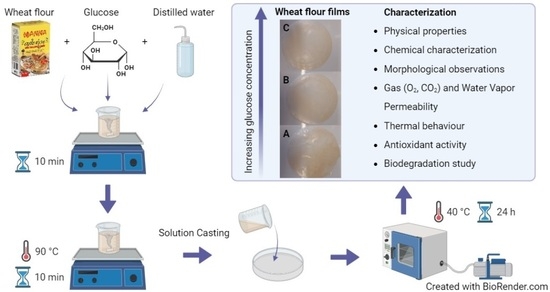
Bioactive Edible Gel Films Based on Wheat Flour and Glucose for Food Packaging Applications
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical Properties
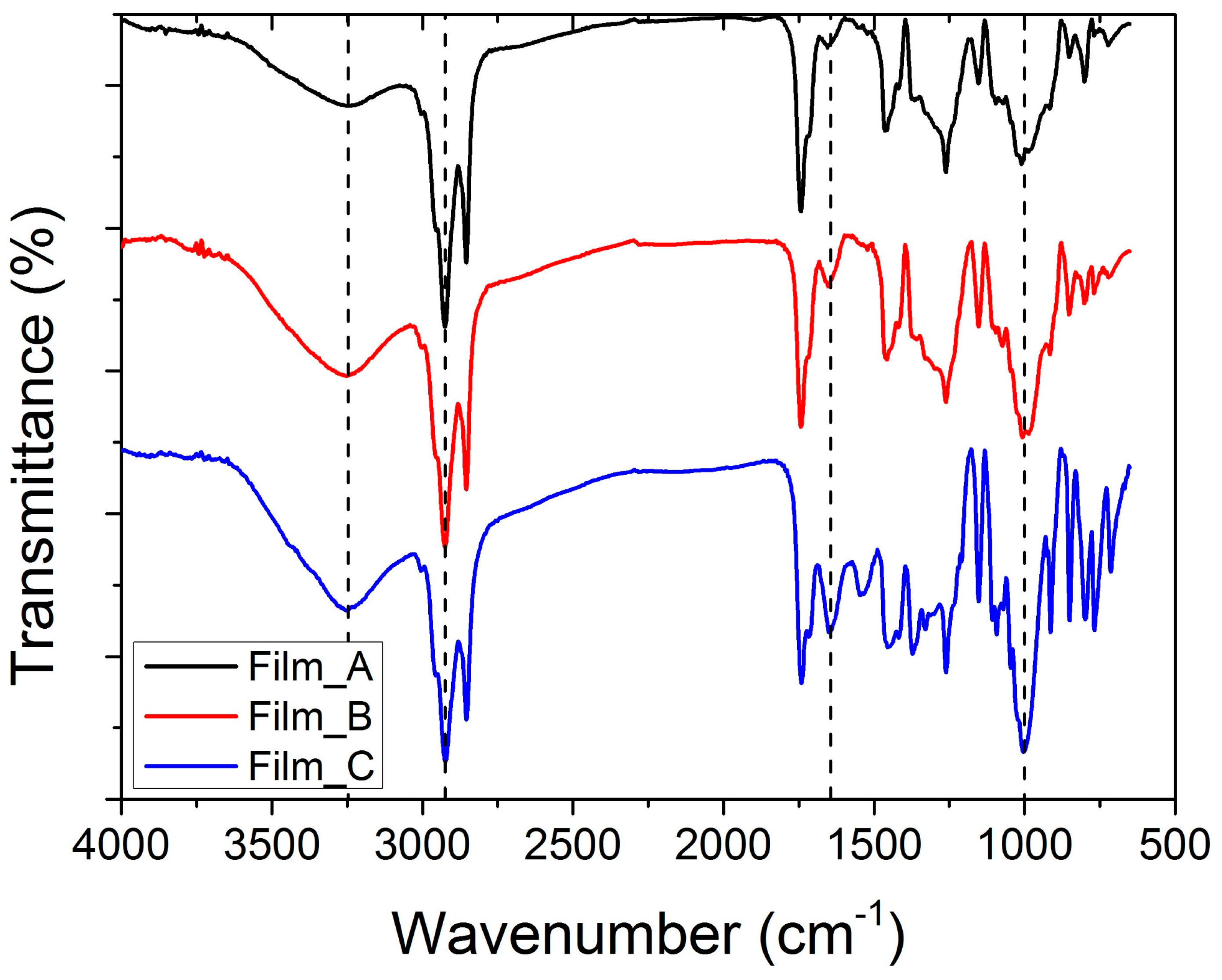
2.2. Chemical Characterization
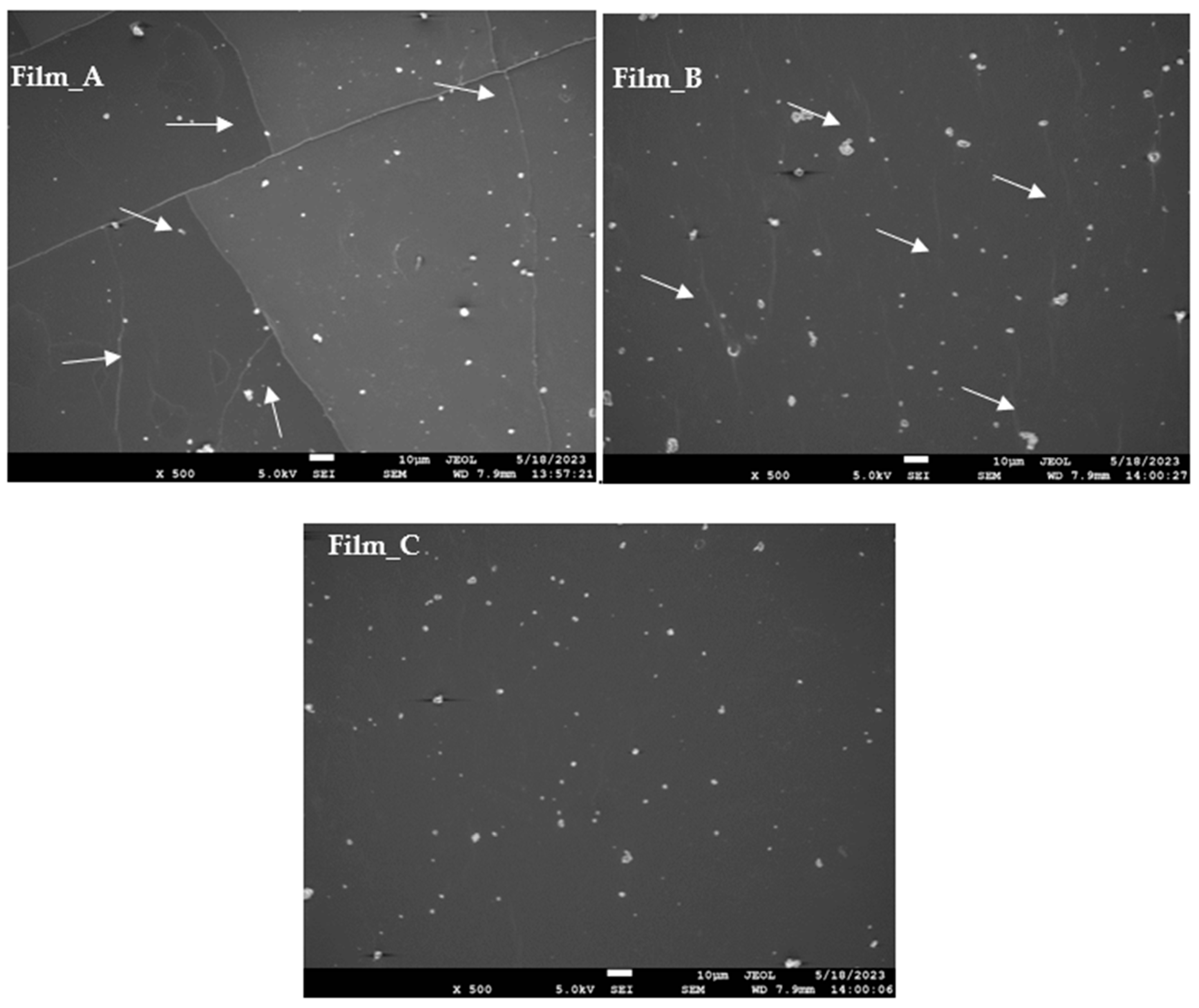
2.3. Morphological Observations
2.4. Gas and Vapor Permeability
2.5. Thermal Behaviour
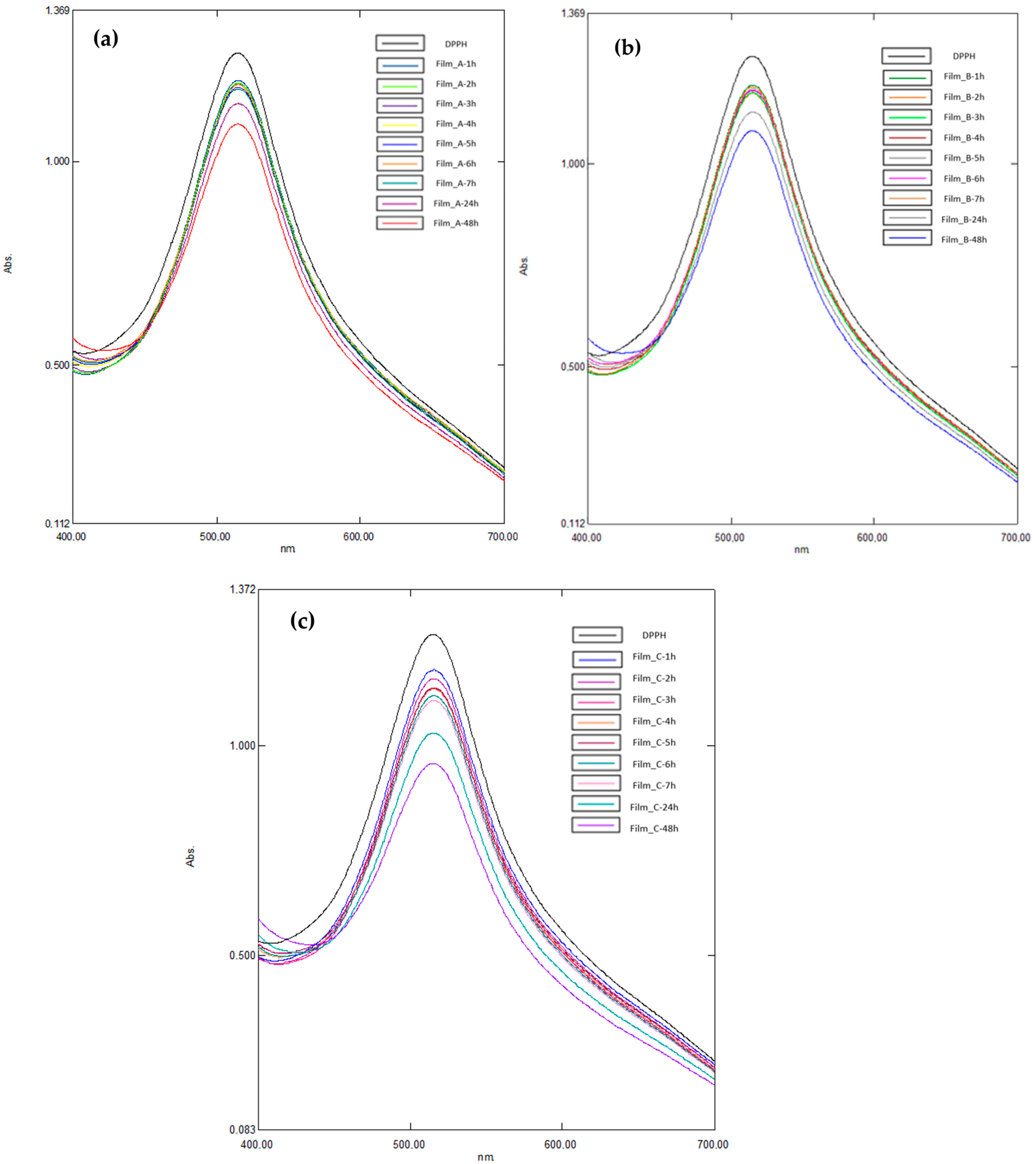
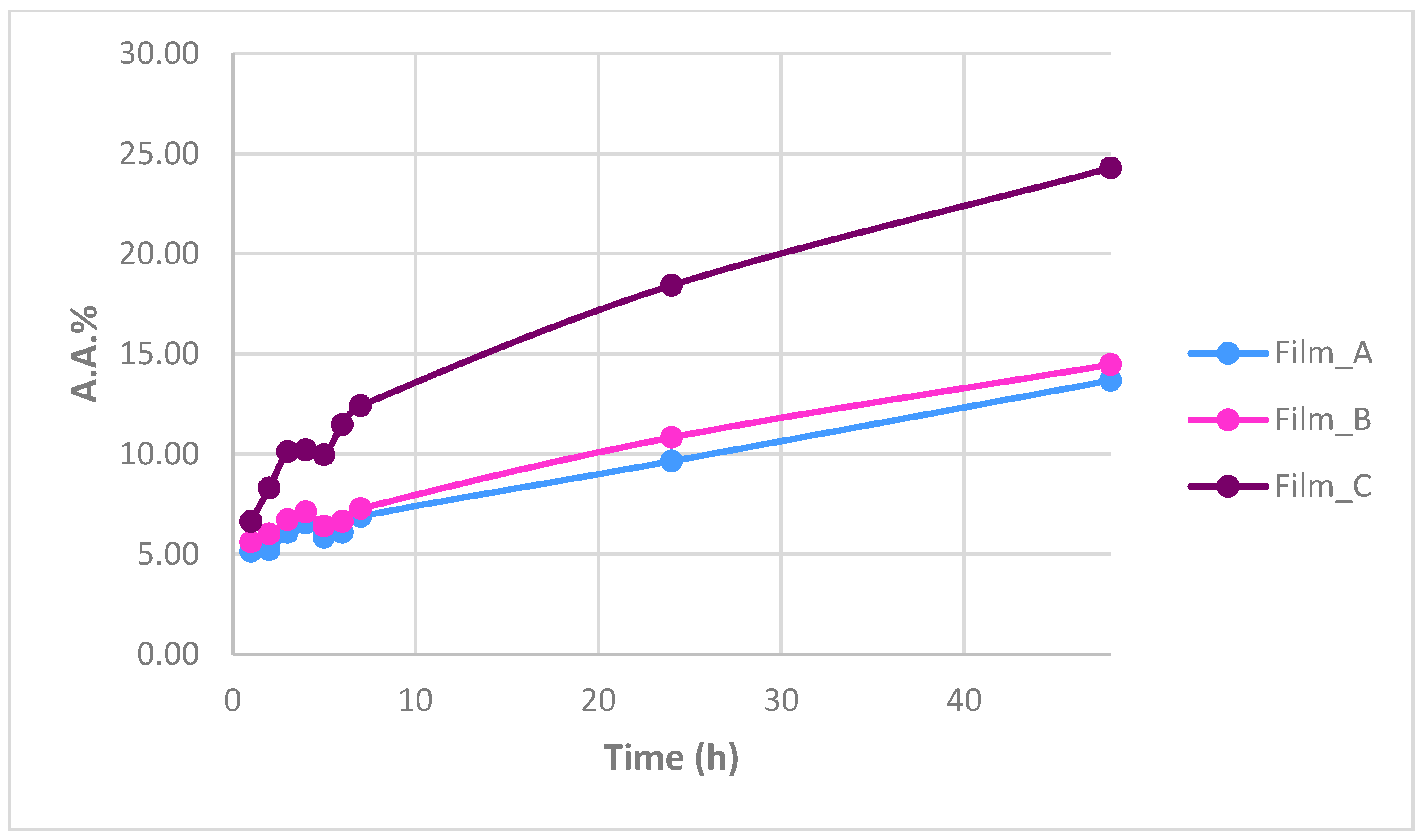
2.6. Antioxidant Activity
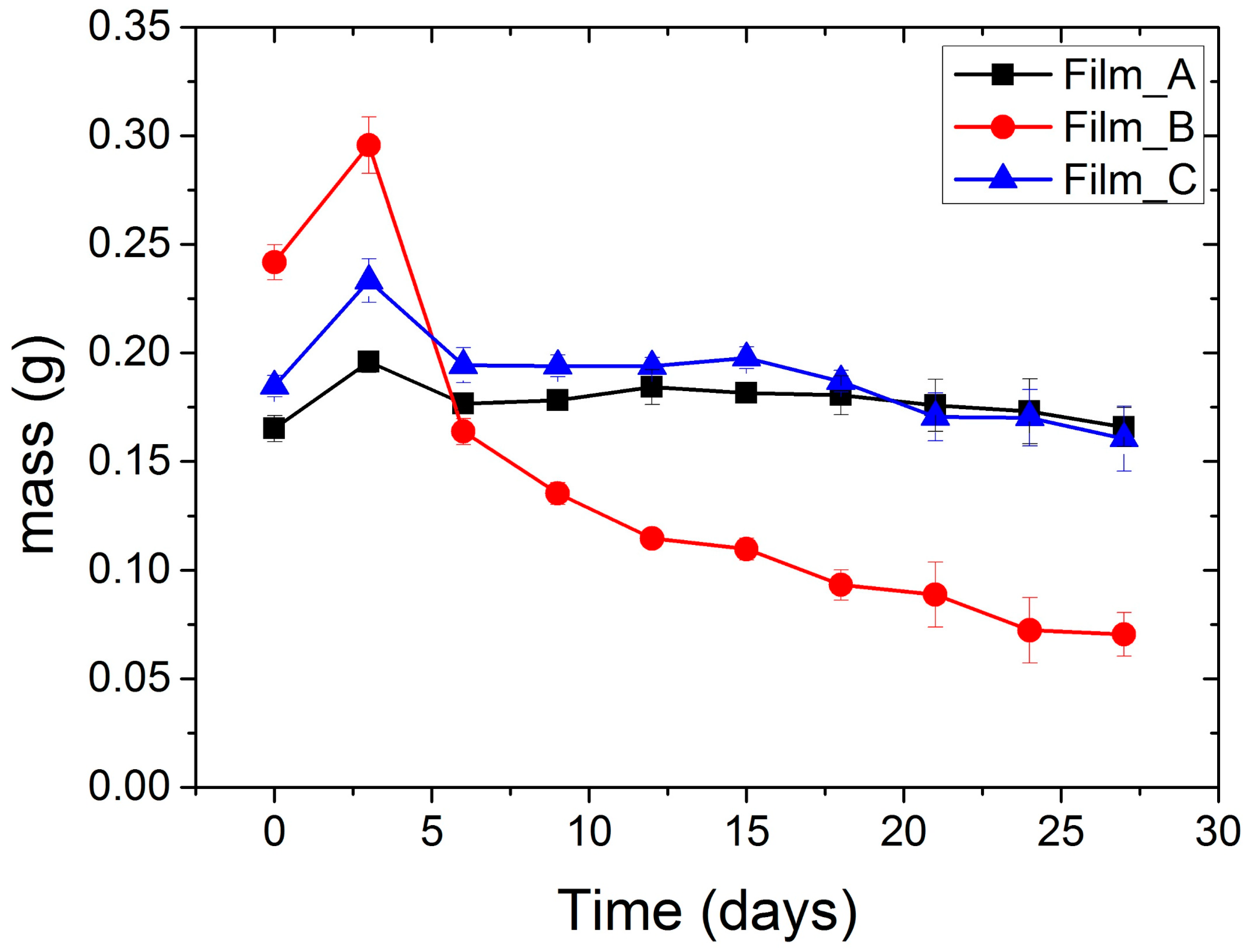
2.7. Biodegradation Study
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Films
4.3. Film Characterization
4.3.1. Thickness
4.3.2. Water Content (WC)
4.3.3. Solubility and Swelling Degree
4.3.4. FT-IR (ATR)
4.3.5. Surface Morphology (SEM)
4.3.6. Gas Permeability
4.3.7. Water Vapor Permeability
4.3.8. Differential Scanning Calorimetry (DSC)
4.3.9. Antioxidant Activity
4.3.10. Aerobic Biodegradation
4.3.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Hedayati, S.; Tarahi, M.; Karaca, A.C.; Hadidi, M.; Assadpour, E.; Jafari, S.M. Advances in transglutaminase cross-linked protein-based food packaging films: A review. Int. J. Biol. Macromol. 2023, 253, 127399. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Manepalli, P.H.; Zhu, L.; Narayan-Sarathy, S.; Alavi, S. Morphological and performance characteristics of nanocomposite films based on poly(lactic acid) compounded with nanocrystalline cellulose and chitin whiskers using melt extrusion. Cellulose 2020, 27, 7523–7534. [Google Scholar] [CrossRef]
