A Potentially Practicable Halotolerant Yeast Meyerozyma guilliermondii A4 for Decolorizing and Detoxifying Azo Dyes and Its Possible Halotolerance Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Isolation and Identification of Halotolerant Azo-Dye-Degrading Yeast
2.3. Conditional Optimization of the Target Yeast for Dye Decolorization and Its Cell Growth
2.4. Acute Toxicity Assessment
2.5. Activity Determination of Key Enzymes
2.6. Presumption of Degradation Pathway of the Target Dye
2.7. Transcriptomic Responses of the Yeast to Salt Stress
2.8. Statistical Analysis
3. Results and Discussion
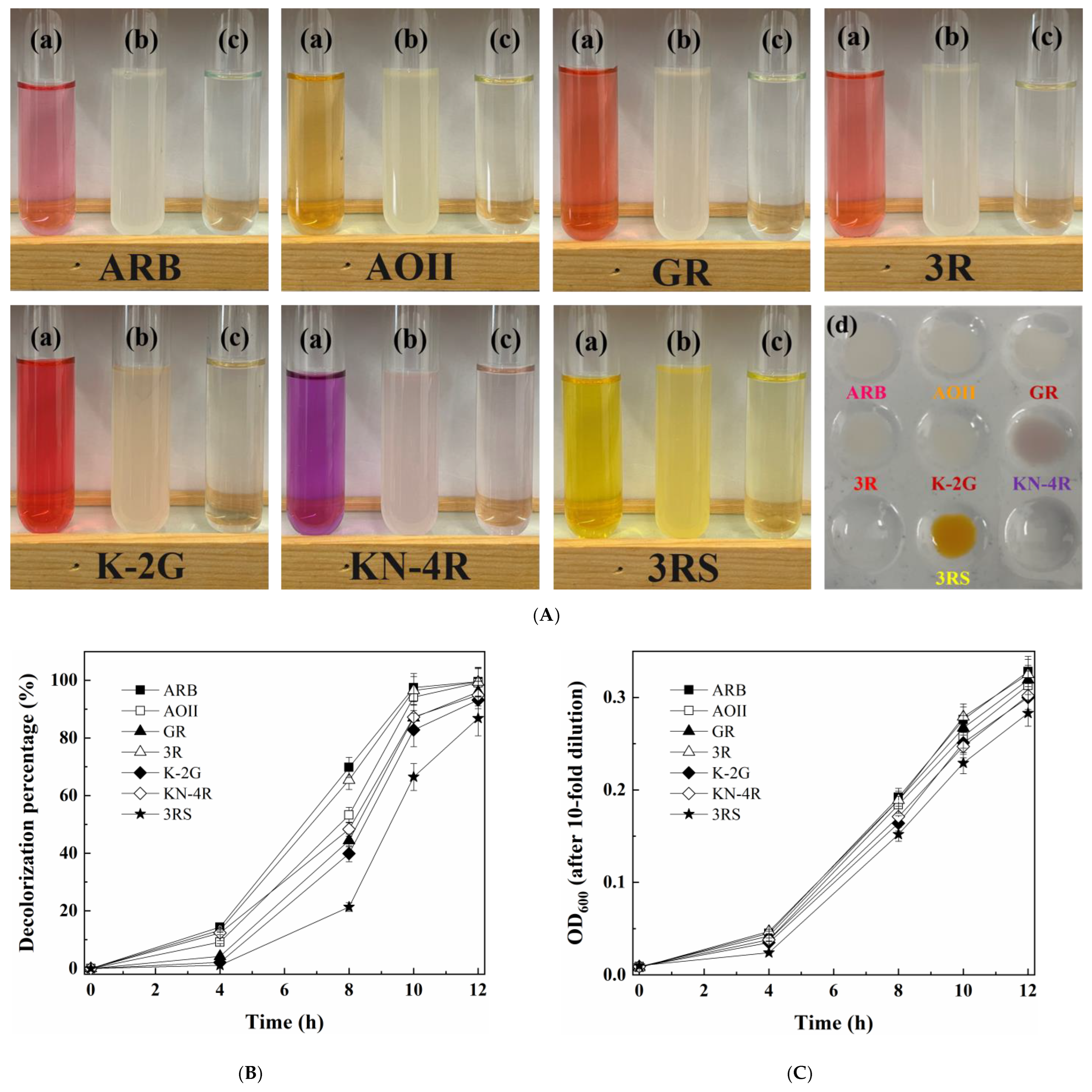
3.1. Isolation and Identification of the Halotolerant Yeast Strain Capable of Decolorizing Various Azo Dyes
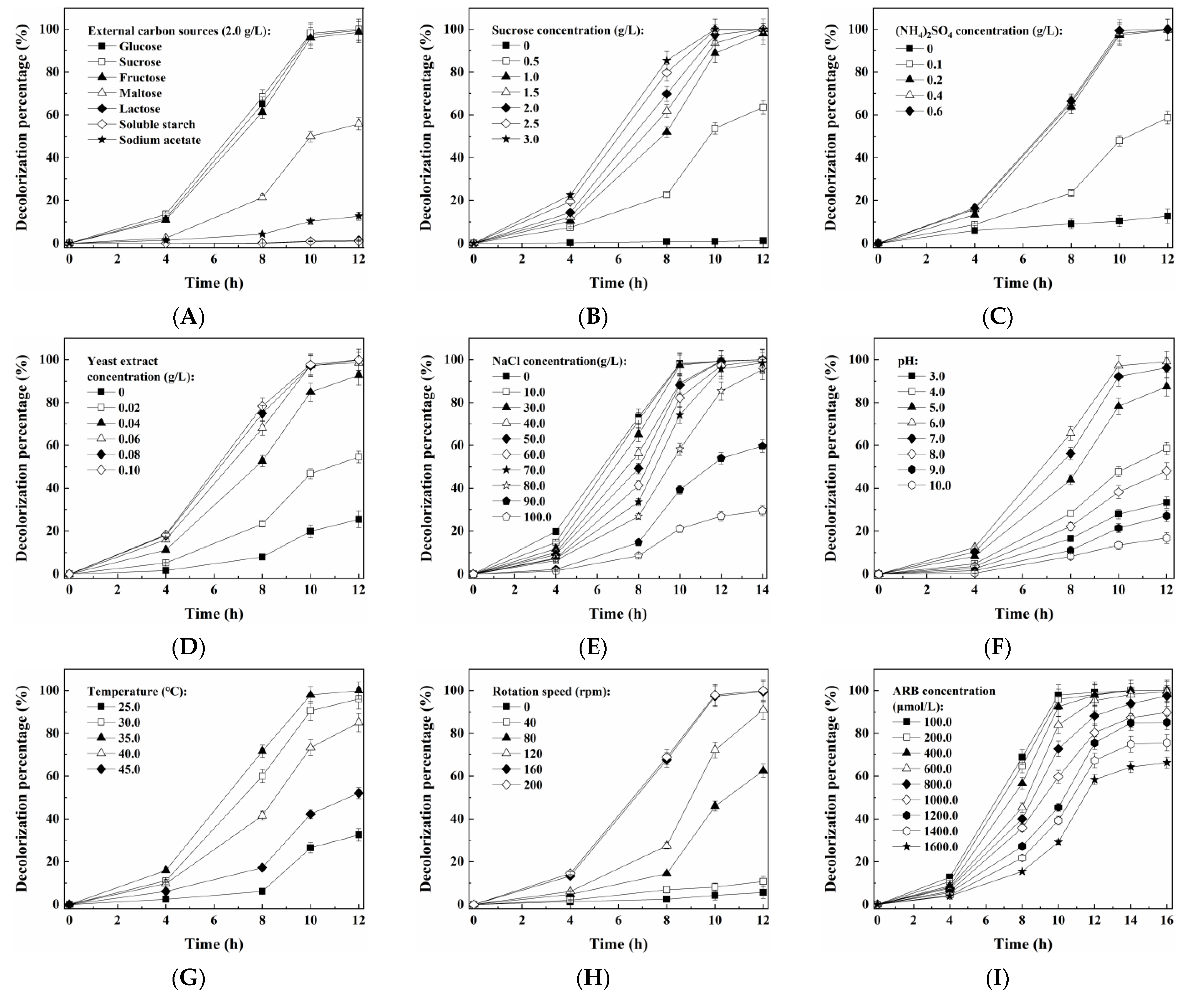
3.2. Optimization of Conditions for Dye Decolorization by the Yeast A4 and Its Cell Growth
3.3. The Detoxification Effect of the Yeast A4 on ARB and Its Metabolic Intermediates
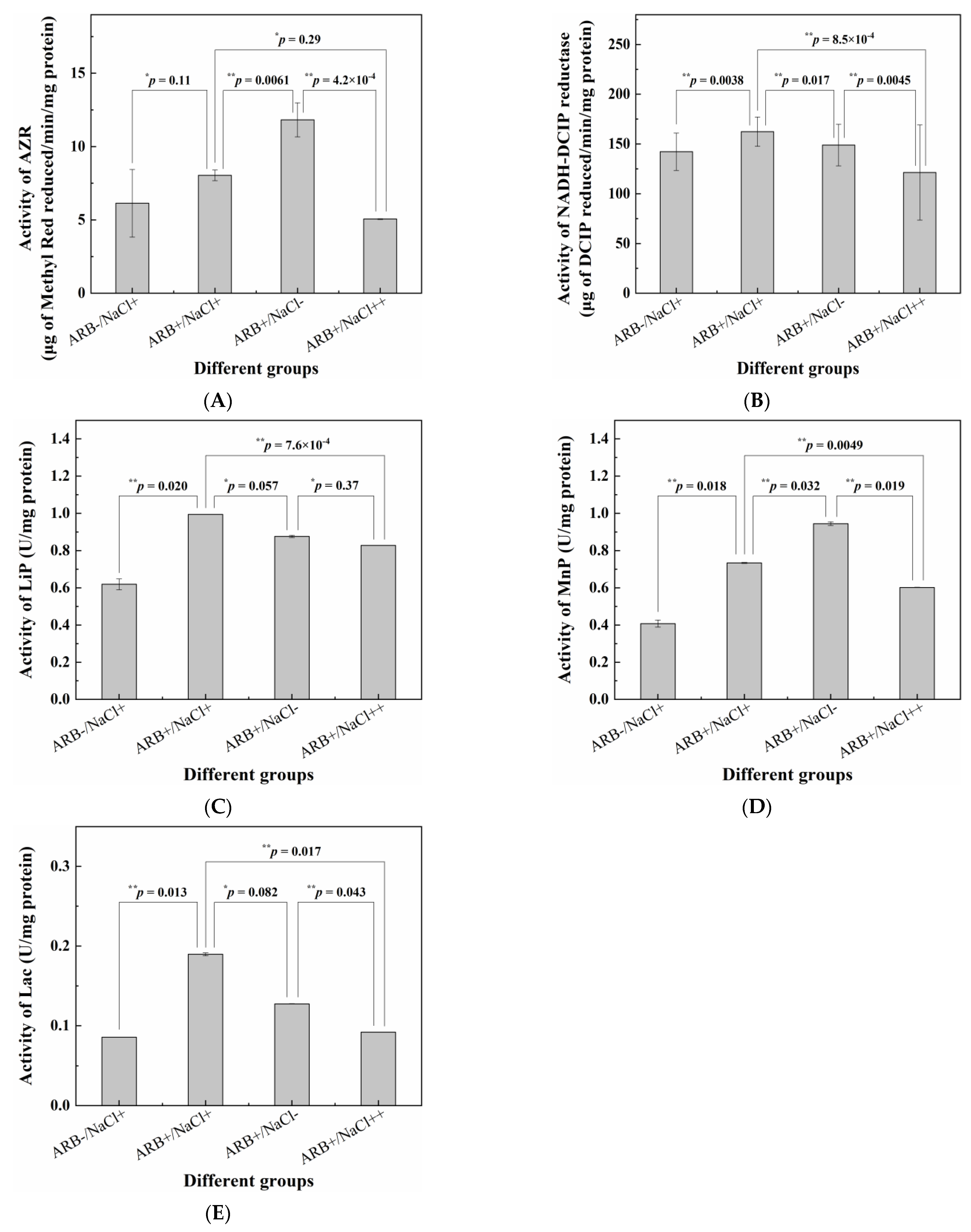
3.4. Determination of Key Enzymes’ Activity
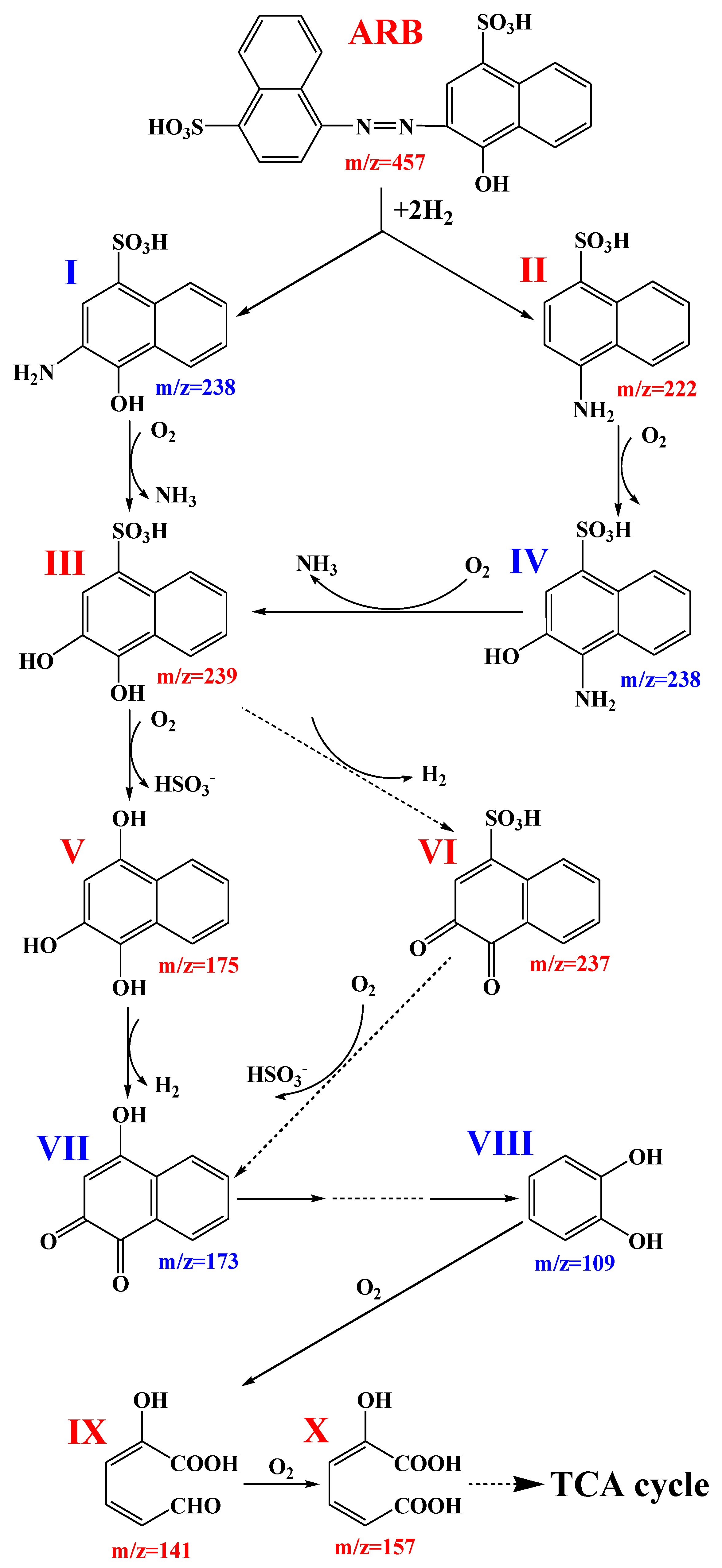
3.5. Degradation Pathways Presumption of ARB by the Yeast A4
3.6. Possible Halotolerant Mechanism of the Yeast A4 Revealed via Comparative Transcriptome Analysis
3.6.1. Transcriptome Sequencing Results
3.6.2. DEGs Associated with Halotolerance and Other Related Responses
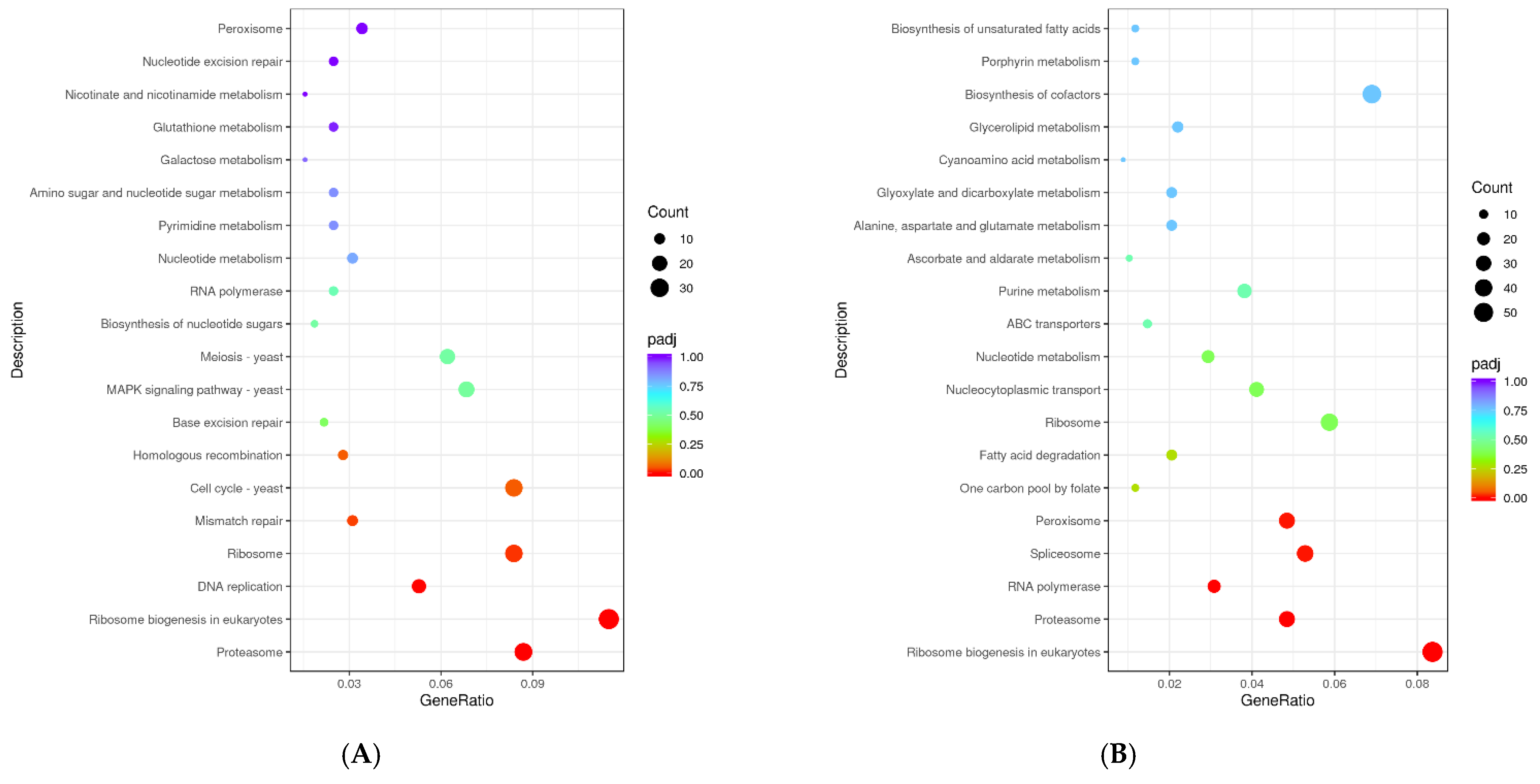
3.6.3. KEGG Enrichment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, M.; Fu, X.; Cui, J.; Feng, Y.; Tan, L. Efficient decolorization and detoxification of azo dyes by a halotolerant yeast Meyerozyma guilliermondii A3 with relatively low external carbon source. J. Water Process Eng. 2022, 47, 102810. [Google Scholar] [CrossRef]
- Stolz, A. Basic and applied aspects in the microbial degradation of azo dyes. Appl. Microbiol. Biotechnol. 2001, 56, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, B.; Ning, S.; Shi, S.; Tan, L. Magnetically stimulated azo dye biodegradation by a newly isolated osmo-tolerant Candida tropicalis A1 and transcriptomic responses. Ecotoxicol. Environ. Saf. 2021, 209, 111791. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Swarna Karthika, T.; Mansiya, C.; Alagar, M. An over review on recently developed techniques, mechanisms and intermediate involved in the advanced azo dye degradation for industrial applications. J. Mol. Struct. 2021, 1224, 129195. [Google Scholar] [CrossRef]
