Recent Developments in 3D-(Bio)printed Hydrogels as Wound Dressings
Abstract
1. Introduction
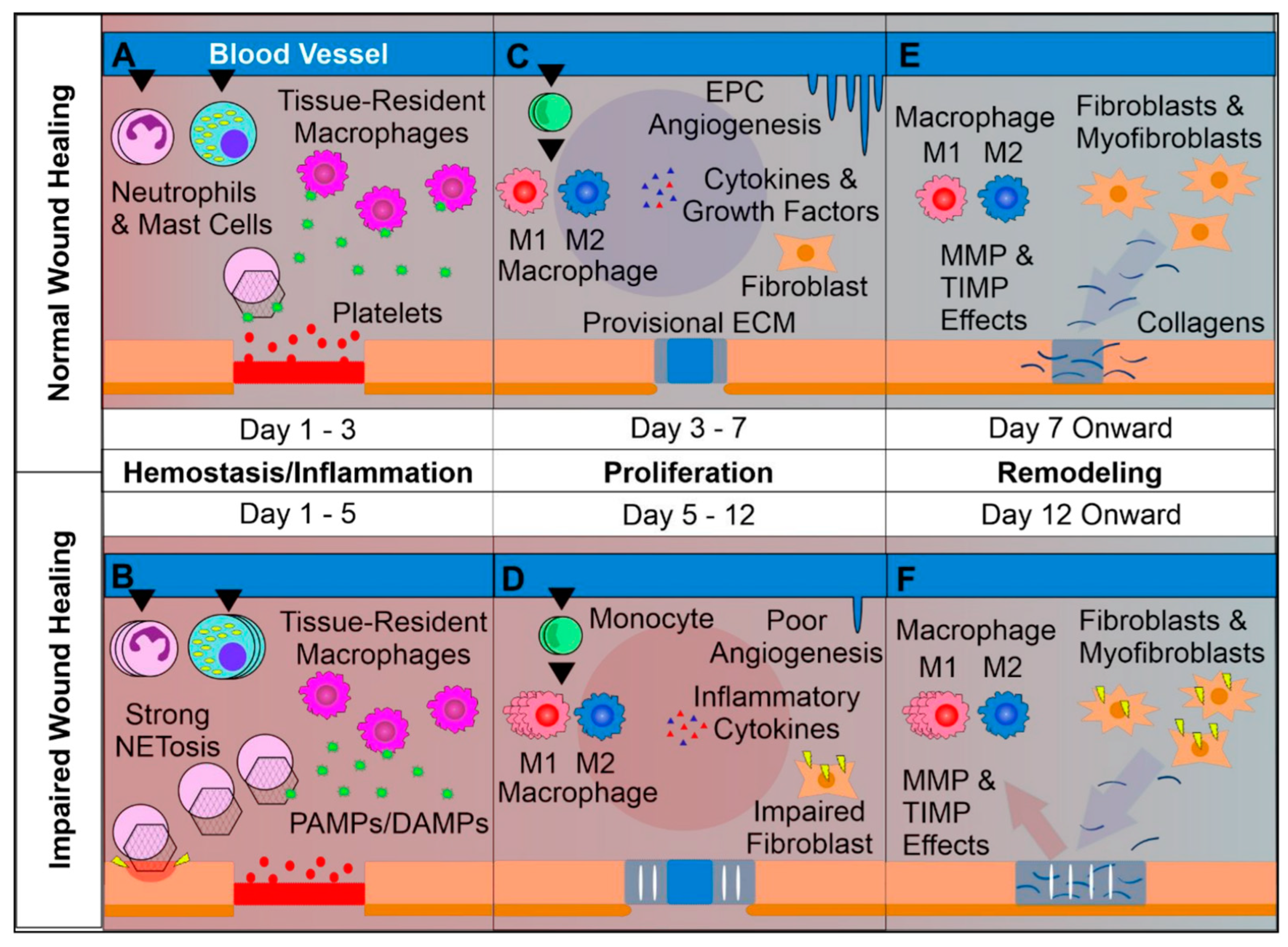
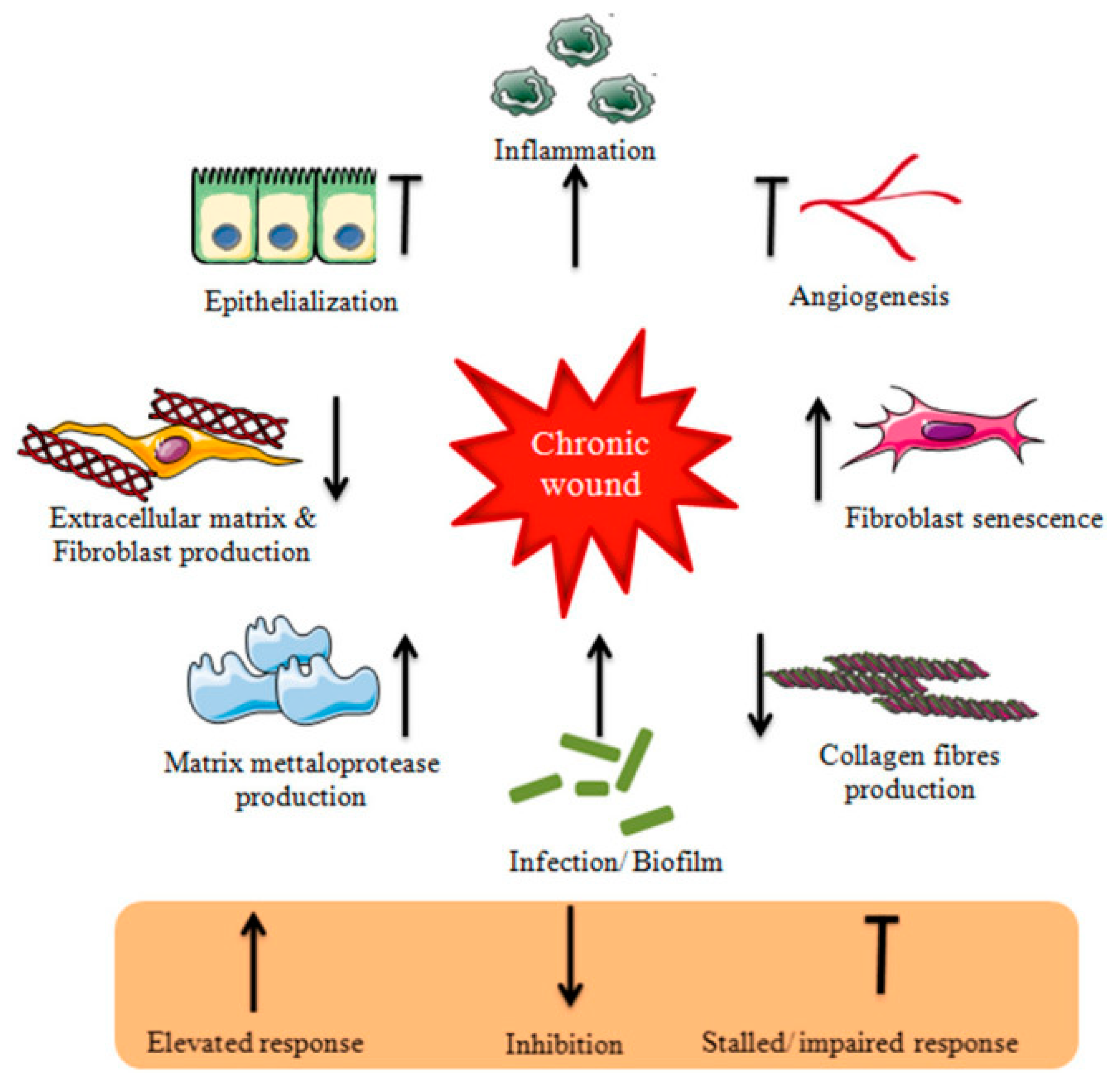
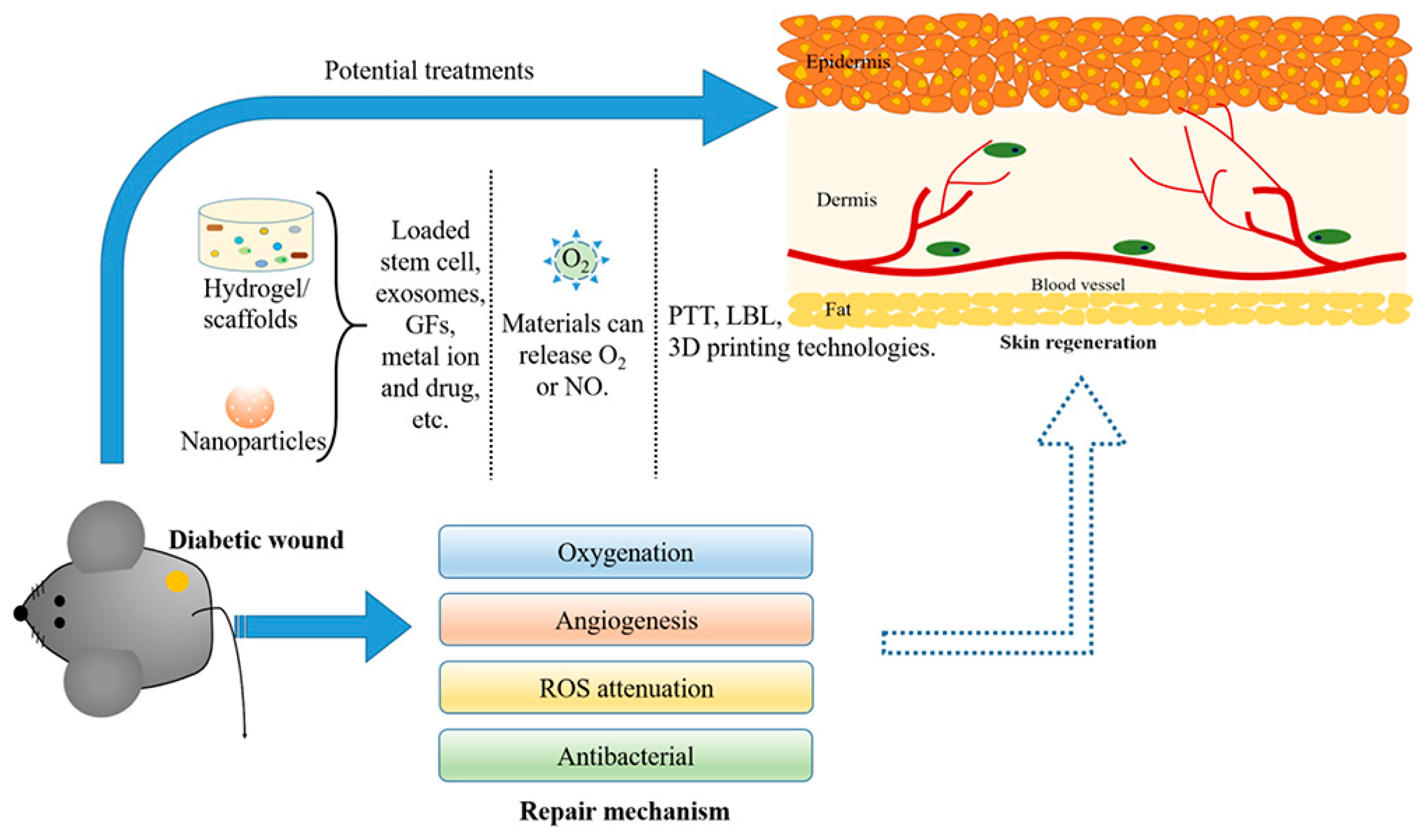
2. Wound Healing
3. Wound Dressings
3.1. Film Dressings
3.2. Foam Dressings
3.3. Hydrocolloid Dressings
3.4. Alginate Dressings
3.5. Hydrogel Dressings
3.6. Cell-Based Dressings
4. Three-Dimensional Printing
3D-Printed Hydrogels
5. Three-Dimensional Bioprinting
5.1. 3D-Bioprinted Hydrogels
5.1.1. Bioinks
5.1.2. Cells
5.1.3. Bioprinting Methods
5.1.4. Encapsulation of Bioactive Agents
6. Conclusions
7. Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Uchida, D.T.; Bruschi, M.L. 3D Printing as a Technological Strategy for the Personalized Treatment of Wound Healing. AAPS Pharm. Sci. Tech. 2023, 24, 41. [Google Scholar] [CrossRef]
- Yang, J.M.; Olanrele, O.S.; Zhang, X.; Hsu, C.C. Fabrication of Hydrogel Materials for Biomedical Applications; Chapter 12. In Novel Biomaterials for Regenerative Medicine, Advances in Experimental Medicine and Biology 1077; Chun, H.J., Park, K., Kim, C.-H., Khang, G., Eds.; Springer: Singapore, 2018. [Google Scholar] [CrossRef]
- Sorg, H.; Sorg, C.G.G. Skin Wound Healing: Of Players, Patterns, and Processes. Eur. Surg. Res. 2023, 64, 141–157. [Google Scholar] [CrossRef]
- Del Amo, C.; Fernández-San Argimiro, X.; Cascajo-Castresana, M.; Perez-Valle, A.; Madarieta, I.; Olalde, B.; Andia, I. Wound-Microenvironment Engineering through Advanced-Dressing Bioprinting. Int. J. Mol. Sci. 2022, 23, 2836. [Google Scholar] [CrossRef]
- Yuan, N.; Shao, K.; Huang, S.; Chen, C. Chitosan, alginate, hyaluronic acid and other novel multifunctional hydrogel dressings for wound healing: A review. Int. J. Biol. Macromol. 2023, 240, 124321. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Le, T.T.N.; Nguyen, A.T.; Le, H.N.T.; Pham, T.T. Biomedical materials for wound dressing: Recent advances and applications. RSC Adv. 2023, 13, 5509. [Google Scholar] [CrossRef]
- Chouhan, D.; Dey, N.; Bhardwaj, N.; Mandala, B.B. Emerging and innovative approaches for wound healing and skin regeneration: Current status and advances. Biomaterials 2019, 216, 119267. [Google Scholar] [CrossRef]
- Tsegay, F.; Elsherif, M.; Butt, H. Smart 3D Printed Hydrogel Skin Wound Bandages: A Review. Polymers 2022, 14, 1012. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.-S.; Gao, Y.; Rui, B.-Y.; Li, X.-R.; Liu, P.-L.; Han, Z.-Y.; Wei, Z.-Y.; Zhang, C.-R.; Wang, F.; Dawes, H.; et al. Double-network hydrogel enhanced by SS31-loaded mesoporous polydopamine nanoparticles: Symphonic collaboration of near-infrared photothermal antibacterial effect and mitochondrial maintenance for full-thickness wound healing in diabetes mellitus. Bioact. Mater. 2023, 27, 409–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, G.; Zhang, H.; Zhao, C.; Sun, L.; Zhao, Y. Emerging Functional Biomaterials as Medical Patches. ACS Nano 2021, 15, 5977–6007. [Google Scholar] [CrossRef] [PubMed]
- Cipriani, F.; Ariño Palao, B.; Gonzalez de Torre, I.; Vega Castrillo, A.; Aguado Hernández, H.J.; Alonso Rodrigo, M.; Àlvarez Barcia, A.J.; Sanchez, A.; García Diaz, V.; Lopez Peña, M.; et al. An elastin-like recombinamer-based bioactive hydrogel embedded with mesenchymal stromal cells as an injectable scaffold for osteochondral repair. Regen. Biomater. 2019, 6, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Tsanaktsidou, E.; Kammona, O.; Labude, N.; Neuss, S.; Krüger, M.; Kock, L.; Kiparissides, C. Biomimetic Cell-Laden MeHA Hydrogels for the Regeneration of Cartilage Tissue. Polymers 2020, 12, 1598. [Google Scholar] [CrossRef]
- Xu, L.; Chen, Y.; Zhang, P.; Tang, J.; Xue, Y.; Luo, H.; Dai, R.; Jin, J.; Liu, J. 3D printed heterogeneous hybrid hydrogel scaffolds for sequential tumor photothermal chemotherapy and wound healing. Biomater. Sci. 2022, 10, 5648. [Google Scholar] [CrossRef]
- Brites, A.; Ferreira, M.; Bom, S.; Grenho, L.; Claudio, R.; Gomes, P.S.; Fernandes, M.H.; Marto, J.; Santos, C. Fabrication of antibacterial and biocompatible 3D printed Manuka-Gelatin based patch for wound healing applications. Int. J. Pharm. 2023, 632, 122541. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.L.; Longaker, M.T. Bioprinted Hydrogels for Fibrosis and Wound Healing: Treatment and Modeling. Gels 2023, 9, 19. [Google Scholar] [CrossRef]
- Holl, J.; Kowalewski, C.; Zimek, Z.; Fiedor, P.; Kaminski, A.; Oldak, T.; Moniuszko, M.; Eljaszewicz, A. Chronic DiabeticWounds and Their Treatment with Skin Substitutes. Cells 2021, 10, 655. [Google Scholar] [CrossRef]
- Bai, Q.; Han, K.; Dong, K.; Zheng, C.; Zhang, Y.; Long, Q.; Lu, T. Potential Applications of Nanomaterials and Technology for Diabetic Wound Healing. Int. J. Nanomed. 2020, 15, 9717–9743. [Google Scholar] [CrossRef] [PubMed]
- Monika, P.; Chandraprabha, M.N.; Rangarajan, A.; Waiker, P.V.; Murthy, K.N.C. Challenges in Healing Wound: Role of Complementary and Alternative Medicine. Front. Nutr. 2022, 8, 791899. [Google Scholar] [CrossRef] [PubMed]
- Elviri, L.; Bianchera, A.; Bergonzi, C.; Bettini, R. Controlled local drug delivery strategies from chitosan hydrogels for wound healing. Expert Opin. Drug Deliv. 2017, 14, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Saifullah, Q.; Sharma, A. Current trends on innovative technologies in topical wound care for advanced healing and management. Curr. Drug Res. Rev. 2023. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Smandri, A.; Nordin, A.; Hwei, N.M.; Chin, K.-Y.; Aziz, I.A.; Fauzi, M.B. Natural 3D-Printed Bioinks for Skin Regeneration and Wound Healing: A Systematic Review. Polymers 2020, 12, 1782. [Google Scholar] [CrossRef]
- Hydrofilm®. Available online: https://www.hartmann.info/en-us/our-products/wound-management/post-surgical-dressings/films/hydrofilm%c2%ae#products (accessed on 30 November 2023).
- BIOCLUSIVETM PLUS. Available online: https://www.acelity.com/-/media/Project/Acelity/Acelity-Base-Sites/shared/PDF/bioclusive-plus-dressing-ifu.pdf (accessed on 30 November 2023).
- Mepore Film. Available online: https://www.molnlycke.com/products-solutions/mepore-film/ (accessed on 30 November 2023).
- Transeal®. Available online: https://www.deroyal.com/products/burn/burn-care-item/!/wc-burn-transeal (accessed on 30 November 2023).
- Tegaderm™ Absorbent Clear Acrylic Dressing, 90800 Series. Available online: https://www.3m.com/3M/en_US/p/d/b00035973/ (accessed on 30 November 2023).