- Assad, I.; Bhat, S.U.; Gani, A.; Shah, A. Protein based packaging of plant origin: Fabrication, properties, recent advances and future perspectives. Int. J. Biol. Macromol. 2020, 164, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Manepalli, P.H.; Zhu, L.; Narayan-Sarathy, S.; Alavi, S. Morphological, barrier and mechanical properties of films from poly (butylene succinate) reinforced with nanocrystalline cellulose and chitin whiskers using melt extrusion. J. Polym. Res. 2019, 26, 188. [Google Scholar] [CrossRef]
- Salgado, P.R.; Ortiz, C.M.; Musso, Y.S.; Di Giorgio, L.; Mauri, A.N. Edible films and coatings containing bioactives. Curr. Opin. Food Sci. 2015, 5, 86–92. [Google Scholar] [CrossRef]
- Maan, A.A.; Ahmed, Z.F.R.; Khan, M.K.I.; Riaz, A.; Nazir, A. Aloe vera gel, an excellent base material for edible films and coatings. Trends Food Sci. Technol. 2021, 116, 329–341. [Google Scholar] [CrossRef]
- Mali, S.; Grossmann, M.V.E.; García, M.A.; Martino, M.N.; Zaritzky, N.E. Barrier, mechanical and optical properties of plasticized yam starch films. Carbohydr. Polym. 2004, 56, 129–135. [Google Scholar] [CrossRef]
- Kaya, S.; Kaya, A. Microwave drying effects on properties of whey protein isolate edible films. J. Food Eng. 2000, 43, 91–96. [Google Scholar] [CrossRef]
- Gao, X.; Tong, J.; Guo, L.; Yu, L.; Li, S.; Yang, B.; Wang, L.; Liu, Y.; Li, F.; Guo, J.; et al. Influence of gluten and starch granules interactions on dough mixing properties in wheat (Triticum aestivum L.). Food Hydrocoll. 2020, 106, 105885. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, S.; Sun, B.; Wang, F.; Huang, J.; Wang, X.; Bao, Q. Effects of thermal properties and behavior of wheat starch and gluten on their interaction: A review. Int. J. Biol. Macromol. 2021, 177, 474–484. [Google Scholar] [CrossRef]
