Progress in Research on the Preparation of Graphene-Based Aerogels Using γ-ray Irradiation Technology
Abstract
1. Introduction
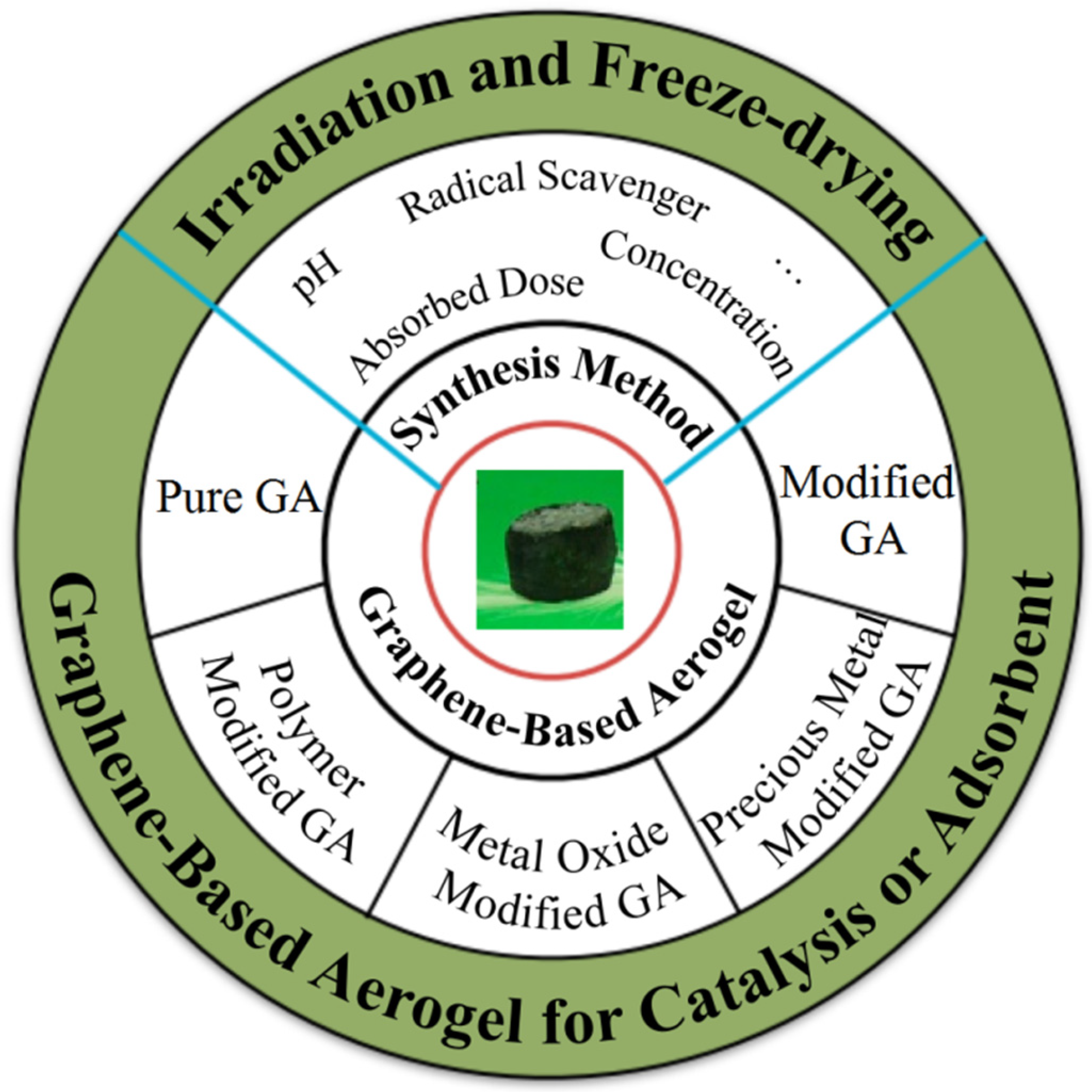
2. Radiation Synthesis of GA
2.1. Pure GA
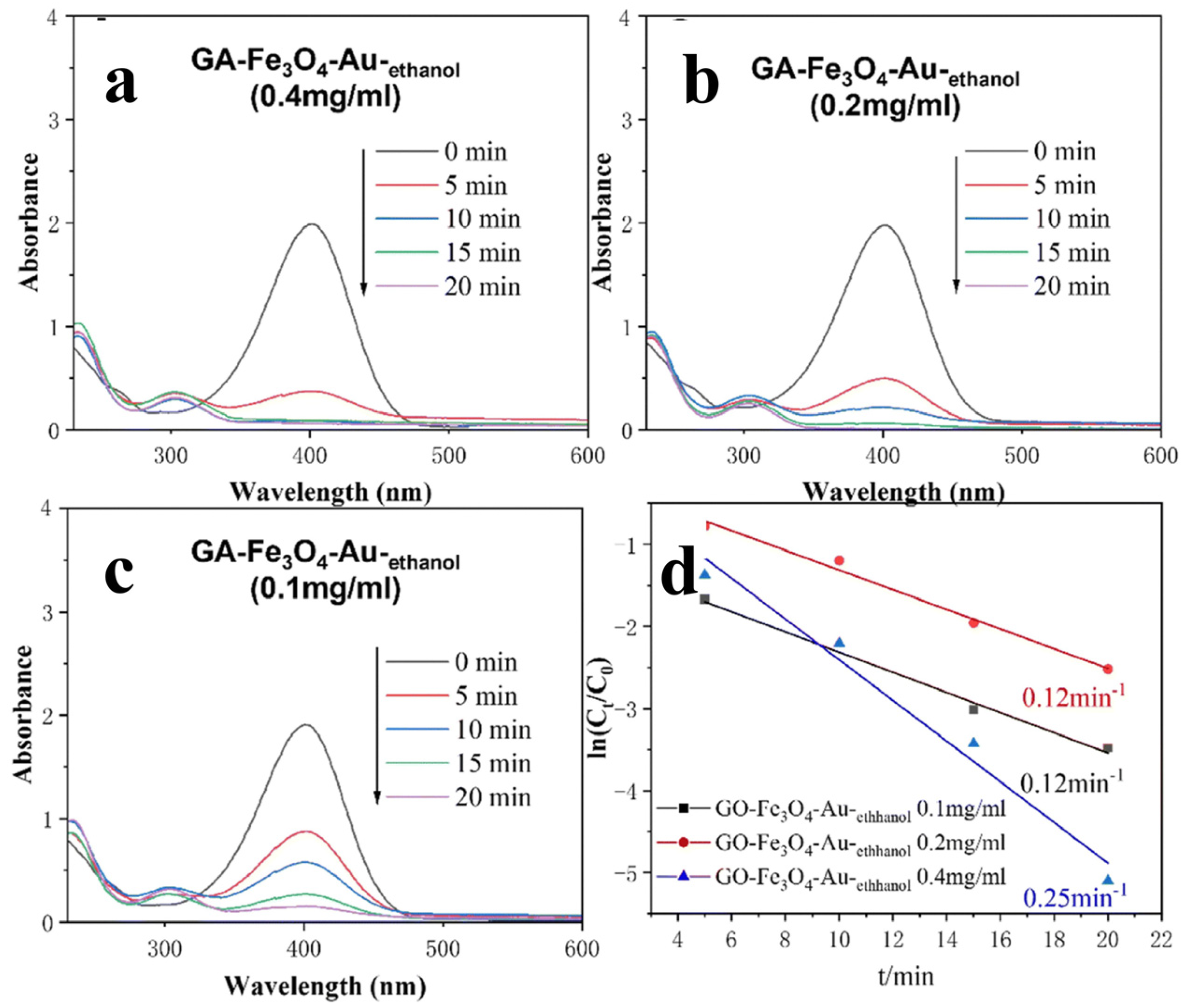
2.2. Modified GA
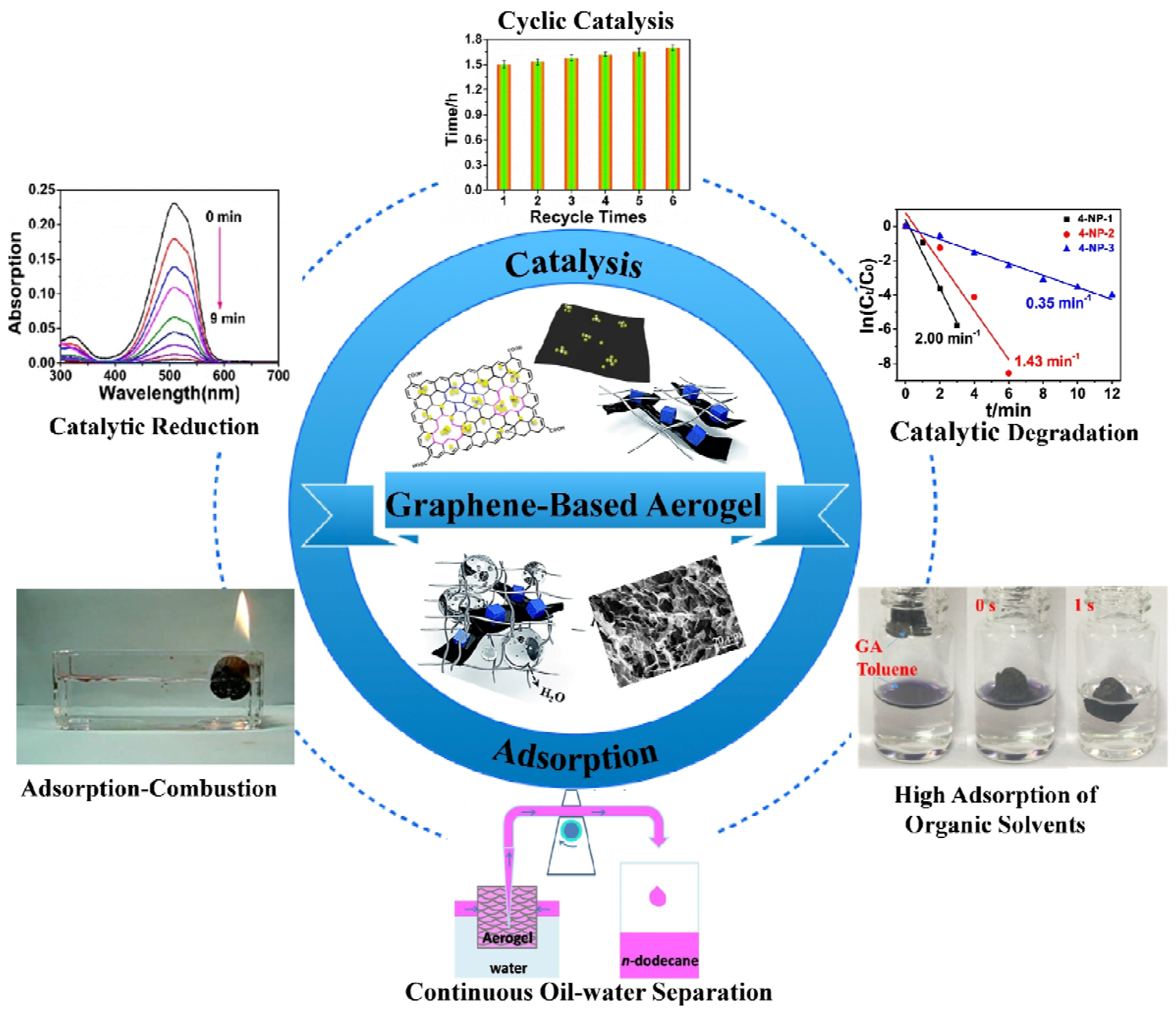
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gholipour-Ranjbar, H.; Ganjali, M.R.; Norouzi, P.; Naderi, H.R. Functionalized graphene aerogel with p-phenylenediamine and its composite with porous MnO2: Investigating the effect of functionalizing agent on supercapacitive performance. J. Mater. Sci. Mater. Electron. 2016, 27, 10163–10172. [Google Scholar] [CrossRef]
- Pawar, R.C.; Kang, S.; Khan, H.; Han, H.; Lee, C.S. Study of multi-faceted CoS2 introduced graphene aerogel hybrids via chemical approach for an effective electrocatalytic water splitting. Curr. Appl. Phys. 2021, 32, 78–85. [Google Scholar] [CrossRef]
- Arumugam, M.; Seralathan, K.-K.; Praserthdam, S.; Tahir, M.; Praserthdam, P. Synthesis of novel graphene aerogel encapsulated bismuth oxyiodide composite towards effective removal of methyl orange azo-dye under visible light. Chemosphere 2022, 303, 135121. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.H.; Kamarudin, S.K.; Basri, S.; Karim, N.A. Three-Dimensional Graphene Aerogel Supported on Efficient Anode Electrocatalyst for Methanol Electrooxidation in Acid Media. Catalysts 2023, 13, 879. [Google Scholar] [CrossRef]
- Vrettos, K.; Spyrou, K.; Georgakilas, V. Graphene Aerogel Growth on Functionalized Carbon Fibers. Molecules 2020, 25, 1295. [Google Scholar] [CrossRef] [PubMed]
- Sawut, A.; Wu, T.; Simayi, R.; Wu, T.; Gong, X.; Wang, Z. Reduced graphene oxide-TiO2/sodium alginate/polyacrylamide composite hydrogel for enhanced adsorption-photocatalytic degradation of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2023, 678, 132531. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Zhang, Y.; Huang, Y.; Liu, J.; Zhang, X.; Liu, X.; Teng, H.; Zhang, X.; Zhang, J.; et al. A Review of Graphene-Based Materials/Polymer Composite Aerogels. Polymers 2023, 15, 1888. [Google Scholar] [CrossRef] [PubMed]
- Rathi, K.; Kim, D. Super-compressible and mechanically stable reduced graphene oxide aerogel for wearable functional devices. Sci. Technol. Adv. Mater. 2023, 24, 2214854. [Google Scholar] [CrossRef]
