Functional Hydrogels for Delivery of the Proteolytic Enzyme Serratiopeptidase
Abstract
1. Introduction
2. Results and Discussion
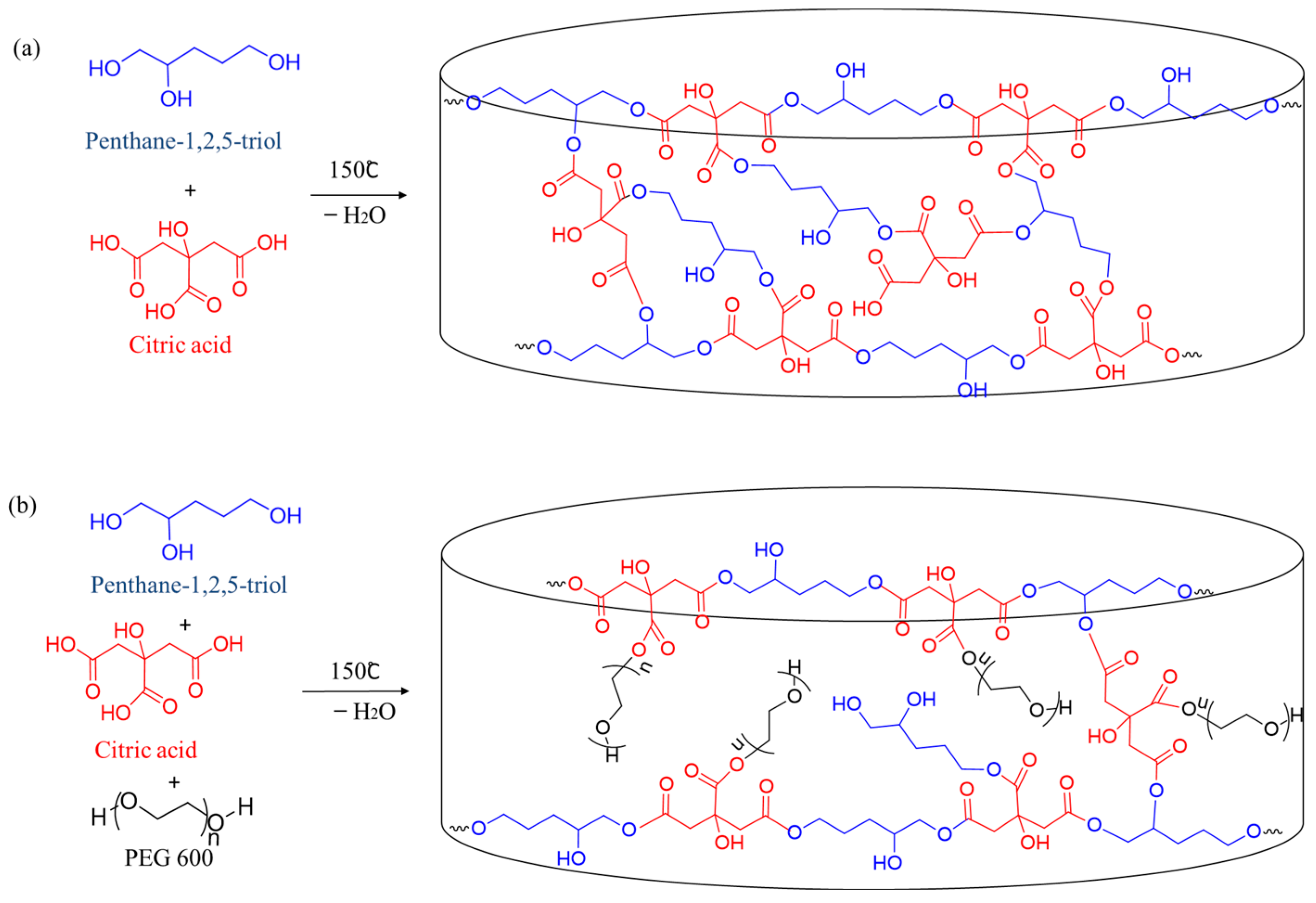
2.1. Synthesis of Hydrogels
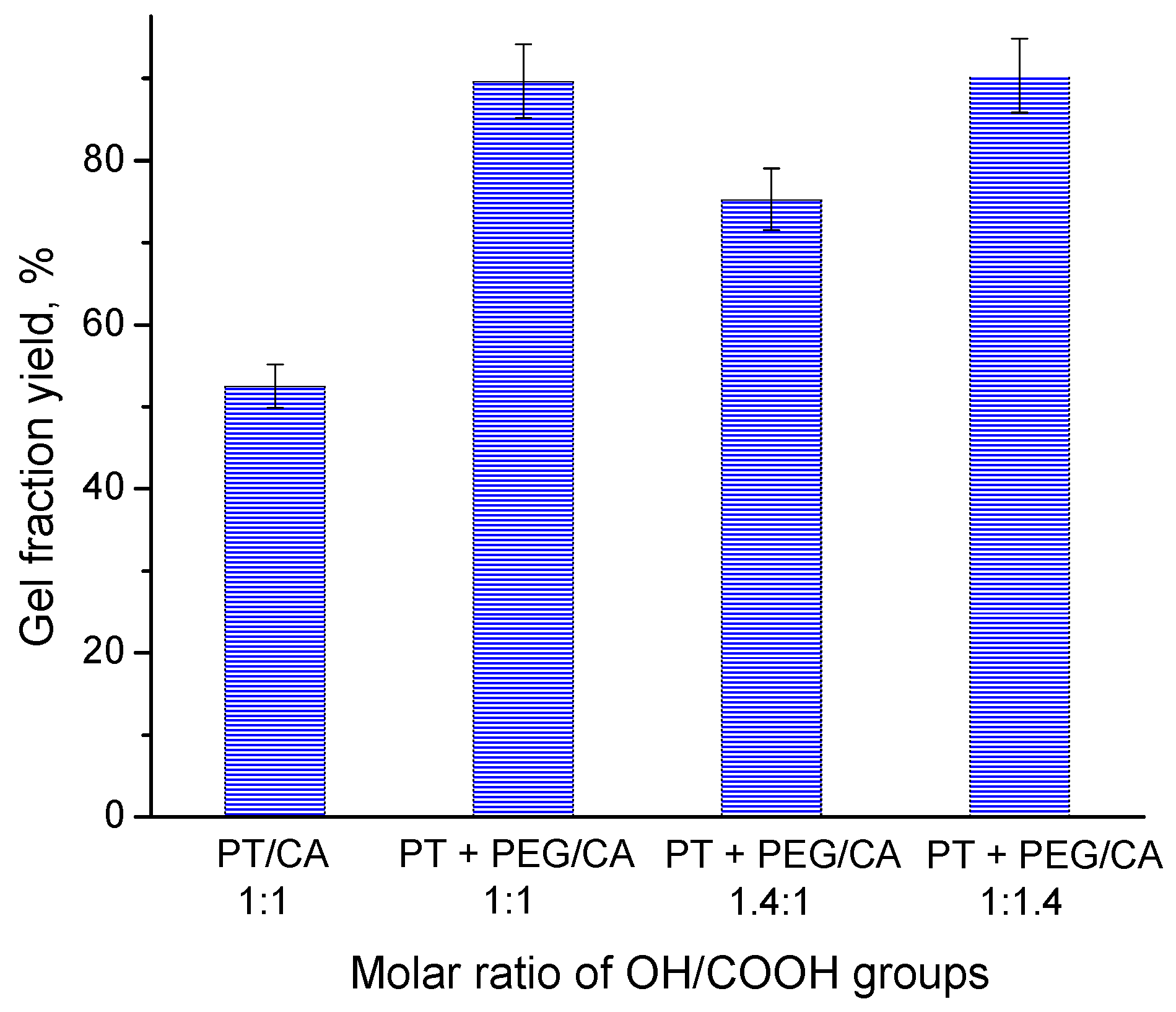
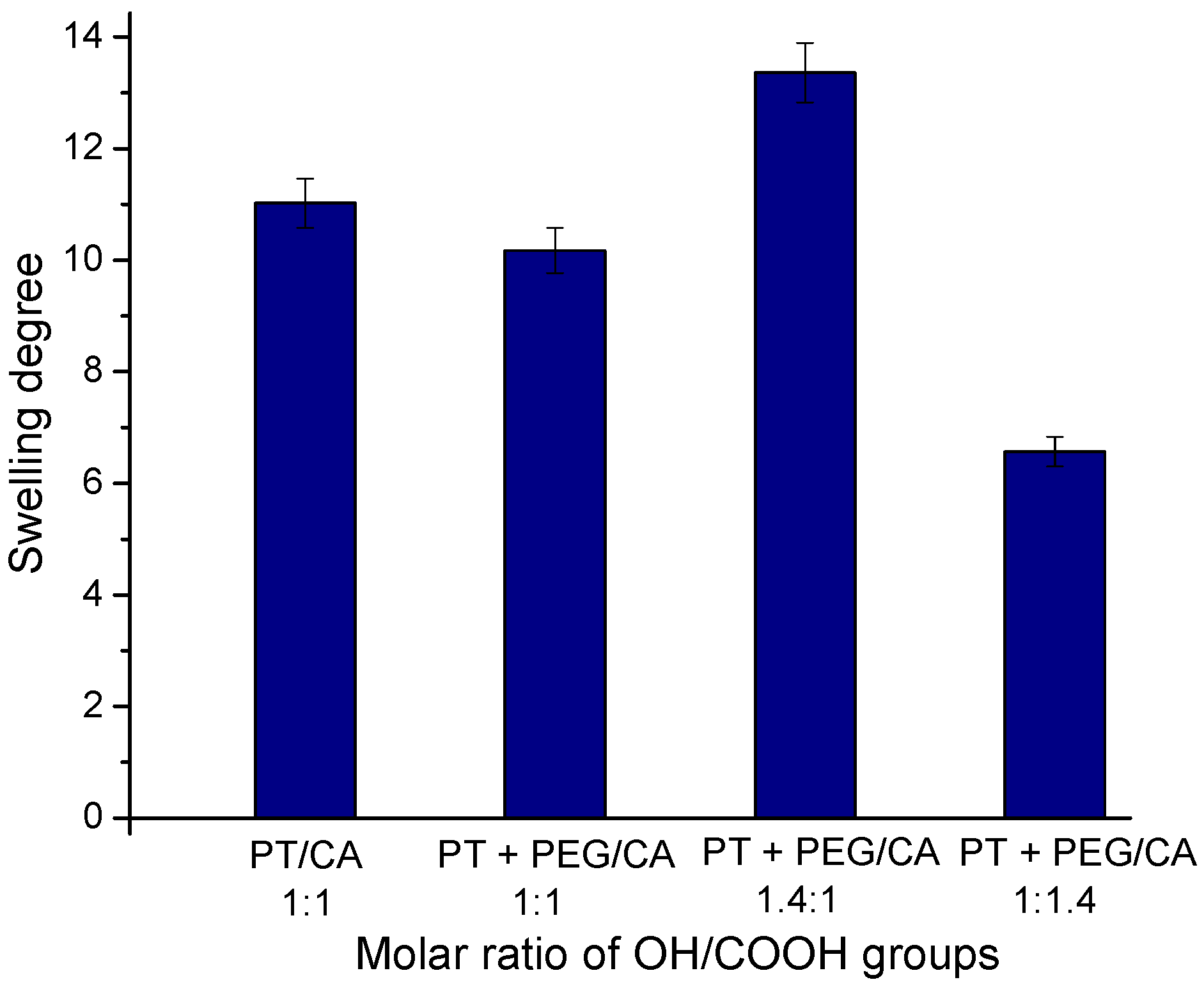
2.2. Effect of Reagents Composition on the Gel Fraction Yield and Swelling Degree
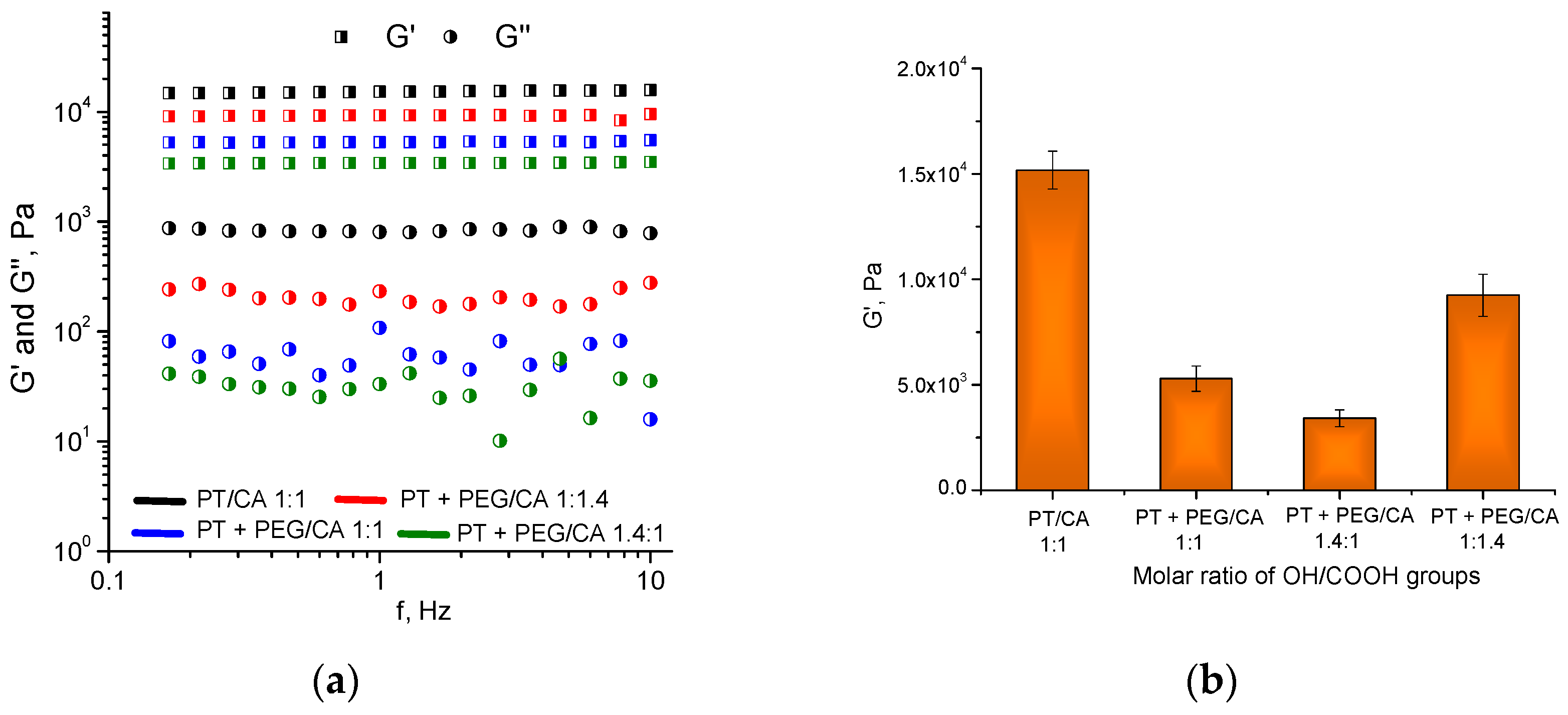
2.3. Dynamic Rheological Studies
2.4. pH-Sensitive Properties and Hydrolitic Degradation of Hydrogels
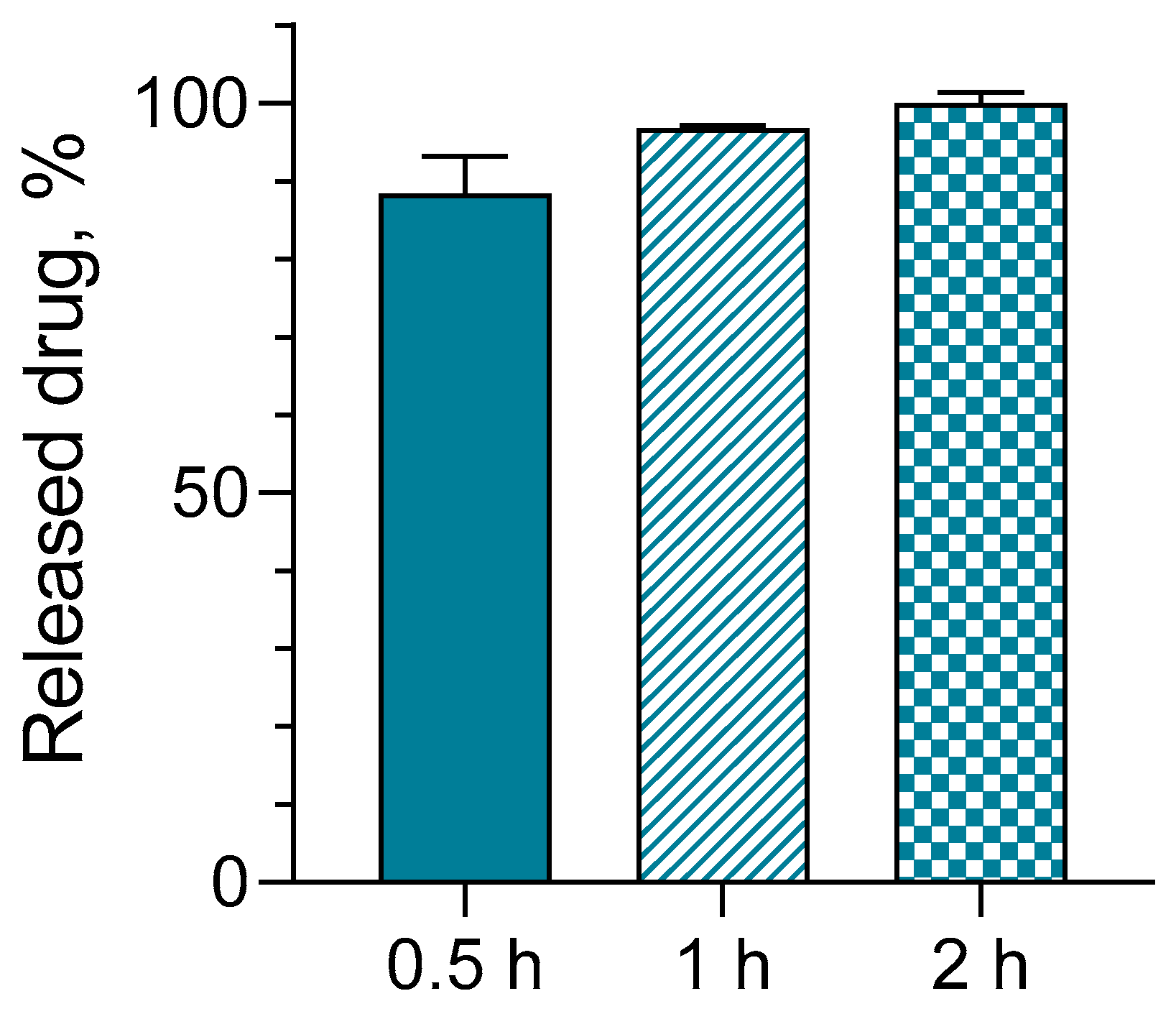
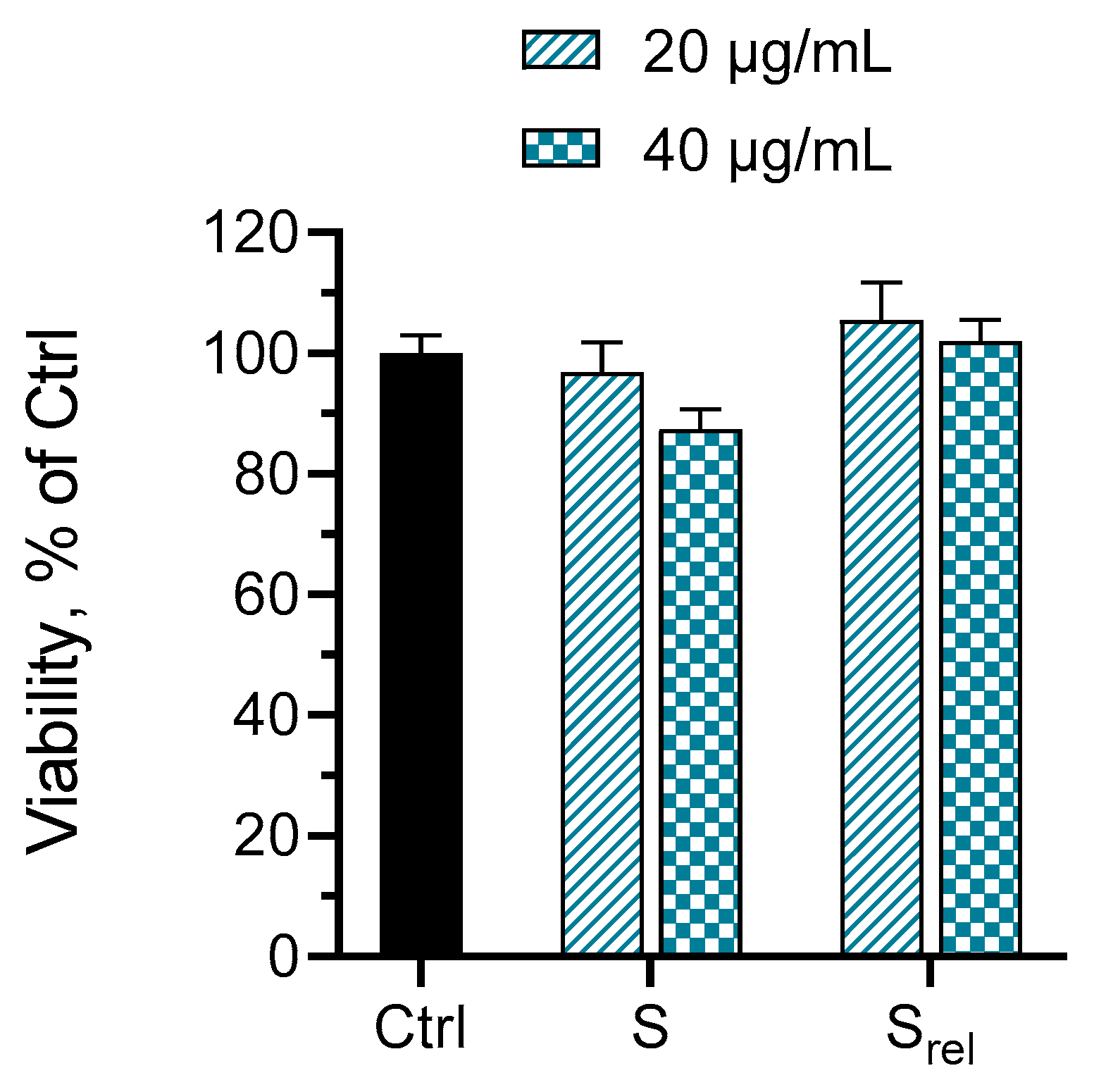
2.5. Loading of Hydrogels with Serratiopeptidase, In Vitro Release and Cytotoxicity Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Extraction, Purification and Proteolytic Activity Assay of Serratiopeptidase Enzyme
4.3. Synthesis of Hydrogels
4.4. Determination of Gel Fraction Yield and Swelling Degree
4.5. Hydrogel Swelling at Different pH of Media
4.6. Hydrolytic Degradation of Hydrogel
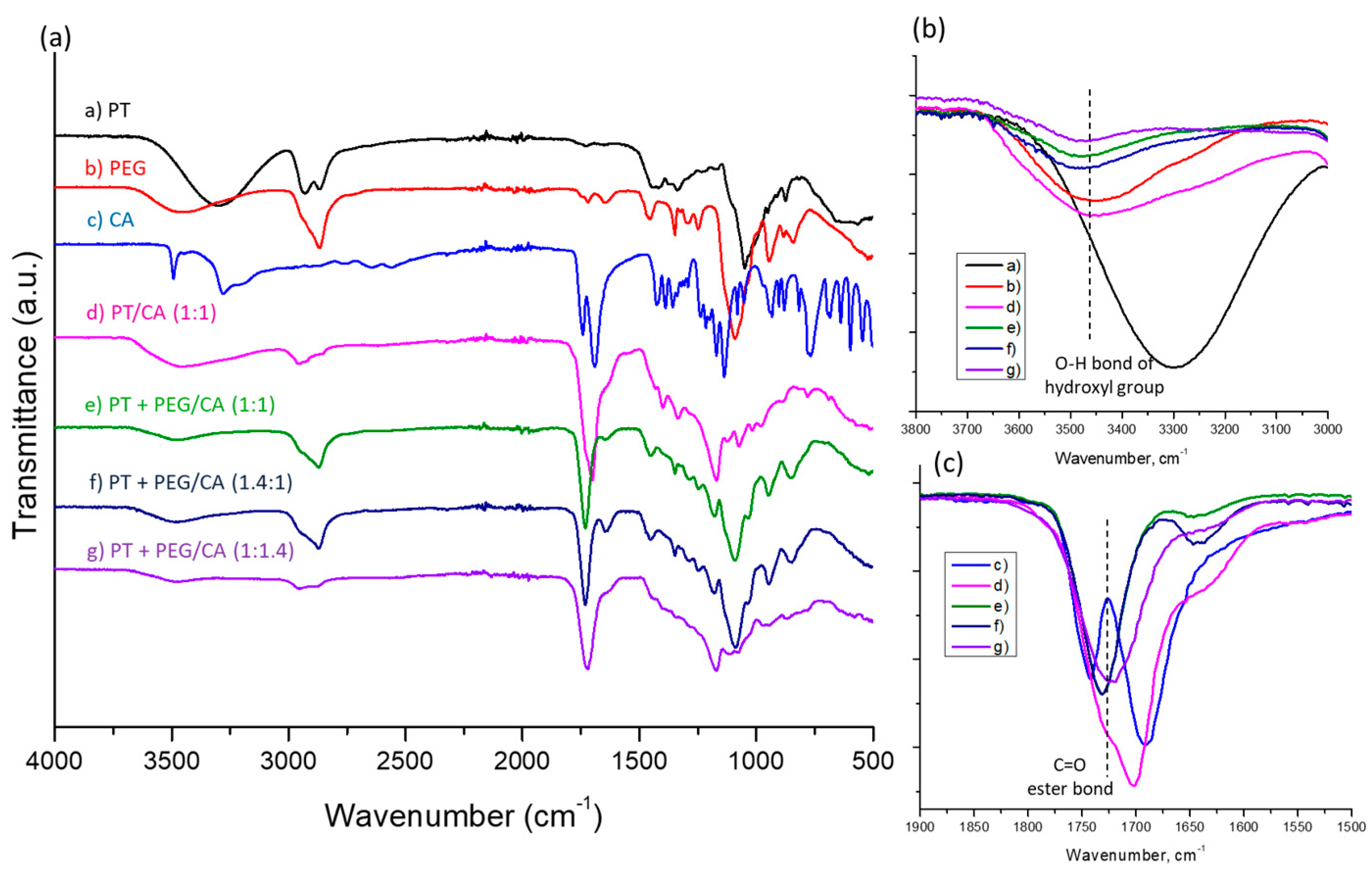
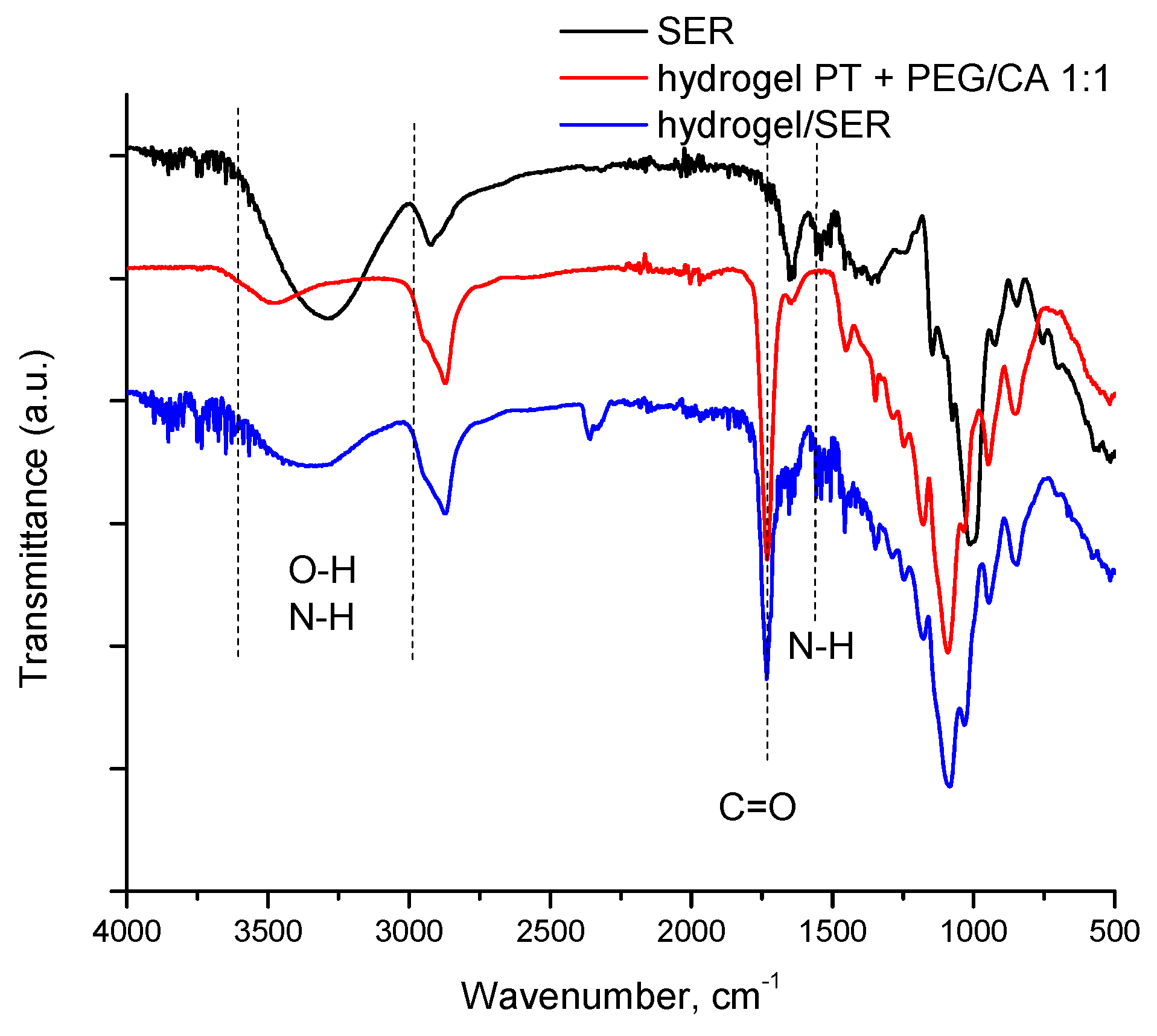
4.7. FTIR Spectroscopy
4.8. Dynamic Rheological Measurements
4.9. Loading of Hydrogels and In Vitro Release Test
4.10. In Vitro Release Test
4.11. In Vitro Cytotoxicity Studies
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maheshwer, B.; Drager, J.; John, N.; Williams, B.; LaPrade, R.; Chahla, J. Incidence of Intraoperative and Postoperative Complications after Posterolateral Corner Reconstruction or Repair: A Systematic Review of the Current Literature. Am. J. Sports Med. 2021, 49, 3443–3452. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.; Chang, C.; Chan, H.; Chung, T.; Shu, C.; Chuang, K.; Duh, T.; Yang, M.; Tyan, Y. Hydrogels: Properties and Applications in Biomedicine. Molecules 2022, 27, 2902. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Li, H.; Chen, J.; Xiong, Y.; Li, X.; Zhou, J.; Li, S.; Wang, S.; Sun, B. Highly Water-Absorptive and Antibacterial Hydrogel Dressings for Rapid Postoperative Detumescence. Front. Bioeng. Biotechnol. 2022, 10, 845345. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Newton, M.; Cheng, H.; Zhang, Q.; Gao, W.; Zheng, Y.; Lu, Z.; Dai, Z.; Zhu, J. Progress of Hydrogel Dressings with Wound Monitoring and Treatment Functions. Gels 2023, 9, 694. [Google Scholar] [CrossRef]
- Peppas, N. Hydrogels and drug delivery. Curr. Opin. Colloid. Interface 1997, 2, 531–537. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N. Methods of synthesis of hydrogels: A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.; Mujtaba, M.; Alghamdi, N.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef]
- Ahmed, E. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Khan, E.; Ozaltin, K.; Bernal-Ballen, A.; Di Martino, A. Renewable Mixed Hydrogels Based on Polysaccharide and Protein for Release of Agrochemicals and Soil Conditioning. Sustainability 2021, 13, 10439. [Google Scholar] [CrossRef]
- Sapuła, P.; Bialik-Wąs, K.; Malarz, K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics 2023, 15, 253. [Google Scholar] [CrossRef]