- OPSITE POST OP. Available online: https://www.smith-nephew.com/en-gb/health-care-professionals/products/advanced-wound-management/opsite-post-op-global#application (accessed on 30 November 2023).
- Mepitel Transparent Wound Contact Layer with Safetac. Available online: https://www.molnlycke.us/products-solutions/mepitel/ (accessed on 30 November 2023).
- PermaFoam® Classic. Available online: https://www.molnlycke.us/products-solutions/mepitel/ (accessed on 30 November 2023).
- HydroTac® and HydroTac® Comfort. Available online: https://www.hartmann.info/en-gb/articles/c/9/hydrotac (accessed on 30 November 2023).
- Mepilex® Ag. Available online: https://www.molnlycke.us/products-solutions/mepilex-ag/ (accessed on 30 November 2023).
- Tegaderm™ Silicone Foam Dressings. Available online: https://www.3m.com/3M/en_US/medical-us/tegaderm-silicone-foam-dressings/ (accessed on 30 November 2023).
- Tegaderm™ High Performance Foam Adhesive Dressing. Available online: https://www.3m.com/3M/en_US/p/d/b00035953/ (accessed on 30 November 2023).
- Tegaderm™ High Performance Foam Non-Adhesive Dressing. Available online: https://www.3m.com/3M/en_US/p/d/b00036071/ (accessed on 30 November 2023).
- Askina Foam™. Available online: https://www.bbraun.com/en/products/b/askina-foam-wound-dressing.html (accessed on 30 November 2023).
- Lyofoam® Max. Available online: https://www.molnlycke.com/products-solutions/lyofoam-max/ (accessed on 30 November 2023).
- ALLEVYN Non-Bordered Dressings. Available online: https://www.smith-nephew.com/en/health-care-professionals/products/advanced-wound-management/allevyn-non-bordered-global#productfeatures (accessed on 30 November 2023).
- ALLEVYN Ag. Available online: https://www.smith-nephew.com/en/health-care-professionals/products/advanced-wound-management/allevyn-ag-range#allevyn-ag-adhesive (accessed on 30 November 2023).
- TIELLETM PHMB. Available online: https://www.acelity.com/-/media/Project/Acelity/Acelity-Base-Sites/shared/PDF/10012895-tielle-phmb-non-adhesive-instructions-for-use.pdf (accessed on 30 November 2023).
- ActivHeal® PHMB. Available online: https://activheal.com/wound-care-dressing-range/phmb-foam-dressing/ (accessed on 30 November 2023).
- TIELLE® LIQUALOCK. Available online: https://www.acelity.com/-/media/Project/Acelity/Acelity-Base-Sites/shared/PDF/519402rc-for-web-viewing.pdf (accessed on 30 November 2023).
- Tromboguard®. Available online: https://tricomed.com/products/tromboguard/#show (accessed on 30 November 2023).
- ActivHeal® Silicone Foam. Available online: https://activheal.com/wound-care-dressing-range/silicone-dressing/#foam (accessed on 30 November 2023).
- Granuflex® Dressing. Available online: https://www.convatec.com/en-gb/products/advanced-wound-care/brand-names/pc-wound-duoderm-granluflex/granuflex-dressing/ (accessed on 30 November 2023).
- Comfeel® Plus Dressing. Available online: https://products.coloplast.co.uk/coloplast/wound-care/comfeel-plus/comfeel-plus-dressing/ (accessed on 30 November 2023).
- Confeel® Plus Transparent. Available online: https://products.coloplast.co.uk/coloplast/wound-care/comfeel-plus-transparent/ (accessed on 30 November 2023).
- Tegaderm™ Hydrocolloid Dressing. Available online: https://multimedia.3m.com/mws/media/653961O/tegaderm-hydrocolloid-dressings-sell-sheet.pdf (accessed on 30 November 2023).
- DuoDERM® Extra Thin Dressing. Available online: https://www.convatec.com/products/advanced-wound-care/wound-type/pc-wound-diabetic-foot-ulcers/duoderm-extra-thin-dressing/ (accessed on 30 November 2023).
- DuoDERM® Signal™ Dressing. Available online: https://www.convatec.com/products/advanced-wound-care/wound-type/pc-wound-diabetic-foot-ulcers/duoderm-signal-dressing/ (accessed on 30 November 2023).
- REPLICARE® Thin Hydrocolloid Dressing. Available online: https://www.woundsource.com/product/replicare-thin-hydrocolloid-dressing (accessed on 30 November 2023).
- Restore Hydrocolloid Dressing. Available online: https://www.hollister.com/-/media/files/pdfs-for-download/wound-care/restore-hydrocolloid-sterile-protocol-910453-1008.ashx (accessed on 30 November 2023).
- Kaltostat® Dressing. Available online: https://www.convatec.com/en-gb/products/advanced-wound-care/wound-type/pc-wound-diabetic-foot-ulcers/kaltostat-alginate-dressing/ (accessed on 30 November 2023).
- ALGICELL® Ag. Available online: http://www.kromh.com/wp-content/uploads/ALGICELL-AG-IFU.pdf (accessed on 30 November 2023).
- Algivon. Available online: https://advancismedical.hr/products/algivon?shpxid=9870809e-dde7-4567-87fd-358e2180ddee (accessed on 30 November 2023).
- Algivon Plus. Available online: https://advancismedical.hr/products/algivon-plus?shpxid=4ee90f53-7349-4146-917b-1584db42e7dc (accessed on 30 November 2023).
- Fibracol™ Plus. Available online: https://www.3m.com/3M/en_US/p/d/b5005265077/ (accessed on 30 November 2023).
- ActivHeal® Alginate. Available online: https://activheal.com/wound-care-dressing-range/alginate-dressing/ (accessed on 30 November 2023).
- Restore™ Calcium Alginate. Available online: https://www.hollister.com/-/media/files/pdfs-for-download/wound-care/restore-calcium-alginate-protocol-910455-1008.ashx (accessed on 30 November 2023).
- Restore™ Calcium Alginate Dressing with Silver. Available online: https://www.hollister.com/-/media/files/pdfs-for-download/wound-care/restore-calcium-alginate-silver-protocol-910444-1008.ashx (accessed on 30 November 2023).
- ActivHeal Aquafiber® Ag. Available online: https://activheal.com/wound-care-dressing-range/aquafiber-ag-dressing/ (accessed on 30 November 2023).
- ACTIVHEAL® HYDROGEL. Available online: https://activheal.com/wound-care-dressing-range/hydrogel-dressing/ (accessed on 30 November 2023).
- Restore Hydrogel Dressing. Available online: https://www.hollister.com/-/media/files/pdfs-for-download/wound-care/hwc_protocol_910452-1008.ashx (accessed on 30 November 2023).
- SUPRASORB® G. Available online: https://www.lohmann-rauscher.com/us-en/products/wound-care/modern-wound-care/suprasorb-g/ (accessed on 30 November 2023).
- AquaDerm™. Available online: https://dermarite.com/product/aquaderm/ (accessed on 30 November 2023).
- Neoheal®. Available online: https://kikgel.com.pl/en/products/neoheal/ (accessed on 30 November 2023).
- INTRASITE◊ GEL. Available online: https://www.smith-nephew.com/en/health-care-professionals/products/advanced-wound-management/intrasite-gel-ppl#reference-materials (accessed on 30 November 2023).
- Nu-Gel™. Available online: https://www.3m.com/3M/en_US/p/d/b5005265144/ (accessed on 30 November 2023).
