In Situ Hydrogel Formulation for Advanced Wound Dressing: Influence of Co-Solvents and Functional Excipient on Tailored Alginate–Pectin–Chitosan Blend Gelation Kinetics, Adhesiveness, and Performance
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Powder Production
4.1.1. Alginate, Pectin, and Chitosan Aqueous Feed
- Chitosan was dissolved in an acidic aqueous solution (1% w/w CH3COOH), at room temperature, under a gentle stirrer overnight.
- Pectin was dispersed in an aqueous solution where it was solubilized using alginate, at room temperature, under a stirrer producing the alginate-pectin (AP) solution.
4.1.2. Alginate, Pectin, and Chitosan with Co-Solvent Feed
4.1.3. Alginate, Pectin, and Chitosan with Functional Excipients
4.1.4. Microencapsulation Process
4.2. Morphology and Particle Size Distribution
4.3. Fluid Uptake Ability
4.4. Rheological Studies
4.5. Adhesion Strength Measurement
4.6. Water Vapor Transmission Rate and Rate of Water Evaporation from the Hydrogels Formed In Situ
4.7. Differential Scanning Calorimetry (DSC)
4.8. Fourier Transform Infrared Spectroscopy (FT-IR)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, B.K.; Siprashvili, Z.; Khavari, P.A. Advances in skin grafting and treatment of cutaneous wounds. Science 2014, 346, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Kumar, D.; Gopalkrishnan, A.; Pathak, N.N.; Kurade, N.P.; Tandan, S.K.; Kumar, D. Topical pluronic F-127 gel application enhances cutaneous wound healing in rats. Acta Histochem. 2014, 116, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Rapp, B.E. Chapter 9—Fluids. In Microfluidics: Modelling, Mechanics and Mathematics; Rapp, B.E., Ed.; Elsevier: Oxford, UK, 2017; pp. 243–263. [Google Scholar]
- Seavey, J.G.; Masters, Z.A.; Balazs, G.C.; Tintle, S.M.; Sabino, J.; Fleming, M.E.; Valerio, I.L. Use of a bioartificial dermal regeneration template for skin restoration in combat casualty injuries. Regen. Med. 2016, 11, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Aramwit, P. 1—Introduction to biomaterials for wound healing. In Wound Healing Biomaterials; Ågren, M.S., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 3–38. [Google Scholar]