- Birajdar, M.; Joo, H.; Koh, W.-G.; Park, H. Natural bio-based monomers for biomedical applications: A review. Biomater. Res. 2021, 25, 8. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Yahya, R.; Hassan, A.; Yar, M.; Azzahari, A.; Selvanathan, V.; Sonsudin, F.; Abouloula, C. pH Sensitive Hydrogels in Drug Delivery: Brief History, Properties, Swelling, and Release Mechanism, Material Selection and Applications. Polymers 2017, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Willner, I. Stimuli-Controlled Hydrogels and Their Applications. Acc. Chem. Res. 2017, 50, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Suhail, M.; Shao, Y.; Vu, Q.; Wu, P. Designing of pH-Sensitive Hydrogels for Colon Targeted Drug Delivery; Characterization and In Vitro Evaluation. Gels. 2022, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. In Emerging Concepts in Analysis and Applications of Hydrogels; IntechOpen: London, UK, 2016; pp. 9–38. [Google Scholar]
- Shi, Q.; Liu, H.; Tang, D.; Li, Y.; Li, X.; Xu, F. Bioactuators based on stimulus-responsive hydrogels and their emerging biomedical applications. NPG Asia Mater. 2019, 11, 64. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- Salihu, R.; Razak, S.; Zawawi, N.; Kadir, M.; Ismail, N.; Jusoh, N.; Mohamad, M.; Nayan, N. Citric acid: A green cross-linker of biomaterials for biomedical applications. Eur. Polym. J. 2021, 146, 110271, ISSN 0014-3057. [Google Scholar] [CrossRef]