- Guo, P.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Mechanisms of starch gelatinization during heating of wheat flour and its effect on in vitro starch digestibility. Food Hydrocoll. 2018, 82, 370–378. [Google Scholar] [CrossRef]
- Chen, G.-X.; Zhou, J.-W.; Liu, Y.-L.; Lu, X.-B.; Han, C.-X.; Zhang, W.-Y.; Xu, Y.-H.; Yan, Y.-M. Biosynthesis and Regulation of Wheat Amylose and Amylopectin from Proteomic and Phosphoproteomic Characterization of Granule-binding Proteins. Sci. Rep. 2016, 6, 33111. [Google Scholar] [CrossRef]
- BeMiller, J.N. Pasting, paste, and gel properties of starch–hydrocolloid combinations. Carbohydr. Polym. 2011, 86, 386–423. [Google Scholar] [CrossRef]
- Waterschoot, J.; Gomand, S.V.; Fierens, E.; Delcour, J.A. Production, structure, physicochemical and functional properties of maize, cassava, wheat, potato and rice starches. Starch/Staerkea 2014, 67, 14–29. [Google Scholar] [CrossRef]
- Zavareze, E.d.R.; Dias, A.R.G. Impact of heat-moisture treatment and annealing in starches: A review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Singh, S.; Singh, N.; Isono, N.; Noda, T. Relationship of granule size distribution and amylopectin structure with pasting, thermal, and retrogradation properties in wheat starch. J. Agric. Food Chem. 2010, 58, 1180–1188. [Google Scholar] [CrossRef]
- Shanbhag, C.; Shenoy, R.; Shetty, P.; Srinivasulu, M.; Nayak, R. Formulation and characterization of starch-based novel biodegradable edible films for food packaging. J. Food Sci. Technol. 