- Kasar, A.K.; Tian, S.; Xiong, G.; Menezes, P.L. Graphene aerogel and its composites: Synthesis, properties and applications. J. Porous Mater. 2022, 29, 1011–1025. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, Z.; Xu, B.; Yue, S.; Luo, Y.; Zhan, W.; Liu, B. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J. Mater. Chem. 2011, 21, 6494–6497. [Google Scholar] [CrossRef]
- Grushevskaya, H.; Timoshchenko, A.; Lipnevich, I. Topological Defects Created by Gamma Rays in a Carbon Nanotube Bilayer. Nanomaterials 2023, 13, 410. [Google Scholar] [CrossRef] [PubMed]
- Abedini, A.; Daud, A.R.; Hamid, M.A.A.; Othman, N.K. Radiolytic Formation of Fe3O4 Nanoparticles: Influence of Radiation Dose on Structure and Magnetic Properties. PLoS ONE 2014, 9, e90055. [Google Scholar] [CrossRef]
- Borodinov, N.L.; Giarnmarco, J.; Patel, N.; Agarwal, A.; O’Donnell, K.R.; Kucera, C.J.; Jacobsohn, L.G.; Luzinov, I. Stability of Grafted Polymer Nanoscale Films toward Gamma Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 19455–19465. [Google Scholar] [CrossRef]
- Misra, N.; Biswal, J.; Gupta, A.; Sainis, J.K.; Sabharwal, S. Gamma radiation induced synthesis of gold nanoparticles in aqueous polyvinyl pyrrolidone solution and its application for hydrogen peroxide estimation. Radiat. Phys. Chem. 2012, 81, 195–200. [Google Scholar] [CrossRef]
