygen electrocatalyst. Catal. Today 2023, 418, 114057. [Google Scholar] [CrossRef]

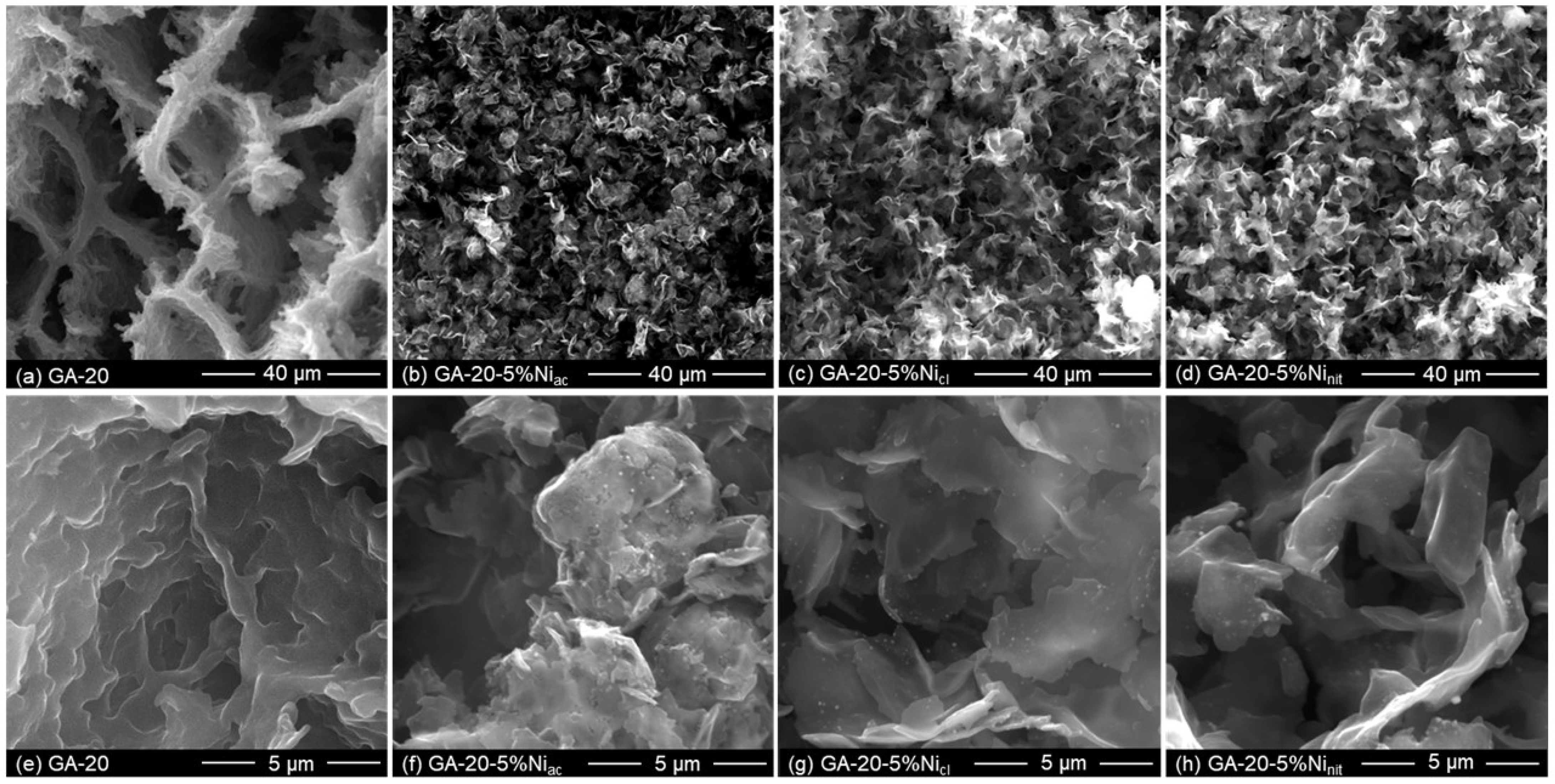
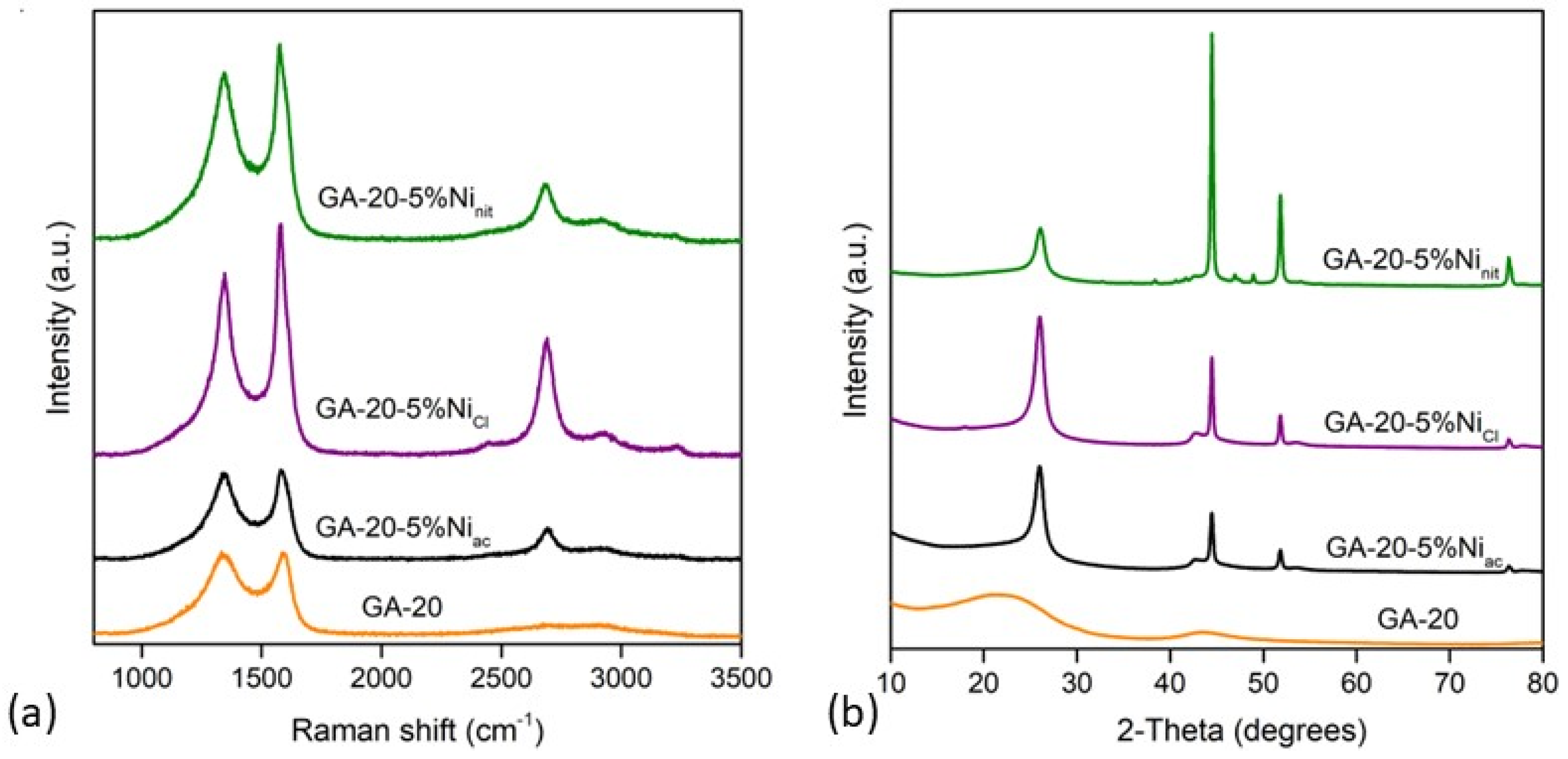
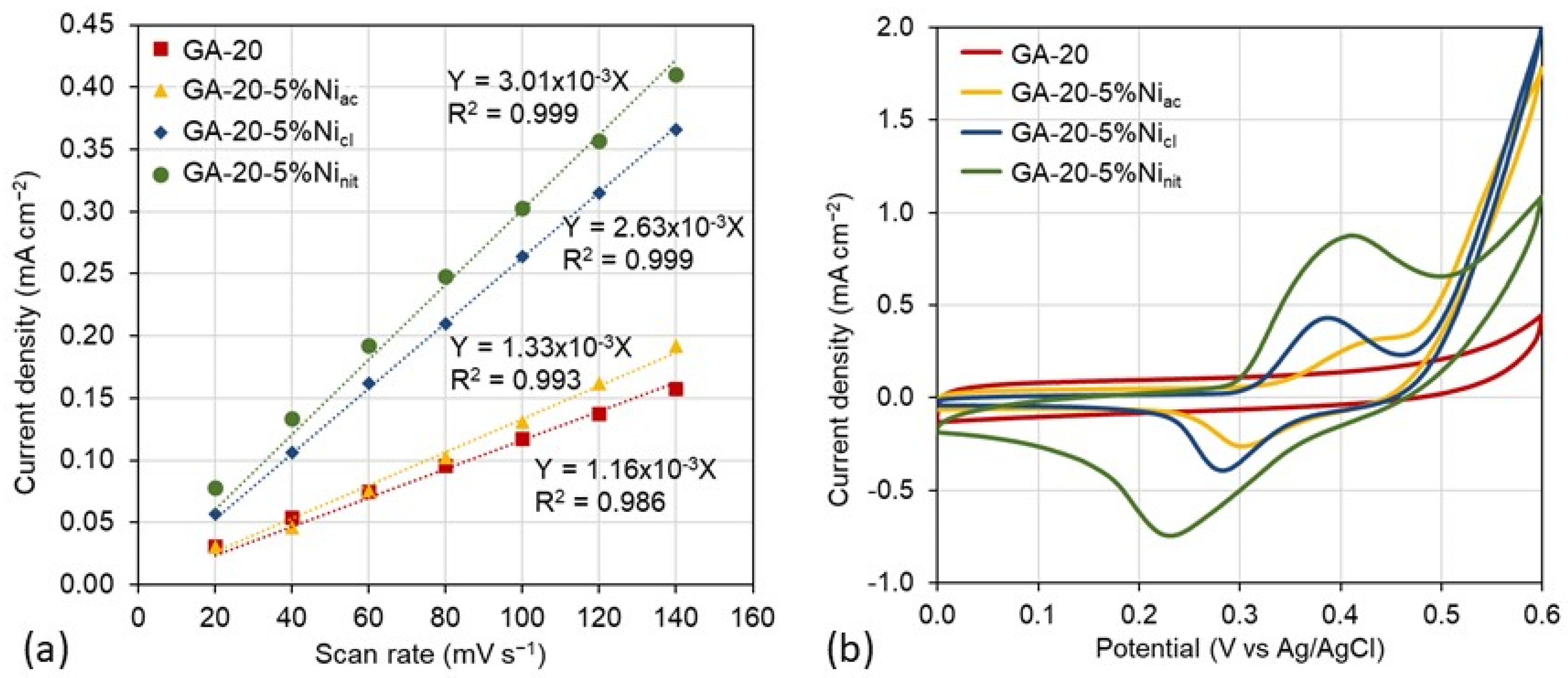
| Material | SBET (m2 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | env (g cm−3) | ε (%) | K (S m−1) |
|---|---|---|---|---|---|---|
| GA-20 | 480 | 0.17 | 0.01 | 0.24 | 87.8 | 231 |
| GA-50 | 143 | 0.05 | 0.01 | 0.14 | 91.4 | 1290 |
| GA-60 | 5 | - a | - a | 0.12 | 91.6 | 1447 |
| GA-20-5%Niac | 408 | 0.14 | 0.10 | 0.11 | 95.1 | 1303 |
| GA-20-5%Nicl | 315 | 0.11 | 0.13 | 0.16 | 92.4 | 2390 |
| GA-20-5%Ninit | 317 | 0.11 | 0.10 | 0.16 | 93.6 | 2772 |