- Sen, S.K.; Raut, S.; Bandyopadhyay, P.; Raut, S. Fungal decolouration and degradation of azo dyes: A review. Fungal Biol. Rev. 2016, 30, 112–133. [Google Scholar] [CrossRef]
- Tan, L.; Ning, S.; Zhang, X.; Shi, S. Aerobic decolorization and degradation of azo dyes by growing cells of a newly isolated yeast Candida tropicalis TL-F1. Bioresour. Technol. 2013, 138, 307–313. [Google Scholar] [CrossRef]
- Pakshirajan, K.; Kheria, S. Continuous treatment of coloured industry wastewater using immobilized Phanerochaete chrysosporium in a rotating biological contactor reactor. J. Environ. Manag. 2012, 101, 118–123. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Nakajima, F.; Fukushi, K. Factors governing performance of continuous fungal reactor during non-sterile operation–the case of a membrane bioreactor treating textile wastewater. Chemosphere 2009, 74, 810–817. [Google Scholar] [CrossRef]
- Yu, Z.; Wen, X. Screening and identification of yeasts for decolorizing synthetic dyes in industrial wastewater. Int. Biodeterior. Biodegrad. 2005, 56, 109–114. [Google Scholar] [CrossRef]
- Song, L.; Shao, Y.; Ning, S.; Tan, L. Performance of a newly isolated salt-tolerant yeast strain Pichia occidentalis G1 for degrading and detoxifying azo dyes. Bioresour. Technol. 2017, 233, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Li, H.; Ning, S.; Xu, B. Aerobic decolorization and degradation of azo dyes by suspended growing cells and immobilized cells of a newly isolated yeast Magnusiomyces ingens LH-F1. Bioresour. Technol. 2014, 158, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Kiayi, Z.; Lotfabad, T.B.; Heidarinasab, A.; Shahcheraghi, F. Microbial degradation of azo dye carmoisine in aqueous medium using Saccharomyces cerevisiae ATCC 9763. J. Hazard. Mater. 2019, 373, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; He, M.; Song, L.; Fu, X.; Shi, S. Aerobic decolorization, degradation and detoxification of azo dyes by a newly isolated salt-tolerant yeast Scheffersomyces spartinae TLHS-SF1. Bioresour. Technol. 2016, 203, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Song, L.; Shao, Y.; Tan, L. Degradation and detoxification of azo dyes by a salt-tolerant yeast Cyberlindnera samutprakarnensis S4 under high-salt conditions. World J. Microbiol. Biotechnol. 2018, 34, 131. [Google Scholar] [CrossRef] [PubMed]