- Bozova, N.; Petrov, P.D. Highly Elastic Super-Macroporous Cryogels Fabricated by Thermally Induced Crosslinking of 2-Hydroxyethylcellulose with Citric Acid in Solid State. Molecules 2021, 26, 6370. [Google Scholar] [CrossRef]
- Sabzi, M.; Afshari, M.; Babaahmadi, M.; Shafagh, N. pH-dependent swelling and antibiotic release from citric acid crosslinked poly(vinyl alcohol) (PVA)/nano silver hydrogels. Colloids Surf. B 2020, 188, 110757. [Google Scholar] [CrossRef]
- Gupte, V.; Luthra, U. Analytical techniques for serratiopeptidase: A review. J. Pharm. Anal. 2017, 7, 203–207. [Google Scholar] [CrossRef]
- Sharma, C.; Jha, N.; Meeran, M.; Patil, C.; Goyal, S.; Ojha, S. Serratiopeptidase, A Serine Protease Anti-Inflammatory, Fibrinolytic, and Mucolytic Drug, Can Be a Useful Adjuvant for Management in COVID-19. Front. Pharmacol. 2021, 12, 1663–9812. Available online: https://www.frontiersin.org/articles/10.3389/fphar.2021.603997 (accessed on 10 January 2024). [CrossRef] [PubMed]
- Jadhav, S.; Shah, N.; Rathi, A.; Rathi, V.; Rathi, A. Serratiopeptidase: Insights into the therapeutic applications. Biotechnol. Rep. 2020, 28, e00544. [Google Scholar] [CrossRef]
- Tiwari, M. The role of serratiopeptidase in the resolution of inflammation. Asian J. Pharm. Sci. 2017, 12, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Verma, D.; Abbot, V. A review on pharmaceutical, pharmacological and chemical aspects of serratiopeptidase as anti-inflammatory agent. Mater. Today Proc. 2023; in press. [Google Scholar] [CrossRef]
- Nair, S.R.; Subathra Devi, C. Serratiopeptidase: An integrated View of Multifaceted Therapeutic Enzyme. Biomolecules 2022, 12, 1468. [Google Scholar] [CrossRef]