- Wang, S.; Ong, P.J.; Liu, S.; Thitsartarn, W.; Tan, M.J.B.H.; Suwardi, A.; Zhu, Q.; Loh, X.J. Recent Advances in Host-Guest Supramolecular Hydrogels for Biomedical Applications. Chem. Asian J. 2022, 17, e202200608. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; De Leon-Oliva, D.; Liviu Boaru, D.; Fraile-Martinez, O.; García-Montero, C.; Diaz, R.; Coca, S.; Barrena-Blázquez, S.; Bujan, J.; García-Honduvilla, N.; et al. Unraveling the New Perspectives on Antimicrobial Hydrogels: State-of-the-Art and Translational Applications. Gels 2023, 9, 617. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.J.; Bae, S.K.; Jung, Y.S.; Kim, J.C.; Kim, J.S.; Park, S.K.; Suh, J.S.; Yi, S.J.; Ahn, S.H.; Lim, J.O. Enhanced wound healing using a 3D printed VEGF-mimicking peptide incorporated hydrogel patch in a pig model. Biomed. Mater. 2021, 16, 045013. [Google Scholar] [CrossRef]
- Giliomee, J.; du Toit, L.C.; Klumperman, B.; Choonara, Y.E. Investigation of the 3D Printability of Covalently Cross-Linked Polypeptide-Based Hydrogels. ACS Omega 2022, 7, 7556–7571. [Google Scholar] [CrossRef]
- Xu, C.; Molino, B.Z.; Wang, X.; Cheng, F.; Xu, W.; Molino, P.; Bacher, M.; Su, D.; Rosenau, T.; Willför, S.; et al. 3D printing of nanocellulose hydrogel scaffolds with tunable mechanical strength towards wound healing application. J. Mater. Chem. B 2018, 6, 7066. [Google Scholar] [CrossRef]
- Alizadehgiashi, M.; Nemr, C.R.; Chekini, M.; Ramos, D.P.; Mittal, N.; Ahmed, S.U.; Khuu, N.; Kelley, S.O.; Kumacheva, E. Multifunctional 3D-Printed Wound Dressings. ACS Nano 2021, 15, 12375–12387. [Google Scholar] [CrossRef]
- Leppiniemi, J.; Lahtinen, P.; Paajanen, A.; Mahlberg, R.; Metsä-Kortelainen, S.; Pinomaa, T.; Pajari, H.; Vikholm-Lundin, I.; Pursula, P.; Hytönen, V.P. 3D-Printable Bioactivated Nanocellulose−Alginate Hydrogels. ACS Appl. Mater. Interfaces 2017, 9, 21959–21970. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, Y.; Wei, Q.; Li, X.; Guo, Y.; Zhang, S. Design and Fabrication of Sodium Alginate/Carboxymethyl Cellulose Sodium Blend Hydrogel for Artificial Skin. Gels 2021, 7, 115. [Google Scholar] [CrossRef]
- Tsegay, F.; Hisham, M.; Elsherif, M.; Schiffer, A.; Butt, H. 3D Printing of pH Indicator Auxetic Hydrogel Skin Wound Dressing. Molecules 2023, 28, 1339. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Lee, H.; Han, G.; Kang, M.; Park, S.; Kim, D.E.; Lee, M.; Kim, M.-J.; Na, Y.; Oh, S.; et al. 3D-Printed Functional Hydrogel by DNA-Induced Biomineralization for Accelerated Diabetic Wound Healing. Adv. Sci. 2023, 10, 2300816. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Duan, X.; Xie, F.; Zheng, D.; Yang, P.; Wang, X.; Hu, Z. 3D-bioprinted double-crosslinked angiogenic alginate/chondroitin sulfate patch for diabetic wound healing. Int. J. Biol. Macromol. 2023, 236, 123952. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xiong, Y.; Chen, J.; Ghanem, A.; Wang, Y.; Yang, J.; Sun, B. Three Dimensional Printing Bilayer Membrane Scaffold Promotes Wound Healing. Front. Bioeng. Biotechnol. 2019, 7, 348. [Google Scholar] [CrossRef]
- Shen, H.-Y.; Liu, Z.-H.; Hong, J.-S.; Wu, M.-S.; Shiue, S.-J.; Lin, H.-Y. Controlled-release of free bacteriophage nanoparticles from 3D-plotted hydrogel fibrous structure as potential antibacterial wound dressing. J. Control. Release 2021, 331, 154–163. [Google Scholar] [CrossRef]
- Chen, B.; Huang, L.; Ma, R.; Luo, Y. 3D printed hollow channeled hydrogel scaffolds with antibacterial and wound healing activities. Biomed. Mater. 2023, 18, 045023. [Google Scholar] [CrossRef] [PubMed]
- Monavari, M.; Homaeigohar, S.; Medhekar, R.; Nawaz, Q.; Monavari, M.; Zheng, K.; Boccaccini, A.R. A 3D-Printed Wound-Healing Material Composed of Alginate Dialdehyde−Gelatin Incorporating Astaxanthin and Borate Bioactive Glass Microparticles. ACS Appl. Mater. Interfaces 2023, 15, 50626–50637. [Google Scholar] [CrossRef]
- Piola, P.; Sabbatini, M.; Gino, S.; Invernizzi, M.; Renò, F. 3D Bioprinting of Gelatin–Xanthan Gum Composite Hydrogels for Growth of Human Skin Cells. Int. J. Mol. Sci. 2022, 23, 539. [Google Scholar] [CrossRef]
- Lin, F.S.; Lee, J.-J.; Lee, A.K.-X.; Ho, C.-C.; Liu, Y.-T.; Shie, M.-Y. Calcium Silicate-Activated Gelatin Methacrylate Hydrogel for Accelerating Human Dermal Fibroblast Proliferation and Differentiation. Polymers 2021, 13, 70. [Google Scholar] [CrossRef]
- Zhou, L.; Ramezani, H.; Sun, M.; Xie, M.; Nie, J.; Lv, S.; Cai, J.; Fu, J.; He, Y. 3D printing of high-strength chitosan hydrogel scaffolds without any organic solvents. Biomater. Sci. 2020, 8, 5020–5028. [Google Scholar] [CrossRef]
- Zarandona, I.; Bengoechea, C.; Álvarez-Castillo, E.; de la Caba, K.; Guerrero, A.; Guerrero, P. 3D Printed Chitosan-Pectin Hydrogels: From Rheological Characterization to Scaffold Development and Assessment. Gels 2021, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Molino, B.Z.; Cheng, F.; Molino, P.; Yue, Z.; Su, D.; Wang, X.; Willför, S.; Xu, C.; Wallace, G.G. On Low-Concentration Inks Formulated by Nanocellulose Assisted with Gelatin Methacrylate (GelMA) for 3D Printing toward Wound Healing Application. ACS Appl. Mater. Interfaces 2019, 11, 8838–8848. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, J.; Zhang, W.; Pu, Y.; Yang, R.; Wang, P.; Liu, S.; Tan, X.; Chi, B. 3D-printed antioxidant antibacterial carboxymethyl cellulose/ε-polylysine hydrogel promoted skin wound repair. Int. J. Biol. Macromol. 2021, 187, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Nizioł, M.; Paleczny, J.; Junka, A.; Shavandi, A.; Dawiec-Lisniewska, A.; Podstawczyk, D. 3D Printing of Thermoresponsive Hydrogel Laden with an Antimicrobial Agent towards Wound Healing Applications. Bioengineering 2021, 8, 79. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Hong, Y. Combination of the Silver−Ethylene Interaction and 3D Printing to Develop Antibacterial Superporous Hydrogels for Wound Management. ACS Appl. Mater. Interfaces 2019, 11, 33734–33747. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wu, T.; Guo, Y.; Wang, L.; Yu, X.; Zhu, B.; Fan, L.; Xin, J.H.; Yu, H. Platelet-rich plasma-loaded bioactive chitosan@sodium alginate@gelatin shell-core fibrous hydrogels with enhanced sustained release of growth factors for diabetic foot ulcer healing. Int. J. Biol. Macromol. 2023, 234, 123722. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.; Yang, C.; Shang, L.; Zhao, Y.; Shen, X. Dynamically Responsive Scaffolds from Microfluidic 3D Printing for Skin Flap Regeneration. Adv. Sci. 2022, 9, 2201155. [Google Scholar] [CrossRef]
- Bashiri, Z.; Fomeshi, M.R.; Hamidabadi, H.G.; Jafari, D.; Alizadeh, S.; Bojnordi, M.N.; Orive, G.; Dolatshahi-Pirouz, A.; Zahiri, M.; Reis, R.L.; et al. 3D-printed placental-derived bioinks for skin tissue regeneration with improved angiogenesis and wound healing properties. Mater. Today Bio 2023, 20, 100666. [Google Scholar] [CrossRef]
- Siebert, L.; Luna-Cerón, E.; García-Rivera, L.E.; Oh, J.; Jang, J.; Rosas-Gómez, D.A.; Pérez-Gómez, M.D.; Maschkowitz, G.; Fickenscher, H.; Oceguera-Cuevas, D.; et al. Light-controlled growth factors release on tetrapodal ZnO-incorporated 3D-printed hydrogels for developing smart wound scaffold. Adv. Funct. Mater. 2021, 31, 2007555. [Google Scholar] [CrossRef]
- Teoh, J.H.; Tay, S.M.; Fuh, J.; Wang, C.-H. Fabricating scalable, personalized wound dressings with customizable drug loadings via 3D printing. J. Control. Release 2022, 341, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Cheng, Y.; Chen, G.; Zhao, Y. 3D-Printed Janus Piezoelectric Patches for Sonodynamic Bacteria Elimination and Wound Healing. Research 2023, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Lee, J., Jr.; Ng, H.Y.; Lin, Y.-H.; Liu, E.-W.; Lin, T.-J.; Chiu, H.-T.; Ho, X.-R.; Yang, H.-A.; Shie, M.-Y. The 3D printed conductive grooved topography hydrogel combined with electrical stimulation for synergistically enhancing wound healing of dermal fibroblast cells. Biomater. Adv. 2022, 142, 213132. [Google Scholar] [CrossRef]
- Ding, N.; Fu, X.; Gui, Q.; Wu, M.; Niu, Z.; Du, A.; Liu, J.; Wu, H.; Wang, Y.; Yue, X.; et al. Biomimetic Structure Hydrogel Loaded with Long-Term Storage Platelet-Rich Plasma in Diabetic Wound Repair. Adv. Healthc. Mater. 2023, 2303192. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Wei, X.; Chen, B.; Luo, Y. 3D printed hydrogel/PCL core/shell fiber scaffolds with NIR-triggered drug release for cancer therapy and wound healing. Acta Biomater. 2021, 131, 314–325. [Google Scholar] [CrossRef]