- Manu, B.; Chauhari, S. Decolorization of indigo and azo dyes in semi continuous reactors with long hydraulic retention time. Process Biochem. 2003, 38, 1213–1221. [Google Scholar] [CrossRef]
- Edbeib, M.F.; Wahab, R.A.; Huyop, F. Halophiles: Biology, adaptation, and their role in decontamination of hypersaline environments. World J. Microbiol. Biotechnol. 2016, 32, 135. [Google Scholar] [CrossRef]
- Zhuang, X.; Han, Z.; Bai, Z.; Zhuang, G.; Shim, H. Progress in decontamination by halophilic microorganisms in saline wastewater and soil. Environ. Pollut. 2010, 158, 1119–1126. [Google Scholar] [CrossRef]
- Kavynifard, A.; Ebrahimipour, G.; Ghasempour, A. Optimization of crude oil degradation by Dietzia cinnamea KA1, capable of biosurfactant production. J. Basic Microbiol. 2016, 56, 566–575. [Google Scholar] [CrossRef]
- Castillo-Carvajal, L.C.; Sanz-Martín, J.L.; Barragán-Huerta, B.E. Biodegradation of organic pollutants in saline wastewater by halophilic microorganisms: A review. Environ. Sci. Pollut. Res. 2014, 21, 9578–9588. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.Y.; Yen, C.Y.; Chen, W.M.; Chang, C.T.; Wang, C.T.; Hu, Y.C. Exploring threshold operation criteria of biostimulation for azo dye decolorization using immobilized cell systems. Bioresour. Technol. 2009, 100, 5763–5770. [Google Scholar] [CrossRef]
- Guo, J.; Zhou, J.; Wang, D.; Tian, C.; Wang, P.; Salah Uddin, M. A novel moderately halophilic bacterium for decolorizing azo dye under high salt condition. Biodegradation 2008, 19, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, F.; Duan, W.; Lin, Y.; Zheng, Y. Biodegradation of phenol in saline or hypersaline environments by bacteria: A review. Ecotoxicol. Environ. Saf. 2019, 184, 109658. [Google Scholar] [CrossRef] [PubMed]
- Asad, S.; Amoozegar, M.A.; Pourbabaee, A.A.; Sarbolouki, M.N.; Dastgheib, S.M. Decolorization of textile azo dyes by newly isolated halophilic and halotolerant bacteria. Bioresour. Technol. 2007, 98, 2082–2088. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Goyal, N.; Gupta, A. Degradation of azo dye methyl red by alkaliphilic, halotolerant Nesterenkonia lacusekhoensis EMLA3: Application in alkaline and salt-rich dyeing effluent treatment. Extremophiles 2017, 21, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Haliru, M.; Hafiz, K.F.; Anas, N.G.A.; Subash, G. Salt-adapted moulds and yeasts: Potentials in industrial and environmental biotechnology. Process Biochem. 2018, 69, 33–44. [Google Scholar]
