Assessing the In Vitro Potential of Glatiramer Acetate (Copaxone®) as a Chemotherapeutic Candidate for the Treatment of Cryptococcus neoformans Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Strain
2.2. Medium
2.2.1. RPMI Medium
2.2.2. Minimal Medium
2.3. Antifungal Activity
2.4. Cell Viability
2.5. Morphometric Analysis
2.6. Immunofluorescence
2.7. Scanning Electron Microscopy
2.8. Extraction and Concentration of Secreted Polysaccharides
2.9. Zeta Potential (ζ) and Conductance
2.10. Dynamic Light Scattering (DLS)
2.11. Passive Microrheology
2.12. Statistical Treatment
3. Results
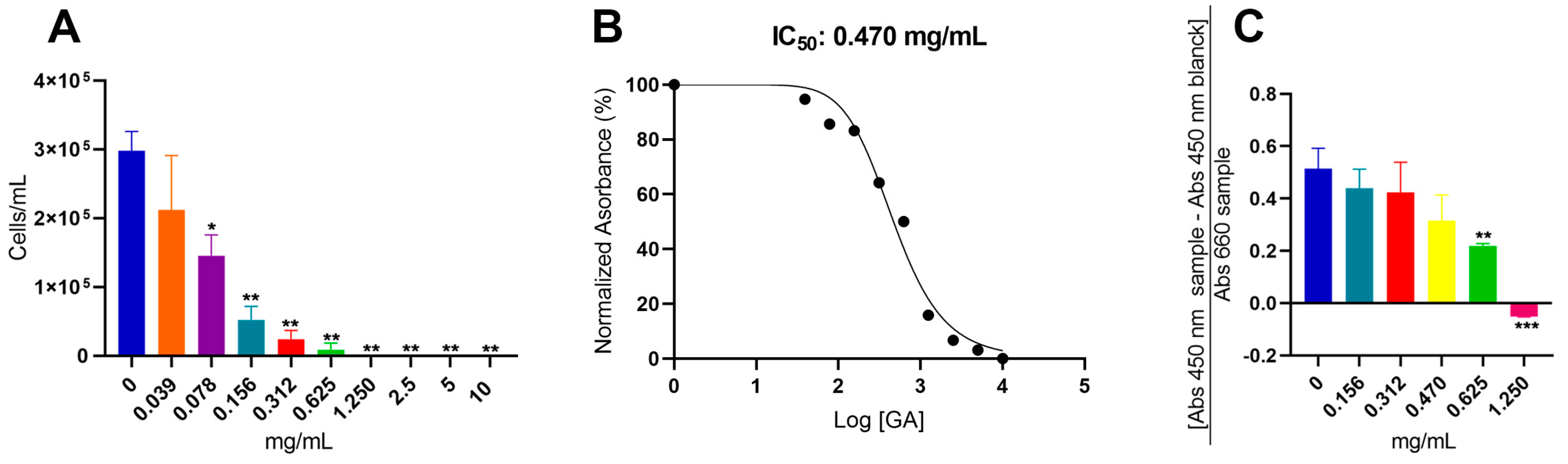
3.1. The Antifungal Potential of Glatiramer Acetate in C. neoformans
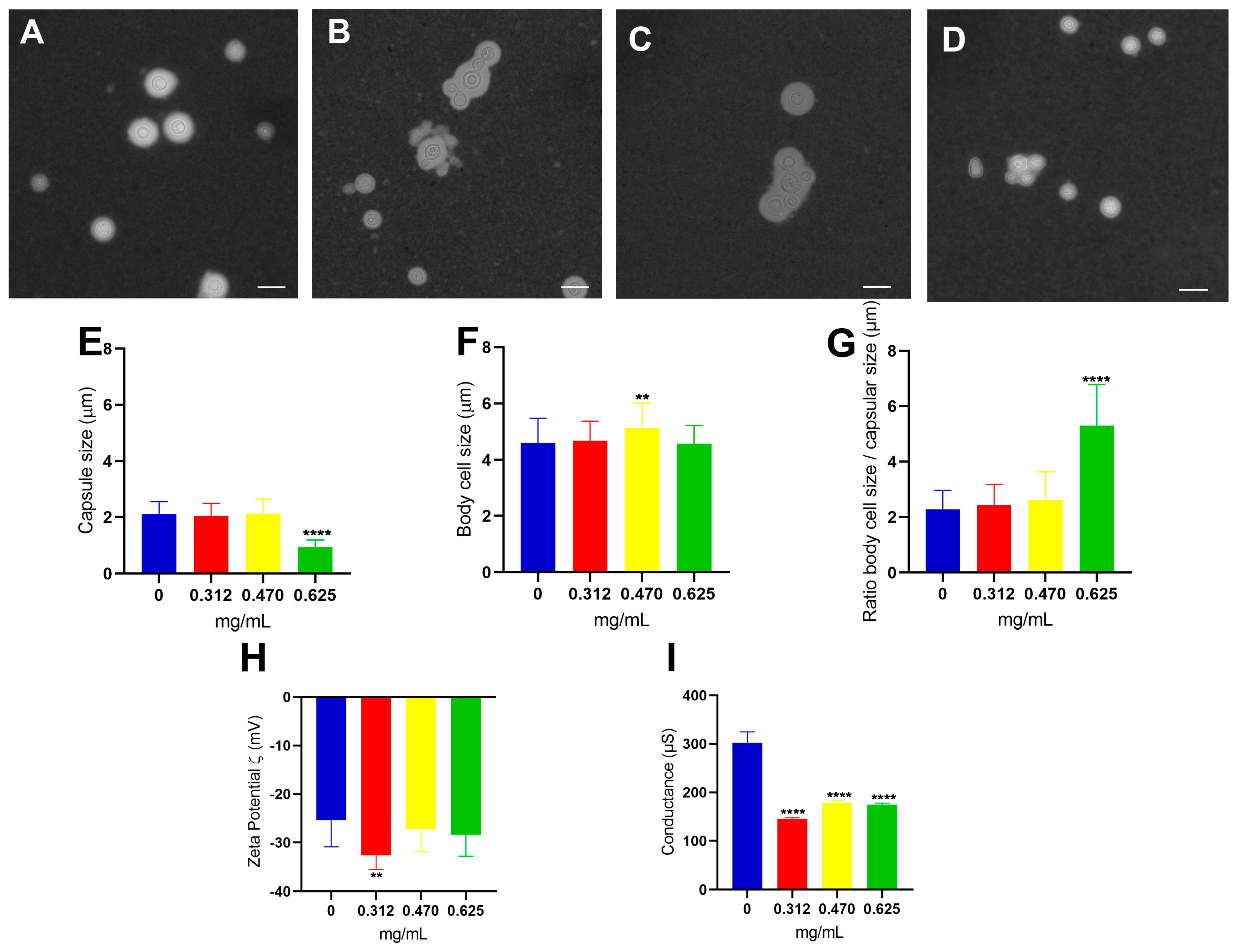
3.2. Effect of Glatiramer Acetate on the Structural and Physical–Chemical Properties of C. neoformans Cells
3.3. Analysis of the Physicochemical Properties of Polysaccharides Secreted (PS) by C. neoformans at Different Concentrations of Glatiramer Acetate
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Idnurm, A.; Bahn, Y.S.; Nielsen, K.; Lin, X.; Fraser, J.A.; Heitman, J. Deciphering the Model Pathogenic Fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 2005, 3, 753–764. [Google Scholar] [CrossRef]
- Martin, T.R.; Frevert, C.W. Innate Immunity in the Lungs. Proc. Am. Thorac. Soc. 2005, 2, 403–411. [Google Scholar] [CrossRef]
- Zaragoza, O. Basic Principles of the Virulence of Cryptococcus. Virulence 2019, 10, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Goldman, J.D.; Vollmer, M.E.; Luks, A.M. Cryptococcosis in the Immunocompetent Patient. Respir. Care 2010, 55, 1499–1503. [Google Scholar]
- Zaragoza, O.; Nielsen, K. Titan Cells in Cryptococcus neoformans: Cells with a Giant Impact. Curr. Opin. Microbiol. 2013, 16, 409–413. [Google Scholar] [CrossRef]
- Li, S.S.; Mody, C.H. Cryptococcus. Proc. Am. Thorac. Soc. 2010, 7, 186–196. [Google Scholar] [CrossRef]
- Kwon-Chung, K.J.; Fraser, J.A.; Doering, T.Á.L.; Wang, Z.A.; Janbon, G.; Idnurm, A.; Bahn, Y.S. Cryptococcus neoformans and Cryptococcus gattii, the Etiologic Agents of Cryptococcosis. Cold Spring Harb. Perspect. Med. 2015, 4, a019760. [Google Scholar] [CrossRef]
- Chayakulkeeree, M.; Perfect, J. Chapter 14. Cryptococcosis. In Diagnosis and Treatment of Human Mycoses; Springer: Berlin/Heidelberg, Germany, 2008; pp. 255–276. [Google Scholar]
- Zhu, X.; Williamson, P.R. Role of Laccase in the Biology and Virulence of Cryptococcus neoformans. FEMS Yeast Res. 2004, 5, 1–10. [Google Scholar] [CrossRef]