- Anson-Casaos, A.; Puertolas, J.A.; Pascual, F.J.; Hernandez-Ferrer, J.; Castell, P.; Benito, A.M.; Maser, W.K.; Martinez, M.T. The effect of gamma-irradiation on few-layered graphene materials. Appl. Surf. Sci. 2014, 301, 264–272. [Google Scholar] [CrossRef]
- Dumee, L.F.; Feng, C.; He, L.; Allioux, F.-M.; Yi, Z.; Gao, W.; Banos, C.; Davies, J.B.; Kong, L. Tuning the grade of graphene: Gamma ray irradiation of free-standing graphene oxide films in gaseous phase. Appl. Surf. Sci. 2014, 322, 126–135. [Google Scholar] [CrossRef]
- Lin, L.; Wang, W.; Li, D.; Xu, S.; Sun, Y.; Li, L.; Fan, K.; Xing, C.; Zhang, L.; Li, J. Multifunctional graphene/Ag hydrogel with antimicrobial and catalytic properties for efficient solar-driven desalination and wastewater purification. Chem. Eng. J. 2023, 478, 147249. [Google Scholar] [CrossRef]
- Jung, C.-H.; Park, Y.-W.; Hwang, I.-T.; Go, Y.-J.; Na, S.-I.; Shin, K.; Lee, J.-S.; Choi, J.-H. Eco-friendly and simple radiation-based preparation of graphene and its application to organic solar cells. J. Phys. D Appl. Phys. 2014, 47, 015105. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Luo, K.; Li, L.; Chen, J.; Li, J. Engineering Reduced Graphene Oxide Aerogel Produced by Effective γ-ray Radiation-Induced Self-Assembly and Its Application for Continuous Oil-Water Separation. Ind. Eng. Chem. Res. 2016, 55, 3775–3781. [Google Scholar] [CrossRef]