- Jiang, Y.; Yang, K.; Wang, H.; Shang, Y.; Yang, X. Characteristics of phenol degradation in saline conditions of a halophilic strain JS3 isolated from industrial activated sludge. Mar. Pollut. Bull. 2015, 99, 230–234. [Google Scholar] [CrossRef]
- Li, C.; Xu, Y.; Jiang, W.; Dong, X.; Wang, D.; Liu, B. Effect of NaCl on the heavy metal tolerance and bioaccumulation of Zygosaccharomyces rouxii and Saccharomyces cerevisiae. Bioresour. Technol. 2013, 143, 46–52. [Google Scholar] [CrossRef]
- Li, H.; Tan, L.; Ning, S.; He, M. Reactor performance and microbial community dynamics during aerobic degradation and detoxification of Acid Red B with activated sludge bioaugmented by a yeast Candida tropicalis TL-F1 in MBR. Int. Biodeterior. Biodegr. 2015, 104, 149–156. [Google Scholar] [CrossRef]
- Varjani, S.; Upasani, V.N. Influence of abiotic factors, natural attenuation, bioaugmentation and nutrient supplementation on bioremediation of petroleum crude contaminated agricultural soil. J. Environ. Manag. 2019, 245, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Xu, B.; Hao, J.; Wang, J.; Shao, Y.; Mu, G. Biodegradation and detoxification of azo dyes by a newly isolated halotolerant yeast Candida tropicalis SYF-1. Environ. Eng. Sci. 2019, 36, 999–1010. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Ning, S.; Shi, S.; Tan, L. Improving azo dye decolorization performance and halotolerance of Pichia occidentalis A2 by static magnetic field and possible mechanisms through comparative transcriptome analysis. Front. Microbiol. 2020, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Kausar, F.; Arshad, M.; Mahmood, T.; Ahmed, I. Accelerated decolorization of reactive azo dyes under saline conditions by bacteria isolated from Arabian seawater sediment. Appl. Microbiol. Biotechnol. 2012, 96, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Martínez, J.D.; Balagurusamy, N.; Montañez, J.; Peralta, R.A.; Moreira, R.F.P.M.; Bracht, A.; Peralta, R.M.; Morales-Oyervides, L. Synthetic dyes biodegradation by fungal ligninolytic enzymes: Process optimization, metabolites evaluation and toxicity assessment. J. Hazard. Mater. 2020, 400, 123254. [Google Scholar] [CrossRef] [PubMed]
- Sorkheh, K.; Shiran, B.; Rouhi, V.; Khodambashi, M.; Sofo, A. Salt stress induction of some key antioxidant enzymes and metabolites in eight Iranian wild almond species. Acta Physiol. Plant. 2012, 34, 203–213. [Google Scholar] [CrossRef]