- Singh, D.; Singh, M. Development of antibiotic and debriding enzyme-loaded PLGA microspheres entrapped in PVA-gelatin hydrogel for complete wound management. Artif. Cell. Blood Sub. Biotechnol. 2012, 40, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Nirale, N.; Menon, M. Topical formulations of serratiopeptidase: Development and pharmacodynamic evaluation. Indian J. Pharm. Sci. 2010, 72, 65–71. [Google Scholar]
- Zhang, Z.; O’Hara, I.M.; Orlando, W.; Doherty, S.; Rackemann, D. Methods for Converting Lignocellulosic Materials to Useful Products. US 2014/0093918 A1, 3 April 2014. [Google Scholar]
- Kamenova, K.; Radeva, L.; Yoncheva, K.; Ublekov, F.; Ravutsov, M.; Marinova, M.; Simeonov, S.; Forys, A.; Trzebicka, B.; Petrov, P. Functional Nanogel from Natural Substances for Delivery of Doxorubicin. Polymers 2022, 14, 3694. [Google Scholar] [CrossRef]
- Thomas, A.; Müller, S.; Frey, H. Beyond Poly(ethylene glycol): Linear polyglycerol as a multifunctional polyether for biomedical and pharmaceutical applications. Biomacromolecules 2014, 15, 1935–1954. [Google Scholar] [CrossRef]
- Sahu, M.; Reddy, V.; Kim, B.; Patro, B.; Park, C.; Kim, W.; Sharma, P. Fabrication of Cu2ZnSnS4 Light Absorber Using a Cost-Effective Mechanochemical Method for Photovoltaic Applications. Materials 2022, 15, 1708. [Google Scholar] [CrossRef]