- He, Y.-L.; Li, J.-H.; Li, L.-F.; Chen, J.-B.; Li, J.-Y. The synergy reduction and self-assembly of graphene oxide via gamma-ray irradiation in an ethanediamine aqueous solution. Nucl. Sci. Techn. 2016, 27, 61. [Google Scholar] [CrossRef]
- He, Y.; Li, J.; Li, L.; Li, J. Gamma-ray irradiation-induced reduction and self-assembly of graphene oxide into three-dimensional graphene aerogel. Mater. Lett. 2016, 177, 76–79. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y.; Jiang, Z.; Wang, M.; Wu, Q.; Zhou, X.; Ge, X. Self-assembly of graphene oxide nanosheets in t-butanol/water medium under gamma-ray radiation. Chin. Chem. Lett. 2018, 29, 931–934. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Y.; Jiang, Z.; Wang, M.; Wu, Q.; Zhou, X.; Ge, X. Formation mechanism of 3D macroporous graphene aerogel in alcohol-water media under gamma-ray radiation. Appl. Surf. Sci. 2018, 427, 1144–1151. [Google Scholar] [CrossRef]
- Luo, K.; Li, J.; Li, L.; Li, J. A facile method for preparing 3D graphene/Ag aerogel via gamma-ray irradiation. Fuller. Nanotub. Carbon Nanostruct. 2016, 24, 720–724. [Google Scholar] [CrossRef]