- Liu, G.; Zhou, J.; Meng, X.; Fu, S.Q.; Wang, J.; Jin, R.; Lv, H. Decolorization of azo dyes by marine Shewanella strains under saline conditions. Appl. Microbiol. Biotechnol. 2013, 97, 4187–4197. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, B.; Phale, P.S. Microbial degradation of naphthalene and substituted naphthalenes: Metabolic diversity and genomic insight for bioremediation. Front. Bioeng. Biotechnol. 2021, 9, 602445. [Google Scholar] [CrossRef]
- Cheng, R.; Xu, L.; Wang, S.; Wang, Y.; Zhang, J. Recombinant expression and characterization of an acid-, alkali- and salt-tolerant beta-1,3–1,4-glucanase from Paenibacillus sp. S09. Biotechnol. Lett. 2014, 36, 797–803. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Ying, S.H.; Feng, M.G. The GPI-anchored protein Ecm33 is vital for conidiation, cell wall integrity, and multi-stress tolerance of two filamentous entomopathogens but not for virulence. Appl. Microbiol. Biotechnol. 2014, 98, 5517–5529. [Google Scholar] [CrossRef]
- Ferreira, C.; Silva, S.; van Voorst, F.; Aguiar, C.; Kielland-Brandt, M.C.; Brandt, A.; Lucas, C. Absence of Gup1p in Saccharomyces cerevisiae results in defective cell wall composition, assembly, stability and morphology. FEMS Yeast Res. 2006, 6, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Gil-Bona, A.; Reales-Calderon, J.A.; Parra-Giraldo, C.M.; Martinez-Lopez, R.; Monteoliva, L.; Gil, C. The cell wall protein Ecm33 of Candida albicans is involved in chronological life span, morphogenesis, cell wall regeneration, stress tolerance, and host-cell interaction. Front. Microbiol. 2016, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Shao, Y.; Mu, G.; Ning, S.; Shi, S. Enhanced azo dye biodegradation performance and halotolerance of Candida tropicalis SYF-1 by static magnetic field (SMF). Bioresour. Technol. 2020, 295, 122283. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Microbial life at high salt concentrations: Phylogenetic and metabolic diversity. Saline Syst. 2008, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Prista, C.; Loureiro-Dias, M.C.; Montiel, V.; García, R.; Ramos, J. Mechanisms underlying the halotolerant way of Debaryomyces hansenii. FEMS Yeast Res. 2005, 5, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.F. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Syst. 2005, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Li, G.; Wang, X.; Jiang, Y.; Yang, Y. Cloning and characterization of a glycerol-3-phosphate dehydrogenase (NAD+) gene from the halotolerant yeast Pichia farinosa. Yeast 2010, 27, 115–121. [Google Scholar] [CrossRef]