- Capanema, N.; Mansur, A.; de Jesus, A.; Carvalho, S.; de Oliveira, L.; Mansur, H. Superabsorbent crosslinked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Koto, N.; Soegijono, B. Effect of rice husk ash filler of resistance against of high-speed projectile impact on polyester-fiberglass double panel composites. J. Phys. Conf. Ser. 2019, 1191, 012058. [Google Scholar] [CrossRef]
- Barleany, D.; Ananta, C.; Maulina, F.; Rochmat, A.; Alwan, H.; Erizal, E. Controlled release of metformin hydrogen chloride from stimuli-responsive hydrogel based on poly(N-Isopropylacrylamide)/Chitosan/Polyvinyl alcohol composite. Int. J. Technol. 2020, 11, 511–521. [Google Scholar] [CrossRef]
- Zuidema, J.; Rivet, C.; Gilbert, R.; Morrison, F. A protocol for rheological characterization of hydrogels for tissue engineering strategies. J. Biomed Mater. Res. B Appl. Biomater. 2014, 102, 1063–1073. [Google Scholar] [CrossRef]
- Maghraby, Y.; El-Shabasy, R.; Ibrahim, A.; Azzazy, H. Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Mohamad, N.; Marzuki, N.; Buang, N.; Huyop, F.; Wahab, R. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Brena, B.; González-Pombo, P.; Batista-Viera, F. Immobilization of enzymes: A literature survey. Methods Mol. Biol. 2013, 1051, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Segger, D.; Aßmus, U.; Brock, M.; Erasmy, J.; Finkel, P.; Fitzner, A.; Heuss, H.; Kortemeier, U.; Munke, S.; Rheinländer, T.; et al. Multicenter Study on Measurement of the Natural pH of the Skin Surface. Int. J. Cosmet. Sci. 2008, 10, 75. [Google Scholar] [CrossRef]
- Kuo, S.-H.; Shen, C.-J.; Shen, C.-F.; Cheng, C.-M. Role of pH Value in Clinically Relevant Diagnosis. Diagnostics 2020, 10, 107. [Google Scholar] [CrossRef]