- Chen, C.-S.; Zeng, F.; Xiao, X.; Wang, Z.; Li, X.-L.; Tan, R.-W.; Liu, W.-Q.; Zhang, Y.-S.; She, Z.-D.; Li, S.-J. Three-Dimensionally Printed Silk-Sericin-Based Hydrogel Scaffold: A Promising Visualized Dressing Material for Real-Time Monitoring of Wounds. ACS Appl. Mater. Interfaces 2018, 10, 33879–33890. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, A.; Mao, Z.; Li, F.; Su, T.; Cao, L.; Wang, W.; Liu, Y.; Wang, C. Silk fibroin–gelatin photo-crosslinked 3D-bioprinted hydrogel with MOF-methylene blue nanoparticles for infected wound healing. Int. J. Bioprint. 2023, 9, 773. [Google Scholar] [CrossRef]
- Metwally, W.M.; El-Habashy, S.E.; El-Hosseiny, L.S.; Essawy, M.M.; Eltaher, H.M.; El-Khordagui, L.K. Bioinspired 3D-printed scaffold embedding DDAB-nano ZnO/nanofibrous microspheres for regenerative diabetic wound healing. Biofabrication 2024, 16, 015001. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, T.; Wang, L.; Zhu, J.; Guo, Y.; Yu, X.; Fan, L.; Xin, J.H.; Yu, H. A multifunctional 3D dressing unit based on the core–shell hydrogel microfiber for diabetic foot wound healing. Biomater. Sci. 2022, 10, 2568–2576. [Google Scholar] [CrossRef]
- Liang, M.; Dong, L.; Guo, Z.; Liu, L.; Fan, Z.; Wei, C.; Mi, S.; Sun, W. Collagen-hyaluronic acid composite hydrogels with applications for chronic diabetic wound repair. ACS Biomater. Sci. Eng. 2023, 9, 5376–5388. [Google Scholar] [CrossRef]
- Zheng, B.; Qiu, Z.; Xu, J.; Zeng, X.; Liub, K.; Chen, L. 3D printing-mediated microporous starch hydrogels for wound hemostasis. J. Mater. Chem. B 2023, 11, 8411. [Google Scholar] [CrossRef]
- Naik, K.; Singh, P.; Yadav, M.; Srivastava, S.K.; Tripathi, S.; Ranjan, R.; Dhar, P.; Verma, A.K.; Chaudhary, S.; Parmar, A.S. 3D printable, injectable amyloid-based composite hydrogel of bovine serum albumin and aloe vera for rapid diabetic wound healing. J. Mater. Chem. B 2023, 11, 8142. [Google Scholar] [CrossRef]
- Cereceres, S.; Lan, Z.; Bryan, L.; Whitely, M.; Wilems, T.; Greer, H.; Alexander, E.R.; Taylor, R.J.; Bernstein, L.; Cohen, N.; et al. Bactericidal activity of 3D-printed hydrogel dressing loaded with gallium maltolate. APL Bioeng. 2019, 3, 026102. [Google Scholar] [CrossRef]
- Liang, J.; Zeng, H.; Qiao, L.; Jiang, H.; Ye, Q.; Wang, Z.; Liu, B.; Fan, Z. 3D Printed Piezoelectric Wound Dressing with Dual Piezoelectric Response Models for Scar-Prevention Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 30507–30522. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Y.; Niu, X.; Su, T.; Huang, X.; Lu, F.; Chang, Q. Direct three-dimensional printed egg white hydrogel wound dressing promotes wound healing with hitching adipose stem cells. Front. Bioeng. Biotechnol. 2022, 10, 930551. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Xiong, Y.; Zhu, Y.; Zhou, F.; Liu, X.; Chen, S.; Li, Z.; Qi, S.; Chen, L. Copper-Epigallocatechin Gallate Enhances Therapeutic Effects of 3D-Printed Dermal Scaffolds in Mitigating Diabetic Wound Scarring. ACS Appl. Mater. Interfaces 2023, 15, 38230–38246. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhou, X.; Zhang, Y.; Ye, D.; Yu, K.; Cao, W.; Zhang, L.; Zheng, H.; Sun, Z.; Guo, C.; et al. White-light crosslinkable milk protein bioadhesive with ultrafast gelation for frst-aid wound treatment. Biomater. Res. 2023, 27, 6. [Google Scholar] [CrossRef] [PubMed]
- Schadte, P.; Rademacher, F.; Andresen, G.; Hellfritzsch, M.; Qiu, H.; Maschkowitz, G.; Gläser, R.; Heinemann, N.; Drücke, D.; Fickenscher, H.; et al. 3D-printed wound dressing platform for protein administration based on alginate and zinc oxide tetrapods. Nano Converg. 2023, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Cleetus, C.M.; Primo, F.A.; Fregoso, G.; Raveendran, N.L.; Noveron, J.C.; Spencer, C.T.; Ramana, C.V.; Joddar, B. Alginate Hydrogels with Embedded ZnO Nanoparticles for Wound Healing Therapy. Int. J. Nanomed. 2020, 15, 5097–5111. [Google Scholar] [CrossRef] [PubMed]
- Remaggi, G.; Bottari, B.; Bancalari, E.; Catanzano, O.; Neviani, E.; Elviri, L. Lactobacillus delbrueckii subsp. bulgaricus derivatives for 3D printed alginate/hyaluronic acid self-crosslinking hydrogels: Manufacturing and wound healing potential. Int. J. Biol. Macromol. 2023, 242, 124454. [Google Scholar] [CrossRef] [PubMed]
- Temirel, M.; Hawxhurst, C.; Tasoglu, S. Shape Fidelity of 3D-Bioprinted Biodegradable Patches. Micromachines 2021, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Rocha, T.; Teixeira, A.M.; Gomes, S.G.; André, A.; Martins, P.; Ferreira, J.; Negrao, R. A 3D printed hydrogel to promote human keratinocytes’ spheroid-based growth. J. Biomed. Mater. Res. 2023, 111, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Karavasili, C.; Tsongas, K.; Andreadis, I.I.; Andriotis, E.G.; Papachristou, E.T.; Papi, R.M.; Tzetzis, D.; Fatouros, D.G. Physico-mechanical and finite element analysis evaluation of 3D printable alginate-methylcellulose inks for wound healing applications. Carbohydr. Polym. 2020, 247, 116666. [Google Scholar] [CrossRef]
- Unalam, I.; Schruefer, S.; Schubert, D.W.; Boccaccini, A.R. 3D-printed multifunctional hydrogels with phytotherapeutic properties: Development of essential oil-incorporated ALG-XAN hydrogels for wound healing applications. ACS Biomater. Sci. Eng. 2023, 9, 4149–4167. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, H.; Lu, Y.; Cao, L.; Cheng, Y.Y.; Tang, X.; Sun, H.; Song, K. Investigation on repairing diabetic foot ulcer based on 3D bio-printing Gel/dECM/Qcs composite scaffolds. Tissue Cell 2023, 85, 102213. [Google Scholar] [CrossRef]
- Lu, W.; Xu, J.; Su, Y.; Fang, H.; Liu, J.; Lv, S.; Cheng, Y.Y.; Nie, Y.; Li, W.; Pan, B.; et al. A biocompatible double-crosslinked gelatin/sodium alginate/dopamine/quaterniazed chitosan hydrogel for wound dressings based on 3D bioprinting technology. Int. J. Bioprint. 2023, 9, 438–452. [Google Scholar] [CrossRef]
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Bettini, R.; Elviri, L. 3D Printed Chitosan/Alginate Hydrogels for the Controlled Release of Silver Sulfadiazine in Wound Healing Applications: Design, Characterization and Antimicrobial Activity. Micromachines 2023, 14, 137. [Google Scholar] [CrossRef] [PubMed]
- Remaggi, G.; Bergamonti, L.; Graiff, C.; Ossiprandi, M.C.; Elviri, L. Rapid Prototyping of 3D-Printed AgNPs- and Nano-TiO2-Embedded Hydrogels as Novel Devices with Multiresponsive Antimicrobial Capability in Wound Healing. Antibiotics 2023, 12, 1104. [Google Scholar] [CrossRef]
- Motealleh, A.; Kehr, N.S. Directed vertical cell migration via bifunctionalized nanomaterials in 3D step-gradient nanocomposite hydrogels. Biomater. Sci. 2020, 8, 5628–5637. [Google Scholar] [CrossRef]
- Miguel, S.P.; Cabral, C.S.D.; Moreira, A.F.; Correia, I.J. Production and characterization of a novel asymmetric 3D printed construct aimed for skin tissue regeneration. Colloids Surf. B Biointerfaces 2019, 181, 994–1003. [Google Scholar] [CrossRef]
- Akkineni, A.R.; Spangenberg, J.; Geissler, M.; Reichelt, S.; Buechner, H.; Lode, A.; Gelinsky, M. Controlled and Local Delivery of Antibiotics by 3D Core/Shell Printed Hydrogel Scaffolds to Treat Soft Tissue Infections. Pharmaceutics 2021, 13, 2151. [Google Scholar] [CrossRef]
- Fratini, C.; Weaver, E.; Moroni, S.; Irwin, R.; Bashi, Y.H.D.; Uddin, S.; Casettari, L.; Wylie, M.P.; Lamprou, D.A. Combining microfluidics and coaxial 3D-bioprinting for the manufacturing of diabetic wound healing dressings. Biomater. Adv. 2023, 153, 213557. [Google Scholar] [CrossRef]
- Long, J.; Etxeberria, A.E.; Nand, A.V.; Bunt, C.R.; Ray, S.; Seyfoddin, A. A 3D printed chitosan-pectin hydrogel wound dressing for lidocaine hydrochloride delivery. Mat. Sci. Eng. C 2019, 104, 109873. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chitrakar, B.; Liu, Z.; Ming, X.; Xu, D.; Mo, H.; Shi, C.; Zhu, X.; Hu, L.; Li, H. Preparation and characterization of 3D-printed antibacterial hydrogel with benzyl isothiocyanate. Int. J. Bioprint. 2023, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Berry, D.; Moran, A.; He, F.; Tam, T.; Chen, L.; Chen, S. Controlled Growth Factor Release in 3D-Printed Hydrogels. Adv. Healthc. Mater. 2020, 9, 1900977. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Wang, L.; Ji, D.; Ren, M.; Ke, D.; Fu, Q.; Zhang, K.; Yang, X. Fabrication of SA/Gel/C scaffold with 3D bioprinting to generate micro-nano porosity structure for skin wound healing: A detailed animal in vivo study. Cell Regen. 2022, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, J.; Zhang, J.; Huang, J.; Luo, L.; Yang, Y.; Shen, K.; Jiao, T.; Jia, Y.; Lian, W.; et al. Functionalizing multi-component bioink with platelet-rich plasma for customized in-situ bilayer bioprinting for wound healing. Mater. Today Bio 2022, 16, 100334. [Google Scholar] [CrossRef] [PubMed]