- Alba-Lois, L.; Segal, C.; Rodarte, B.; Valdés-López, V.; DeLuna, A.; Cárdenas, R. NADP-glutamate dehydrogenase activity is increased under hyperosmotic conditions in the halotolerant yeast Debaryomyces hansenii. Curr. Microbiol. 2004, 48, 68–72. [Google Scholar] [CrossRef]
- De Nadal, E.; Calero, F.; Ramos, J.; Ariño, J. Biochemical and genetic analyses of the role of yeast casein kinase 2 in salt tolerance. J. Bacteriol. 1999, 181, 6456–6462. [Google Scholar] [CrossRef]
- Geisler, M.; Frangne, N.; Gomès, E.; Martinoia, E.; Palmgren, M.G. The ACA4 gene of Arabidopsis encodes a vacuolar membrane calcium pump that improves salt tolerance in yeast. Plant Physiol. 2000, 124, 1814–1827. [Google Scholar] [CrossRef]
- Park, S.Y.; Seo, S.B.; Lee, S.J.; Na, J.G.; Kim, Y.J. Mutation in PMR1, a Ca2+-ATPase in Golgi, confers salt tolerance in Saccharomyces cerevisiae by inducing expression of PMR2, an Na+-ATPase in plasma membrane. J. Biol. Chem. 2001, 276, 28694–28699. [Google Scholar] [CrossRef]
- Morrison, J.M.; John, G.H. Non-classical azoreductase secretion in Clostridium perfringens in response to sulfonated azo dye exposure. Anaerobe 2015, 34, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Nagai, Y.; Kawagishi, H.; Hirai, H. Functional characterization of the manganese transporter smf2 homologue gene, PsMnt, of Phanerochaete sordida YK-624 via homologous overexpression. FEMS Microbiol. Lett. 2018, 365, fny050. [Google Scholar] [CrossRef] [PubMed]
- Kunishima, N.; Fukuyama, K.; Matsubara, H.; Hatanaka, H.; Shibano, Y.; Amachi, T. Crystal structure of the fungal peroxidase from Arthromyces ramosus at 1.9 A resolution. Structural comparisons with the lignin and cytochrome c peroxidases. J. Mol. Biol. 1994, 235, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, Y.; Duan, X.; Luan, Y.; Li, Q.; Pang, Y.; Sun, F.; Gou, M. A complete prostaglandin pathway from synthesis to inactivation in the oral gland of the jawless vertebrate lamprey, Lethenteron camtschaticum. Dev. Comp. Immunol. 2023, 148, 104903. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.T.; Liu, D.Y.; Hseu, T.H. Cell growth restoration and high level protein expression by the promoter of hexose transporter, HXT7, from Saccharomyces cerevisiae. Biotechnol. Lett. 2007, 29, 1287–1292. [Google Scholar] [CrossRef] [PubMed]