- Luessen, H.L.; Verhoef, J.C.; Borchard, G.; Lehr, C.M.; de Boer, A.G.; Junginger, H.E. Mucoadhesive polymers in peroral peptide drug delivery. II. Carbomer and polycarbophil are potent inhibitors of the intestinal proteolytic enzyme trypsin. Pharm. Res. 1995, 12, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yang, M.; Liu, W.; Li, J. Cell-Free Expression of a Therapeutic Protein Serratiopeptidase. Molecules 2023, 28, 3132. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Desmoulière, A.; Tredget, E.E. 45—Molecular and Cellular Basis of Hypertrophic Scarring. In Total Burn Care, 5th ed.; Herndon, D.N., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 455–465.e4. ISBN 978-0-323-47661-4. [Google Scholar]
- Piipponen, M.; Li, D.; Landén, N.X. The Immune Functions of Keratinocytes in Skin Wound Healing. Int. J. Mol. Sci. 2020, 21, 8790. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Krieg, T.; Smola, H. Keratinocyte–Fibroblast Interactions in Wound Healing. J. Invest. Dermatol. 2007, 127, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, S.P.; Ravutsov, M.A.; Mihovilovic, M.D. Biorefinery via achmatowicz rearrangement: Synthesis of pentane-1,2,5-triol from furfuryl alcohol. ChemSusChem 2019, 12, 2748–2754. [Google Scholar] [CrossRef]
- Cupp-Enyard, C. Sigma’s Non-specific Protease Activity Assay—Casein as a Substrate. J. Vis. Exp. 2008, 17, 899. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods. 1983, 65, 55–63. [Google Scholar] [CrossRef]