- Colombo, A.C.; Rodrigues, M.L. Fungal Colonization of the Brain: Anatomopathological Aspects of Neurological Cryptococcosis Epidemic in HIV Patients (Armstrong-James et al. People Die Each Year Because of Systemic Fungal Cryptococcosis Presented in the Last (9 Th) Edition and Cryp. Acad. Bras. Cienc. 2015, 87, 1293–1309. [Google Scholar] [CrossRef] [PubMed]
- Nosanchuk, J.D.; Rosas, A.L.; Lee, S.C.; Casadevall, A. Melanisation of Cryptococcus neoformans in Human Brain Tissue. Lancet 2000, 355, 2049–2050. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for Diagnosing, Preventing and Managing Cryptococcal Disease among Adults, Adolescents and Children Living with HIV; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Perfect, J.R.; Dismukes, W.E.; Dromer, F.; Goldman, D.L.; Graybill, J.R.; Hamill, R.J.; Harrison, T.S.; Larsen, R.A.; Lortholary, O.; Nguyen, M.-H.; et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2010, 10, 161–166. [Google Scholar] [CrossRef]
- Yoon, H.A.; Nakouzi, A.; Chang, C.C.; Kuniholm, M.H.; Carreño, L.J.; Wang, T.; Ndung’u, T.; Lewin, S.R.; French, M.A.; Pirofski, L.A. Association Between Plasma Antibody Responses and Risk for Cryptococcus-Associated Immune Reconstitution Inflammatory Syndrome. J. Infect. Dis. 2019, 219, 420–428. [Google Scholar] [CrossRef]
- Chang, Y.L.; Yu, S.J.; Heitman, J.; Wellington, M.; Chen, Y.L. New Facets of Antifungal Therapy. Virulence 2017, 8, 222–236. [Google Scholar] [CrossRef]
- Morschhäuser, J. The Development of Fluconazole Resistance in Candida albicans—An Example of Microevolution of a Fungal Pathogen. J. Microbiol. 2016, 54, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Langedijk, J.; Mantel-Teeuwisse, A.K.; Slijkerman, D.S.; Schutjens, M.H.D.B. Drug Repositioning and Repurposing: Terminology and Definitions in Literature. Drug Discov. Today 2015, 20, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Cockell, S.J.; Weile, J.; Lord, P. An Integrated Dataset for in Silico Drug Discovery. J. Integr. Bioinform. 2010, 7, 15–27. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, Y.; Chen, L. Network-Based Drug Repositioning. Mol. Biosyst. 2013, 9, 1268–1281. [Google Scholar] [CrossRef] [PubMed]
- Dudley, J.T.; Deshpande, T.; Butte, A.J. Exploiting Drug-Disease Relationships for Computational Drug Repositioning. Brief. Bioinform. 2011, 12, 303–311. [Google Scholar] [CrossRef]