- Deptuła, M.; Zawrzykraj, M.; Sawicka, J.; Banach-Kope’c, A.; Tylingo, R.; Pikuła, M. Application of 3D-printed hydrogels in wound healing and regenerative medicine. Biomed. Pharmacother. 2023, 167, 115416. [Google Scholar] [CrossRef] [PubMed]
- Somasekharan, L.T.; Raju, R.; Kumar, S.; Geevarghese, R.; Nair, R.P.; Kasoju, N.; Bhatt, A. Biofabrication of skin tissue constructs using alginate, gelatin and diethylaminoethyl cellulose bioink. Int. J. Biol. Macromol. 2021, 189, 398–409. [Google Scholar] [CrossRef]
- Balavigneswaran, C.K.; Selvaraj, S.; Vasudha, T.K.; Iniyan, S.; Muthuvijayan, V. Tissue engineered skin substitutes: A comprehensive review of basic design, fabrication using 3D printing, recent advances and challenges. Biomater. Adv. 2023, 153, 213570. [Google Scholar] [CrossRef]
- Hao, L.; Zhao, S.; Hao, S.; He, Y.; Feng, M.; Zhou, K.; He, Y.; Yang, J.; Mao, H.; Gu, Z. Functionalized gelatin-alginate based bioink with enhanced manufacturability and biomimicry for accelerating wound healing. Int. J. Biol. Macromol. 2023, 240, 124364. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Willis, J.; Aluri, A.; Awad, S.; Smith, M.; Banker, Z.; Mitchell, M.; Macias, L.; Berry, J.; King, T. Three-Dimensionally Printed Skin Substitute Using Human Dermal Fibroblasts and Human Epidermal Keratinocytes. Ann. Plas. Surg. 2021, 86, S628. [Google Scholar] [CrossRef] [PubMed]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-D.; Dai, N.-T.; Liao, C.-Y.; Kang, L.-Y.; Tseng, Y.-W.; Hsu, S.-H. Planar-/Curvilinear-Bioprinted Tri-Cell-Laden Hydrogel for Healing Irregular Chronic Wounds. Adv. Healthc. Mater. 2022, 11, 2201021. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Jahangiri, S.; Kiadehd, S.Z.H.; Rezvaninejade, S.; Ahmadi, Z.; Ahmadi, S.; Safarkhanij, M.; Rabiee, N. Stimuli-responsive biomaterials: Smart avenue toward 4D bioprinting. Crit. Rev. Biotechnol. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Rastin, H.; Ramezanpour, M.; Hassan, K.; Mazinani, A.; Tung, T.T.; Vreugde, S.; Losic, D. 3D bioprinting of a cell-laden antibacterial polysaccharide hydrogel composite. Carbohyd. Polym. 2021, 264, 117989. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Jammalamadaka, U.; Tappa, K. Nanogels for Pharmaceutical and Biomedical Applications and Their Fabrication Using 3D Printing Technologies. Materials 2018, 11, 302. [Google Scholar] [CrossRef]
- Lee, J.; Dutta, S.D.; Acharya, R.; Park, H.; Kim, H.; Randhawa, A.; Patil, T.V.; Ganguly, K.; Luthfikasari, R.; Lim, K.-T. Stimuli-Responsive 3D Printable Conductive Hydrogel: A Step toward Regulating Macrophage Polarization and Wound Healing. Adv. Healthc. Mater. 2023, 13, 2302394. [Google Scholar] [CrossRef]
- Choi, K.Y.; Ajiteru, Q.; Hong, H.; Suh, Y.J.; Sultan, M.T.; Lee, H.; Lee, J.S.; Lee, Y.J.; Lee, O.J.; Kim, S.H.; et al. A digital light processing 3D-printed artificial skin model and full-thickness wound models using silk fibroin bioink. Acta Biomater. 2023, 164, 159–174. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Chen, D.; Su, T.; Chang, Q.; Huang, W.; Lu, F. 3D bioprinting of a gradient stiffened gelatin–alginate hydrogel with adipose-derived stem cells for full-thickness skin regeneration. J. Mater. Chem. B 2023, 11, 2989. [Google Scholar] [CrossRef]
- Hao, L.; Tao, X.; Feng, M.; Zhou, K.; He, Y.; Yang, J.; Mao, H.; Gu, Z. Stepwise Multi-Cross-Linking Bioink for 3D Embedded Bioprinting to Promote Full-Thickness Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 24034–24046. [Google Scholar] [CrossRef]
- Sarmin, A.M.; El Moussaid, N.; Suntornnond, R.; Tyler, E.J.; Kim, Y.-H.; Di Cio, S.; Megone, W.V.; Pearce, O.; Gautrot, J.E.; Dawson, J.; et al. Multi-Scale Analysis of the Composition, Structure, and Function of Decellularized Extracellular Matrix for Human Skin and Wound Healing Models. Biomolecules 2022, 12, 837. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, D.; Zeng, J.; Fu, Q.; Chen, Z.; Sun, X.; Yang, Y.; Li, S.; Chen, M. Application of 3D-printed tissue-engineered skin substitute using innovative biomaterial loaded with human adipose-derived stem cells in wound healing. Int. J. Bioprint. 2023, 9, 674. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, R.; Wang, C.; Guo, Y.; Sun, W.; Ouyang, L. Recombinant Human Collagen-Based Bioinks for the 3D Bioprinting of Full-thickness Human Skin Equivalent. Int. J. Bioprint. 2022, 8, 611. [Google Scholar] [CrossRef]
- Xia, S.; Weng, T.; Jin, R.; Yang, M.; Yu, M.; Zhang, W.; Wang, X.; Han, C. Curcumin-incorporated 3D bioprinting gelatin methacryloyl hydrogel reduces reactive oxygen species-induced adipose-derived stem cell apoptosis and improves implanting survival in diabetic wounds. Burn. Trauma 2022, 10, tkac001. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Hasan, A.; Kaarela, O.; Byambaa, B.; Sheikhi, A.; Gaharwar, A.K.; Khademhosseini, A. Advancing Frontiers in Bone Bioprinting. Adv. Healthc. Mater. 2019, 8, 1801048. [Google Scholar] [CrossRef] [PubMed]
- Puertas-Bartolome, M.; Włodarczyk-Biegun, M.K.; del Campo, A.; Vazquez-Lasa, B.; San Roman, J. Development of bioactive catechol functionalized nanoparticles applicable for 3D bioprinting. Mat. Sci. Eng. C 2021, 131, 112515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhang, Z.; Cao, G.; Jin, Q.; Xu, L.; Li, J.; Liu, Z.; Xu, C.; Le, Y.; Fu, Y.; et al. Engineered dermis loaded with confining forces promotes full-thickness wound healing by enhancing vascularization and epithelialization. Acta Biomater. 2023, 170, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Huyan, Y.; Lian, Q.; Zhao, T.; Li, D.; He, J. Pilot Study of the Biological Properties and Vascularization of 3D Printed Bilayer Skin Grafts. Int. J. Bioprint. 2020, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, R.; Jiao, J.; Li, Z.; Wang, P.; Liu, Y.; Li, S.; Chen, C.; Li, Z.; Qu, G.; et al. A click chemistry-mediated all-peptide cell printing hydrogel platform for diabetic wound healing. Nat. Commun. 2023, 14, 7856. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.P.F.; Teixeira, M.C.; Lameirinhas, N.S.; Matos, F.S.; Luís, J.L.; Pires, L.; Oliveira, H.; Oliveira, M.; Silvestre, A.J.D.; Vilela, C.; et al. Hydrogel Bioinks of Alginate and Curcumin-Loaded Cellulose Ester-Based Particles for the Biofabrication of Drug-Releasing Living Tissue Analogs. ACS Appl. Mater. Interfaces 2023, 15, 40898–40912. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, A.; Al Kayal, T.; Mero, A.; Mezzetta, A.; Guazzelli, L.; Soldani, G.; Losi, P. Fibrinogen-Based Bioink for Application in Skin Equivalent 3D Bioprinting. J. Funct. Biomater. 2023, 14, 459. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yue, Z.; Winberg, P.C.; Dinoro, J.N.; Hayes, P.; Beirne, S.; Wallace, G.G. Development of rhamnose-rich hydrogels based on sulfated xylorhamno-uronic acid toward wound healing applications. Biomater. Sci. 2019, 7, 3497. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, Ö.; Arslan-Yildiz, A. Development of a hydrocolloid bio-ink for 3D bioprinting. Biomater. Sci. 2022, 10, 6707. [Google Scholar] [CrossRef] [PubMed]


| Required Characteristics of Wound Dressings |
|---|
|
|
|
|
|
|
|
|
|
|
| Dressing Characteristics | Activity | Wound Type | Commercial Products |
| Film dressings | |||
| Transparent semi-permeable polyurethane and/or polyacrylate film dressings coated with polyacrylate adhesive. Two-sided wound contact layer with Safetac®. |
| Superficial wounds, closed surgical incisions, minor burns, dry/slightly exuding wounds, cuts and abrasions, donor sites. | Hydrofilm (Hartmann USA, Inc., Rock Hill, SC, USA) [24], Bioclusive™ (KCI, Acelity Inc., San Antonio, TX, USA) [25], Mepore (Mölnlycke, Gothenburg, Sweden) [26], Transeal (Mölnlycke) [27], Tegaderm™ (3M, Maplewood, NJ, USA) [28], OPSITE POST-OP (Smith + Nephew, Watford, UK) [29] Mepitel® (Mölnlycke) [30] |
| Foam dressings | |||