| Material | R (g) | GO Suspension (g) | F (g) | C4H6NiO4 (g) | NiCl2 (g) | Ni(NO3)2 (g) |
|---|---|---|---|---|---|---|
| GA-20 | 4.5 | 40.4 | 6.7 | - | - | - |
| GA-50 | 1.9 | 45.3 | 2.8 | - | - | - |
| GA-60 | 1.6 | 46.0 | 2.4 | - | - | - |
| GA-20-5%Niac | 4.5 | 40.4 | 6.7 | 0.4 | - | - |
| GA-20-5%Nicl | 4.5 | 40.4 | 6.7 | - | 0.5 | - |
| GA-20-5%Ninit | 4.5 | 40.4 | 6.7 | - | - | 3.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Barriuso, M.; Sánchez-Suárez, M.; González-Lavín, J.; Arenillas, A.; Rey-Raap, N. Synthesis of Ni-Doped Graphene Aerogels for Electrochemical Applications. Gels 2024, 10, 180. https://doi.org/10.3390/gels10030180
González-Barriuso M, Sánchez-Suárez M, González-Lavín J, Arenillas A, Rey-Raap N. Synthesis of Ni-Doped Graphene Aerogels for Electrochemical Applications. Gels. 2024; 10(3):180. https://doi.org/10.3390/gels10030180
Chicago/Turabian StyleGonzález-Barriuso, Marina, Mario Sánchez-Suárez, Judith González-Lavín, Ana Arenillas, and Natalia Rey-Raap. 2024. "Synthesis of Ni-Doped Graphene Aerogels for Electrochemical Applications" Gels 10, no. 3: 180. https://doi.org/10.3390/gels10030180
APA StyleGonzález-Barriuso, M., Sánchez-Suárez, M., González-Lavín, J., Arenillas, A., & Rey-Raap, N. (2024). Synthesis of Ni-Doped Graphene Aerogels for Electrochemical Applications. Gels, 10(3), 180. https://doi.org/10.3390/gels10030180