- Lu, M.; Li, J.; Li, L.; Lin, J.; Li, J. Fabrication of ultralight 3D graphene/Pt aerogel via in situ gamma-ray irradiation and its application for the catalytic degradation of methyl orange. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 425–434. [Google Scholar] [CrossRef]
- Li, D.; Lin, L.; Lu, M.; Li, L.; Li, J. Magnetic graphene noble metal aerogels: Preparation and application for catalytic degradation of 4-NP. New J. Chem. 2023, 47, 10751–10758. [Google Scholar] [CrossRef]
- Lu, M.; Li, J.; Li, L.; Lin, J.; Jiang, H.; Li, J. Synthesis of graphene/silver aerogel composites via gamma ray irradiation and its catalytic ability for 4-nitrophenol. J. Radiat. Res. Radiat. Process. 2020, 38, 020201. [Google Scholar]
- Lu, M.; Li, J.; Song, S.; Li, L.; Lin, J.; Zhang, B.; Li, J. The synthesis of 3D graphene/Au composites via γ-ray irradiation and their use for catalytic reduction of 4-nitrophenol. Nanotechnology 2020, 31, 235604. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, C.; Liu, X.; Xu, J.; Yang, X.; Zhang, Z. Facile γ-ray irradiation synthesis of Pt/GA nanocomposite for catalytic reduction of 4-nitrophenol. Green Energy Environ. 2021, 6, 734–742. [Google Scholar] [CrossRef]
- Fan, K.; Liu, C.; Li, L.; Li, J. Fabrication of 3D Porous Graphene@SnO2 Aerogel via In Situ Gamma Ray Irradiation Induced Self-Assembly. Nanobiotechnol. Rep. 2022, 17, 59–63. [Google Scholar] [CrossRef]
