Preparation and Characterization of Amphoteric Cellulose Hydrogels as Adsorbents for the Anionic Dyes in Aqueous Solutions
Abstract
:1. Introduction

2. Results and Discussion
2.1. Preparation and Structural Characterization of CAms
| Sample | Initial Feed Amount | DSA | DSC | Yields/g (%) | |
|---|---|---|---|---|---|
| CMC/g (mmol) a | EPTMAC/g (mmol) | ||||
| CAm 1 | 12.0 (55.5) | 16.8 (111) | 0.68 | 0.41 | 13.5 (87) |
| CAm 2 | 12.0 (55.5) | 33.6 (222) | 0.68 | 0.79 | 16.9 (91) |
| CAm 3 | 12.0 (55.5) | 67.3 (444) | 0.68 | 1.08 | 18.0 (85) |
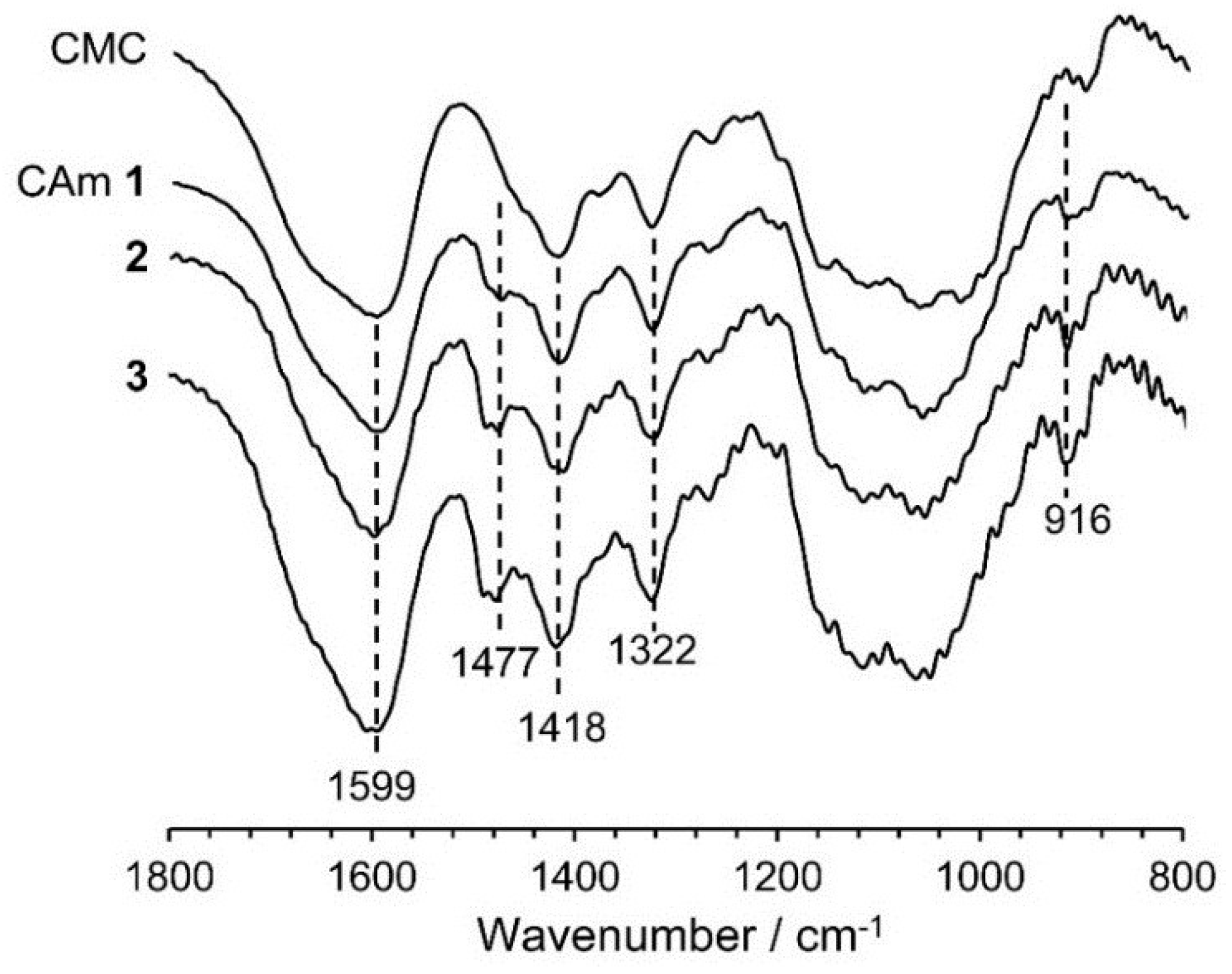
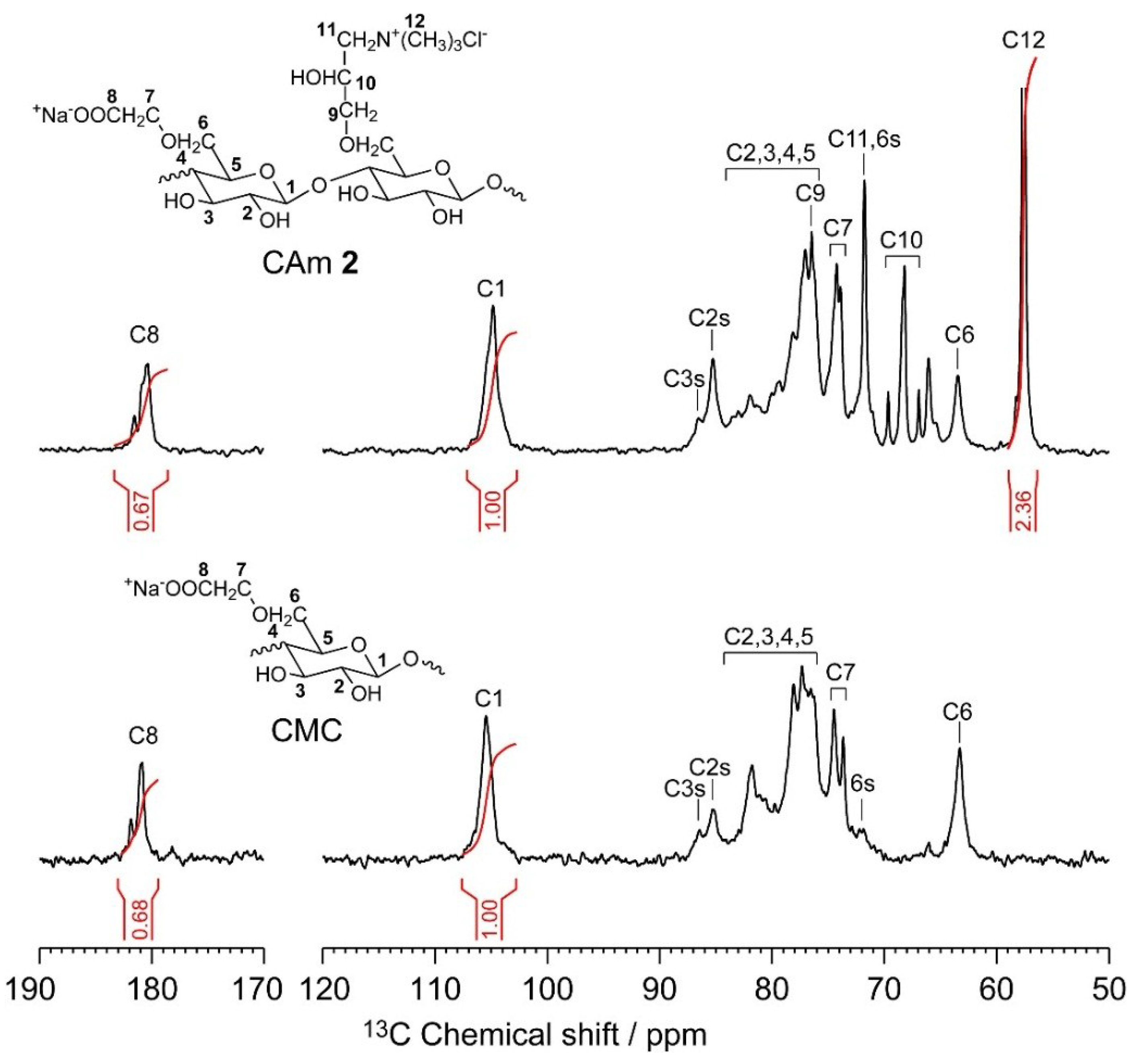
2.2. Preparation and Structural Characterization of CAmGs

| Sample | Initial Feed Amount | CD | Yields/g (%) | ||||