| Polyurethane foam. | Medium to highly exuding, acute and chronic wounds (e.g., diabetic foot ulcers, pressure ulcers II-IV, venous ulcers such as leg ulcers, donor sites, incisions, abrasions). | PermaFoam® (Hartmann) [31] | |
| Hydroresponsive dressing comprising polyurethane foam combined with AquaClear® Technology HydroTac® Comfort is further coated with a waterproof, bacteria-resistant protective film. |
| Wide range of acute and chronic wounds. | HydroTac® (Hartmann) [32] HydroTac® Comfort (Hartmann) [32] |
| Antimicrobial foam dressing with Safetac technology layer. |
| Low to medium exuding wounds (e.g., leg/foot ulcers, pressure ulcers). Partial-thickness burns. | Mepilex® Ag (Mölnlycke) [33] |
| Five-layer polyurethane foam dressing with silicone adhesive. |
| Low to highly exuding wounds (e.g., venous leg ulcers, pressure ulcers, arterial ulcers, neuropathic ulcers, surgical wounds, etc.). | Tegaderm™ Silicone Foam Dressings (3M) [34] |
| Four-layer design including polyurethane foam and acrylate adhesive. |
| Low to highly exuding partial to full-thickness wounds, such as pressure ulcers, diabetic foot ulcers, arterial ulcers, venous leg ulcers, neuropathic ulcers, surgical wounds, abrasions, trauma and skin tears. | Tegaderm™ High-performance Foam Adhesive Dressing [35] |
| Four-layer design including polyurethane foam. |
| Low to highly exuding partial and full-thickness wounds (e.g., pressure ulcers, ulcers of the lower extremity, donor sites, surgical wounds, abrasions and skin tears). Superficial burns of partial thickness. | Tegaderm™ High-performance Foam Non-adhesive Dressing [36] |
| Polyurethane foam with an outer layer of vapor-permeable and water/bacteria-resistant polyurethane film. |
| Moderate to highly exuding wounds (e.g., pressure ulcers, surgical wounds). First and second-degree burns. | Askina Foam™ (B. Braun, Melsungen, Germany) [37] |
| Polyurethane foam combined with a highly permeable backing film. |
| Moderate to highly exuding wounds like leg ulcers and pressure ulcers. | Lyofoam® Max (Mölnlycke) [38] |
| Hydrocellular foam featuring a low-tack silicone. |
| Chronic wounds (e.g., diabetic foot ulcers, venous leg ulcers, pressure injuries). Acute wounds (e.g., burns, surgical wounds, donor sites and skin tears). | ALLEVYN Non-bordered Dressings (Smith + Nephew) [39] |
| Antimicrobial foam dressing containing silver sulfadiazine which provides sustained anti-microbial action. |
| Chronic and acute partial/full-thickness or shallow granulating exuding wounds (e.g., pressure ulcers, diabetic ulcers, venous ulcers, fungating/malignant wounds, burns, surgical wounds, donor sites). Could be used on infected wounds. | ALLEVYN Ag (Smith + Nephew) [40] |
| Hydrophilic polyurethane foam combined with a waterproof polyurethane film and containing polyhexamethylene biguanide (PHMB). |
| Moderate to highly exuding chronic and acute wounds, infected or at risk of being infected (e.g., pressure ulcers, leg/foot ulcers, diabetic ulcers, surgical wounds). | TIELLETM PHMB (Acelity Inc., San Antonio, TX, USA) [41] ActivHeal® PHMB [42] |
| Hydropolymer adhesive dressing. |
| Low to moderately exuding wounds (pressure, venous, arterial ulcers, lower extremity ulcers, diabetic ulcers, donor sites). Could be used on infected wounds. | TIELLE® LIQUALOCK (Acelity) [43] |
| Hydrophilic polyurethane foam combined with an active layer comprising chitosan/alginate, and an outer layer consisting of a semipermeable polyurethane film. |
| Traumatic, postoperative wounds. | Tromboguard® (TRICOMED SA, Lodz, Poland) [44] |
| Polyurethane pad with high absorption capacity combined with a silicone adhesive layer and an outer waterproof polyurethane film. |
| Moderate to heavily exuding chronic and acute wounds (e.g., pressure, diabetic, venous and arterial leg ulcers, trauma and surgical wounds, skin abrasions, superficial/partial-thickness burns). Can be used on cavity wounds as a secondary dressing. | ActivHeal® Silicone Foam [45] |
| Hydrocolloid dressing | |||
| Hydrocolloid dressing comprising sodium CMC, gelatin, pectin and adhesive polymers. |
| Partial and full-thickness low to moderately exudating wounds. Chronic wounds (e.g., stage I-IV pressure ulcers, leg ulcers). Acute wounds (e.g., traumatic wounds such as minor abrasions, lacerations) partial-thickness burns and donor sites. | Granuflex™ (ConvaΤec, London, UK) [46] |
| Hydrocolloid dressing comprising an elastic/sticky mass encapsulating moisture-absorbing sodium carboxymethylcellulose (CMC) particles and calcium alginate and covered by a semi-permeable polyurethane film. |
| Low to moderately exuding wounds (e.g., leg ulcers, pressure ulcers, superficial (partial-thickness) burns, surgical wounds, donor sites and skin abrasions). | Confeel® Plus Dressing (Coloplast, Humlebæk, Denmark) [47] |
| Hydrocolloid dressing comprising an elastic/sticky mass encapsulating moisture-absorbing sodium carboxymethylcellulose (CMC) particles and covered by a semi-permeable polyurethane film. |
| No to low exuding chronic wounds and superficial acute wounds. Superficial (partial-thickness) burns, surgical wounds, donor sites and skin abrasions. | Confeel® Plus Transparent (Coloplast) [48] |
| Hydrocolloid dressing consisting of an inner layer that adheres to the skin in the presence of moisture and highly absorbs exudate. Outer water- and bacteria-resisting film. |
| Low to moderately exuding wounds like partial and full-thickness dermal and leg ulcers, superficial wounds, abrasions, donor sites and superficial and partial-thickness burns. | Tegaderm™ Hydrocolloid Dressing (3M) [49] |
| Hydrocolloid dressing comprising a crosslinked honeycomb matrix of sodium carboxymethylcellulose, gelatin, pectin and adhesive polymers, and an outer waterproof polyurethane film. |
| Lightly exuding acute (e.g., minor burns, surgical wounds, abrasions and lacerations) and chronic wounds (e.g., stage I–II pressure ulcers, leg ulcers). | DuoDERM® Extra Thin Dressing (ConvaTec) [50] |
| Hydrocolloid dressing comprising a crosslinked honeycomb matrix of sodium carboxymethylcellulose, gelatin, pectin and adhesive polymers, and an outer waterproof polyurethane film. Green line indicator to show when the dressing needs to be changed. |
| Lightly to moderately exuding chronic (e.g., pressure and leg ulcers) and acute (e.g., minor burns, surgical and traumatic wounds and donor sites) wounds. | DuoDERM® Signal™ Dressing (ConvaTec) [51] |
| Hydrocolloid dressing featuring a waterproof polyurethane cover film. |
| Partial to full-thickness wounds (e.g., venous, arterial, pressure and diabetic ulcers) donor sites, surgical incisions/excisions, and burns (first and second degree). | REPLICARE® Thin Hydrocolloid Dressing (Smith + Nephew) [52] |
| Hydrocolloid dressing. |
| Minimal to moderate exuding partial to full-thickness wounds. | Restore Hydrocolloid Dressing (Hollister, New Albany, OH, USA) [53] |
| Alginate dressings | |||
| Calcium sodium alginate (80% Ca and 20% Na) with a high content of guluronic acid. |
| Moderate to highly exuding chronic (e.g., leg, pressure and diabetic ulcers, fungating lesions) and acute (e.g., donor sites, post-surgical wounds, abrasions and lacerations) wounds. Wounds with negligible bleeding. | Kaltostat® Dressing (ConvaTec) [54] |
| Calcium alginate containing silver (1.4%). |
| Moderate to heavily exuding wounds like diabetic foot ulcers, leg ulcers (e.g., venous, arterial ulcers, etc), pressure ulcers, traumatic/surgical wounds and donor sites. | ALGICELL® Ag (Gentell, Yardley, PA, USA) [55] |
| Alginate dressing containing medical grade Manuka honey. |
| Various types of wounds including pressure, leg and diabetic ulcers, burns, surgical wounds, graft sites. Suitable for infected wounds. | Algivon and Algivon Plus (Advancis Medical, Hamburg, Germany) [56,57] |
| Combination of alginate (10%) and collagen (90%). |
| Exuding partial and full-thickness wounds, e.g., venous ulcers, pressure injuries, diabetic ulcers, traumatic wounds healing by secondary intention, abrasions, surgical incisions, second-degree burns, donor sites, etc. | Fibracol™ Plus (3M) [58] |
| Calcium alginate. |
| Moderate to heavily exuding granulating wounds or having areas of slough (e.g., pressure ulcers, venous leg ulcers, arterial ulcers, diabetic ulcers, post-operative surgical wounds, cavity wounds, graft and donor sites, superficial/partial-thickness burns). | ActivHeal® Alginate [59] |