- Lee, I.; Kang, S.-M.; Jang, S.-C.; Lee, G.-W.; Shim, H.E.; Rethinasabapathy, M.; Roh, C.; Huh, Y.S. One- pot gamma ray- induced green synthesis of a Prussian blue- laden polyvinylpyrrolidone/reduced graphene oxide aerogel for the removal of hazardous pollutants. J. Mater. Chem. A 2019, 7, 1737–1748. [Google Scholar] [CrossRef]
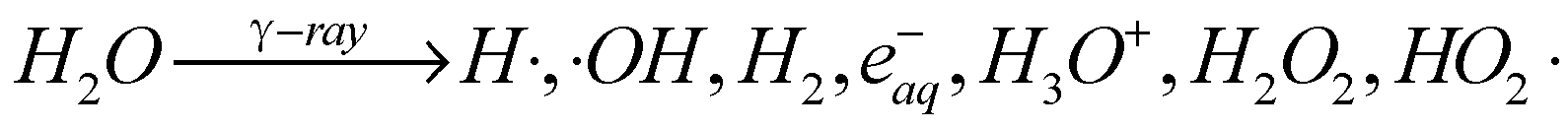

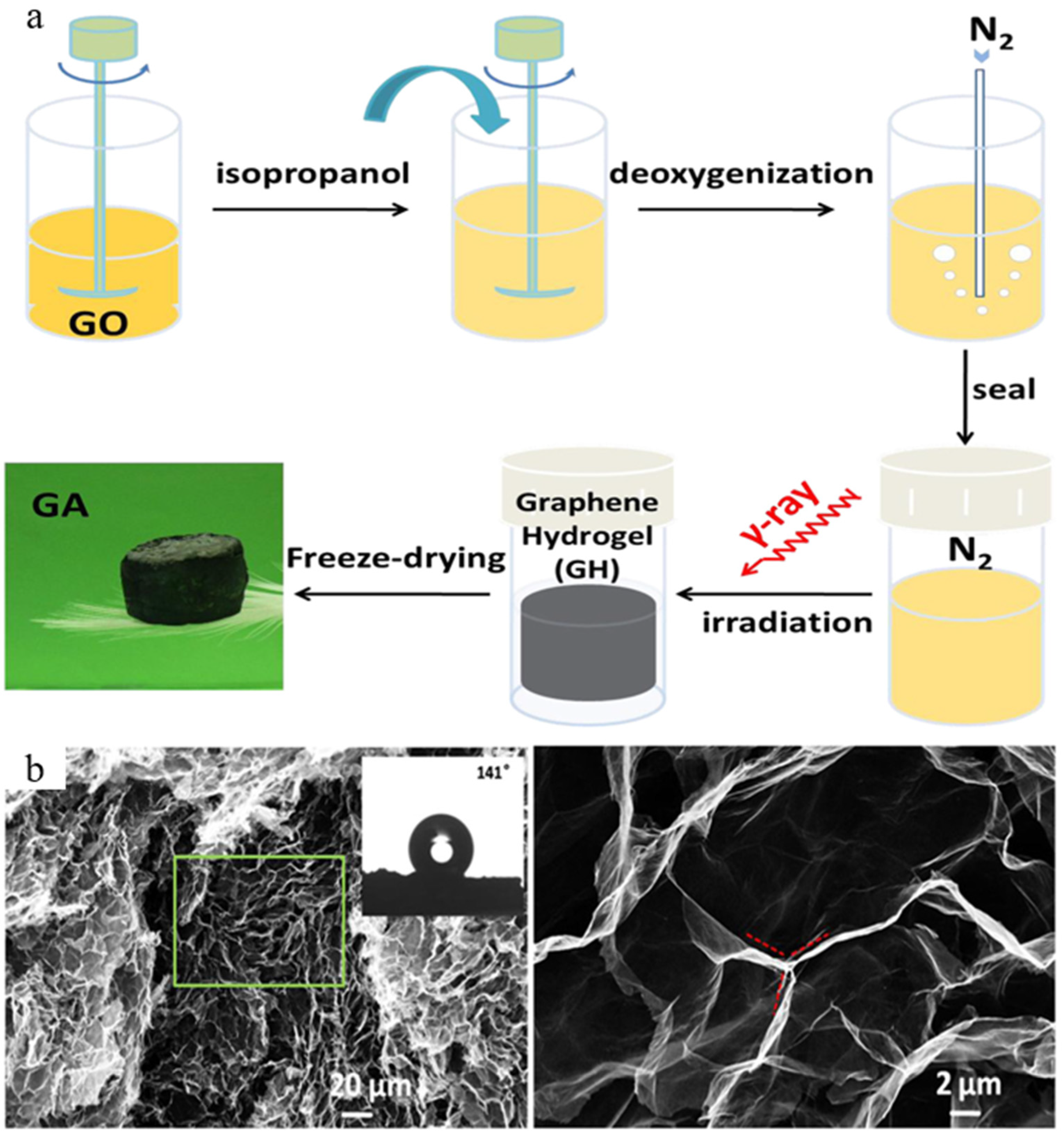
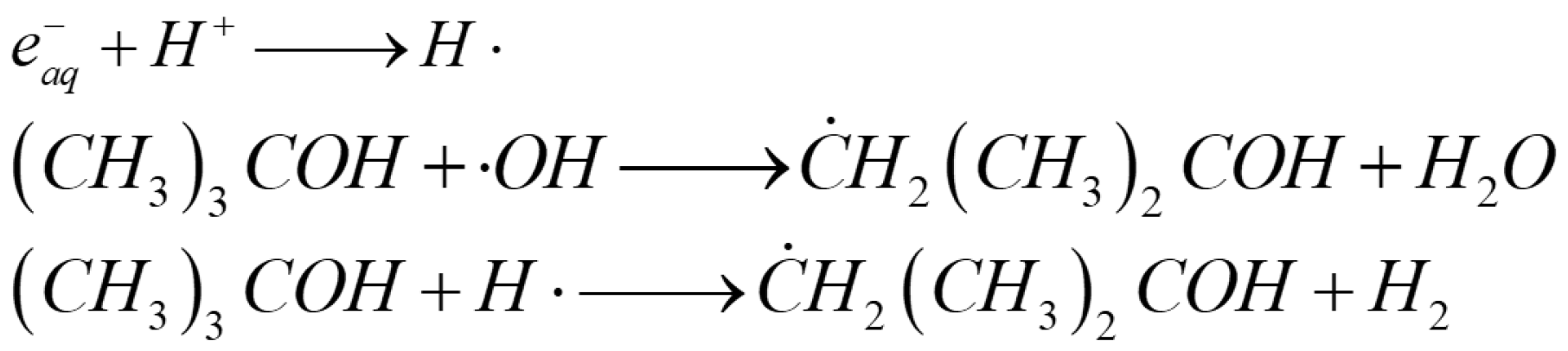
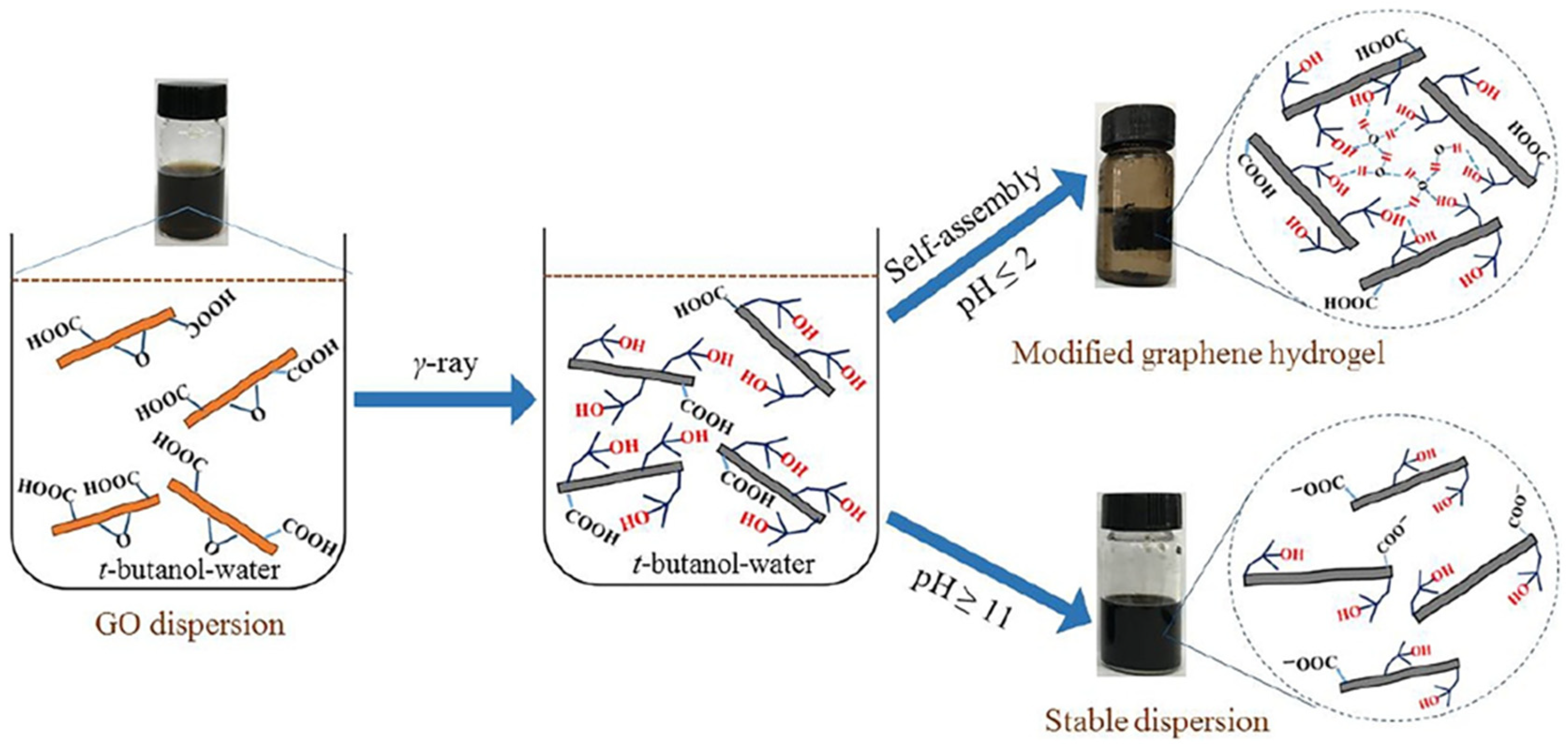
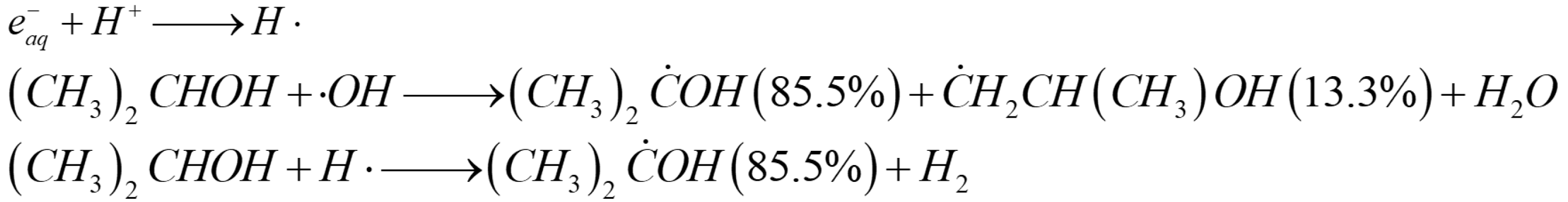

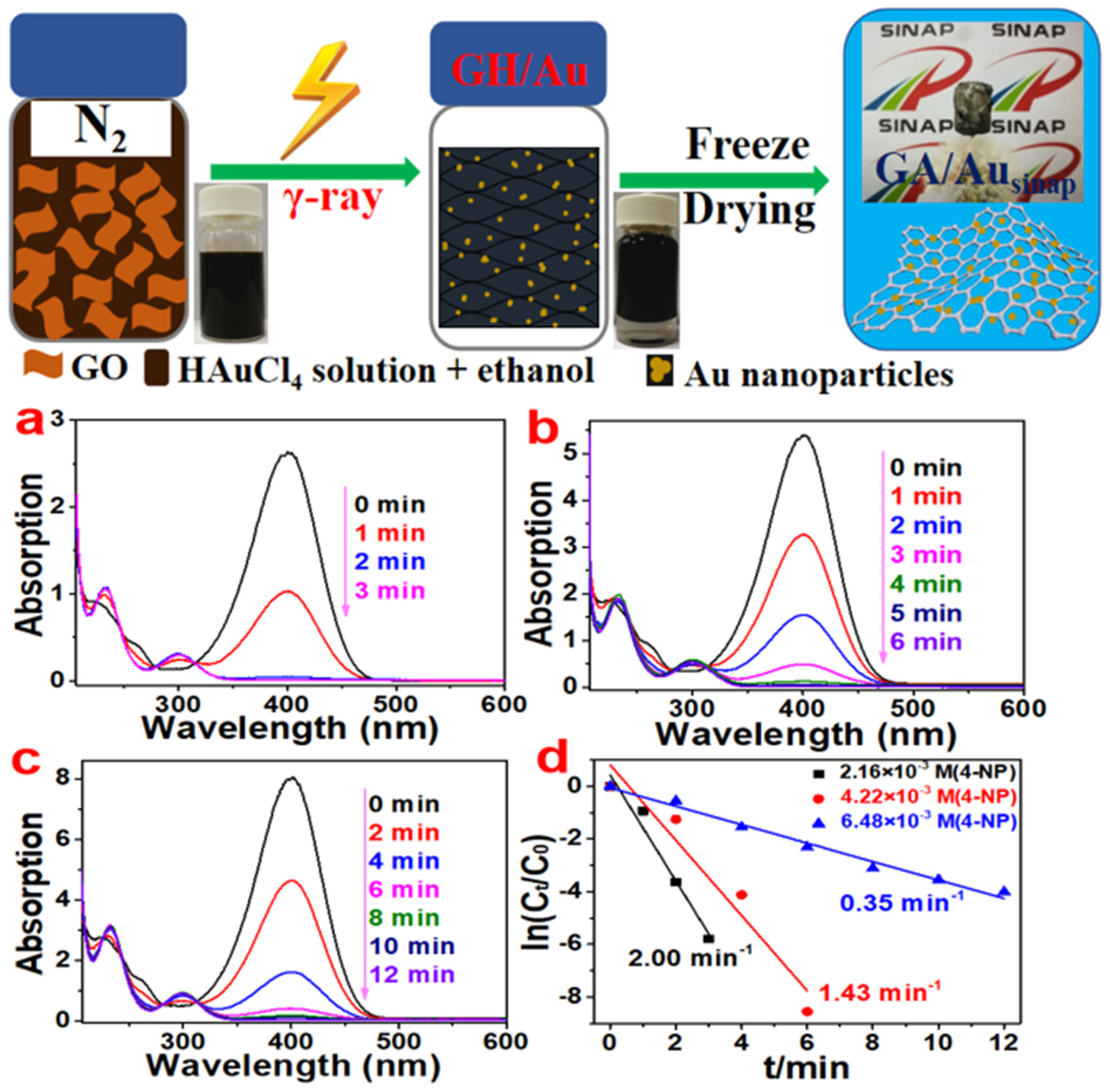
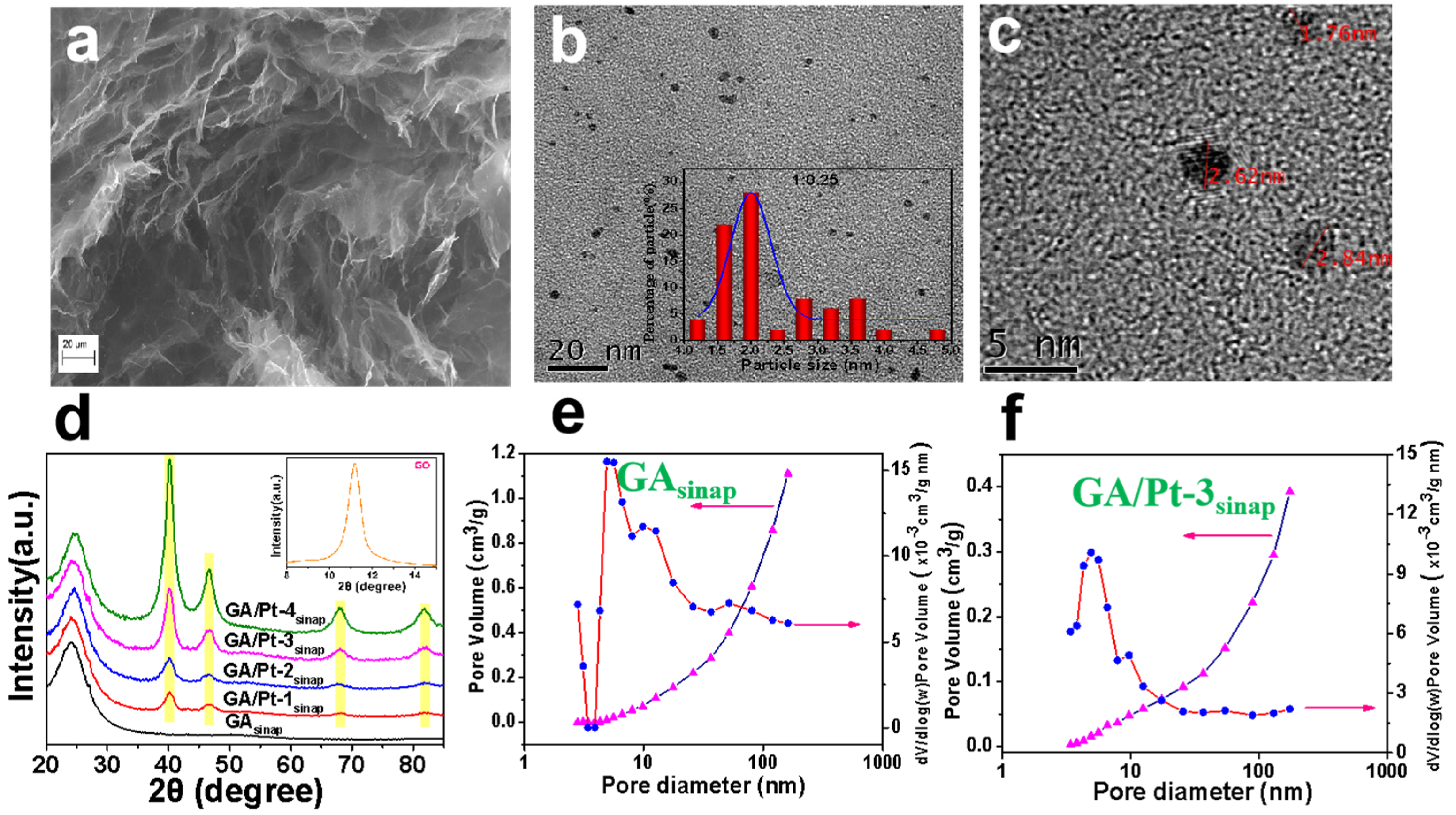
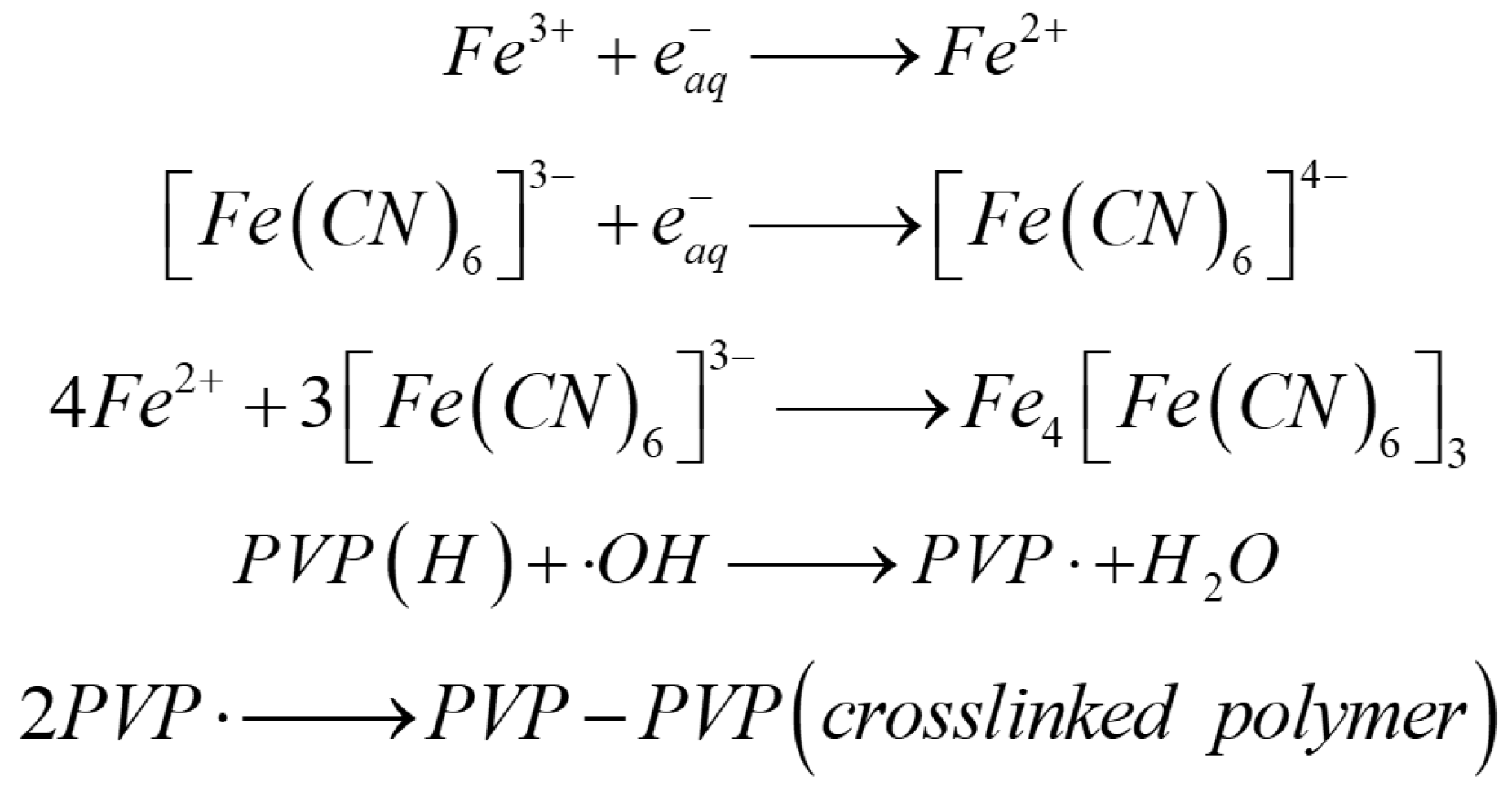
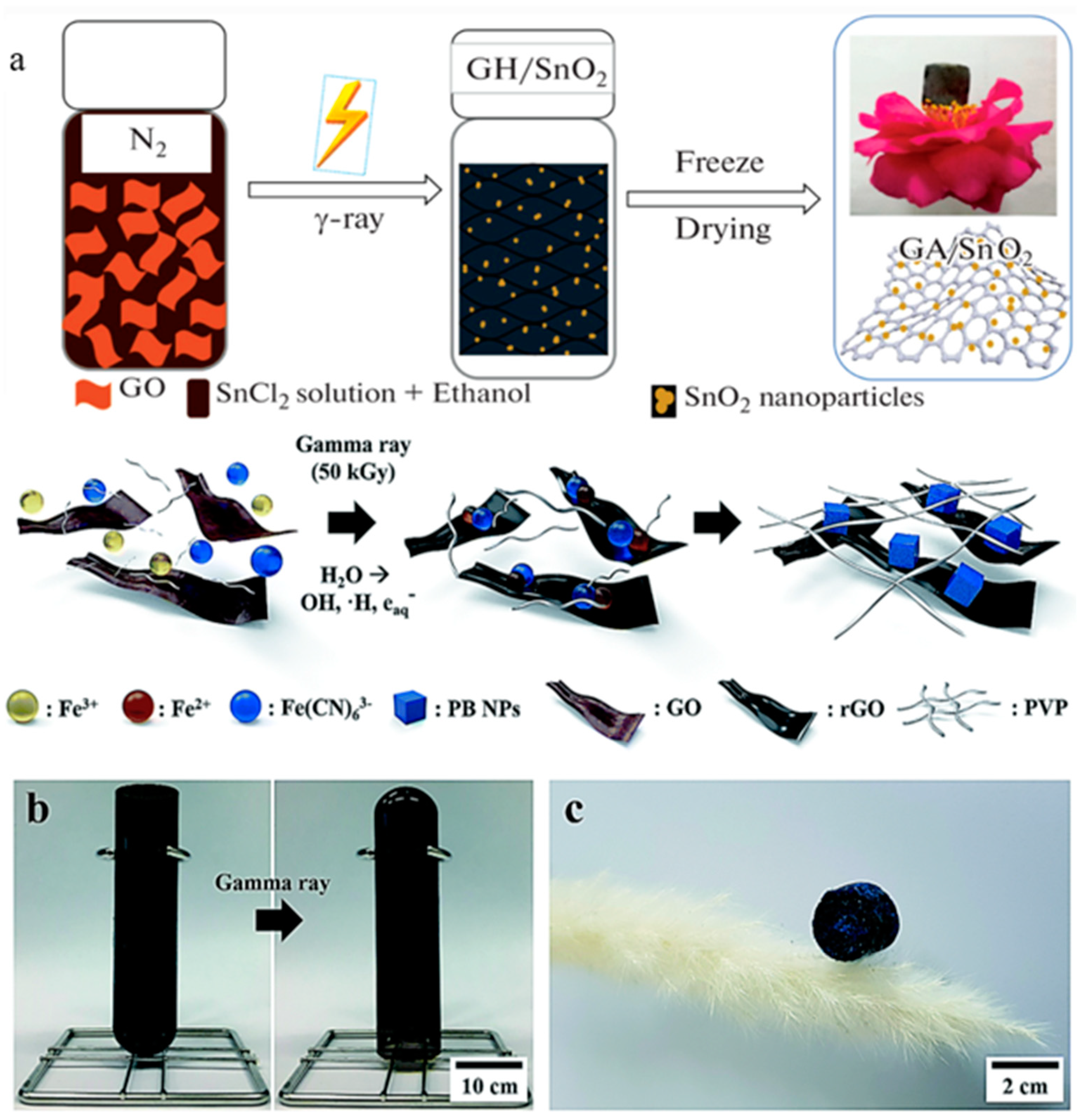
| Architectures | Adsorbed Dose [kGy] | Radical Scavenger | C/O Radio | Pore Size [µm] | NPs Size (1) | Mass Density [mg cm−3] |
|---|---|---|---|---|---|---|
| RGO aerogel 19,20 | 400 | Ethylenediamine | 9.56 | 8–15 | None | 3.5–4.0 |
| GA 21 | 200 | Isopropanol | 12.0 | 10 | None | 3.82 |
| GA 22 | >250 | t-butanol | 5.6 | — | None | 4 |
| GA 23 | 54.7 | Isopropanol | 13.0 | — | None | 4.0 |
| GA/Ag 24 | 200 | Isopropanol | — | 2–15 | 20–80 | — |
| GA/Ag 25 | 300 | Ethanol | — | — | 20–60 | 3.2–5.7 |