- Xue, J.; Wang, X.; Wang, E.; Li, T.; Chang, J.; Wu, C. Bioinspired multifunctional biomaterials with hierarchical microstructure for wound dressing. Acta Biomater. 2019, 100, 270–279. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Gong, C.; Wu, Q.; Wang, Y.; Zhang, D.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. A biodegradable hydrogel system containing curcumin encapsulated in micelles for cutaneous wound healing. Biomaterials 2013, 34, 6377–6387. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, B.; Wang, J.; Zhang, S.; Lin, Q.; Gong, P.; Ma, L.; Yang, S. A Novel Wound Dressing Based on Ag/Graphene Polymer Hydrogel: Effectively Kill Bacteria and Accelerate Wound Healing. Adv. Funct. Mater. 2014, 24, 3933–3943. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, B.; Wu, H.; Liang, Y.; Ma, P.X. Injectable antibacterial conductive nanocomposite cryogels with rapid shape recovery for noncompressible hemorrhage and wound healing. Nat. Commun. 2018, 9, 2784. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Ribeiro, M.P.; Brancal, H.; Coutinho, P.; Correia, I.J. Thermoresponsive chitosan–agarose hydrogel for skin regeneration. Carbohydr. Polym. 2014, 111, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Benbow, M. Best practice in wound assessment. Nurs. Stand. 2016, 30, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Cutting, K.F. Wound exudate: Composition and functions. Br. J. Community Nurs. 2003, 8, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Ski. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Baker, A.B. Biomaterials and Nanotherapeutics for Enhancing Skin Wound Healing. Front. Bioeng. Biotechnol. 2016, 4, 82. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.M.; Campelo, M.d.S.; Câmara Neto, J.F.; Lima, A.B.N.; Silva, G.d.A.; Dias, A.T.d.F.F.; Ricardo, N.M.P.S.; Kaplan, D.L.; Ribeiro, M.E.N.P. Alginate/polyvinyl alcohol films for wound healing: Advantages and challenges. J. Biomed. Mater. Res. Part B Appl. Biomater. 2023, 111, 220–233. [Google Scholar] [CrossRef]