- Lotfi Shahreza, M.; Ghadiri, N.; Mousavi, S.R.; Varshosaz, J.; Green, J.R. A Review of Network-Based Approaches to Drug Repositioning. Brief. Bioinform. 2018, 19, 878–892. [Google Scholar] [CrossRef]
- Alves, V.; Araújo, G.R.S.; Frases, S. Off-Label Treatments as Potential Accelerators in the Search for the Ideal Antifungal Treatment of Cryptococcosis. Future Microbiol. 2023, 18, 127–135. [Google Scholar] [CrossRef]
- Teitelbaum, D.; Aharoni, R.; Sela, M.; Arnon, R. Cross-Reactions and Specificities of Monoclonal Antibodies against Myelin Basic Protein and against the Synthetic Copolymer 1. Proc. Natl. Acad. Sci. USA 1991, 88, 9528–9532. [Google Scholar] [CrossRef]
- Teitelbaum, D.; Meshorer, A.; Hirshfeld, T.; Arnon, R.; Sela, M. Suppression of Experimental Allergic Encephalomyelitis by a Synthetic Polypeptide. Eur. J. Immunol. 1971, 1, 242–248. [Google Scholar] [CrossRef]
- Landa, G.; Butovsky, O.; Shoshani, J.; Schwartz, M.; Pollack, A. Weekly Vaccination with Copaxone (Glatiramer Acetate) as a Potential Therapy for Dry Age-Related Macular Degeneration. Curr. Eye Res. 2008, 33, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Bakalash, S.; Pham, M.; Koronyo, Y.; Salumbides, B.C.; Kramerov, A.; Seidenberg, H.; Berel, D.; Black, K.L.; Koronyo-Hamaoui, M. Egr1 Expression Is Induced Following Glatiramer Acetate Immunotherapy in Rodent Models of Glaucoma and Alzheimer’s Disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9033–9046. [Google Scholar] [CrossRef]
- Schwartz, M.; Bukshpan, S.; Kunis, G. Application of Glatiramer Acetate to Neurodegenerative Diseases beyond Multiple Sclerosis: The Need for Disease-Specific Approaches. BioDrugs 2008, 22, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Belokopytov, M.; Ben-Shlomo, G.; Rosner, M.; Belkin, M.; Dubinski, G.; Epstein, Y.; Ofri, R. Functional Efficacy of Glatiramer Acetate Treatment for Laser-Induced Retinal Damage in Rats. Lasers Surg. Med. 2008, 40, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Corey-Bloom, J.; Aikin, A.M.; Gutierrez, A.; Salam, J.; Howell, T.; Thomas, E.A. Beneficial Effects of Glatiramer Acetate in Huntington’s Disease Mouse Models: Evidence for BDNF-Elevating and Immunomodulatory Mechanisms. Brain Res. 2017, 1673, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Angelov, D.N.; Waibel, S.; Guntinas-Lichius, O.; Lenzen, M.; Neiss, W.F.; Tomov, T.L.; Yoles, E.; Kipnis, J.; Schori, H.; Reuter, A.; et al. Therapeutic Vaccine for Acute and Chronic Motor Neuron Diseases: Implications for Amyotrophic Lateral Sclerosis. Proc. Natl. Acad. Sci. USA 2003, 100, 4790–4795. [Google Scholar] [CrossRef]