| Calcium sodium alginate. Calcium sodium alginate with silver. |
| Moderate to highly exuding wounds. | Restore™ Calcium Alginate (Hollister) [60] Restore™ Calcium Alginate Dressing with Silver (Hollister) [61] |
| Non-woven dressing comprising (mannuronic acid) calcium alginate and carboxymethylcellulose. |
| Moderately to heavily exuding partial and full-thickness wounds (e.g., post-surgical and trauma wounds, cavity wounds leg, pressure and diabetic ulcers, burns). | ActivHeal Aquafiber® Ag [62] |
| Hydrogel dressings | |||
| Amorphous gel delivered into the wound through a tube. |
| No to low exuding dry/sloughy wounds such as pressure ulcers, leg ulcers, diabetic ulcers, cavity wounds, post-surgical wounds, graft and donor sites, lacerations and abrasions. | ACTIVHEAL® HYDROGEL [63] |
| Amorphous gel delivered into the wound through a tube. |
| Partial to full-thickness wounds dry and minimally exuding wounds. Fill dead space in wounds. Could be applied to infected wounds. | Restore Hydrogel Dressing (Hollister) [64] |
| Smart, ionic hydrogel dressing (70% water and 30% acrylic polymers) combined with a transparent polyethylene film. |
| Dry to moderately exuding chronic and acute wounds (e.g., venous leg ulcers (also under compression), arterial ulcers, diabetic foot ulcers, malignant wounds, moderate burns like those from radiotherapy, skin tears, etc.). | SUPRASORB® G (Lohmann-Rauscher, L&R, Milwaukee, WI, USA) [65] |
| Hydrogel Sheet Dressing. |
| Dry to lightly exuding partial and full-thickness wounds (e.g., pressure ulcers, tissue damaged by radiation, minor burns). | AquaDerm™ (DermaRite, North Bergen, NJ, USA) [66] |
| Hydrogel Sheet Dressing (3 mm thick) consisting of natural and synthetic polymers like polyvinyl pyrrolidone, polyethylene glycol and agar and containing 90% water. |
| First-, second- and third-degree burns, ulcers and bedsores. | Neoheal® KIKGEL [67] |
| Clear hydrogel dressing containing modified carboxymethyl cellulose, propylene glycol and water [2]. |
| Shallow, undermined, and deep wounds, granulating cavity wounds, excoriated skin and radiation burns. | INTRASITE◊ GEL (Smith + Nephew) [68] |
| Transparent, amorphous hydrogel containing sodium alginate. |
| Necrotic and sloughy wounds. | Nu-Gel™ (3M™) [69] |
| Category | Commercial Product |
|---|---|
| Amniotic/placental membranes | Dehydrated EpiFix® (MiMedx Group, Marietta, GA, USA) Cryopreserved Grafix* (Osiris Therapeutics, Inc., Columbia, SC, USA) |
| Bovine type I collagen seeded with human allogeneic skin cells | OrCel® (Ortec International, New York, NY, USA) Apligraf® (Organogenesis Inc., Canton, OH, USA) Polyglactin mesh scaffold Dermagraft® (Organogenesis Inc.) |
| Allogeneic fibrin patches containing leukocytes and platelets | LeucoPatch® (Reapplix, Birkerød, Denmark) |
| Materials | Crosslinking Method | 3D Printing Method | Bioactive Agents | Dressing | Animal Model/Wound Type (In Vivo) or Cell Type (In Vitro) | Outcome |
|---|---|---|---|---|---|---|
| In vivo | ||||||
| FSA [79] | Ionic | Extrusion | DNA, biomineralized silica | Bioactive hydrogel | Normal and diabetic mice Male BALB/c mice (8 weeks) Male C57BL/6 mice (8 weeks)/full-thickness wounds |
|
| SA, CHSMA [80] | Ionic and photocrosslinking | Extrusion | Acrylylated VEGF | Double-crosslinked angiogenic hydrogel | T1DM/full-thickness wound |
|
| SA, GG, PDA NPs [13] | Ionic | SA-GG@PDA hydrogel | Female C57BL/6 mice (3–5 weeks) injected with B16F10 melanoma cells (5 × 105 cells)/full-thickness wound (Ø 10 mm) |
| ||
| SA, CMC [98] | Ionic | Pneumatic extrusion | SA/CMC hydrogel scaffolds with two-tier structures | New Zealand rabbits/full-thickness wound (Ø 2.5 cm) |
| |
| PLGA (upper layer), SA (lower layer) [81] | Ionic | Bilayer membrane scaffold | Female SD rats (10 weeks)/10 mm biopsy punch wounds |
| ||
| SA-DA, gelatin [83] | Ionic | Co-axial | Hollow channeled hydrogel scaffold | BALB/c mice/infected wound (Ø 8 mm) |
| |
| Gelatin [14] | Extrusion | MH | Chicken embryo |
| ||
| Gelatin, PRP, SA, CS [93] | Ionic dual crosslinking | Coaxial microfluidic bioprinting | Βioactive shell-core fibrous hydrogels | SD rats with Type 2 diabetes/full-thickness skin wounds |
| |
| GelMA [72] | Photocrosslinking | VEGF mimicking peptide | Hydrogel patches | Micropigs (30–35 kg)/full-thickness excision wound |
| |
| GelMA-alginate (bottom layer) PEGDA-alginate (top layer) [99] | Photocrosslinking and ionic | Extrusion | Au-decorated BTO nanocubes, i.e., piezoelectric nanomaterials (top layer) VEGF (bottom layer) | Janus hydrogel patch | SD rats/full-thickness excision wound (infected dorsal wounds) |
|
| GelMa, PEDOT, PSS [100] | Photopolymerization | Electrical stimulation | Electro-conductive hydrogels with grooved topography | New Zealand rabbits/full-thickness wound |
| |
| GelMAl [96] | Photocrosslinking | VEGF decorated photoactive and antibacterial t-ZnO MPs | Hydrogel with t-ZnO-VEGF | Male SKH-1 hairless mice (6 weeks)/circle defects (9 mm) |
| |
| GelMA, HAMA, PRP [101] | Photocrosslinking | DLP | FDPBH | Diabetic male SD rats (4 weeks) |
| |
| Dopamine (coating), Gelatin/SA (core), PCL (shell) [102] | Ionic | Extrusion | Dopamine-coated core–shell (gelatin/SA/PCL) hydrogel | Balb/C female mice/full-thickness wound |
| |
| SS, GelMA [103] | Photocrosslinking | Extrusion | Hybrid IPN SS/GelMA hydrogel | Female SD rats |
| |
| SF, gelatin [104] | Photocrosslinking | Extrusion | MB-loaded UiO-66(Ce) NPs | SF/gelatin hydrogel loaded with MB@UiO-66(Ce) NPs | Kunming mice (8 weeks)/ full-thickness infected skin defect (Ø 5 mm) |
|
| CS [87] | Thermal gelation | DIW extrusion | Green CS hydrogels of increased strength | Male SD rats/full-thickness skin wounds (Ø 15 mm) |
| |
| HA/CS (top layer), HA/CS and PLA nanofibrous MS (bottom layer) [105] | Chemical | Extrusion | ZNP or D-ZNP | Hydrogel construct | Diabetic male Wistar rats (225 ± 25 g)/full-thickness excision wounds (Ø 1.5 cm) |
|
| CS, SA, PVA [106] | Coaxial biological 3D printing technology | MMP inhibitor, indomethacin | Dressings based on core–shell hydrogel fibers | Type 2 diabetic SD rats/wounds of sufficient depth to reach fascia |
| |
| CNCs, CS-MA [75] | Photocrosslinking | Extrusion | VEGF, gentamicin, silver NPs | Mesh-like hydrogel 3D printed on Tegaderm | Female C57BL/6J mice (6–8 weeks)/full-thickness excision wound |
|
| CMC, GMA, ε- PL [90] | Photocrosslinking | Extrusion | Bionic antibacterial and antioxidant hydrogels | SD rats/full-thickness infected skin wound |
| |
| PAM, HPMC [92] | Silver–ethylene interaction | FDM 3D printing (for the PLA template) | Silver NPs | Superporous antibacterial hydrogels | SD rats/full-thickness wound (1 × 1 cm) |
|
| RHCMA, HAMA [107] | Photocrosslinking | AgNCs | Diabetic model |
| ||
| OMS [108] | Ionic | Green hot extrusion | Male C57BL/6 mice (6–8 weeks)/skin wounds (5 × 5 mm) |
| ||
| BSA and AV gel [109] | Thermal | Extrusion | Diabetic rats/full-thickness open-excision wound |
| ||
| NIPAAm, SA [94] | Ionic and photocrosslinking | Microfluidic-aided 3D printing | MXene, VEGF | Dynamically responsive channeled hydrogel scaffolds | Male C57BL/6 mice (22–24 g) |
|
| PEGDA [110] | Photocrosslinking | GaM | Hydrogel dressing with hierarchical porosity | C57BL/6 mice (2 months)/splinted-wound |
| |
| PVDF, SA [111] | Ionic | ZnO NPs | Piezoelectric hydrogel | Adult female SD rats/ full-skin wound |
| |
| EW [112] | NaOH, DMEM (secondary crosslinking) | Extrusion | ASCs L929 Fbs | C57 mice/full-thickness skin defect (Ø 1 cm) |
| |
| ECM, SA, gelatin [95] | Ionic and chemical | Extrusion | Male BALB/c, (25–30 g)/full-thickness circle wound (area~100 mm2) |
| ||
| ECMMA [113] | Photocrosslinking | Extrusion | Cu-EGCG capsules | ECMMA/Cu-EGCG dermal scaffold | Male SD rats (200−250 g)/a circular full-thickness skin wound (Ø 20 mm) |
|
| Casein [114] | Photocrosslinking with white light | DLP | Male C57BL/6 N mice (8 weeks, 20 ± 2.5 g) |
| ||
| Ex vivo | ||||||
| SA [115] | Ionic | t-ZnO | Human skin explants |
| ||
| In vitro | ||||||
| SA [116] | Ionic | Extrusion | ZnO NPs | SA hydrogel with embedded ZnO NPs | Mitomycin-C-treated STO fibroblasts |
|
| SA [82] | Ionic | Extrusion | HZJ bacteriophage that targets DH5α E. coli | Bacteriophage-based SA hydrogel | L929 fibroblasts |
|