| GA/Au 26 | — | Ethanol | 14.13 | — | 6–28 | 4.5–5.6 |
| GA/Pt 27 | — | Ethanol | 8.8 | 2–10 | 2–10 | 3.4–5.9 |
| Pt/GA 28 | 200 | Isopropanol | Decreased significantly | — | 3 | — |
| GA@SnO2 29 | — | Ethanol | — | 10 | 3–5 | 10.82 |
| PB@PVP/rGO aerogel 30 | 50 | No | — | 38.66 ± 4.20 98.28 ± 7.77 | 57.94 ± 3.10 | 3.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, K.; Lin, L.; Li, D.; Wang, F.; Li, J. Progress in Research on the Preparation of Graphene-Based Aerogels Using γ-ray Irradiation Technology. Gels 2024, 10, 90. https://doi.org/10.3390/gels10020090
Fan K, Lin L, Li D, Wang F, Li J. Progress in Research on the Preparation of Graphene-Based Aerogels Using γ-ray Irradiation Technology. Gels. 2024; 10(2):90. https://doi.org/10.3390/gels10020090
Chicago/Turabian StyleFan, Kai, Lin Lin, Danyi Li, Fangzheng Wang, and Jihao Li. 2024. "Progress in Research on the Preparation of Graphene-Based Aerogels Using γ-ray Irradiation Technology" Gels 10, no. 2: 90. https://doi.org/10.3390/gels10020090
APA StyleFan, K., Lin, L., Li, D., Wang, F., & Li, J. (2024). Progress in Research on the Preparation of Graphene-Based Aerogels Using γ-ray Irradiation Technology. Gels, 10(2), 90. https://doi.org/10.3390/gels10020090