|---|---|---|---|---|---|---|---|
| CAm 1/g (mmol) a | CAm 2/g (mmol) a | CAm 3/g (mmol) a | CMC/g (mmol) a | EGDE/g (mmol) | |||
| CAmG 1 | 5.0 (18.0) | 3.3 (19.2) | 0.27 | 4.8 (82) | |||
| CAmG 2 | 5.0 (14.9) | 3.3 (19.2) | 0.24 | 4.9 (87) | |||
| CAmG 3 | 5.0 (13.2) | 3.3 (19.2) | 0.17 | 4.8 (89) | |||
| CMCG | 5.0 (23.1) | 3.3 (19.2) | 0.30 | 5.1 (82) | |||
2.3. Water Absorbency of CAmGs
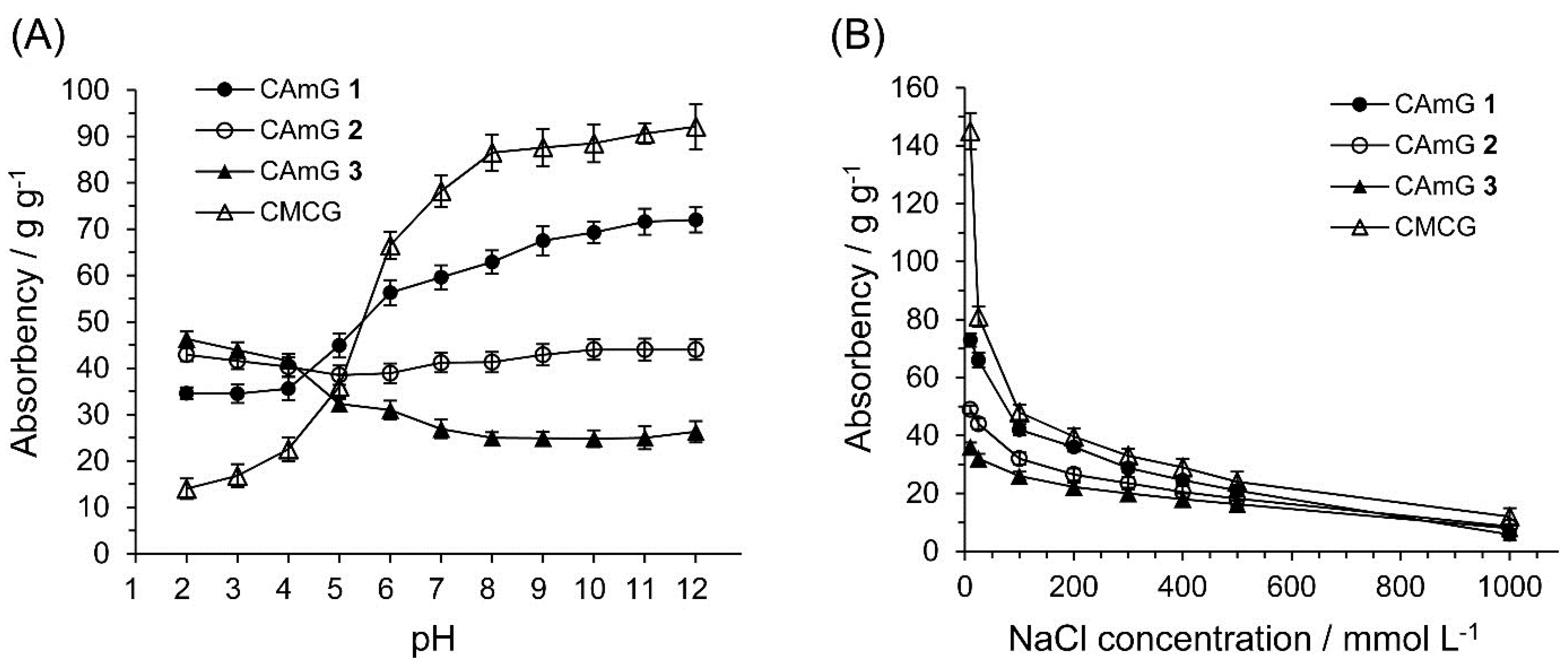
2.4. Adsorption of Anionic Dyes onto CAmGs
2.4.1. Effects of pH and Cationic Degree of CAmGs

2.4.2. Effect of the Adsorbent Dosage

2.4.3. Adsorption Kinetics
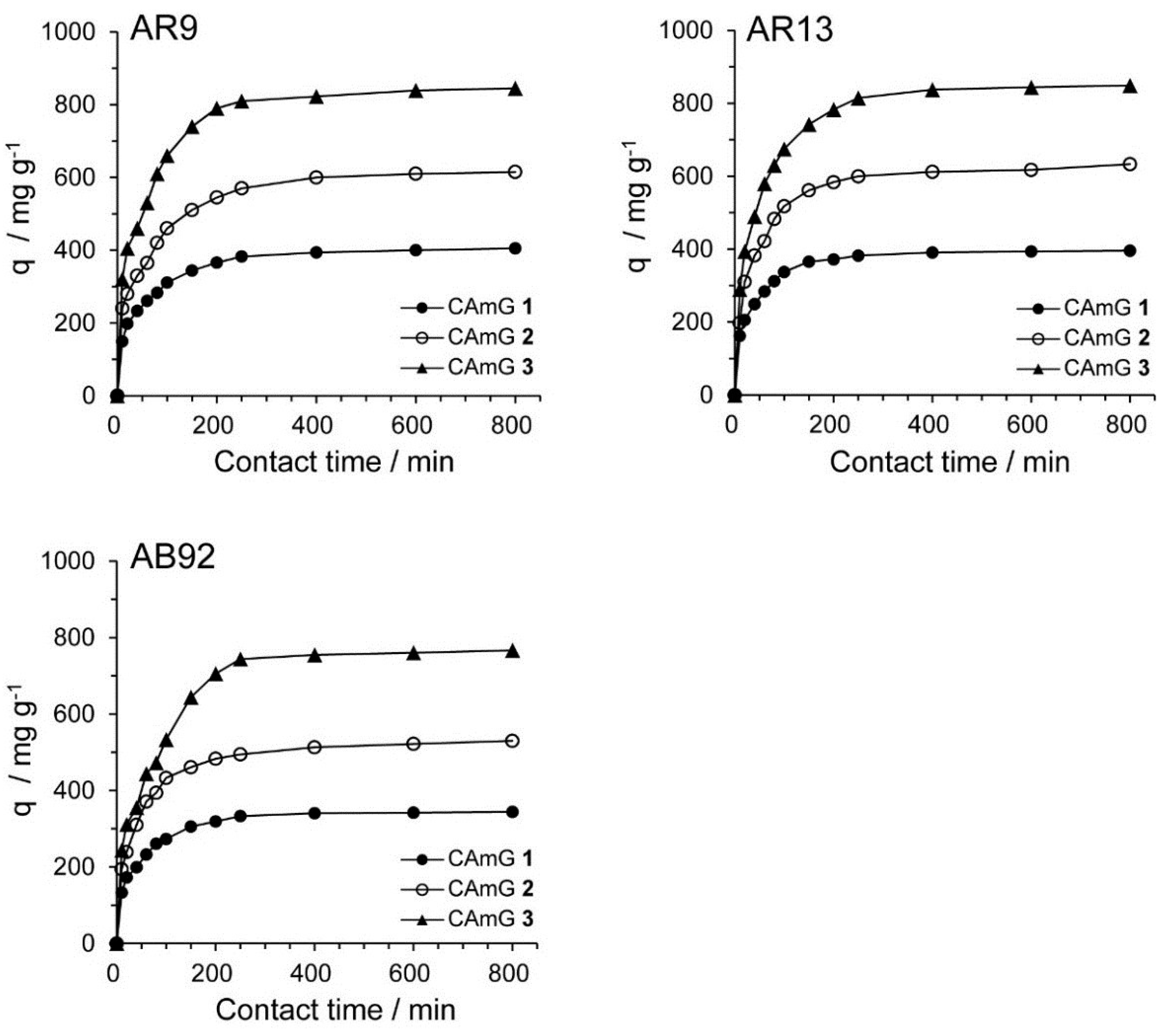

| Dye | Adsorbent | qe, t = 800 min a/mg·g−1 | k1/min−1 | qe,cal/mg·g−1 | R2 |
|---|---|---|---|---|---|
