QbD Approach for the Development of Tea Tree Oil-Enhanced Microemulgel Loaded with Curcumin and Diclofenac for Rheumatoid Arthritis Treatment
Abstract
1. Introduction
2. Results and Discussion
2.1. Screening and Selection of Oils, Surfactant, and Cosurfactant
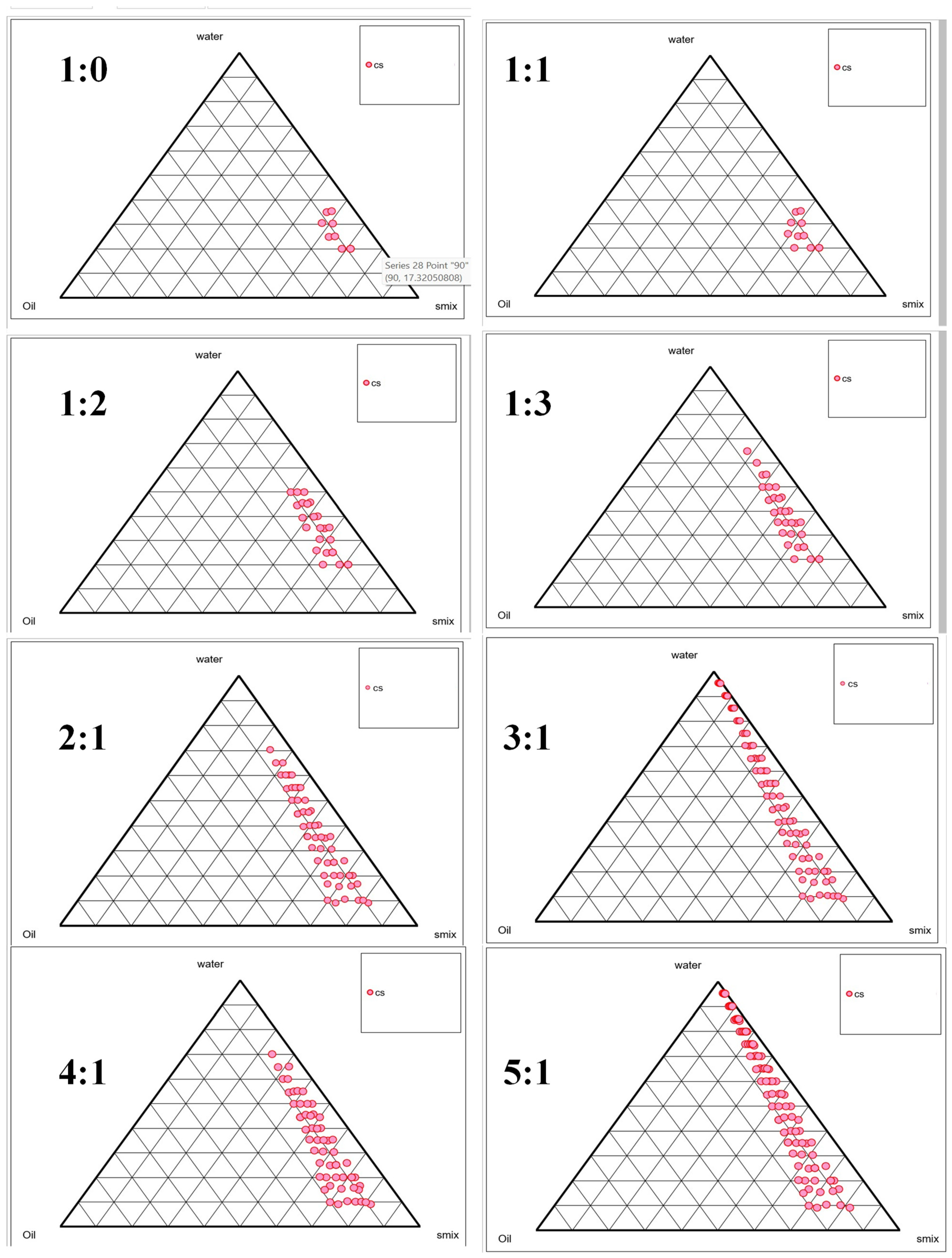
2.2. Selection of Smix Using Phase Titration Method
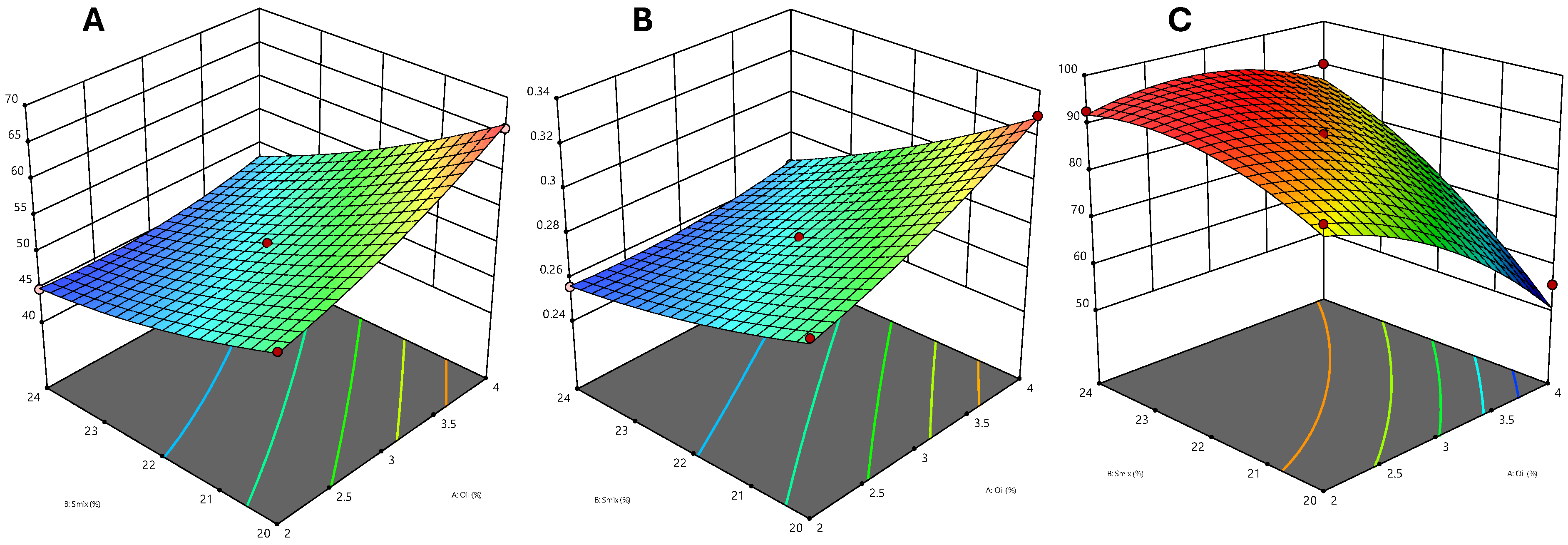
2.3. Optimization of Nanoemulsion Using Central Composite Rotatable Design
2.4. Data Analysis
2.5. Effect of the Independent Variable on the Particle Size
2.6. Effect of Independent Variable on PDI
2.7. Effect of Independent Variable on Transmittance
2.8. Selection of Optimized Formulation
2.9. Physical Stability Studies of the Microemulsion