2023, 60, 2858–2867. [Google Scholar] [CrossRef] [PubMed]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical properties, modifications and applications of starches from different botanical sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Yaradoddi, J.S.; Banapurmath, N.R.; Ganachari, S.V.; Soudagar, M.E.M.; Sajjan, A.M.; Kamat, S.; Mujtaba, M.; Shettar, A.S.; Anqi, A.E.; Safaei, M.R.; et al. Bio-based material from fruit waste of orange peel for industrial applications. J. Mater. Res. Technol. 2021, 17, 3186–3197. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Amritkumar, P. Synthesis and characterization of eco-friendly bioplastic from low-cost plant resources. SN Appl. Sci. 2019, 1, 1432. [Google Scholar] [CrossRef]
- Marichelvam, M.K.; Jawaid, M.; Asim, M. Corn and rice starch-based bio-plastics as alternative packaging materials. Fibers 2019, 7, 32. [Google Scholar] [CrossRef]
- Xu, J.; Li, Y. Wheat gluten–based coatings and films: Preparation, properties, and applications. J. Food Sci. 2023, 88, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Melanie, S.; Fransiska, D.; Darmawan, M.; Irianto, H.E. Barrier and physical properties of arrowroot starch-carrageenan-based biofilms. J. Bio-Sci. 2017, 25, 45–56. [Google Scholar]
- Shafqat, A.; Tahir, A.; Ullah Khan, W.; Mahmood, A.; Abbasi, G.H. Production and characterisation of rice starch and corn starch-based biodegradable bioplastics using various plasticizers and natural reinforcing fillers. Cellul. Chem. Technol. 2021, 55, 867–881. [Google Scholar] [CrossRef]
- Cortes-Trivino, E.; Martinez, I. Wheat gluten/montmorillonite biocomposites: Effect of pH on the mechanical properties and clay dispersion. Express Polym. Lett. 2018, 12, 616–627. [Google Scholar] [CrossRef]