- Koronyo, Y.; Salumbides, B.C.; Sheyn, J.; Pelissier, L.; Li, S.; Ljubimov, V.; Moyseyev, M.; Daley, D.; Fuchs, D.T.; Pham, M.; et al. Therapeutic Effects of Glatiramer Acetate and Grafted CD115+ Monocytes in a Mouse Model of Alzheimer’s Disease. Brain 2015, 138, 2399–2422. [Google Scholar] [CrossRef]
- Yao, Y.; Han, W.; Liang, J.; Ji, J.; Wang, J.; Cantor, H.; Lu, L. Glatiramer Acetate Ameliorates Inflammatory Bowel Disease in Mice through the Induction of Qa-1-Restricted CD8+ Regulatory Cells. Eur. J. Immunol. 2013, 43, 125–136. [Google Scholar] [CrossRef]
- Christiansen, S.H.; Murphy, R.A.; Juul-Madsen, K.; Fredborg, M.; Hvam, M.L.; Axelgaard, E.; Skovdal, S.M.; Meyer, R.L.; Sørensen, U.B.S.; Möller, A.; et al. The Immunomodulatory Drug Glatiramer Acetate Is Also an Effective Antimicrobial Agent That Kills Gram-Negative Bacteria. Sci. Rep. 2017, 7, 15653. [Google Scholar] [CrossRef]
- Arnon, R.; Aharoni, R. Mechanism of Action of Glatiramer Acetate in Multiple Sclerosis and Its Potential for the Development of New Applications. Proc. Natl. Acad. Sci. USA 2004, 101, 14593–14598. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, R.; Teitelbaum, D.; Sela, M.; Arnon, R. Copolymer 1 Induces T Cells of the T Helper Type 2 That Crossreact with Myelin Basic Protein and Suppress Experimental Autoimmune Encephalomyelitis. Proc. Natl. Acad. Sci. USA 1997, 94, 10821–10826. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for Accurate EC50/IC50 Estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Ibrahim, A.S.; Fu, Y.; Shafiq, M.C.; Edwards, J.E.; Criddle, R.S. Susceptibility Testing of Cryptococcus neoformans: A Microdilution Technique. J. Clin. Microbiol. 1992, 30, 2881–2886. [Google Scholar] [CrossRef]
- Rodriguez-Tudela, J.L. EUCAST Definitive Document EDef 7.1: Method for the Determination of Broth Dilution MICs of Antifungal Agents for Fermentative Yeasts. Clin. Microbiol. Infect. 2008, 14, 398–405. [Google Scholar] [CrossRef]
- Thermo Fisher Scientific. CyQUANT TM XTT Cell Viability Assay; Thermo Fisher Scientific: Waltham, MA, USA, 2018; Pub. No. MAN0017857 Rev. A.0; Available online: https://assets.thermofisher.com/TFS-Assets%2FLSG%2Fmanuals%2FMAN0017857_CyQUANT_XTT_Cell_Viability_Assay_UG.pdf (accessed on 24 July 2023).
- ImageJ. Available online: https://imagej.nih.gov/ij/ (accessed on 26 April 2023).
- Goldman, D.L.; Fries, B.C.; