L-Theanine Improves the Gelation of Ginkgo Seed Proteins at Different pH Levels
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Properties
2.1.1. Solubility and Surface Hydrophobicity
2.1.2. Particle Size and ζ Potential
2.2. Fluorescence Spectra
2.3. Rheological Characteristics
2.4. Analysis of Texture Profiles
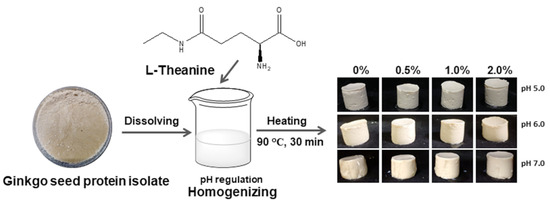
2.5. Water Holding Capacity (WHC) and Gel Appearance
2.6. Gel Microstructure
2.7. Fourier Transform Infrared Spectrum (FT-IR)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Extraction of Ginkgo Seed Protein Isolate (GSPI)
4.3. Preparation of the GSPI Sols and Gels
4.4. Solubility and Surface Hydrophobicity of the GSPI
4.5. Particle Size and ζ Potential of the Sols
4.6. Intrinsic Fluorescence
4.7. Rheology
4.8. Texture Profile Analysis (TPA)
4.9. Water Holding Capacity (WHC) of Gels
4.10. Microstruture of the Gels
4.11. Fourier Transform Infrared Spectrum (FT-IR)
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, M.; Tomar, M.; Punia, S.; Dhakane-Lad, J.; Dhumal, S.; Changan, S.; Senapathy, M.; Berwal, M.K.; Sampathrajan, V.; Sayed, A.A.S.; et al. Plant-based proteins and their multifaceted industrial applications. LWT—Food Sci. Technol. 2022, 154, 112620. [Google Scholar] [CrossRef]
- Avelar, Z.; Vicente, A.A.; Saraiva, J.A.; Rodrigues, R.M. The role of emergent processing technologies in tailoring plant protein functionality: New insights. Trends Food Sci. Technol. 2021, 113, 219–231. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Moreno, Y.M.F.; Carciofi, B.A.M. Plant proteins as high-quality nutritional source for human diet. Trends Food Sci. Technol. 2020, 97, 170–184. [Google Scholar] [CrossRef]
- Wu, G.; Fanzo, J.; Miller, D.D.; Pingali, P.; Post, M.; Steiner, J.L.; Thalacker-Mercer, A.E. Production and supply of high-quality food protein for human consumption: Sustainability, challenges, and innovations. Ann. N. Y. Acad. Sci. 2014, 1321, 1–19. [Google Scholar] [CrossRef]
- Tan, M.; Nawaz, M.A.; Buckow, R. Functional and food application of plant proteins—A review. Food Rev. Int. 2023, 39, 2428–2456. [Google Scholar] [CrossRef]
- Nasrabadi, M.N.; Doost, A.S.; Mezzenga, R. Modification approaches of plant-based proteins to improve their techno-functionality and use in food products. Food Hydrocoll. 2021, 118, 106789. [Google Scholar] [CrossRef]
- Lin, D.; Zhang, L.; Li, R.; Zheng, B.; Rea, M.C.; Miao, S. Effect of plant protein mixtures on the microstructure and rheological properties of myofibrillar protein gel derived from red sea bream (Pagrosomus major). Food Hydrocoll. 2019, 96, 537–545. [Google Scholar] [CrossRef]
- Sim, S.Y.; Srv, A.; Chiang, J.H.; Henry, C.J. Plant proteins for future foods: A roadmap. Foods 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Klost, M.; Drusch, S. Structure formation and rheological properties of pea protein-based gels. Food Hydrocoll. 2019, 94, 622–630. [Google Scholar] [CrossRef]
- Tomczyńska-Mleko, M.; Terpiłowski, K.; Pérez-Huertas, S.; Sapiga, V.; Polischuk, G.; Sołowiej, B.; Nastaj, M.; Wesołowska-Trojanowska, M.; Mleko, S. Co-gelation of pumpkin-seed protein with egg-white protein. Foods 2023, 12, 2030. [Google Scholar] [CrossRef] [PubMed]
- Langyan, S.; Yadava, P.; Khan, F.N.; Dar, Z.A.; Singh, R.; Kumar, A. Sustaining protein nutrition through plant-based foods. Front. Nutr. 2022, 8, 772573. [Google Scholar] [CrossRef]
- Deng, Q.; Wang, L.; Wei, F.; Xie, B.; Huang, F.; Huang, W.; Shi, J.; Huang, Q.; Tian, B.; Xue, S. Functional properties of protein isolates, globulin and albumin extracted from Ginkgo biloba seeds. Food Chem. 2011, 124, 1458–1465. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Zhang, Y.-Q. The main active constituents and detoxification process of Ginkgo biloba seeds and their potential use in functional health foods. J. Food Compos. Anal. 2019, 83, 103247. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.M. Effect of different drying methods on product quality, bioactive and toxic components of Ginkgo biloba L. seed. J. Sci. Food Agric. 2021, 101, 3290–3297. [Google Scholar] [CrossRef] [PubMed]
- Grollino, M.G.; Raschella, G.; Cordelli, E.; Villani, P.; Pieraccioli, M.; Paximadas, I.; Malandrino, S.; Bonassi, S.; Pacchierotti, F. Cytotoxicity, genotoxicity and gene expression changes elicited by exposure of human hepatic cells to Ginkgo biloba leaf extract. Food Chem. Toxicol. 2017, 109, 486–496. [Google Scholar] [CrossRef]
- Shen, D.; Labreche, F.; Wu, C.; Fan, G.; Li, T.; Shi, H.; Ye, C. Preparation and aroma analysis of flavonoid-rich ginkgo seeds fermented using rice wine starter. Food Biosci. 2021, 44, 101459. [Google Scholar] [CrossRef]
- Yu, M.; Aoki, D.; Akita, T.; Fujiyasu, S.; Takada, S.; Matsushita, Y.; Yoshida, M.; Fukushima, K. Distribution of lignans and lignan mono/diglucosides within Ginkgo biloba L. stem. Phytochemistry 2022, 196, 11310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, C.; Ye, J.; Chen, H.; Tao, R.; Cao, F. Effects of high hydrostatic pressure treatment on structural, allergenicity, and functional properties of proteins from ginkgo seeds. Innov. Food Sci. Emerg. Technol. 2016, 34, 187–195. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.-M. Ginkgo biloba L. seed; A comprehensive review of bioactives, toxicants, and processing effects. Ind. Crops Prod. 2022, 176, 114281. [Google Scholar] [CrossRef]
- Liu, W.; Zou, M.; Wang, Y.; Cao, F.; Su, E. Ginkgo seed proteins: Characteristics, functional properties and bioactivities. Plant Foods Hum. Nutr. 2021, 76, 281–291. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Zhao, J.; Ma, T.; He, Z.; Huang, M.; Wang, Y. Modification of structure and functionalities of ginkgo seed proteins by pH-shifting treatment. Food Chem. 2021, 358, 129862. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, C.; Zhao, J.; Li, W.; Wang, Y. Physicochemical properties of a ginkgo seed protein-pectin composite gel. Food Hydrocoll. 2021, 118, 106781. [Google Scholar] [CrossRef]
- Williams, R.A. Opportunities and challenges for the introduction of new food proteins. Annu. Rev. Food Sci. Technol. 2021, 12, 75–91. [Google Scholar] [CrossRef]
- Guan, T.; Zhang, Z.; Li, X.; Cui, S.; McClements, D.J.; Wu, X.; Chen, L.; Long, J.; Jiao, A.; Qiu, C.; et al. Preparation, characteristics, and advantages of plant protein-based bioactive molecule delivery systems. Foods 2022, 11, 1562. [Google Scholar] [CrossRef]
- Ogawa, S.; Ota, M.; Ogura, J.; Kato, K.; Kunugi, H. Effects of L-theanine on anxiety-like behavior, cerebrospinal fluid amino acid profile, and hippocampal activity in Wistar Kyoto rats. Psychopharmacology 2018, 235, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wen, S.; Li, Q.; Lai, X.; Chen, R.; Zhang, Z.; Li, D.; Sun, S. L-theanine relieves acute alcoholic liver injury by regulating the TNF-α/NF-κB signaling pathway in C57BL/6J mice. J. Funct. Foods 2021, 86, 104699. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, A.; Roy, S.; Bera, B.; Bandyopadhyay, S.K. L-Theanine healed NSAID-induced gastric ulcer by modulating pro/antioxidant balance in gastric ulcer margin. J. Nat. Med. 2014, 68, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ravi, C.; Zaved Ahmed, K.; Abul Kalam Azad, M. Fabrication of poly(D, L-lactic acid) nanoparticles as delivery system for sustained release of L-theanine. IET Nanobiotechnol. 2019, 13, 742–747. [Google Scholar] [CrossRef]
- Xue, X.; He, H.; Liu, C.; Wang, L.; Wang, L.; Wang, Y.; Wang, L.; Yang, C.; Wang, J.; Hou, R. L-Theanine improves emulsification stability and antioxidant capacity of diacylglycerol by hydrophobic binding β-lactoglobulin as emulsion surface stabilizer. Food Chem. 2022, 366, 130557. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, J.; Zheng, B.; Lu, Q.; Chen, L. Effects of matcha and its active components on the structure and rheological properties of gluten. LWT—Food Sci. Technol. 2020, 124, 109197. [Google Scholar] [CrossRef]
- Roslan, A.A.; Mohamad, S.B.; Tayyab, S. Docking evaluation of the interaction between green tea active ingredient, L-theanine and human serum albumin. Proc. Natl. Sci. Lett. 2021, 44, 17–19. [Google Scholar] [CrossRef]
- Wijaya, W.; Van der Meeren, P.; Patel, A.R. Cold-set gelation of whey protein isolate and low-methoxyl pectin at low pH. Food Hydrocoll. 2017, 65, 35–45. [Google Scholar] [CrossRef]
- Tanger, C.; Engel, J.; Kulozik, U. Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll. 2020, 107, 105949. [Google Scholar] [CrossRef]
- Yan, S.Z.; Xu, J.W.; Zhang, S.; Li, Y. Effects of flexibility and surface hydrophobicity on emulsifying properties: Ultrasound-treated soybean protein isolate. LWT—Food Sci. Technol. 2021, 142, 110881. [Google Scholar] [CrossRef]
- Chong, S.H.; Ham, S. Interaction with the surrounding water plays a key role in determining the aggregation propensity of proteins. Angew. Chem. Int. Ed. 2014, 53, 3961–3964. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Advances in fabrication of emulsions with enhanced functionality using structural design principles. Curr. Opin. Colloid Interface Sci. 2012, 17, 235–245. [Google Scholar] [CrossRef]
- Chao, D.; Aluko, R.E. Modification of the structural, emulsifying, and foaming properties of an isolated pea protein by thermal pretreatment. CyTA—J. Food. 2018, 16, 357–366. [Google Scholar] [CrossRef]
- Malik, M.A.; Saini, C.S. Heat treatment of sunflower protein isolates near isoelectric point: Effect on rheological and structural properties. Food Chem. 2019, 276, 554–561. [Google Scholar] [CrossRef]
- Dai, Z.; Tian, L.; Song, B.; Liu, X.; Yuan, J. Development of a novel lysosome-targetable time-gated luminescence probe for ratiometric and luminescence lifetime detection of nitric oxide in vivo. Chem. Sci. 2017, 8, 1969–1976. [Google Scholar] [CrossRef]
- Liu, Q.; Geng, R.; Zhao, J.; Chen, Q.; Kong, B. Structural and gel textural properties of soy protein isolate when subjected to extreme acid pH-shifting and mild heating processes. J. Agric. Food. Chem. 2015, 63, 4853–4861. [Google Scholar] [CrossRef]
- Keerati-u-rai, M.; Miriani, M.; Iametti, S.; Bonomi, F.; Corredig, M. Structural changes of soy proteins at the oil-water interface studied by fluorescence spectroscopy. Colloids Surf. B 2012, 93, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, C.; Zhao, J.; Guo, F.; You, J.; Zhang, L.; Wang, Y. Alkali-induced phenolic acid oxidation enhanced gelation of ginkgo seed protein. Foods 2023, 12, 1506. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, W.; Feizollahi, E.; Roopesh, M.S.; Chen, L. Improvement of pea protein gelation at reduced temperature by atmospheric cold plasma and the gelling mechanism study. Innov. Food Sci. Emerg. Technol. 2021, 67, 102567. [Google Scholar] [CrossRef]
- Zhao, H.B.; Yu, B.; Hemar, Y.; Chen, J.; Cui, B. Improvement of calcium sulfate-induced gelation of soy protein via incorporation of soy oil before and after thermal denaturation. LWT—Food Sci. Technol. 2020, 117, 110881. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, S.; Zhong, M.; Qi, B.; Li, Y. Soy and whey protein isolate mixture/calcium chloride thermally induced emulsion gels: Rheological properties and digestive characteristics. Food Chem. 2022, 380, 132212. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Kong, X.L.; Li, Q.M.; Zhang, H.L.; Zha, X.Q.; Luo, J.P. Stability and bioavailability of vitamin D3 encapsulated in composite gels of whey protein isolate and lotus root amylopectin. Carbohydr. Polym. 2020, 227, 115337. [Google Scholar] [CrossRef] [PubMed]