2.10. Characterization of the Optimized Microemulsion
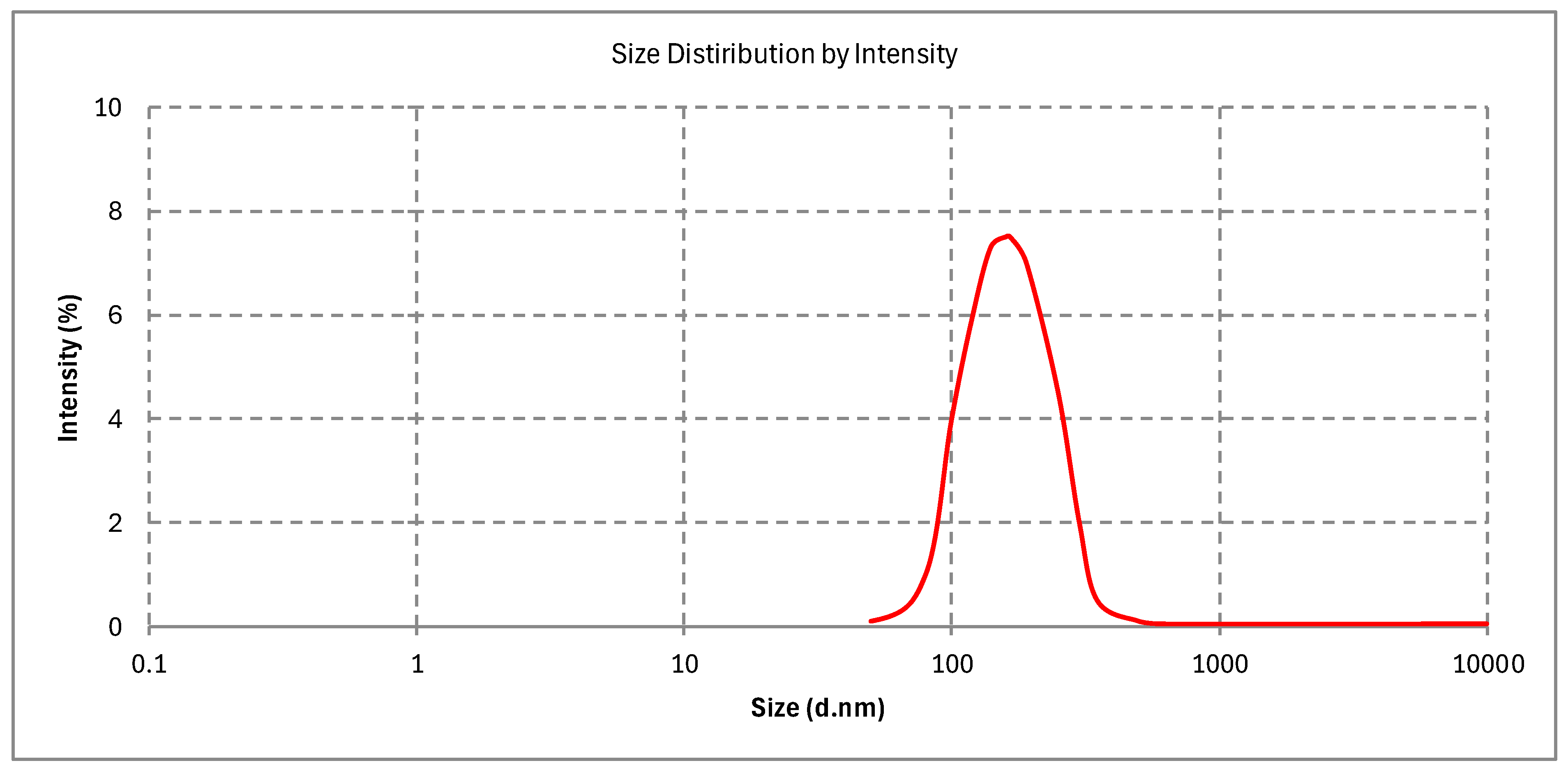
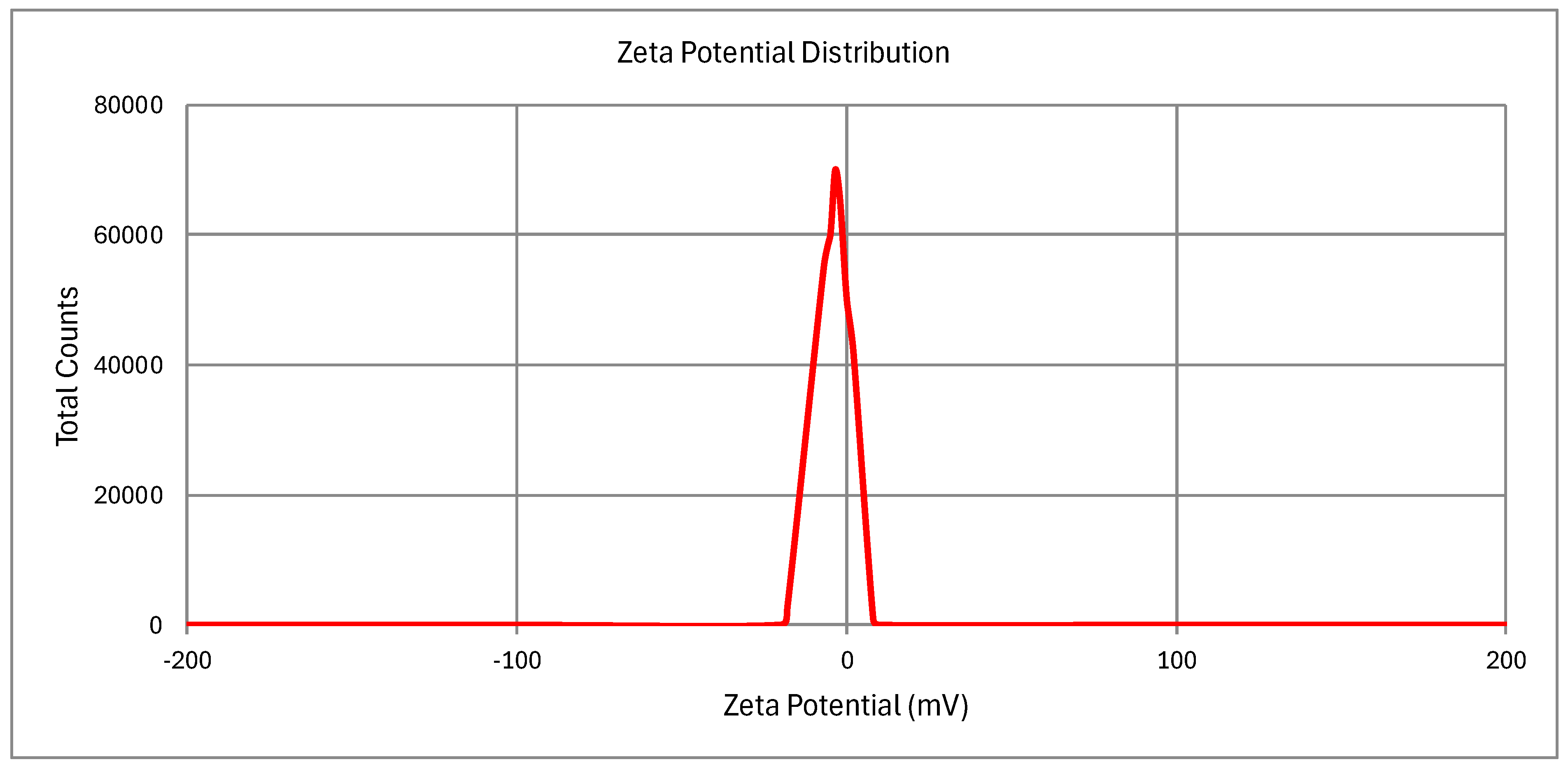
2.10.1. Particle Size, PDI, and Zeta Potential
2.10.2. Percentage Transmittance, pH, and Refractive Index
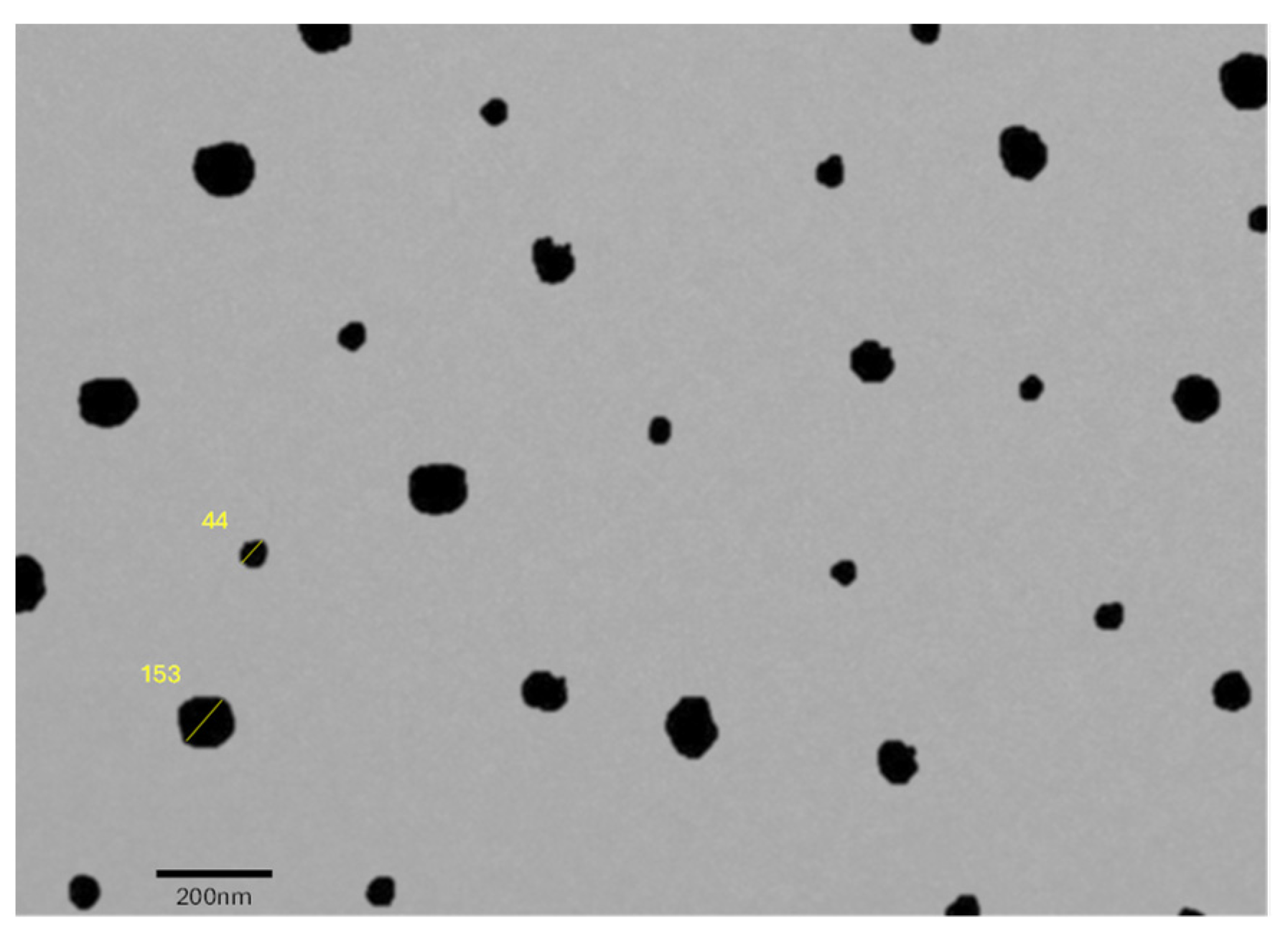
2.10.3. Surface Morphology
2.11. Incorporation of Drug in Optimized Formulation
2.12. Drug Content Uniformity of Optimized Gel
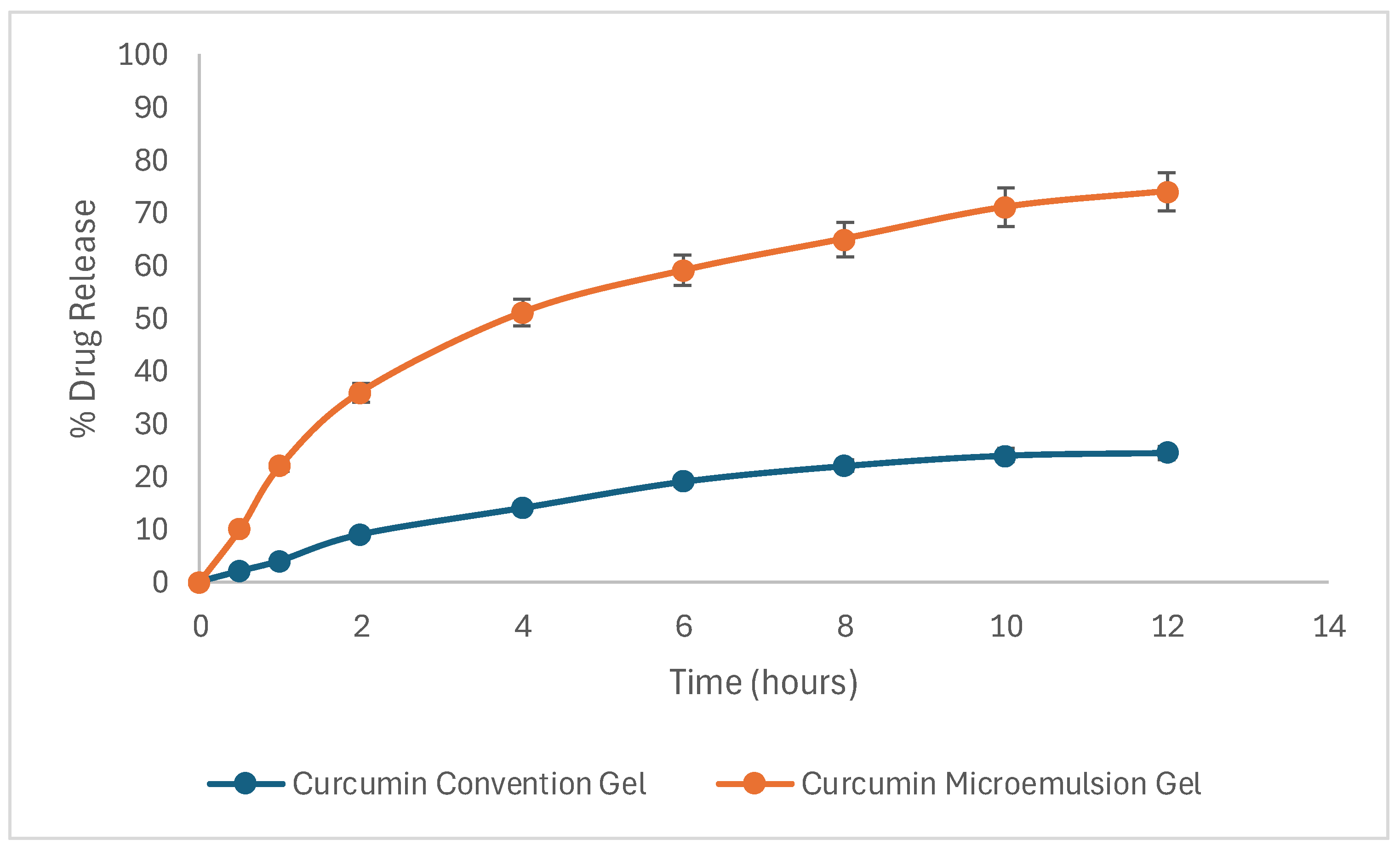
2.13. Skin Permeation Studies
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Screening and Selection of Excipients
4.3. Smix Determination via the Phase Titration Technique
4.4. Experimental Design for Formulation Optimization