| AR9 | CAmG 1 | 405 | 0.00716 | 223 | 0.943 |
| CAmG 2 | 615 | 0.00775 | 386 | 0.980 | |
| CAmG 3 | 845 | 0.00796 | 469 | 0.943 | |
| AR13 | CAmG 1 | 396 | 0.00859 | 187 | 0.935 |
| CAmG 2 | 633 | 0.00613 | 291 | 0.842 | |
| CAmG 3 | 849 | 0.00857 | 477 | 0.955 | |
| AB92 | CAmG 1 | 344 | 0.00877 | 185 | 0.941 |
| CAmG 2 | 530 | 0.00666 | 266 | 0.918 | |
| CAmG 3 | 767 | 0.00859 | 503 | 0.931 |
| Dye | Adsorbent | qe, t = 800 min a/mg·g−1 | k2/g mg−1·min−1 | qe,cal/mg·g−1 | R2 |
|---|---|---|---|---|---|
| AR9 | CAmG 1 | 405 | 8.32 × 10−5 | 411 | 0.999 |
| CAmG 2 | 615 | 4.79 × 10−5 | 621 | 0.998 | |
| CAmG 3 | 845 | 4.15 × 10−5 | 850 | 0.999 | |
| AR13 | CAmG 1 | 396 | 1.23 × 10−5 | 398 | 1.000 |
| CAmG 2 | 633 | 6.35 × 10−5 | 644 | 1.000 | |