- Heher, P.; Mühleder, S.; Mittermayr, R.; Redl, H.; Slezak, P. Fibrin-based delivery strategies for acute and chronic wound healing. Adv. Drug Deliv. Rev. 2018, 129, 134–147. [Google Scholar] [CrossRef]
- Ehterami, A.; Salehi, M.; Farzamfar, S.; Samadian, H.; Vaez, A.; Ghorbani, S.; Ai, J.; Sahrapeyma, H. Chitosan/alginate hydrogels containing Alpha-tocopherol for wound healing in rat model. J. Drug Deliv. Sci. Technol. 2019, 51, 204–213. [Google Scholar] [CrossRef]
- Abbas, M.; Hussain, T.; Arshad, M.; Ansari, A.R.; Irshad, A.; Nisar, J.; Hussain, F.; Masood, N.; Nazir, A.; Iqbal, M. Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int. J. Biol. Macromol. 2019, 140, 871–876. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Xu, F.-J. Versatile Functionalization of Polysaccharides via Polymer Grafts: From Design to Biomedical Applications. Acc. Chem. Res. 2017, 50, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Auriemma, G.; Russo, P.; Del Gaudio, P.; García-González, C.A.; Landín, M.; Aquino, R.P. Technologies and Formulation Design of Polysaccharide-Based Hydrogels for Drug Delivery. Molecules 2020, 25, 3156. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. Alginate dressings in surgery and wound management—Part 1. J. Wound Care 2000, 9, 56–60. [Google Scholar] [CrossRef]
- Giri, T.K.; Thakur, D.; Alexander, A.; Ajazuddin; Badwaik, H.; Tripathi, D.K. Alginate based hydrogel as a potential biopolymeric carrier for drug delivery and cell delivery systems: Present status and applications. Curr. Drug Deliv. 2012, 9, 539–555. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, M.R.; Amante, C.; Aquino, R.P.; Russo, P.; Rodríguez-Dorado, R.; Neagu, M.; García-González, C.A.; Adami, R.; Del Gaudio, P. Hollow Particles Obtained by Prilling and Supercritical Drying as a Potential Conformable Dressing for Chronic Wounds. Gels 2023, 9, 492. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chen, H.H.; Chang, S.H.; Ni, T.S. Pectin-chitosan-PVA nanofibrous scaffold made by electrospinning and its potential use as a skin tissue scaffold. J. Biomater. Sci. Polym. Ed. 2013, 24, 470–484. [Google Scholar] [CrossRef]