- El-Wakil, N.A.; Hassan, E.A.; Abou-Zeid, R.E.; Dufresne, A. Development of wheat gluten/nanocellulose/titanium dioxide nanocomposites for active food packaging. Carbohydr. Polym. 2015, 124, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Blácido, D.; Sobral, P.D.A.; Menegalli, F. Optimization of amaranth flour films plasticized with glycerol and sorbitol by multi-response analysis. LWT 2011, 44, 1731–1738. [Google Scholar] [CrossRef]
- Araujo-Farro, P.C.; Podadera, G.; Sobral, P.J.A.; Menegalli, F.C. Development of films based on quinoa (Chenopodium quinoa, Willdenow) starch. Carbohydr. Polym. 2010, 81, 839–848. [Google Scholar] [CrossRef]
- Dias, A.B.; Müller, C.M.; Larotonda, F.D.; Laurindo, J.B. Biodegradable films based on rice starch and rice flour. J. Cereal Sci. 2010, 51, 213–219. [Google Scholar] [CrossRef]
- Tapia-Blácido, D.; Mauri, A.; Menegalli, F.; Sobral, P.; Añón, M. Contribution of the starch, protein, and lipid fractions to the physical, thermal, and structural properties of amaranth (Amaranthus caudatus) flour films. J. Food Sci. 2007, 72, E293–E300. [Google Scholar] [CrossRef]
- Drakos, A.; Pelava, E.; Evageliou, V. Properties of flour films as affected by the flour’s source and particle size. Food Res. Int. 2018, 107, 551–558. [Google Scholar] [CrossRef]
- Solano, A.C.V.; de Rojas Gante, C. Two different processes to obtain antimicrobial packaging containing natural oils. Food Bioprocess Technol. 2012, 5, 2522–2528. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Andrade-Mahecha, M.M.; Sobral, P.J.D.A.; Menegalli, F.C. Comparative study on the properties of flour and starch films of plantain bananas (Musa paradisiaca). Food Hydrocoll. 2013, 30, 681–690. [Google Scholar] [CrossRef]
- Mossavi, M.P.; Kashiri, M.; Maghsoudlou, Y.; Khomiri, M.; Alami, M. Development and characterization of a novel multifunctional film based on wheat filter flour incorporated with carvacrol: Antibacterial, antifungal, and insecticidal potentials. Food Sci. Technol. Int. 2021, 28, 603–612. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Y.; Li, Y. Development of tea extracts and chitosan composite films for active packaging materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef]
- Luo, S.; Chen, J.; He, J.; Li, H.; Jia, Q.; Hossen, A.; Dai, J.; Qin, W.; Liu, Y. Preparation of corn starch/rock bean protein edible film loaded with d-limonene particles and their application in glutinous rice cake preservation. Int. J. Biol. Macromol. 2022, 206, 313–324. [Google Scholar] [CrossRef]
- Tao, R.; Sedman, J.; Ismail, A. Characterization and in vitro antimicrobial study of soy protein isolate films incorporating carvacrol. Food Hydrocoll. 2021, 122, 107091. [Google Scholar] [CrossRef]
- Xiao, Q.; Woo, M.W.; Hu, J.; Xiong, H.; Zhao, Q. The role of heating time on the characteristics, functional properties and antioxidant activity of enzyme-hydrolyzed rice proteins-glucose Maillard reaction products. Food Biosci. 2021, 43, 101225. [Google Scholar] [CrossRef]
- Chen, X.; Cui, F.; Zi, H.; Zhou, Y.; Liu, H.; Xiao, J. Development and characterization of a hydroxypropyl starch/zein bilayer edible film. Int. J. Biol. Macromol. 2019, 141, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Furer, V.; Vandyukov, A.; Zaripov, S.; Solovieva, S.; Antipin, I.; Kovalenko, V. FT-IR and FT-Raman study of hydrogen bonding in p-alkylcalix[8]arenes. Vib. Spectrosc. 2018, 95, 38–43. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the development and application of edible coatings for fresh and minimally processed fruits and vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Zuo, M.; Song, Y.; Zheng, Q. Preparation and properties of wheat gluten/methylcellulose binary blend film casting from aqueous ammonia: A comparison with compression molded composites. J. Food Eng. 2009, 91, 415–422. [Google Scholar] [CrossRef]
- Giannakas, A. Na-montmorillonite vs. organically modified montmorillonite as essential oil nanocarriers for melt-extruded low-density poly-ethylene nanocomposite active packaging films with a controllable and long-life antioxidant activity. Nanomaterials 2020, 10, 1027. [Google Scholar] [CrossRef]
- Bastioli, C. Handbook of Biodegradable Polymers; Rapra Technology: Telford, UK, 2005. [Google Scholar]
- Bakhshizadeh, M.; Ayaseh, A.; Hamishehkar, H.; Kafil, H.S.; Moghaddam, T.N.; Haghi, P.B.; Tavassoli, M.; Amjadi, S.; Lorenzo, J.M. Multifunctional performance of packaging system based on gelatin/alove vera gel film containing of rosemary essential oil and common poppy anthocyanins. Food Control 2023, 154, 110017. [Google Scholar] [CrossRef]
- da Silva, M.A.; Bierhalz, A.C.K.; Kieckbusch, T.G. Alginate and pectin composite films crosslinked with Ca2+ ions: Effect of the plasticizer concentration. Carbohydr. Polym. 2009, 77, 736–742. [Google Scholar] [CrossRef]
- Zhong, Y.; Song, X.; Li, Y. Antimicrobial, physical and mechanical properties of kudzu starch–chitosan composite films as a function of acid solvent types. Carbohydr. Polym. 2011, 84, 335–342. [Google Scholar] [CrossRef]
- ASTM E96/E96M-16; Standard Test Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2016.