| CAmG 3 | 849 | 4.36 × 10−5 | 852 | 0.999 | |
| AB92 | CAmG 1 | 344 | 1.15 × 10−5 | 346 | 0.999 |
| CAmG 2 | 530 | 7.34 × 10−5 | 532 | 1.000 | |
| CAmG 3 | 767 | 3.28 × 10−5 | 772 | 0.997 |
2.4.4. Adsorption Isotherms
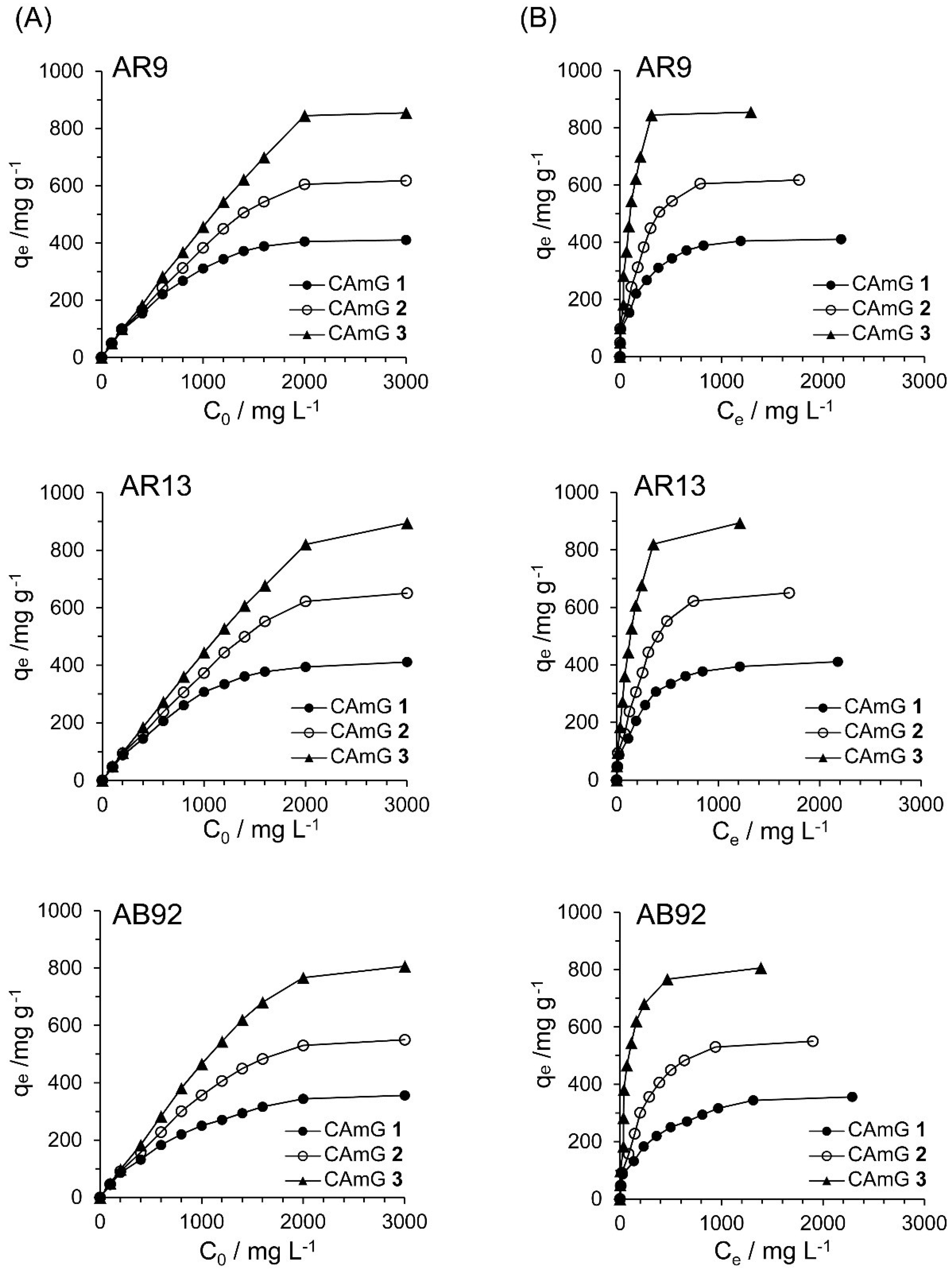
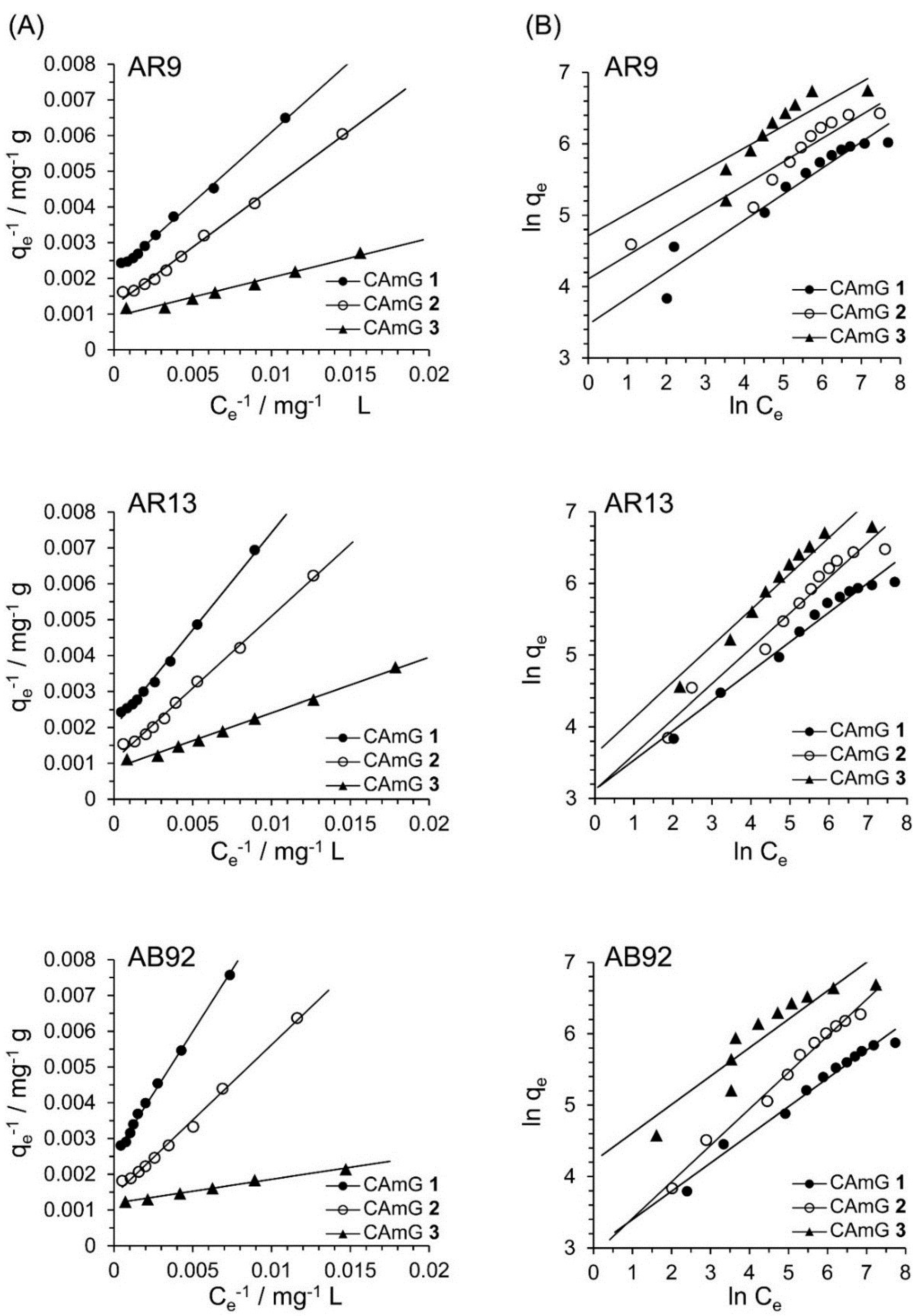
| Dye | Adsorbent | Q0/mg·g−1 | b/mg−1·L | RL | R2 |
|---|---|---|---|---|---|
| AR9 | CAmG 1 | 419 | 0.00604 | 0.0523 | 0.997 |
| CAmG 2 | 644 | 0.00474 | 0.0658 | 0.996 | |
| CAmG 3 | 866 | 0.01051 | 0.0307 | 0.976 | |
| AR13 | CAmG 1 | 410 | 0.00450 | 0.0690 | 0.996 |
| CAmG 2 | 651 | 0.00383 | 0.0801 | 0.996 | |
| CAmG 3 | 911 | 0.00710 | 0.0448 | 0.994 | |
| AB92 | CAmG 1 | 371 | 0.00389 | 0.0789 | 0.997 |
| CAmG 2 | 561 | 0.00424 | 0.0729 | 0.996 | |