- De Cicco, F.; Reverchon, E.; Adami, R.; Auriemma, G.; Russo, P.; Calabrese, E.C.; Porta, A.; Aquino, R.P.; Del Gaudio, P. In situ forming antibacterial dextran blend hydrogel for wound dressing: SAA technology vs. spray drying. Carbohydr. Polym. 2014, 101, 1216–1224. [Google Scholar] [CrossRef]
- Xu, J.; Strandman, S.; Zhu, J.X.X.; Barralet, J.; Cerruti, M. Genipin-crosslinked catechol-chitosan mucoadhesive hydrogels for buccal drug delivery. Biomaterials 2015, 37, 395–404. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef] [PubMed]
- Moeini, A.; Pedram, P.; Makvandi, P.; Malinconico, M.; Gomez d’Ayala, G. Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: A review. Carbohydr. Polym. 2020, 233, 115839. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Liow, S.S.; Dou, Q.; Kai, D.; Li, Z.; Sugiarto, S.; Yu, C.Y.Y.; Kwok, R.T.K.; Chen, X.; Wu, Y.-L.; Ong, S.T.; et al. Long-Term Real-Time In Vivo Drug Release Monitoring with AIE Thermogelling Polymer. Small 2017, 13, 1603404. [Google Scholar] [CrossRef] [PubMed]
- Paomephan, P.; Assavanig, A.; Chaturongakul, S.; Cady, N.C.; Bergkvist, M.; Niamsiri, N. Insight into the antibacterial property of chitosan nanoparticles against Escherichia coli and Salmonella Typhimurium and their application as vegetable wash disinfectant. Food Control 2018, 86, 294–301. [Google Scholar] [CrossRef]
- Amante, C.; Andretto, V.; Rosso, A.; Augusti, G.; Marzocco, S.; Lollo, G.; Del Gaudio, P. Alginate-pectin microparticles loaded with nanoemulsions as nanocomposites for wound healing. Drug Deliv. Transl. Res. 2023, 13, 1343–1357. [Google Scholar] [CrossRef] [PubMed]
- Gull, N.; Khan, S.M.; Zahid Butt, M.T.; Khalid, S.; Shafiq, M.; Islam, A.; Asim, S.; Hafeez, S.; Khan, R.U. In vitro study of chitosan-based multi-responsive hydrogels as drug release vehicles: A preclinical study. RSC Adv. 2019, 9, 31078–31091. [Google Scholar] [CrossRef]
- Amante, C.; Esposito, T.; Del Gaudio, P.; Di Sarno, V.; Porta, A.; Tosco, A.; Russo, P.; Nicolais, L.; Aquino, R.P. A Novel Three-Polysaccharide Blend In Situ Gelling Powder for Wound Healing Applications. Pharmaceutics 2021, 13, 1680. [Google Scholar] [CrossRef]