4.5. Physical Stability of Microemulsion
4.6. Preparation and Analysis of Optimized Microemulsion
4.7. Particle Size, Zeta Potential, and PDI
4.8. Percentage Transmittance, pH, and Refractive Index
4.9. Examining Surface Morphology via Transmission Electron Microscopy (TEM)
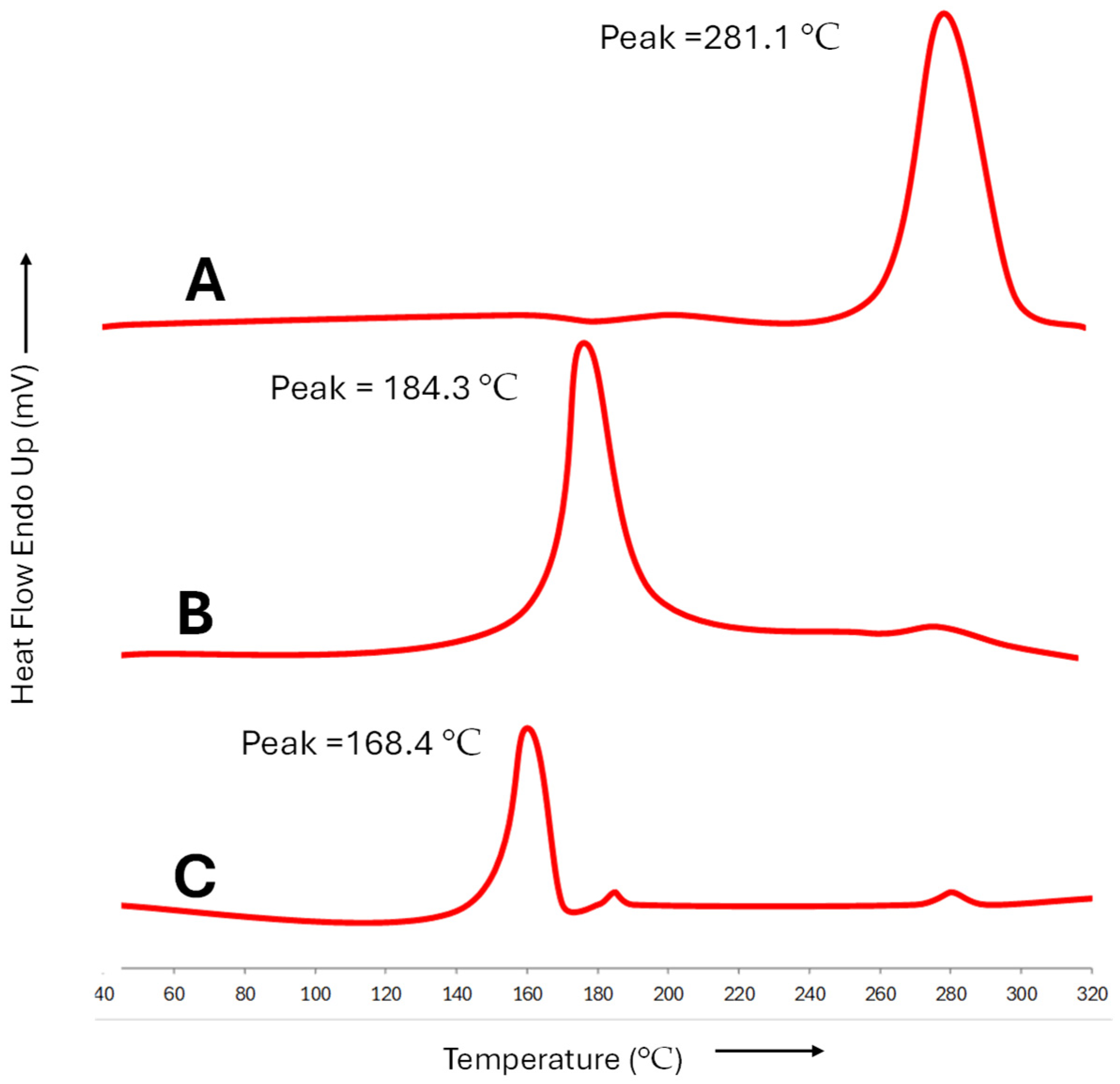
4.10. Examining the Integration of Diclofenac and Curcumin in Microemulsion: Insights from Differential Scanning Calorimetry (DSC)
4.11. Conversion of Microemulsion into Gel
4.12. Drug Content Uniformity and pH of Optimized Gel
4.13. Skin Permeation Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COX | Cyclooxygenase |
| CCRD | Central Composite Rotatable Design |
| RA | Rheumatoid Arthritis |
| NSAID | Nonsteroidal Anti-Inflammatory Drug |
| PDI | Polydispersity Index |
| DSC | Differential Scanning Calorimetry |
| HLB | Hydrophilic-Lipophilic Balance |
| Smix | Surfactant and Cosurfactant Mixture |
| TNF-α | Tumor Necrosis Factor Alpha |
| IL-1β | Interleukin-1 Beta |
| NF-κB | Nuclear Factor Kappa B |
| TEM | Transmission Electron Microscopy |
| QbD | Quality by Design |
| DMARD | Disease-Modifying Antirheumatic Drugs |
| DMSO | Dimethyl Sulfoxide |
| RI | Refractive Index |
| TEM | Transmission Electron Microscopy |
| DSC | Differential Scanning Calorimetry |
| UV | Ultraviolet |
| MCT | Microcentrifuge Tubes |
| PTPD | Pseudo-Ternary Phase Diagram |
| BBD | Box–Behnken Design |
| RSM | Response Surface Methodology |
| PEG | Polyethylene Glycol |
| RPM | Revolutions Per Minute |
References
- Ding, Q.; Hu, W.; Wang, R.; Yang, Q.; Zhu, M.; Li, M.; Cai, J.; Rose, P.; Mao, J.; Zhu, Y.Z. Signaling pathways in rheumatoid arthritis: Implications for targeted therapy. Signal Transduct. Target. Ther. 2023, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Wang, Y.; Xu, D.; Nossent, J.; Pavlos, N.J.; Xu, J. Rheumatoid arthritis: Pathological mechanisms and modern pharmacologic therapies. Bone Res. 2018, 6, 15. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Jandu, J.S.; Brent, L.H.; Al-Dhahir, M.A. Rheumatoid Arthritis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK441999/ (accessed on 3 April 2024).
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID Development: Evolution of Diclofenac Products Using Pharmaceutical Technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef]
- Bermas, B.L. Non-steroidal anti inflammatory drugs, glucocorticoids and disease modifying anti-rheumatic drugs for the management of rheumatoid arthritis before and during pregnancy. Curr. Opin. Rheumatol. 2014, 26, 334. [Google Scholar] [CrossRef]
- Bullock, J.; Rizvi, S.A.A.; Saleh, A.M.; Ahmed, S.S.; Do, D.P.; Ansari, R.A.; Ahmed, J. Rheumatoid Arthritis: A Brief Overview of the Treatment. Med. Princ. Pract. 2019, 27, 501–507. [Google Scholar] [CrossRef]
- Crofford, L.J. Use of NSAIDs in treating patients with arthritis. Arthritis Res. Ther. 2013, 15, S2. [Google Scholar] [CrossRef]
- Ben Mrid, R.; Bouchmaa, N.; Ainani, H.; El Fatimy, R.; Malka, G.; Mazini, L. Anti-rheumatoid drugs advancements: New insights into the molecular treatment of rheumatoid arthritis. Biomed. Pharmacother. 2022, 151, 113126. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gauri; Sharma, R. Advancement in the management of rheumatoid arthritis. RPS Pharm. Pharmacol. Rep. 2023, 2, rqad005. [Google Scholar] [CrossRef]
- Köhler, B.M.; Günther, J.; Kaudewitz, D.; Lorenz, H.-M. Current Therapeutic Options in the Treatment of Rheumatoid Arthritis. J. Clin. Med. 2019, 8, 938. [Google Scholar] [CrossRef]
- Radu, A.-F.; Bungau, S.G. Management of Rheumatoid Arthritis: An Overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Alfaro, R.A.; Davis, D.D. Diclofenac. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK557879/ (accessed on 3 April 2024).
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Efficacy and safety of combination of curcuminoid complex and diclofenac versus diclofenac in knee osteoarthritis. Medicine 2020, 99, e19723. [Google Scholar] [CrossRef]
- Kou, H.; Huang, L.; Jin, M.; He, Q.; Zhang, R.; Ma, J. Effect of curcumin on rheumatoid arthritis: A systematic review and meta-analysis. Front. Immunol. 2023, 14, 1121655. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Ashhar, M.U.; Qizilbash, F.F.; Qamar, Z.; Narang, J.K.; Kumar, S.; Ali, J.; Baboota, S. Ligand Conjugated Targeted Nanotherapeutics for Treatment of Neurological Disorders. Curr. Pharm. Des. 2020, 26, 2291–2305. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chauhan, S.B.; Gupta, C.; Singh, I.; Gupta, A.; Sharma, S.; Kawish, S.M.; Rahman, S.; Iqbal, M. Design and Characterization of Citronella Oil-Loaded Micro-Emulgel for the Treatment of Candida Albicans Infection. Gels 2023, 9, 799. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Lebaz, N.; Fessi, H.; Elaissari, A. Exploring the Versatility of Microemulsions in Cutaneous Drug Delivery: Opportunities and Challenges. Nanomaterials 2023, 13, 1688. [Google Scholar] [CrossRef] [PubMed]
- Bagwe, R.; Kanicky, J.; Palla, B.; Patanjali, P.; Shah, D. Improved Drug Delivery Using Microemulsions: Rationale, Recent Progress, and New Horizons. Crit. Rev. Ther. Drug Carrier Syst. 2001, 18, 77–140. [Google Scholar] [CrossRef]
- Souto, E.B.; Cano, A.; Martins-Gomes, C.; Coutinho, T.E.; Zielińska, A.; Silva, A.M. Microemulsions and Nanoemulsions in Skin Drug Delivery. Bioengineering 2022, 9, 158. [Google Scholar] [CrossRef]
- Yadav, K.S.; Soni, G.; Choudhary, D.; Khanduri, A.; Bhandari, A.; Joshi, G. Microemulsions for enhancing drug delivery of hydrophilic drugs: Exploring various routes of administration. Med. Drug Discov. 2023, 20, 100162. [Google Scholar] [CrossRef]
- De Cássia da Silveira e Sá, R.; Andrade, L.N.; De Sousa, D.P. A Review on Anti-Inflammatory Activity of Monoterpenes. Molecules 2013, 18, 1227–1254. [Google Scholar] [CrossRef]
- Hart, P.H.; Brand, C.; Carson, C.F.; Riley, T.V.; Prager, R.H.; Finlay-Jones, J.J. Terpinen-4-ol, the main component of the essential oil of Melaleuca alternifolia (tea tree oil), suppresses inflammatory mediator production by activated human monocytes. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2000, 49, 619–626. [Google Scholar] [CrossRef]
- Nogueira, M.N.M.; Aquino, S.G.; Rossa Junior, C.; Spolidorio, D.M.P. Terpinen-4-ol and alpha-terpineol (tea tree oil components) inhibit the production of IL-1β, IL-6 and IL-10 on human macrophages. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 2014, 63, 769–778. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Ashhar, M.U.; Ahuja, A.; Ali, J.; Baboota, S. Role of Natural Bioactives and their Nanocarriers for Overcoming Oxidative Stress Induced Cancer. Curr. Med. Chem. 2021, 28, 7477–7512. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50. [Google Scholar] [CrossRef]
- Kairey, L.; Agnew, T.; Bowles, E.J.; Barkla, B.J.; Wardle, J.; Lauche, R. Efficacy and safety of Melaleuca alternifolia (tea tree) oil for human health—A systematic review of randomized controlled trials. Front. Pharmacol. 2023, 14, 1116077. [Google Scholar] [CrossRef]
- Koh, K.J.; Pearce, A.L.; Marshman, G.; Finlay-Jones, J.J.; Hart, P.H. Tea tree oil reduces histamine-induced skin inflammation. Br. J. Dermatol. 2002, 147, 1212–1217. [Google Scholar] [CrossRef]
- Gupta, P.; Sharma, S.; Gupta, A.; Kawish, S.M.; Iqbal, M.; Rahman, S.; Aqil, M.; Kohli, K.; Sultana, Y. Development and Validation of a Robust RP-HPLC Method for the Simultaneous Analysis of Exemestane and Thymoquinone and Its Application to Lipid-Based Nanoformulations. ACS Omega 2024, 9, 30120–30130. [Google Scholar] [CrossRef]
- Azeem, A.; Rizwan, M.; Ahmad, F.J.; Iqbal, Z.; Khar, R.K.; Aqil, M.; Talegaonkar, S. Nanoemulsion Components Screening and Selection: A Technical Note. AAPS PharmSciTech 2009, 10, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gurram, A.K.; Deshpande, P.B.; Kar, S.S.; Nayak, U.Y.; Udupa, N.; Reddy, M.S. Role of Components in the Formation of Self-microemulsifying Drug Delivery Systems. Indian J. Pharm. Sci. 2015, 77, 249–257. [Google Scholar] [PubMed]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Ali, J.; Baboota, S. Enhanced anti-psoriatic activity of tacrolimus loaded nanoemulsion gel via omega 3—Fatty acid (EPA and DHA) rich oils-fish oil and linseed oil. J. Drug Deliv. Sci. Technol. 2021, 63, 102458. [Google Scholar] [CrossRef]
- Osborne, D.W.; Musakhanian, J. Skin Penetration and Permeation Properties of Transcutol®—Neat or Diluted Mixtures. AAPS PharmSciTech 2018, 19, 3512–3533. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Gull, A.; Alam, M.; Aqil, M.; Sultana, Y. Ultrasonically tailored, chemically engineered and “QbD” enabled fabrication of agomelatine nanoemulsion; optimization, characterization, ex-vivo permeation and stability study. Ultrason. Sonochem. 2018, 41, 213–226. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Ahmad, R.; Al-Qudaihi, A.; Alaseel, S.E.; Fita, I.Z.; Khalid, M.S.; Pottoo, F.H. Correction: Preparation of a novel curcumin nanoemulsion by ultrasonication and its comparative effects in wound healing and the treatment of inflammation. RSC Adv. 2020, 10, 27322. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Ali, J.; Baboota, S. DoE Engineered Development and Validation of an RP-HPLC Method for Simultaneous Estimation of Temozolomide and Resveratrol in Nanostructured Lipid Carrier. J. AOAC Int. 2022, 105, qsac045. [Google Scholar] [CrossRef]
- Mittal, S.; Ali, J.; Baboota, S. Overcoming the Challenges in the Treatment of Glioblastoma via Nanocarrier- based Drug Delivery Approach. Curr. Pharm. Des. 2021, 27, 4539–4556. [Google Scholar] [CrossRef]
- Mittal, S.; Shah, S.; Yadav, H.N.; Ali, J.; Gupta, M.M.; Baboota, S. Quality by design engineered, enhanced anticancer activity of temozolomide and resveratrol coloaded NLC and brain targeting via lactoferrin conjugation in treatment of glioblastoma. Eur. J. Pharm. Biopharm. Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik EV 2023, 191, 175–188. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, P.; Gupta, A.; Kawish, S.M.; Iqbal, Z.; Vohora, D.; Kohli, K. Rapid Analytical Method Development and Validation of RP-HPLC Method for the Simultaneous Estimation of Exemestane and Genistein with Specific Application in Lipid-Based Nanoformulations. ACS Omega 2023, 8, 25101–25113. [Google Scholar] [CrossRef]
- Khan, M.A.; Ansari, M.M.; Arif, S.T.; Raza, A.; Choi, H.-I.; Lim, C.-W.; Noh, H.-Y.; Noh, J.-S.; Akram, S.; Nawaz, H.A.; et al. Eplerenone nanocrystals engineered by controlled crystallization for enhanced oral bioavailability. Drug Deliv. 2021, 28, 2510–2524. [Google Scholar] [CrossRef]
- Thakral, S.; Sonje, J.; Suryanarayanan, R. Anomalous behavior of mannitol hemihydrate: Implications on sucrose crystallization in colyophilized systems. Int. J. Pharm. 2020, 587, 119629. [Google Scholar] [CrossRef]
- Thakral, S.; Sonje, J.; Munjal, B.; Bhatnagar, B.; Suryanarayanan, R. Mannitol as an Excipient for Lyophilized Injectable Formulations. J. Pharm. Sci. 2023, 112, 19–35. [Google Scholar] [CrossRef]
- Ashhar, M.U.; Kumar, S.; Ali, J.; Baboota, S. CCRD based development of bromocriptine and glutathione nanoemulsion tailored ultrasonically for the combined anti-parkinson effect. Chem. Phys. Lipids 2021, 235, 105035. [Google Scholar] [CrossRef] [PubMed]
- Raut, P.; Kumar, S.; Iqbal, B.; Ali, J.; Baboota, S. Development of nanoemulsion gel based formulation of terbinafine for the synergistic antifungal activity: Dermatokinetic experiment for investigation of epidermal terbinafine deposition enhancement. Inorg. Nano-Met. Chem. 2021, 51, 1867–1881. [Google Scholar] [CrossRef]
- Kreilgaard, M. Influence of microemulsion on cutaneous drug delivery. Adv. Drug Deliv. Rev. 2002, 54 (Suppl. S1), S77–S98. [Google Scholar] [CrossRef]
- Shinde, U.; Pokharkar, S.; Modani, S. Design and Evaluation of Microemulsion Gel System of Nadifloxacin. Indian J. Pharm. Sci. 2012, 74, 237–247. [Google Scholar] [CrossRef]
- Shukla, T.; Upmanyu, N.; Agrawal, M.; Saraf, S.; Saraf, S.; Alexander, A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed. Pharmacother. 2018, 108, 1477–1494. [Google Scholar] [CrossRef]
- Fatima, N.; Rehman, S.; Nabi, B.; Baboota, S.; Ali, J. Harnessing nanotechnology for enhanced topical delivery of clindamycin phosphate. J. Drug Deliv. Sci. Technol. 2019, 54, 101253. [Google Scholar] [CrossRef]
- Gupta, A.; Dahima, R.; Panda, S.K.; Gupta, A.; Singh, G.D.; Wani, T.A.; Hussain, A.; Rathore, D. QbD-Based Development and Evaluation of Pazopanib Hydrochloride Extrudates Prepared by Hot-Melt Extrusion Technique: In Vitro and In Vivo Evaluation. Pharmaceutics 2024, 16, 764. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Meher, J.G.; Gupta, A.; Pawar, V.K.; Singh, Y.; Chourasia, M.K. Delivery of methotrexate executed by engineered polymeric micelles intended to improve chemotherapy. J. Biomater. Tissue Eng. 2014, 4, 700–709. [Google Scholar] [CrossRef]
- Nair, S.C.; Vinayan, K.P.; Mangalathillam, S. Nose to Brain Delivery of Phenytoin Sodium Loaded Nano Lipid Carriers: Formulation, Drug Release, Permeation and In Vivo Pharmacokinetic Studies. Pharmaceutics 2021, 13, 1640. [Google Scholar] [CrossRef]
- Salamanca, C.H.; Barrera-Ocampo, A.; Lasso, J.C.; Camacho, N.; Yarce, C.J. Franz Diffusion Cell Approach for Pre-Formulation Characterisation of Ketoprofen Semi-Solid Dosage Forms. Pharmaceutics 2018, 10, 148. [Google Scholar] [CrossRef]

| Independent Variable | Response | ||||
|---|---|---|---|---|---|
| Run | Factor 1 A: Oil (%) | Factor 2 B: Smix (v/v) (%) | Y1: Particle Size | Y2: % PDI | Y3: Transmittance |
| 1 | 1.58579 | 22 | 47.6 | 0.264 | 90.2 |
| 2 | 2 | 20 | 53.7 | 0.286 | 85.7 |
| 3 | 2 | 24 | 44.8 | 0.256 | 92.6 |
| 4 | 3 | 19.1716 | 62.8 | 0.313 | 58.9 |
| 5 | 3 | 22 | 50.8 | 0.277 | 87.8 |
| 6 | 3 | 22 | 50.8 | 0.277 | 87.8 |
| 7 | 3 | 22 | 50.8 | 0.277 | 87.7 |
| 8 | 3 | 22 | 50.8 | 0.277 | 87.9 |
| 9 | 3 | 22 | 50.8 | 0.277 | 87.9 |
| 10 | 3 | 24.8284 | 44.3 | 0.254 | 92.8 |
| 11 | 4 | 20 | 65.9 | 0.329 | 55.6 |
| 12 | 4 | 24 | 47.3 | 0.265 | 90.2 |
| 13 | 4.41421 | 22 | 60.1 | 0.305 | 59 |
| Source | Adjusted R2 | Predicted R2 | SD | CV % | Model Suggested |
|---|---|---|---|---|---|
| R1 (particle size) | 0.9919 | 0.9664 | 0.6015 | 1.15 | Quadratic |
| R2 (PDI) | 0.9947 | 0.9778 | 0.0016 | 0.5766 | Quadratic |
| R3 (% Transmittance) | 0.9335 | 0.7244 | 3.57 | 4.37 | Quadratic |
| Independent Variable | Levels | ||||
|---|---|---|---|---|---|
| Axial (−2) | Low (−1) | Medium (0) | High (+1) | Axial (+2) | |
| A= Oil (%, v/v) | 1.58 | 2 | 3 | 4 | 4.41 |
| B = Smix (%, v/v) | 19.17 | 20 | 22 | 24 | 24.82 |
| Constraint | Importance | ||||
| Independent variable | |||||
| A = Oil (%, v/v) | In range | +++ | |||
| B = Smix (%, v/v) | In range | +++ | |||
| Dependent variable | +++ | ||||
| Y1 = Particle Size (nm) | Minimum | +++ | |||
| Y2 = PDI | Minimum | +++ | |||
| Y3 = Percentage Transmittance | Maximum | +++ | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pathak, S.; Singh, R.; Hussain, A.; Siddiqui, N.A.; Mittal, S.; Gupta, A. QbD Approach for the Development of Tea Tree Oil-Enhanced Microemulgel Loaded with Curcumin and Diclofenac for Rheumatoid Arthritis Treatment. Gels 2024, 10, 634. https://doi.org/10.3390/gels10100634
Pathak S, Singh R, Hussain A, Siddiqui NA, Mittal S, Gupta A. QbD Approach for the Development of Tea Tree Oil-Enhanced Microemulgel Loaded with Curcumin and Diclofenac for Rheumatoid Arthritis Treatment. Gels. 2024; 10(10):634. https://doi.org/10.3390/gels10100634
Chicago/Turabian StylePathak, Shivam, Ruhi Singh, Afzal Hussain, Nasir Ali Siddiqui, Saurabh Mittal, and Annie Gupta. 2024. "QbD Approach for the Development of Tea Tree Oil-Enhanced Microemulgel Loaded with Curcumin and Diclofenac for Rheumatoid Arthritis Treatment" Gels 10, no. 10: 634. https://doi.org/10.3390/gels10100634
APA StylePathak, S., Singh, R., Hussain, A., Siddiqui, N. A., Mittal, S., & Gupta, A. (2024). QbD Approach for the Development of Tea Tree Oil-Enhanced Microemulgel Loaded with Curcumin and Diclofenac for Rheumatoid Arthritis Treatment. Gels, 10(10), 634. https://doi.org/10.3390/gels10100634