| CAmG 3 | 816 | 0.01831 | 0.0179 | 0.994 |
| Dye | Adsorbent | KF/mg·L−1 | n−1 | R2 |
|---|---|---|---|---|
| AR9 | CAmG 1 | 32.1 | 0.365 | 0.931 |
| CAmG 2 | 60.8 | 0.328 | 0.956 | |
| CAmG 3 | 111.4 | 0.307 | 0.866 | |
| AR13 | CAmG 1 | 22.7 | 0.412 | 0.968 |
| CAmG 2 | 22.6 | 0.493 | 0.961 | |
| CAmG 3 | 37.2 | 0.504 | 0.934 | |
| AB92 | CAmG 1 | 20.2 | 0.395 | 0.977 |
| CAmG 2 | 18.4 | 0.510 | 0.986 | |
| CAmG 3 | 67.5 | 0.398 | 0.844 |
2.4.5. Adsorption Mechanism
- The entire surface of the adsorbent is uniform, and all the adsorption sites are equivalent.
- There is no interaction between the adsorbed molecules.
- All adsorbate molecules absorb onto the adsorbent by the same mechanism.
- The adsorbate molecules adsorb only onto the surface of the adsorbent, not onto previously adsorbed molecules.
3. Conclusions
- The adsorption ability of a CAmG strongly depends on its DSC and on the pH of the adsorption medium: the maximum adsorption of anionic dyes occurred using CAmGs with higher DSC values, and under lower pH (less than 3) conditions.
- The adsorption of AR9, AR13, and AB13 onto the CAmGs occurs via a pseudo-second order kinetic mechanism.
- The adsorption isotherms of the three anionic dyes by the CAmGs could be well fitted by the Langmuir adsorption isotherm model rather than the Freundlich adsorption isotherm model, indicating that the adsorption of the anionic dyes onto the CAmGs predominantly occurred via the charge neutralization mechanism.