- Wan, F.; Bohr, A.; Maltesen, M.J.; Bjerregaard, S.; Foged, C.; Rantanen, J.; Yang, M. Critical Solvent Properties Affecting the Particle Formation Process and Characteristics of Celecoxib-Loaded PLGA Microparticles via Spray-Drying. Pharm. Res. 2013, 30, 1065–1076. [Google Scholar] [CrossRef]
- Wang, Z.; Ordoubadi, M.; Wang, H.; Vehring, R. Morphology and formation of crystalline leucine microparticles from a co-solvent system using multi-orifice monodisperse spray drying. Aerosol Sci. Technol. 2021, 55, 901–919. [Google Scholar] [CrossRef]
- Cal, K.; Sollohub, K. Spray Drying Technique. I: Hardware and Process Parameters. J. Pharm. Sci. 2010, 99, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Nandiyanto, A.B.D.; Okuyama, K. Progress in developing spray-drying methods for the production of controlled morphology particles: From the nanometer to submicrometer size ranges. Adv. Powder Technol. 2011, 22, 1–19. [Google Scholar] [CrossRef]
- Kulig, D.; Zimoch-Korzycka, A.; Jarmoluk, A.; Marycz, K. Study on Alginate–Chitosan Complex Formed with Different Polymers Ratio. Polymers 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Vehring, R.; Foss, W.R.; Lechuga-Ballesteros, D. Particle formation in spray drying. J. Aerosol Sci. 2007, 38, 728–746. [Google Scholar] [CrossRef]
- Ji, S.; Thulstrup, P.; Mu, H.; Hansen, S.; van de Weert, M.; Rantanen, J.; Yang, M. Effect of ethanol as a co-solvent on the aerosol performance and stability of spray-dried lysozyme. Int. J. Pharm. 2016, 513, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Bazunova, M.V.; Shurshina, A.S.; Chernova, V.V.; Kulish, E.I. A rheological study of molecular and supramolecular organization of chitosan succinamide in a mixed water–etanol solvent. Russ. J. Phys. Chem. B 2016, 10, 1014–1021. [Google Scholar] [CrossRef]
- Hermansson, E.; Schuster, E.; Lindgren, L.; Altskär, A.; Ström, A. Impact of solvent quality on the network strength and structure of alginate gels. Carbohydr. Polym. 2016, 144, 289–296. [Google Scholar] [CrossRef]