- Kolekar, Y.M.; Nemade, H.N.; Markad, V.L.; Adav, S.S.; Patole, M.S.; Kodam, K.M. Decolorization and biodegradation of azo dye, reactive blue 59 by aerobic granules. Bioresour. Technol. 2012, 104, 818–822. [Google Scholar] [CrossRef]
- Yang, H.L.; Liao, Y.Y.; Zhang, J.; Wang, X.L. Comparative transcriptome analysis of salt tolerance mechanism of Meyerozyma guilliermondii W2 under NaCl stress. 3 Biotech 2019, 9, 286. [Google Scholar] [CrossRef]
- Ishii, T.; Funakoshi, M.; Kobayashi, H.; Sekiguchi, T. Yeast Irc22 is a novel Dsk2-interacting protein that is involved in salt tolerance. Cells 2014, 3, 180–198. [Google Scholar] [CrossRef]
- Manzanares-Estreder, S.; Espí-Bardisa, J.; Alarcón, B.; Pascual-Ahuir, A.; Proft, M. Multilayered control of peroxisomal activity upon salt stress in Saccharomyces cerevisiae. Mol. Microbiol. 2017, 104, 851–868. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Yong, X.; Zhang, R.; Zhang, N.; Chen, Y.; Huang, X.; Zhao, J.; Shen, Q. Development of a specific real-time PCR assay targeting the poly-γ-glutamic acid synthesis gene, pgsB, for the quantification of Bacillus amyloliquefaciens in solid-state fermentation. Bioresour. Technol. 2013, 129, 477–484. [Google Scholar] [CrossRef] [PubMed]

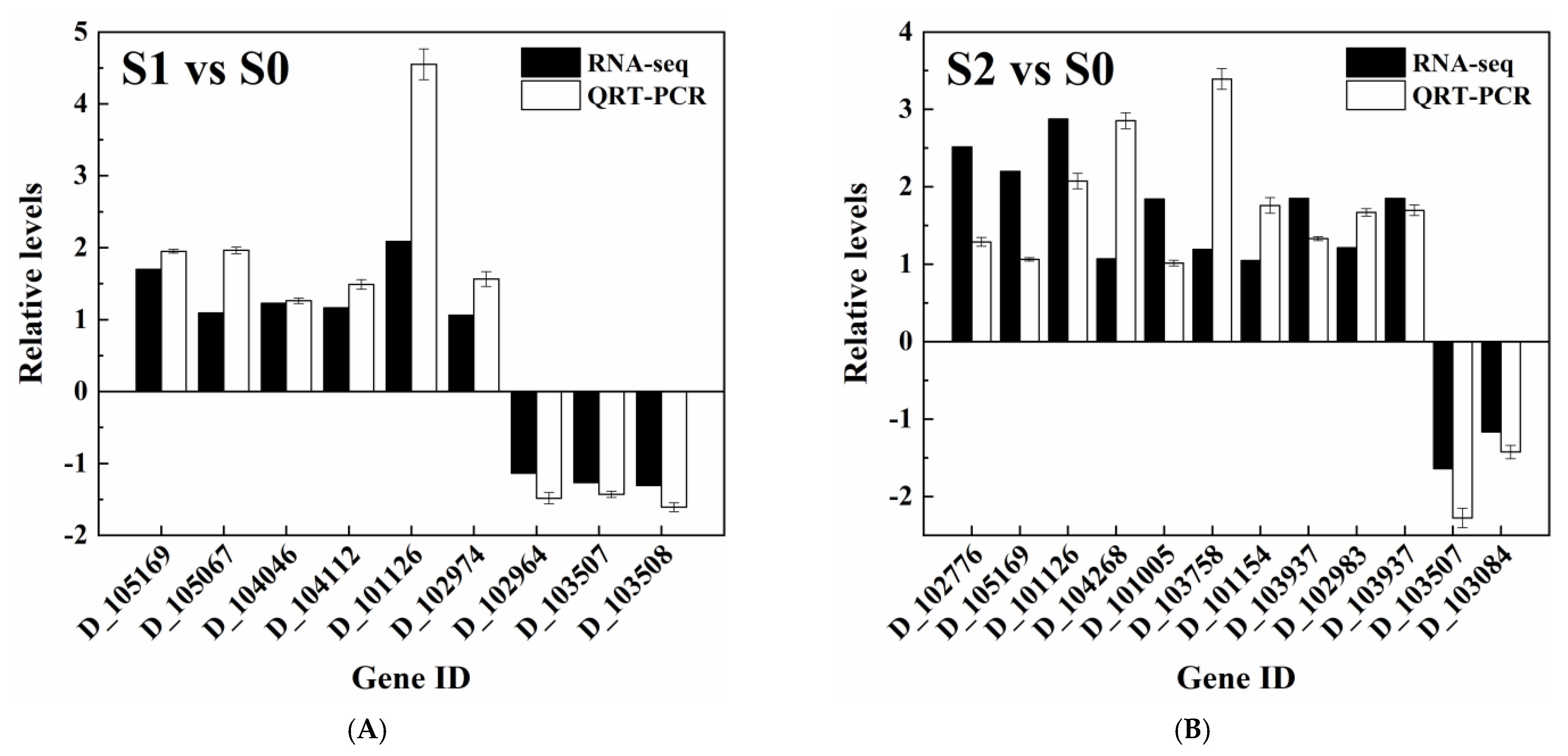
| Description | Gene ID | Annotation (Protein ID) | log2 FC ** | |
|---|---|---|---|---|
| S1 vs. S0 | S2 vs. S0 | |||
| Cell wall regulation | D_102776 | Essential for maintenance of the cell wall protein 1 (P42842) | —— | 2.514 * |
| D_105169 | 1,3-beta-glucan synthase component GSC2 (P40989) | 1.697 * | 2.201 * | |
| D_105067 | 1,3-beta-glucan synthase component FKS3 (Q04952) | 1.092 * | —— | |
| D_104046 | Endo-1,3(4)-beta-glucanase 1 (Q5AIR7) | 1.228 * | —— | |
| D_104112 | Cell wall protein ECM33 (C7GQJ1) | 1.165 * | —— | |
| D_101126 | Cell surface GPI-anchored protein ECM33 (Q5AGC4) | 2.088 * | 2.877 * | |
| D_101744 | Chitin synthase 3 (P30573) | 1.514 | 2.918 | |
| D_100586 | Chitin synthase 2 (P30572) | 1.160 | —— | |
| D_100803 | Chitin synthase 1 (P23316) | —— | 1.638 | |
| D_103766 | Chitin synthase 2 (P30572) | —— | 1.405 | |
| Potassium transporter (high-salt-in strategy) | D_102974 | High-affinity potassium transporter (P50505) | 1.063 * | —— |