4. Experimental Section
4.1. Materials
4.2. Preparation of CAms
4.3. Preparation of CAmGs
4.4. FTIR Spectroscopy
4.5. NMR Spectroscopy
4.6. Water Absorbency
4.7. Dye Adsorption
Acknowledgments
Conflicts of Interest
References
- Sethuraman, V.V.; Raymahashay, B.C. Color removal by clays. Kinetic study of adsorption of cationic and anionic dyes. Environ. Sci. Technol. 1975, 9, 1139–1140. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.K.; Afroze, S.; Ang, H.M. Equilibrium, kinetics and mechanism of removal of methylene blue from aqueous solution by adsorption onto pine cone biomass of Pinus radiate. Water Air Soil Pollut. 2011, 218, 499–515. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Ang, H.M. Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves. Water Air Soil Pollut. 2012, 223, 5267–5282. [Google Scholar] [CrossRef]
- Fu, Y.; Viraraghavan, T. Fungal decolorization of dye waste waters: A review. Bioresour. Technol. 2001, 79, 251–262. [Google Scholar] [CrossRef]
- Lazar, T. Color chemistry: Synthesis, properties, and applications of organic dyes and pigments, 3rd revised edition. Color Res. Appl. 2005, 30, 313–314. [Google Scholar] [CrossRef]
- Kadirvelu, K.; Kavipriya, M.; Karthika, C.; Radhika, M.; Vennilamani, N.; Pattabhi, S. Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour. Technol. 2003, 87, 129–132. [Google Scholar] [CrossRef]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Sailaja, P.; Srimurali, M.; Karthikeyan, J. Color removal of monoazo acid dye from aqueous solution by adsorption and chemical coagulation. Environ. Eng. Policy 1998, 1, 149–154. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Lau, Y.Y.; Wong, Y.S.; Teng, T.T.; Morad, N.; Rafatullah, M.; Ong, S.A. Coagulation-flocculation of azo dye Acid Orange 7 with green refined laterite soil. Chem. Eng. J. 2014, 246, 383–390. [Google Scholar] [CrossRef]
- Galindo, C.; Jacques, P.; Kalt, A. Photochemical and photocatalytic degradation of an indigoid dye: A case study of acid blue 74 (AB74). J. Photochem. Photobiol. A Chem. 2001, 141, 47–56. [Google Scholar] [CrossRef]
- Malik, P.K. Dye removal from wastewater using activated carbon developed from sawdust: Adsorption equilibrium and kinetics. J. Hazard. Mat. 2004, 113, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Rocher, V.; Siaugue, J.M.; Cabuil, V.; Bee, A. Removal of organic dyes by magnetic alginate beads. Water Res. 2008, 42, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Bazmizeynabad, F.; Seyyedi, B. Kappa-Carrageenan beads as new adsorbent to remove crystal violet dye from water: Adsorption kinetics and isotherm. Desalination Water Treat. 2015, 53, 2529–2539. [Google Scholar] [CrossRef]
- Pavan, F.A.; Mazzocato, A.C.; Gushikem, Y. Removal of methylene blue dye from aqueous solutions by adsorption using yellow passion fruit peel as adsorbent. Bioresour. Technol. 2008, 99, 3162–3165. [Google Scholar] [CrossRef] [PubMed]
- Crini, G.; Badot, P.-M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Annadurai, G.; Juang, R.S.; Lee, D.J. Use of cellulose-based wastes for adsorption of dyes from aqueous solutions. J. Hazard. Mat. 2002, 92, 263–274. [Google Scholar] [CrossRef]
- Gupta, V.K.; Suhas. Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Shang, Y.; Lu, Y.; Chen, Y.; Huang, X.; Chen, A.; Jiang, Y.; Gu, W.; Qian, X.; Yang, H.; et al. Flocculation properties of biodegradable amphoteric chitosan-based flocculants. Chem. Eng. J. 2011, 172, 287–295. [Google Scholar] [CrossRef]
- Kono, H.; Fujita, S.; Oeda, I. Comparative study of homogeneous solvents for the esterification crosslinking of cellulose with 1,2,3,4-butanetetracarboxylic dianhydride and water absorbency of the reaction products. J. Appl. Polym. Sci. 2013, 127, 478–486. [Google Scholar] [CrossRef]
- Kono, H.; Kusumoto, R. Preparation, structural characterization, and flocculation ability of amphoteric cellulose. React. Funct. Polym. 2014, 82, 111–119. [Google Scholar] [CrossRef]
- Kono, H.; Otaka, F.; Ozaki, M. Preparation and characterization of guar gum hydrogels as carrier materials for controlled protein drug delivery. Carbohydr. Polym. 2014, 111, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Hara, H.; Hashimoto, H.; Shimizu, Y. Nonionic gelation agents prepared from hydroxypropyl guar gum. Carbohydr. Polym. 2015, 117, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Kono, H. Characterization and properties of carboxymethyl cellulose hydrogels crosslinked by polyethylene glycol. Carbohydr. Polym. 2014, 106, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.G. Dye removal from colored textile wastewater using synthesized chitosan. Int. J. Sci. Technol. 2013, 2, 359–364. [Google Scholar]