| SA, HA [117] | CaCO3, GDL | Extrusion-based 3D printing on a stainless steel cold (−14 °C) Peltier cell | LD derivatives | Self-crosslinked SA-HA hydrogels functionalized with LD derivatives | Primary human skin fibroblasts |
|
| SA/CNCs and SA/T-CNF [118] | Ionic | Extrusion | Hybrid hydrogels | NIH/3T3 mouse fibroblasts |
| |
| SA, gelatin [119] | Ionic | Extrusion | Keratinocytes (HaCaT) |
| ||
| ADA-GEL [84] | Ionic | ASX and BBG MPs | NIH 3T3 fibroblasts Keratinocytes (HaCaT) |
| ||
| SA, MC [120] | Ionic | Extrusion | MH, eucalyptus essential oil, AV gel | SA-MC hydrogels | HDFs |
|
| SA, XG [121] | Ionic | Extrusion | CLV | Raw 264.7 macrophage-like cells |
| |
| GelMA [86] | Photocrosslinking | Extrusion | CaS extracts with various concentrations of Si ions | - |
| |
| Gelatin, dECM, QCS [122] | Chemical | Extrusion | Gelatin–dECM–QCS composite scaffolds | L929 cells (5 × 105 cells) |
| |
| Gelatin, SA, QCS, dopamine [123] | EDC/NHS and ionic | Extrusion | Double-crosslinked gelatin/SA/ dopamine/ QCS hydrogel | L929 fibroblasts |
| |
| T-CNF, GelMA [89] | Ionic and photocrosslinking | Extrusion | T-CNF/GelMA hydrogel | 3T3 fibroblasts |
| |
| CS, SA [124] | Ionic | Extrusion | SSD | CS/SA hydrogels | Human fibroblasts |
|
| CS or SA [125] | Ionic | Extrusion | Ag NPs and TiO2 | Human fibroblasts |
| |
| CS, P(KA), 4-PEG [73] | Chemical (P(KA)/4-PEG MPs) Photocrosslinking (CS)-P(KA)/4-PEG | Extrusion | - | NIH 3T3 mouse fibroblast cells |
| |
| Calcium D-gluconate monohydrate, SA [126] | Ionic | Printing in a vertical orientation | Bifunctional NMs: PMO functionalized with Dex and PDL | 3D step-gradient nanocomposite hydrogels comprising different hydrogel layers with increasing amounts of bifunctional NMs | Primary dermal fibroblasts |
|
| CNFs [72] | Double crosslinking: ionic and chemical BDDE | Nanocellulose hydrogel | HDFs |
| ||
| PCL, SS (epidermis layer) CS, SA (dermis layer) [127] | Ionic | Electrospinning Extrusion | 3D skin asymmetric construct |
| ||
| PNIPAAm, SA, MC [91] | Photocrosslinking and ionic | Temperature-controlled pneumatic-based extrusion printing | Octeisept® | Thermoresponsive hydrogel with antimicrobial properties | Fibroblasts L929 |
|
| Physicochemical characterization | ||||||
| SA, MC and SA, MC, Laponite (core/shell) [128] | Core/shell extrusion-based printing | Antibiotics (clindamycin vancomycin, gentamicin) | Antibiotic-loaded core/shell scaffolds |
| ||
| SA, CNC (shell), HEC (core) [129] | Coaxial | TO loaded pegylated liposomes (core), TO (shell) | Composite core/shell scaffolds |
| ||
| CS, PEC [130] | Physical (hydrogen bonding) | Extrusion | LDC | Lyophilized hydrogel with sponge-like morphology |
| |
| CS, PEC [88] | Physical crosslinking | CS/PEC hydrogels |
| |||
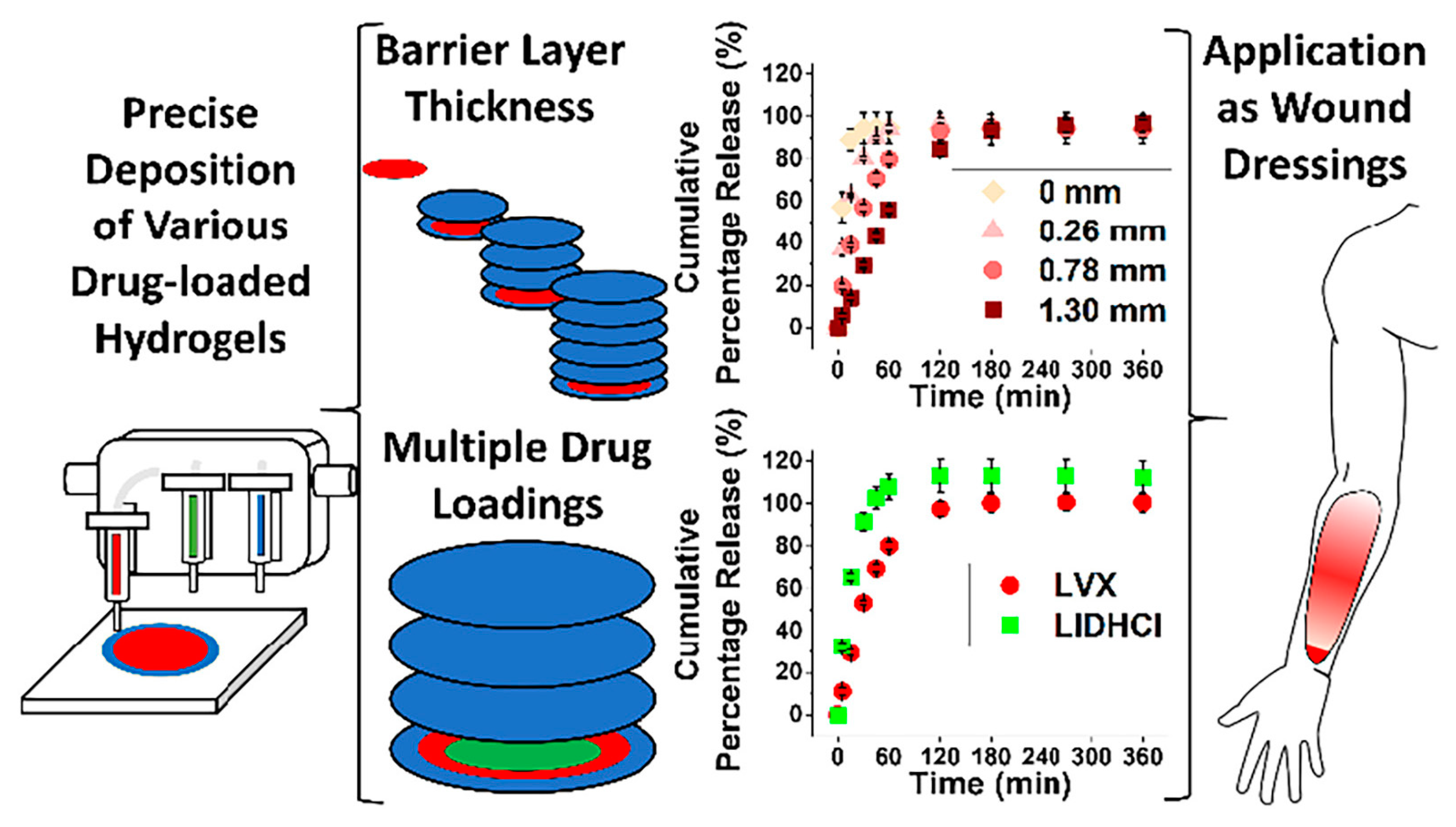
| CSMeA [97] | Photocrosslinking | LIDHCl, LVX | Drug-loaded hydrogels of customizable shape |
| ||
| XG, LBG, KG, CA [131] | Physical crosslinking | BITC |
| |||
| Avidin/T-CNF, SA [76] | Ionic | Microdispensing | Biofunctionalized T-CNF/SA hydrogel |
| ||
| Hep-SH, HA-GM [132] | Photocrosslinking | DLP | GFs | Hydrogels of complex geometry |
| |
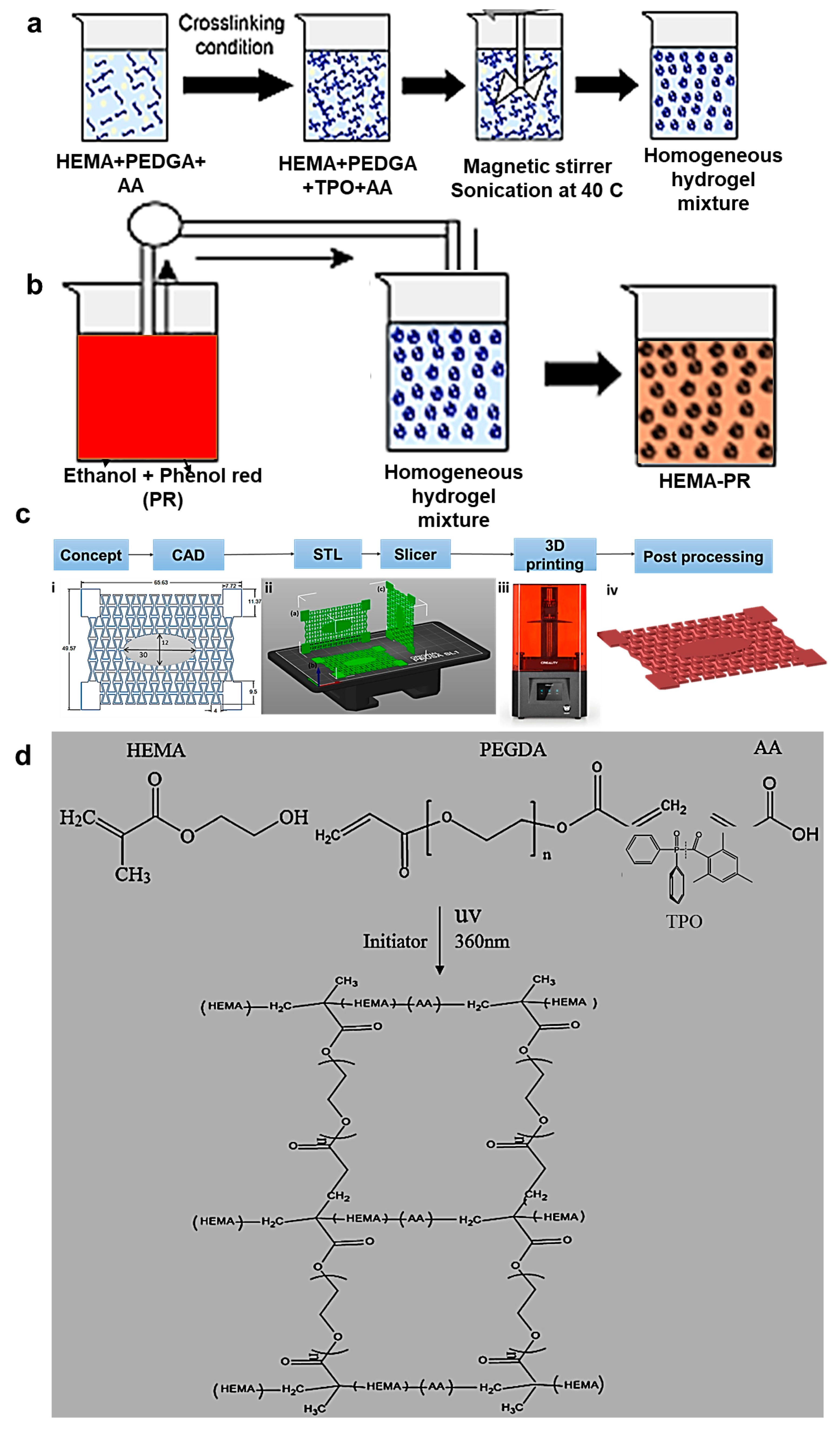
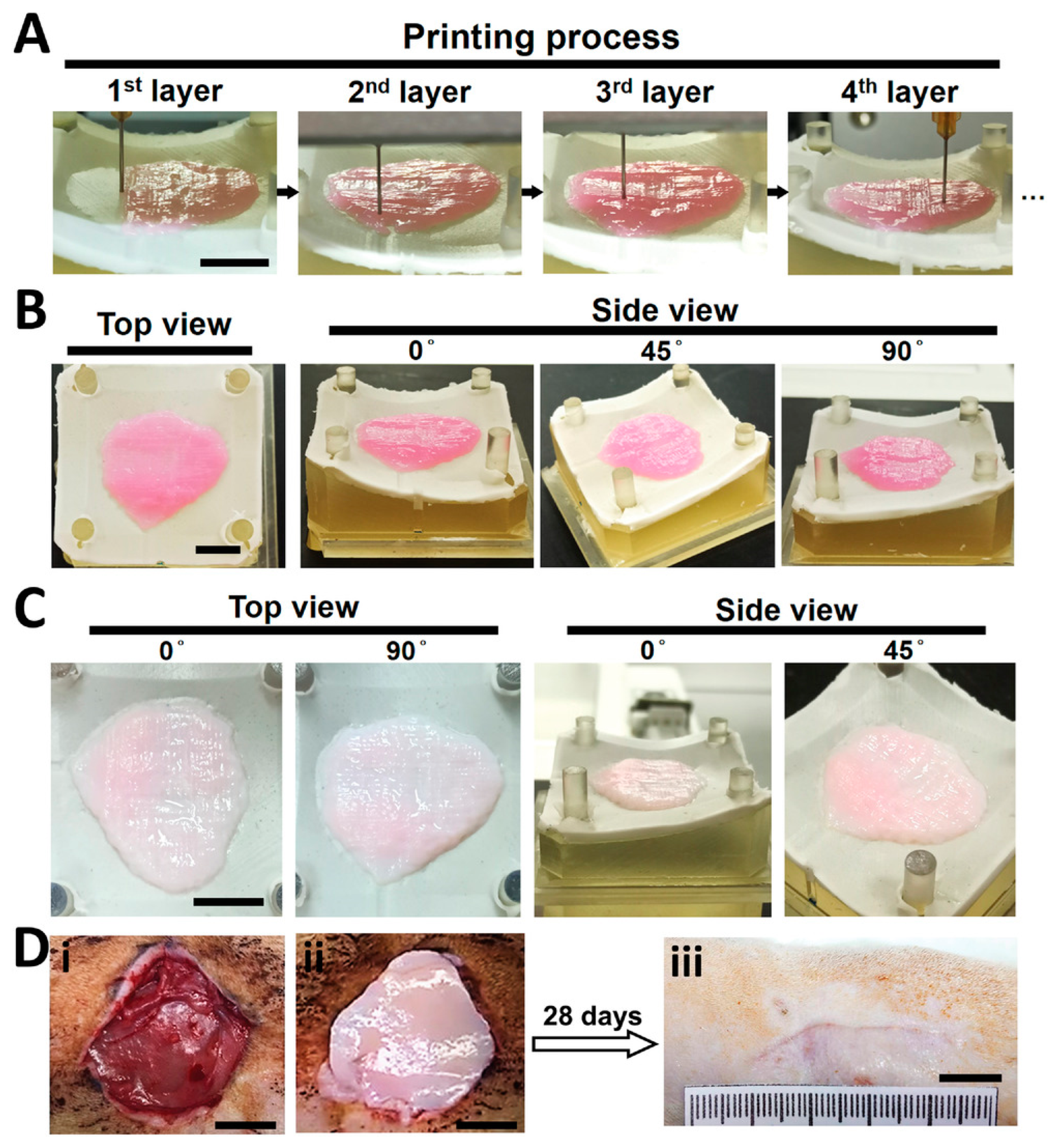
| HEMA [78] | Photocrosslinking | pH indicator | Auxetic wound dressing |
| ||
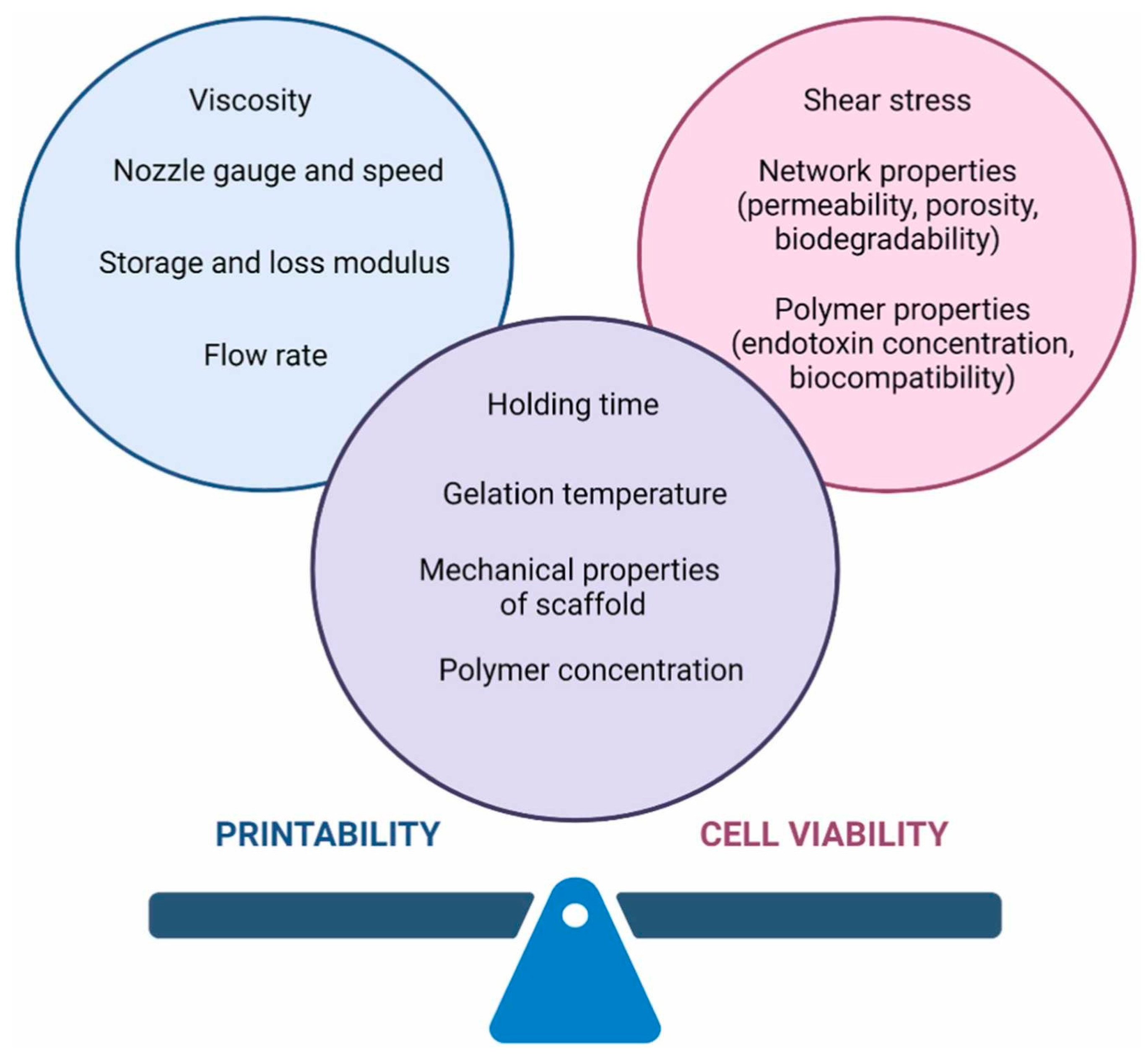
| Bioprinting Technical Challenges |
|---|
|
|
|
|
|
|
| Characteristics | Bioprinting Method | |||
|---|---|---|---|---|
| Extrusion | Inkjet | Laser Assisted | Digital Light Processing | |
| Bioink viscosity (mPa s−1) | 30–6 × 107 | 3.5–12 | 1–300 | |
| Crosslinking method | Photopolymerization, chemical, shear thinning, temperature | Photopolymerization, chemical | Photopolymerization, chemical | Photopolymerization |
| Bioprinter speed | Slow | Fast | Medium fast | Fast |
| Droplet size | ||||
| Cell density (cells/mL) | High (cell spheroids) | Low (<106) | Medium (108) | High |
| Cell viability (%) | 40–80 | >85 | >95 | High |
| Advantages | ||||
|
|
|
| |
| Disadvantages | ||||
|
|
| ||
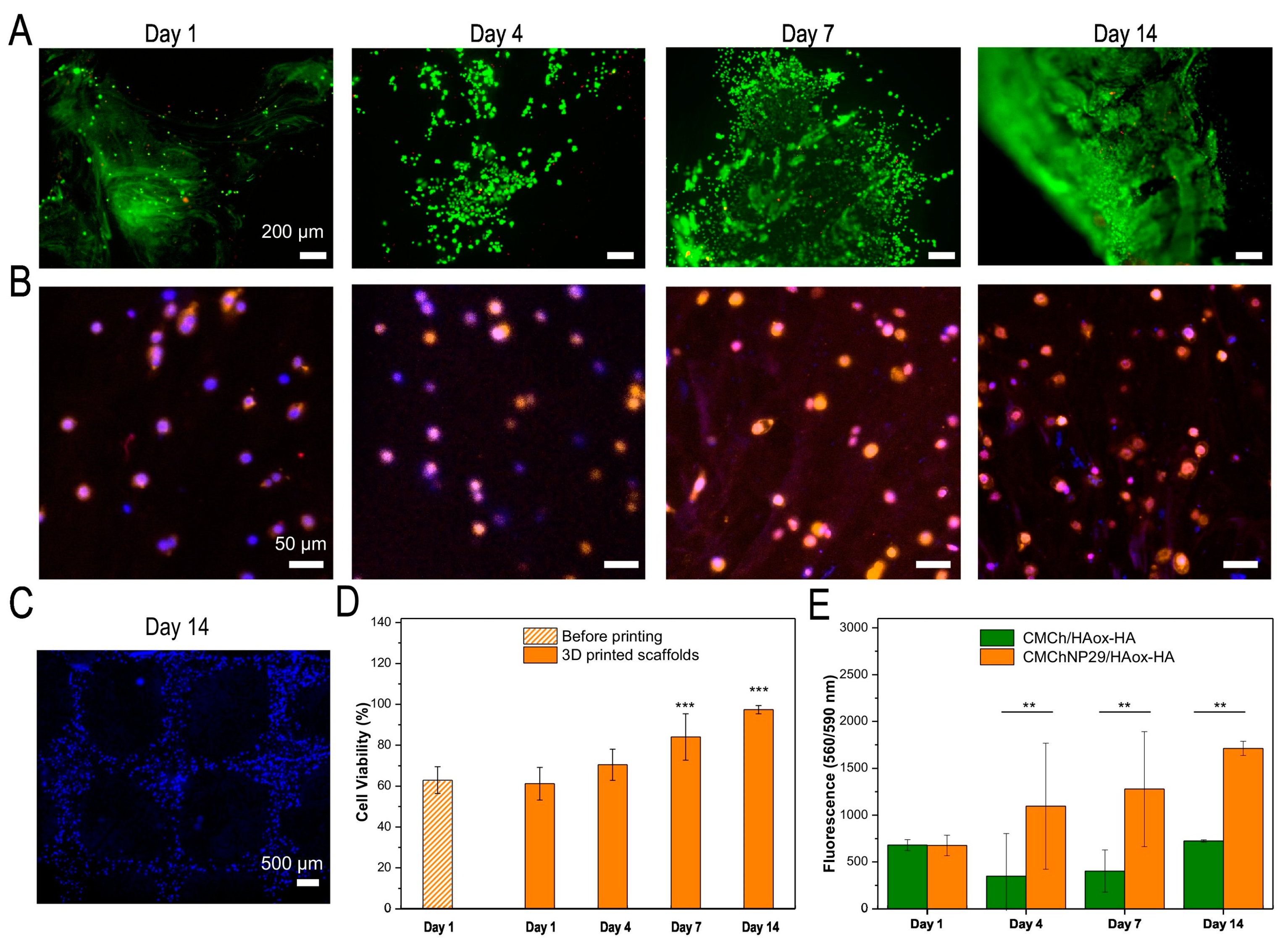
| Materials | Crosslinking Method | 3D Printing Method | Bioactive Agents | Cells | Dressing | Animal Model /Wound Type | Outcome |
|---|---|---|---|---|---|---|---|
| In Vivo | |||||||
| GelMAl [152] | Photocrosslinking | Extrusion | CUR | ADSCs | nu/nu athymic nude mice induced with diabetes/full-skin defect |
| |
| GelMAl/rhCol3 [151] | Photocrosslinking | Extrusion | HDFs (1 × 106/mL) HaCaTs (2 × 105/cm2) seeded on the upper surface of the printed skin construct | SD rats (8 weeks)/full-thickness wounds (Ø 5 mm) |
| ||
| GelMAm, HAMA, fibrin (bioink), PCL frame, gelatin lower layer [155] | Photocrosslinking and polymerization with thrombin | Extrusion | HUVECs and NHDFs | Nude mice (6–8 wks)/full-thickness wounds (Ø 10 mm) |
| ||
| GAH [147] | Ionic | Extrusion | ADSCs | GSH | Male C57BL/6 mice/full-thickness skin defects (Ø 10 mm) |
| |
| Gelatin, SA [156] | Ionic | Extrusion | NHDFs, HMVECs (bottom layer) and NHEKs (top layer) | Nude mice/full-thickness wound |
| ||
| GH/AT [138] | Photocrosslinking and ionic | Extrusion | NIH 3 T3 and hUC-MSCs | ICR male mice/full-thickness skin defect |
| ||
| PU, gelatin [141] | Ionic | Extrusion | Fibroblasts, keratinocytes and EPCs | Planar-/curvilinear bio-printable hydrogel | Normal, DM rats/full-thickness circular (Ø 1.5 cm) and irregular wounds |
| |
| γ-PGA-GMA, γ-PGA-SH, RGDC [157] | Photocrosslinking | DLP | HUVEC vegf165+ | HUVECvegf165+-laden microporous hydrogel | Diabetic male SD rats (7–8 weeks, ~220 g)/full-thickness skin defects (Ø 10 mm) |
| |
| PF127-SH and HAMA [148] | Michael addition, self-assembly and photocrosslinking | Extrusion | MSCs | Male ICR mice (~20 g)/ Full-thickness skin defects (Ø 7 mm) |
| ||
| dECM pre-gel, GelMA and HAMA [150] | Photocrosslinking | Extrusion | hADSCs | Nude mice/full-thickness skin wound |
| ||
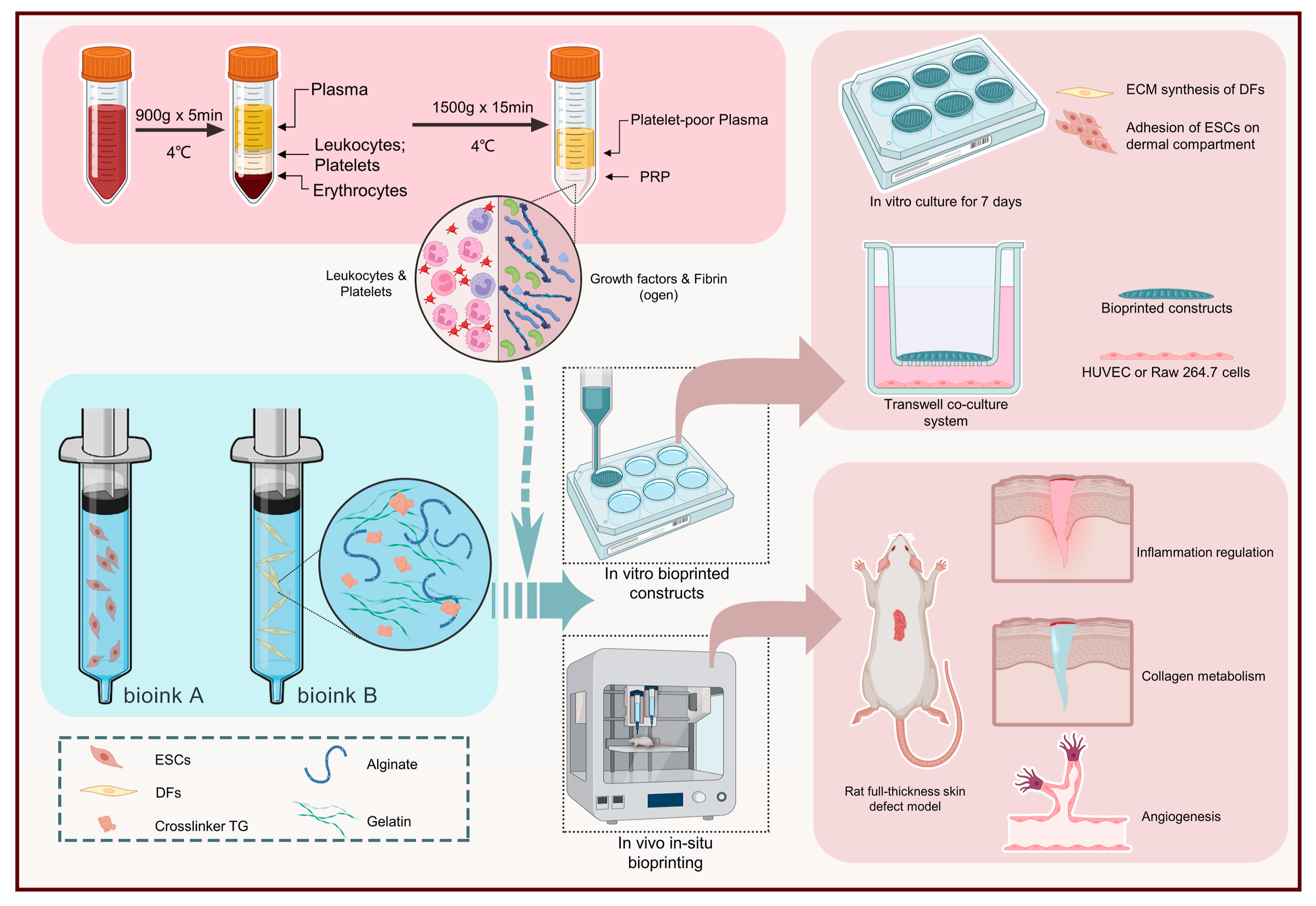
| PRP, AG [134] | Ionic and enzymatic | In situ extrusion | DFs and ESCs | Male SD rats/full-thickness cutaneous defects (Ø 15 mm) |
| ||
| In Vitro | |||||||
| SF-GMA, gelatin-GMA [146] | Photocrosslinking | DLP | HUVEC, HDF and NHEK | Artificial skin | Printed scars of various shapes (skin wound model) |
| |
| FSA [79] | Ionic | Extrusion | DNA, biomineralized silica | NIH3T3/GFP fibroblasts | Bioactive cell-laden hydrogel |
| |
| SA [158] | Ionic | Extrusion | CEpCUR | HaCaTs | SA:CEpCUR living construct |
| |
| SA, fibrinogen [159] | Ionic, enzymatic polymerization with thrombin | Extrusion | NHDFs, HaCaTs (seeded on top of the bioprinted construct) | Skin equivalent |
| ||
| SA, gelatin, collagen [133] | Ionic | Extrusion | Fibroblasts (2 × 106 cells/mL) | Full-thickness hydrogel with gradient pore structure |
| ||
| SA, gelatin and DCEL [136] | Ionic | Pneumatic-based extrusion | Primary adult fibroblasts and primary epidermal keratinocytes |
| |||
| MC, SA [143] | Ionic | Extrusion | gallium (Ga+3) | Foreskin fibroblast cells |
| ||
| pDAM2, SA [160] | Extrusion | blood derivatives (platelets, plasma growth factors) | Platelets, human dermal cells | Interactive composite hydrogels |
| ||
| Gelatin, XG [85] | Chemical (GTA) | Extrusion | HDFs and human keratinocytes | Hybrid composite hydrogels |
| ||
| CMCh, HA [154] | Chemical (formation of Schiff-bases) | Reactive mixing extrusion | polymer NPs containing catechol | HDFs |
| ||
| XRU-MA [160] | Photocrosslinking | Extrusion | penicillin–streptomycin | HDFs (1 × 106 mL−1) |
| ||
| QSHs [161] | EDC-NHS coupling | NIH-3T3 |
| ||||
| CELLINK Skin [139] | Submersion in a thrombin-based solution | Pneumatic bioprinting | HDFa and HEKa | Fibrinogen scaffold |
| ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kammona, O.; Tsanaktsidou, E.; Kiparissides, C. Recent Developments in 3D-(Bio)printed Hydrogels as Wound Dressings. Gels 2024, 10, 147. https://doi.org/10.3390/gels10020147
Kammona O, Tsanaktsidou E, Kiparissides C. Recent Developments in 3D-(Bio)printed Hydrogels as Wound Dressings. Gels. 2024; 10(2):147. https://doi.org/10.3390/gels10020147
Chicago/Turabian StyleKammona, Olga, Evgenia Tsanaktsidou, and Costas Kiparissides. 2024. "Recent Developments in 3D-(Bio)printed Hydrogels as Wound Dressings" Gels 10, no. 2: 147. https://doi.org/10.3390/gels10020147
APA StyleKammona, O., Tsanaktsidou, E., & Kiparissides, C. (2024). Recent Developments in 3D-(Bio)printed Hydrogels as Wound Dressings. Gels, 10(2), 147. https://doi.org/10.3390/gels10020147