- Diaz, C.A.; Shah, R.K.; Evans, T.; Trabold, T.A.; Draper, K. Thermoformed Containers Based on Starch and Starch/Coffee Waste Biochar Composites. Energies 2020, 13, 6034. [Google Scholar] [CrossRef]



| Thickness (μm) | Water Content (%) | Solubility | Swelling Degree | |
|---|---|---|---|---|
| Film_A | 146.7 ± 11 a | 7.98 ± 1.74 a | 0.38 ± 0.15 a | 2.33 ± 0.21 a |
| Film_Β | 180.8 ± 14 b | 12.77 ± 0.87 b | 0.40 ± 0.12 a | 2.07 ± 0.18 a |
| Film_C | 182.5 ± 7 b | 12.91 ± 0.94 b | 0.54 ± 0.16 b | 1.61 ± 0.15 b |
| Oxygen Permeability, OTR [cm³/(m2 × days × 0.1 MPa)] | Carbon Dioxide Permeability, CO2TR [cm³/(m2 × days × 0.1 MPa)] | |
|---|---|---|
| Film_B | 14.147 ± 0.82 a | 16.302 ± 1.10 a |
| Film_C | 11.583 ± 0.78 b | 9.925 ± 0.93 b |
| Water Vapor Transmission Rate WVTR, (g/(m2 × h) | Water Vapor Permeability WVP. (g/m × h × bar) | Percentage of Water That Penetrates (24 h) (%) | |
|---|---|---|---|
| Film_B | 16.596 ± 0.44 a | 1040×10−6 ± 0.08 a | 2190 ± 0.23 a |
| Film_C | 16.277 ± 0.31 a | 1247×10−6 ± 0.15 b | 2275 ± 0.17 a |
| Time (Days) | Film_A | Film_B | Film_C |
|---|---|---|---|
| 0 | 0.1652 ± 0.006 | 0.2417 ± 0.008 | 0.1847 ± 0.005 |
| 3 | 0.1959 ± 0.005 | 0.2956 ± 0.013 | 0.2332 ± 0.010 |
| 6 | 0.1767 ± 0.003 | 0.1638 ± 0.006 | 0.1943 ± 0.008 |
| 9 | 0.1781 ± 0.003 | 0.1353 ± 0.005 | 0.1940 ± 0.005 |
| 12 | 0.1843 ± 0.008 | 0.1146 ± 0.004 | 0.1939 ± 0.004 |
| 15 | 0.1816 ± 0.004 | 0.1097 ± 0.005 | 0.1977 ± 0.005 |
| 18 | 0.1805 ± 0.009 | 0.0932 ± 0.007 | 0.1869 ± 0.005 |
| 21 | 0.1759 ± 0.012 | 0.0887 ± 0.015 | 0.1705 ± 0.011 |
| 24 | 0.1731 ± 0.015 | 0.0723 ± 0.015 | 0.1702 ± 0.013 |
| 27 | 0.1659 ± 0.009 | 0.0704 ± 0.010 | 0.1605 ± 0.015 |
| Time (Days) | Film_A | Film_B | Film_C |
|---|---|---|---|
| 3 |  |  |  |
| 6 |  |  |  |
| 9 |  |  |  |
| 12 |  |  |  |
| 15 |  |  |  |
| 18 |  |  |  |
| 21 |  |  |  |
| 24 |  |  |  |
| 27 |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petaloti, A.-I.; Makri, S.; Achilias, D.S. Bioactive Edible Gel Films Based on Wheat Flour and Glucose for Food Packaging Applications. Gels 2024, 10, 105. https://doi.org/10.3390/gels10020105
Petaloti A-I, Makri S, Achilias DS. Bioactive Edible Gel Films Based on Wheat Flour and Glucose for Food Packaging Applications. Gels. 2024; 10(2):105. https://doi.org/10.3390/gels10020105
Chicago/Turabian StylePetaloti, Argyri-Ioanna, Styliani Makri, and Dimitris S. Achilias. 2024. "Bioactive Edible Gel Films Based on Wheat Flour and Glucose for Food Packaging Applications" Gels 10, no. 2: 105. https://doi.org/10.3390/gels10020105
APA StylePetaloti, A.-I., Makri, S., & Achilias, D. S. (2024). Bioactive Edible Gel Films Based on Wheat Flour and Glucose for Food Packaging Applications. Gels, 10(2), 105. https://doi.org/10.3390/gels10020105