| D_104268 | Low-affinity K+ transporter 1 (P47114) | —— | 1.071 * | |
| Compatible solutes (organic-solute-in strategy) | D_103937 | NADP-specific glutamate dehydrogenase (P29507) | —— | 1.850 * |
| D_102983 | Glycerol-3-phosphate dehydrogenase, mitochondrial (O14400) | —— | 1.213 * | |
| D_102964 | Glycerol-3-phosphate dehydrogenase [NAD+] (Q7ZA43) | −1.134 * | —— | |
| D_103937 | NADP-specific glutamate dehydrogenase (P29507) | —— | 1.850 * | |
| Other possible halotolerant mechanisms | D_101005 | Calcium-transporting ATPase 1 (P13586) | —— | 1.841 * |
| D_103758 | Calcium-transporting ATPase 2 (P38929) | —— | 1.193 * | |
| D_101154 | Casein kinase II subunit alpha (P21868) | —— | 1.050 * | |
| Possible key enzymes | D_103507 | Probable NADPH dehydrogenase (NADH:flavin oxidoreductase) (P43084) | −1.268 * | −1.637 * |
| D_103508 | Probable NADPH dehydrogenase (NADH:flavin oxidoreductase) (P43084) | −1.308 * | —— | |
| D_103084 | YEAST manganese transporter SMF1 (P38925) | −0.178 | −1.165 * | |
| D_100122 | Laccase PFICI_06862 (W3X732) | 0.402 | −0.283 | |
| D_104858 | Cytochrome c peroxidase (Q6BKY9) | 0.678 | −0.078 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Cui, J.; Xu, B.; Jiang, Y.; Fu, C.; Tan, L. A Potentially Practicable Halotolerant Yeast Meyerozyma guilliermondii A4 for Decolorizing and Detoxifying Azo Dyes and Its Possible Halotolerance Mechanisms. J. Fungi 2023, 9, 851. https://doi.org/10.3390/jof9080851
Feng Y, Cui J, Xu B, Jiang Y, Fu C, Tan L. A Potentially Practicable Halotolerant Yeast Meyerozyma guilliermondii A4 for Decolorizing and Detoxifying Azo Dyes and Its Possible Halotolerance Mechanisms. Journal of Fungi. 2023; 9(8):851. https://doi.org/10.3390/jof9080851
Chicago/Turabian StyleFeng, Yue, Jingru Cui, Bingwen Xu, Yifan Jiang, Chunqing Fu, and Liang Tan. 2023. "A Potentially Practicable Halotolerant Yeast Meyerozyma guilliermondii A4 for Decolorizing and Detoxifying Azo Dyes and Its Possible Halotolerance Mechanisms" Journal of Fungi 9, no. 8: 851. https://doi.org/10.3390/jof9080851
APA StyleFeng, Y., Cui, J., Xu, B., Jiang, Y., Fu, C., & Tan, L. (2023). A Potentially Practicable Halotolerant Yeast Meyerozyma guilliermondii A4 for Decolorizing and Detoxifying Azo Dyes and Its Possible Halotolerance Mechanisms. Journal of Fungi, 9(8), 851. https://doi.org/10.3390/jof9080851