- Renuka, M.; Nishadh, P.; Shah, J.; Tejal, M. Mucoadhesive wound healing film of Doxycycline Hydrochloride. Int. J. Drug Dev. Res. 2012, 4, 128–140. [Google Scholar]
- Romić, M.D.; Klarić, M.Š.; Lovrić, J.; Pepić, I.; Cetina-Čižmek, B.; Filipović-Grčić, J.; Hafner, A. Melatonin-loaded chitosan/Pluronic® F127 microspheres as in situ forming hydrogel: An innovative antimicrobial wound dressing. Eur. J. Pharm. Biopharm. 2016, 107, 67–79. [Google Scholar] [CrossRef]
- Xu, R.; Xia, H.; He, W.; Li, Z.; Zhao, J.; Liu, B.; Wang, Y.; Lei, Q.; Kong, Y.; Bai, Y. Controlled water vapor transmission rate promotes wound-healing via wound re-epithelialization and contraction enhancement. Sci. Rep. 2016, 6, 24596. [Google Scholar] [CrossRef]
- Lin, N.; Zuo, B. Silk sericin/fibroin electrospinning dressings: A method for preparing a dressing material with high moisture vapor transmission rate. J. Biomater. Sci. Polym. Ed. 2021, 32, 1983–1997. [Google Scholar] [CrossRef] [PubMed]
- Mladenovska, K.; Cruaud, O.; Richomme, P.; Belamie, E.; Raicki, R.S.; Venier-Julienne, M.C.; Popovski, E.; Benoit, J.P.; Goracinova, K. 5-ASA loaded chitosan–Ca–alginate microparticles: Preparation and physicochemical characterization. Int. J. Pharm. 2007, 345, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Drożdż, E.; Stachura, M.; Wyrwa, J.; Rekas, M. Effect of the addition of pore former: Graphite and ammonium bicarbonate on the properties of Ni/Al2O3–3YSZ composite materials. J. Therm. Anal. Calorim. 2015, 122, 157–166. [Google Scholar] [CrossRef]
- Zeng, L.; Qin, C.; Wang, L.; Li, W. Volatile compounds formed from the pyrolysis of chitosan. Carbohydr. Polym. 2011, 83, 1553–1557. [Google Scholar] [CrossRef]
- Wahba, M.I. Sodium bicarbonate-gelled chitosan beads as mechanically stable carriers for the covalent immobilization of enzymes. Biotechnol. Prog. 2018, 34, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Del Gaudio, P.; Amante, C.; Civale, R.; Bizzarro, V.; Petrella, A.; Pepe, G.; Campiglia, P.; Russo, P.; Aquino, R.P. In situ gelling alginate-pectin blend particles loaded with Ac2-26: A new weapon to improve wound care armamentarium. Carbohydr. Polym. 2020, 227, 115305. [Google Scholar] [CrossRef]
- Assaad, E.; Maire, M.; Lerouge, S. Injectable thermosensitive chitosan hydrogels with controlled gelation kinetics and enhanced mechanical resistance. Carbohydr. Polym. 2015, 130, 87–96. [Google Scholar] [CrossRef]
- Tang, Y.; Wang, X.; Li, Y.; Lei, M.; Du, Y.; Kennedy, J.F.; Knill, C.J. Production and characterisation of novel injectable chitosan/methylcellulose/salt blend hydrogels with potential application as tissue engineering scaffolds. Carbohydr. Polym. 2010, 82, 833–841. [Google Scholar] [CrossRef]
- Falk, M.; Miller, A.G. Infrared spectrum of carbon dioxide in aqueous solution. Vib. Spectrosc. 1992, 4, 105–108. [Google Scholar] [CrossRef]
- Montembault, A.; Viton, C.; Domard, A. Rheometric study of the gelation of chitosan in aqueous solution without cross-linking agent. Biomacromolecules 2005, 6, 653–662. [Google Scholar] [CrossRef]
- Mazzoli, A.; Favoni, O. Particle size, size distribution and morphological evaluation of airborne dust particles of diverse woods by Scanning Electron Microscopy and image processing program. Powder Technol. 2012, 225, 65–71. [Google Scholar] [CrossRef]
- Bowler, P.G.; Welsby, S.; Towers, V.; Booth, R.; Hogarth, A.; Rowlands, V.; Joseph, A.; Jones, S.A. Multidrug-resistant organisms, wounds and topical antimicrobial protection. Int. Wound J. 2012, 9, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Said, J.; Dodoo, C.C.; Walker, M.; Parsons, D.; Stapleton, P.; Beezer, A.E.; Gaisford, S. An in vitro test of the efficacy of silver-containing wound dressings against Staphylococcus aureus and Pseudomonas aeruginosa in simulated wound fluid. Int. J. Pharm. 2014, 462, 123–128. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, F.; Porta, A.; Sansone, F.; Aquino, R.P.; Del Gaudio, P. Nanospray technology for an in situ gelling nanoparticulate powder as a wound dressing. Int. J. Pharm. 2014, 473, 30–37. [Google Scholar] [CrossRef]
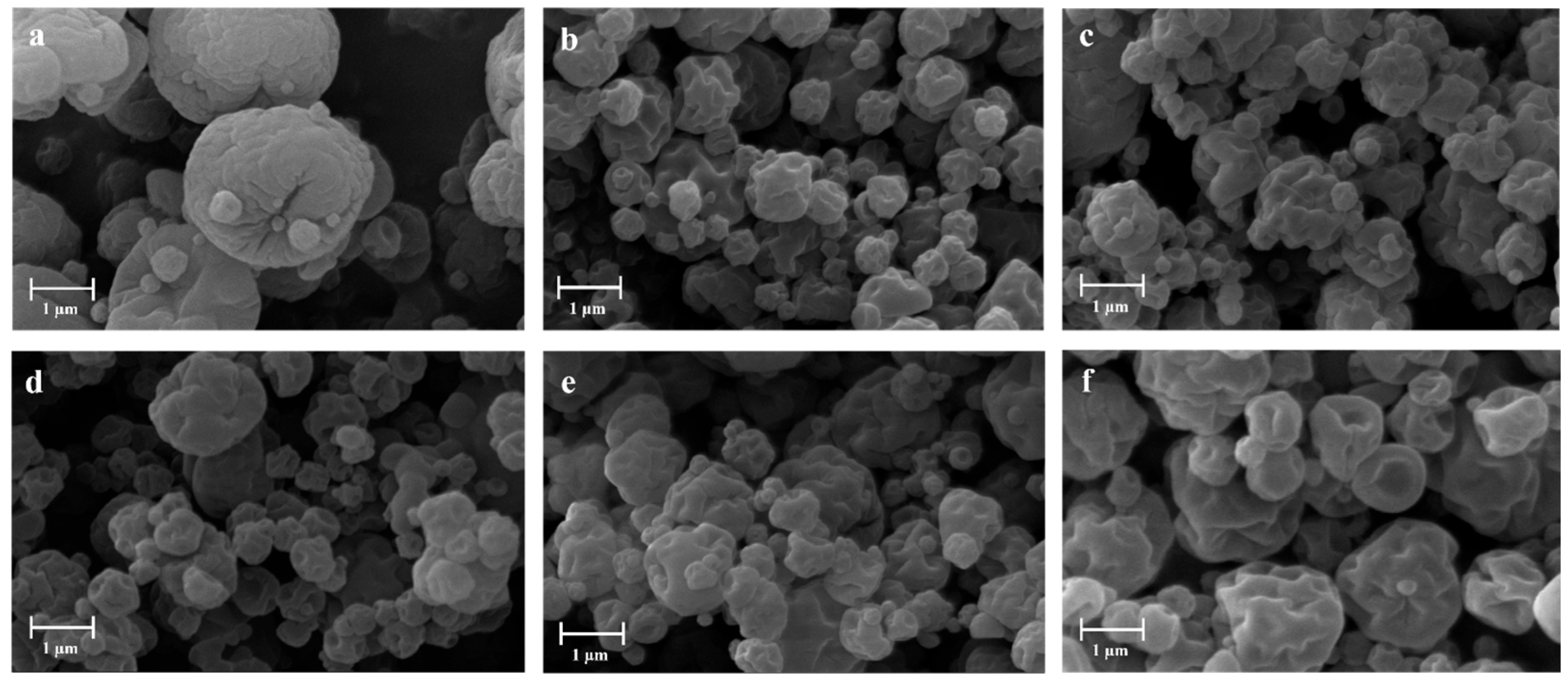
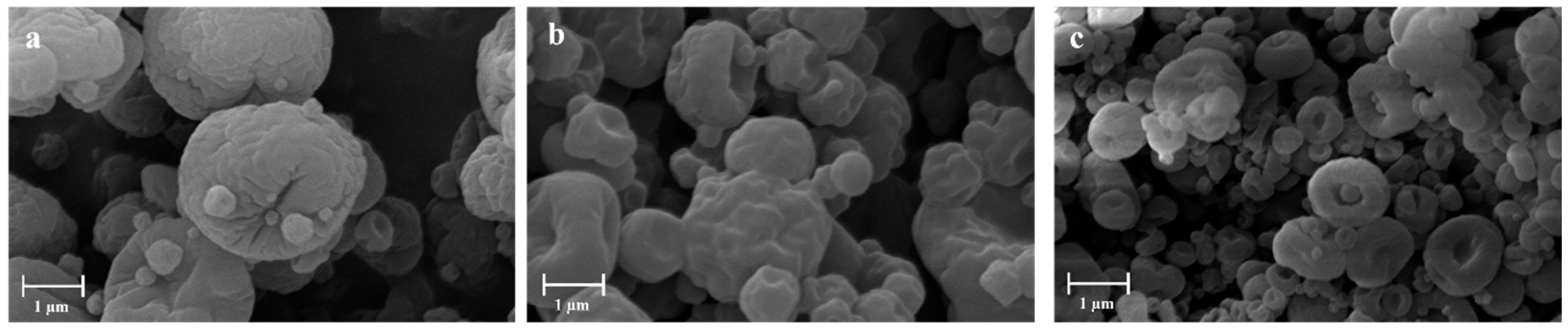
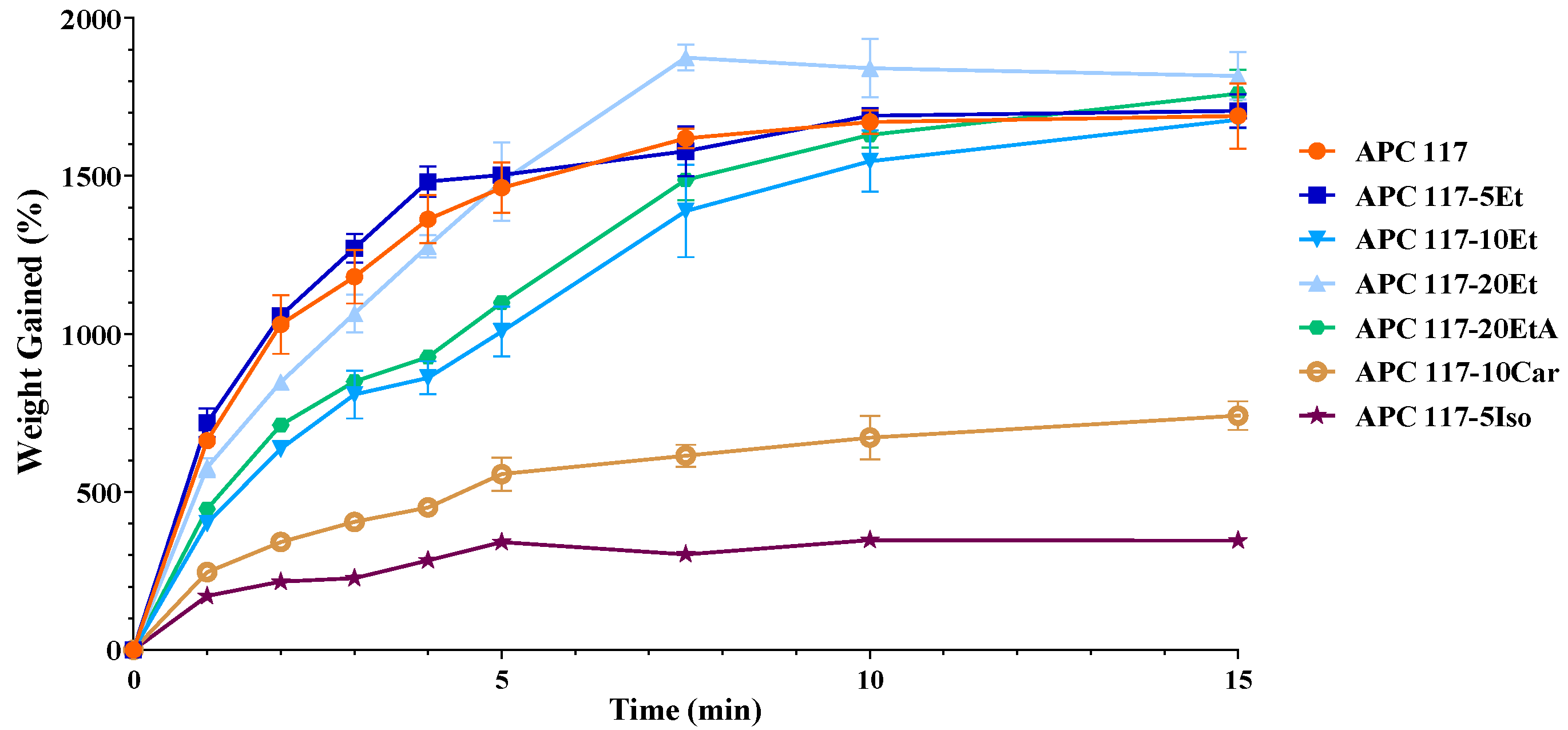
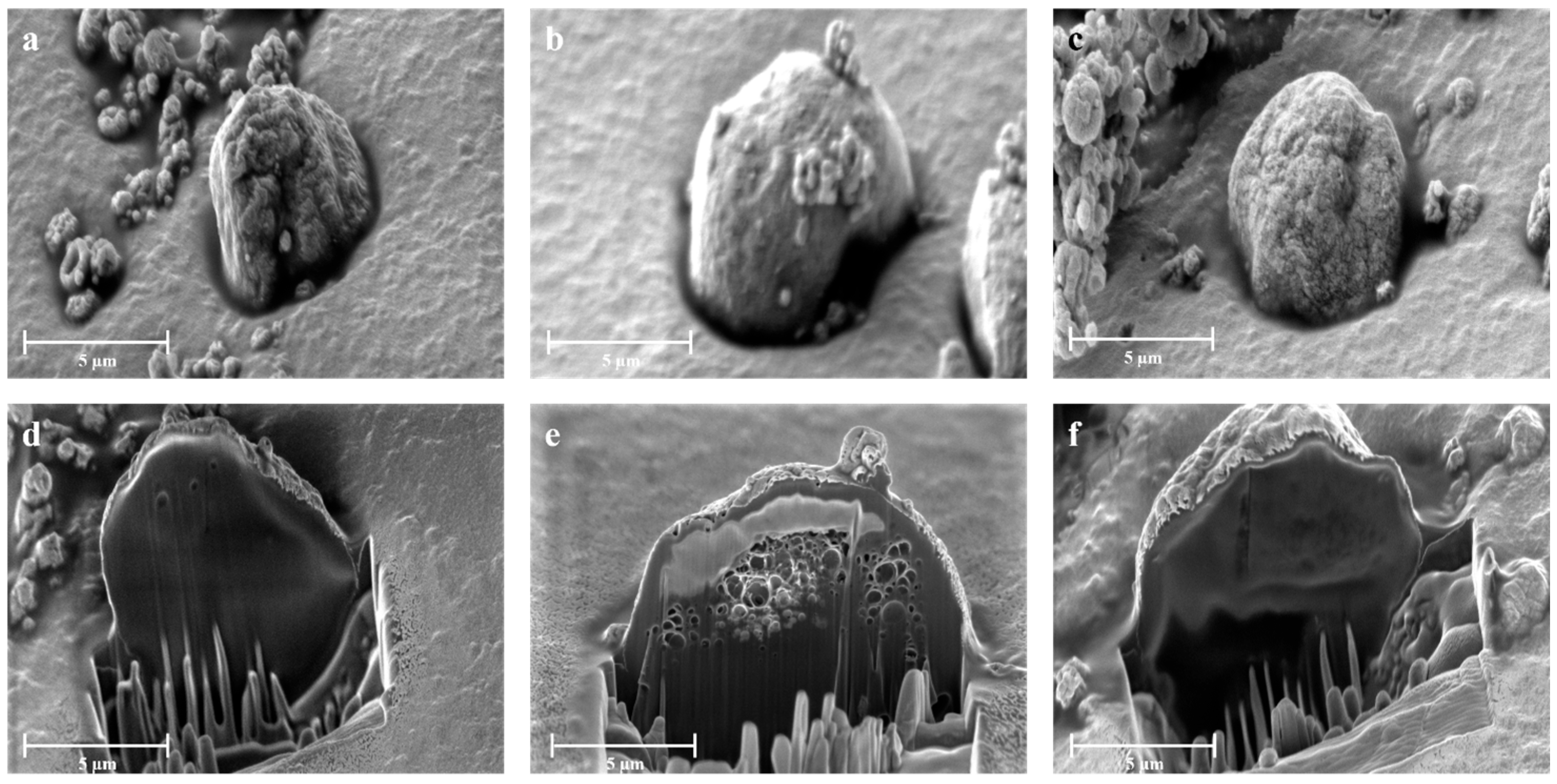
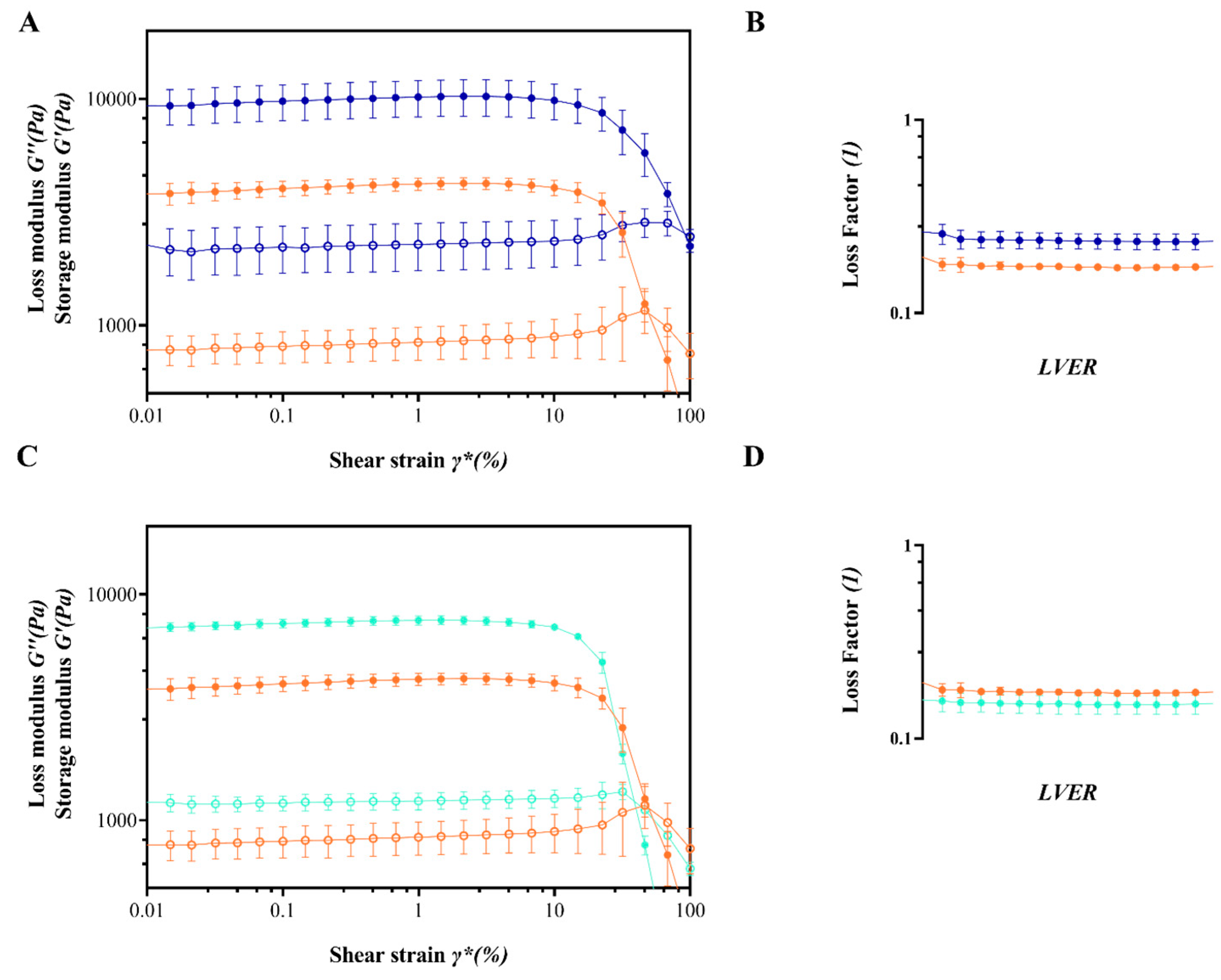