- 14:00–17:00 ISO 10993-5:2009. Available online: https://www.iso.org/standard/36406.html (accessed on 19 January 2024).

| Concentration of SER (mg/mL) | U/mL |
|---|---|
| 0.1 | 0.36 |
| 0.25 | 0.88 |
| 0.5 | 1.72 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamenova, K.; Prancheva, A.; Stoyanova, S.; Radeva, L.; Tibi, I.P.-E.; Yoncheva, K.; Ravutsov, M.A.; Marinova, M.K.; Simeonov, S.P.; Mitova, S.; et al. Functional Hydrogels for Delivery of the Proteolytic Enzyme Serratiopeptidase. Gels 2024, 10, 156. https://doi.org/10.3390/gels10030156
Kamenova K, Prancheva A, Stoyanova S, Radeva L, Tibi IP-E, Yoncheva K, Ravutsov MA, Marinova MK, Simeonov SP, Mitova S, et al. Functional Hydrogels for Delivery of the Proteolytic Enzyme Serratiopeptidase. Gels. 2024; 10(3):156. https://doi.org/10.3390/gels10030156
Chicago/Turabian StyleKamenova, Katya, Anna Prancheva, Stiliyana Stoyanova, Lyubomira Radeva, Ivanka Pencheva-El Tibi, Krassimira Yoncheva, Martin A. Ravutsov, Maya K. Marinova, Svilen P. Simeonov, Simona Mitova, and et al. 2024. "Functional Hydrogels for Delivery of the Proteolytic Enzyme Serratiopeptidase" Gels 10, no. 3: 156. https://doi.org/10.3390/gels10030156
APA StyleKamenova, K., Prancheva, A., Stoyanova, S., Radeva, L., Tibi, I. P.-E., Yoncheva, K., Ravutsov, M. A., Marinova, M. K., Simeonov, S. P., Mitova, S., Eneva, R., Zaharieva, M. M., Najdenski, H., & Petrov, P. D. (2024). Functional Hydrogels for Delivery of the Proteolytic Enzyme Serratiopeptidase. Gels, 10(3), 156. https://doi.org/10.3390/gels10030156