- Yan, L.; Tao, H.; Bangal, P.R. Synthesis and flocculation behavior of cationic cellulose prepared in a NaOH/urea aqueous solution. Clean Soil Air Water 2009, 37, 39–44. [Google Scholar] [CrossRef]
- Singh, R.P.; Pal, S.; Rana, V.K.; Ghorai, S. Amphoteric amylopectin: A novel polymeric flocculant. Carbohydr. Polym. 2013, 91, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Qian, S.; Li, C.; Pan, H.; Wu, Z.; Liu, G. Synthesis, flocculation and adsorption performance of amphoteric starch. Carbohydr. Polym. 2012, 90, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.L.; Pinto, L.A.A. Adsorption of food dyes onto chitosan: Optimization process and kinetic. Carbohydr. Polym. 2011, 84, 231–238. [Google Scholar] [CrossRef]
- Koner, S.; Saha, B.K.; Kumar, R.; Adak, A. Adsorption kinetics and mechanism of methyl orange dye on modified silica gel factory waste. Int. J. Sci. Technol. 2011, 3, 128–133. [Google Scholar]
- Hameed, B.H.; Din, A.T.M.; Ahmad, A.L. Adsorption of methylene blue onto bamboo-based activated carbon: Kinetics and equilibrium studies. J. Hazard. Mat. 2007, 141, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Dalaran, M.; Emik, S.; Güçlü, G.; İyim, T.B.; Özgümüş, S. Study on a novel polyampholyte nanocomposite superabsorbent hydrogels: Synthesis, characterization and investigation of removal of indigo carmine from aqueous solution. Desalination 2011, 279, 170–182. [Google Scholar] [CrossRef]
- Kono, H.; Nakamura, T.; Hashimoto, H.; Shimizu, Y. Characterization, molecular dynamics, and encapsulation ability of β-cyclodextrin polymers crosslinked by polyethylene glycol. Carbohydr. Polym. 2015, 128, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Omidian, H.; Rocca, J.G.; Park, K. Advances in superporous hydrogel. J. Control. Release 2005, 102, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Kinetic and equilibrium studies on the removal of cationic dyes from aqueous solution by adsorption onto a cyclodextrin polymer. Dyes Pigment. 2008, 77, 415–426. [Google Scholar] [CrossRef]
- Weber, T.W.; Chakravorti, R.K. Pore and solid diffusion models for fixed-bed adsorbers. AIChE J. 1974, 20, 228–238. [Google Scholar] [CrossRef]
- Chipot, C.; Jaffe, R.; Maigret, B.; Pearlman, D.A.; Kollman, P.A. Benzene dimer: A good model for π−π interactions in proteins? A comparison between the benzene and the toluene dimers in the gas phase and in an aqueous solution. J. Am. Chem. Soc. 1996, 118, 11217–11224. [Google Scholar] [CrossRef]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Modeling the drug release from hydrogel-based matrices. Mol. Pharm. 2015, 12, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Caccavo, D.; Cascone, S.; Lamberti, G.; Barba, A.A. Controlled drug release from hydrogel-based matrices: Experiments and modeling. Int. J. Pharm. 2015, 486, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Galdi, I.; Barba, A.A. Controlled release from hydrogel-based solid matrices. A model accounting for water up-take, swelling and erosion. Int. J. Pharm. 2011, 407, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Kusumoto, R. Removal of anionic dyes in aqueous solution by flocculation with cellulose ampholytes. J. Water Process Eng. 2015, 7, 83–93. [Google Scholar] [CrossRef]
- Kono, H.; Hashimoto, H.; Shimizu, Y. NMR characterization of cellulose acetate: Chemical shift assignments, substituent effects, and chemical shift additivity. Carbohydr. Polym. 2015, 118, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Fung, B.M.; Khitrin, A.K.; Ermolaev, K. An improved broadband decoupling sequence for liquid crystals and solids. J. Magn. Reson. 2000, 142, 97–101. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kono, H. Preparation and Characterization of Amphoteric Cellulose Hydrogels as Adsorbents for the Anionic Dyes in Aqueous Solutions. Gels 2015, 1, 94-116. https://doi.org/10.3390/gels1010094
Kono H. Preparation and Characterization of Amphoteric Cellulose Hydrogels as Adsorbents for the Anionic Dyes in Aqueous Solutions. Gels. 2015; 1(1):94-116. https://doi.org/10.3390/gels1010094
Chicago/Turabian StyleKono, Hiroyuki. 2015. "Preparation and Characterization of Amphoteric Cellulose Hydrogels as Adsorbents for the Anionic Dyes in Aqueous Solutions" Gels 1, no. 1: 94-116. https://doi.org/10.3390/gels1010094
APA StyleKono, H. (2015). Preparation and Characterization of Amphoteric Cellulose Hydrogels as Adsorbents for the Anionic Dyes in Aqueous Solutions. Gels, 1(1), 94-116. https://doi.org/10.3390/gels1010094