- Picone, C.S.F.; Takeuchi, K.P.; Cunha, R.L. Heat-induced whey protein gels: Effects of pH and the addition of sodium caseinate. Food Biophys. 2010, 6, 77–83. [Google Scholar] [CrossRef]
- Chantrapornchai, W.; McClements, D.J. Influence of NaCl on optical properties, large-strain rheology and water holding capacity of heat-induced whey protein isolate gels. Food Hydrocoll. 2002, 16, 467–476. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Liu, C.; Li, W. Influence of γ-aminobutyric acid on gelling properties of heat-induced whey protein gels. Food Hydrocoll. 2019, 94, 287–293. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, R.; Xu, X.; Zhou, G.; Liu, D. Structural modification by high-pressure homogenization for improved functional properties of freeze-dried myofibrillar proteins powder. Food Res. Int. 2017, 100, 193–200. [Google Scholar] [CrossRef]
- Pereira, R.N.; Souza, B.W.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Effects of electric fields on protein unfolding and aggregation: Influence on edible films formation. Biomacromolecules 2010, 11, 2912–2918. [Google Scholar] [CrossRef]
- Rahman, M.S.; Go, G.-W.; Seo, J.-K.; Gul, K.; Choi, S.-G.; Yang, H.-S. Thiol concentration, structural characteristics and gelling properties of bovine heart protein concentrates. LWT—Food Sci. Technol. 2019, 111, 175–181. [Google Scholar] [CrossRef]
- Xu, Y.; Dong, M.; Tang, C.; Han, M.; Xu, X.; Zhou, G. Glycation-induced structural modification of myofibrillar protein and its relation to emulsifying properties. LWT—Food Sci. Technol. 2020, 117, 108664. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, T.; Liu, C.; Guo, F.; Zhao, J. L-Histidine improves solubility and emulsifying properties of soy proteins under various ionic strengths. LWT—Food Sci. Technol. 2021, 152, 112382. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, C.; Ma, T.; Zhao, J. Physicochemical and functional properties of γ-aminobutyric acid-treated soy proteins. Food Chem. 2019, 295, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Wu, J.; Cheng, Y.; Chen, T.; Ren, X.; Ma, H. Physicochemical properties and digestive kinetics of whey protein gels filled with potato and whey protein mixture emulsified oil droplets: Effect of protein ratios. Food Funct. 2021, 12, 5927–5939. [Google Scholar] [CrossRef]
- Xie, Y.; Yu, X.; Wei, S.; Zheng, J.; Prakash, S.; Dong, X. Impact of homogenization on the physicochemical properties of the cod protein gel. LWT—Food Sci. Technol. 2021, 149, 111841. [Google Scholar] [CrossRef]
- He, Z.; Liu, C.; Zhao, J.; Guo, F.; Wang, Y. Enhanced gelling properties and hydration capacity of ginkgo seed proteins by genipin cross-linking. Food Chem. 2023, 399, 133924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, J.; Cheng, Y. Mechanical properties, microstructure, and in vitro digestion of transglutaminase-crosslinked whey protein and potato protein hydrolysate composite gels. Foods 2023, 12, 2040. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Ge, H.; Zhao, J.; Liu, C.; Wang, Y. L-Theanine Improves the Gelation of Ginkgo Seed Proteins at Different pH Levels. Gels 2024, 10, 131. https://doi.org/10.3390/gels10020131
Zhang L, Ge H, Zhao J, Liu C, Wang Y. L-Theanine Improves the Gelation of Ginkgo Seed Proteins at Different pH Levels. Gels. 2024; 10(2):131. https://doi.org/10.3390/gels10020131
Chicago/Turabian StyleZhang, Luyan, Huifang Ge, Jing Zhao, Changqi Liu, and Yaosong Wang. 2024. "L-Theanine Improves the Gelation of Ginkgo Seed Proteins at Different pH Levels" Gels 10, no. 2: 131. https://doi.org/10.3390/gels10020131
APA StyleZhang, L., Ge, H., Zhao, J., Liu, C., & Wang, Y. (2024). L-Theanine Improves the Gelation of Ginkgo Seed Proteins at Different pH Levels. Gels, 10(2), 131. https://doi.org/10.3390/gels10020131