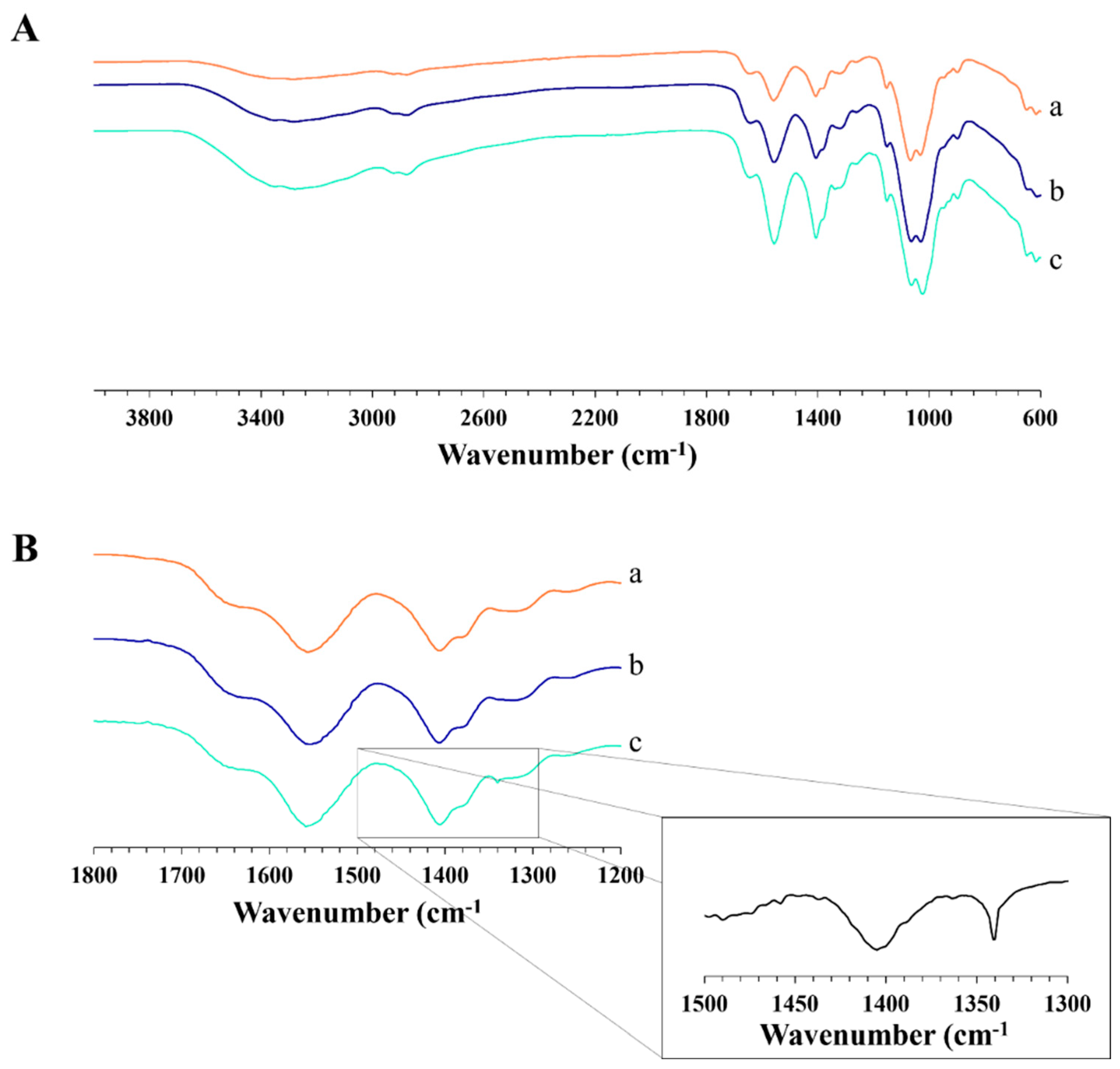
| Sample | H2O—Co-Solvent Ratio | Salt Concentration (w/w) | Process Yield (%) | Mean Diameter (µm) ± SD |
|---|---|---|---|---|
| APC 117 | 100:00 | - | 61.5 | 3.65 ± 0.01 |
| APC 117-5Et | 95:5 Ethanol | - | 55.8 | 5.05 ± 0.06 |
| APC 117-10Et | 90:10 Ethanol | - | 55.8 | 4.09 ± 0.02 |
| APC 117-20Et | 80:20 Ethanol | - | 45.7 | 4.80 ± 0.19 |
| APC 117-20EtA | 80:10:10 Ethanol/Acetone | - | 55.6 | 2.91 ± 0.01 |
| APC 117-5Iso | 95:5 Isopropanol | - | 50.3 | 3.27 ± 0.05 |
| APC 117-10Bic | 100:00 | 10% Sodium Bicarbonate | 64.1 | 2.89 ± 0.11 |
| APC 117-10Car | 100:00 | 10% Ammonium Carbonate | 59.3 | 2.11 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amante, C.; Falcone, G.; Aquino, R.P.; Russo, P.; Nicolais, L.; Del Gaudio, P. In Situ Hydrogel Formulation for Advanced Wound Dressing: Influence of Co-Solvents and Functional Excipient on Tailored Alginate–Pectin–Chitosan Blend Gelation Kinetics, Adhesiveness, and Performance. Gels 2024, 10, 3. https://doi.org/10.3390/gels10010003
Amante C, Falcone G, Aquino RP, Russo P, Nicolais L, Del Gaudio P. In Situ Hydrogel Formulation for Advanced Wound Dressing: Influence of Co-Solvents and Functional Excipient on Tailored Alginate–Pectin–Chitosan Blend Gelation Kinetics, Adhesiveness, and Performance. Gels. 2024; 10(1):3. https://doi.org/10.3390/gels10010003
Chicago/Turabian StyleAmante, Chiara, Giovanni Falcone, Rita P. Aquino, Paola Russo, Luigi Nicolais, and Pasquale Del Gaudio. 2024. "In Situ Hydrogel Formulation for Advanced Wound Dressing: Influence of Co-Solvents and Functional Excipient on Tailored Alginate–Pectin–Chitosan Blend Gelation Kinetics, Adhesiveness, and Performance" Gels 10, no. 1: 3. https://doi.org/10.3390/gels10010003
APA StyleAmante, C., Falcone, G., Aquino, R. P., Russo, P., Nicolais, L., & Del Gaudio, P. (2024). In Situ Hydrogel Formulation for Advanced Wound Dressing: Influence of Co-Solvents and Functional Excipient on Tailored Alginate–Pectin–Chitosan Blend Gelation Kinetics, Adhesiveness, and Performance. Gels, 10(1), 3. https://doi.org/10.3390/gels10010003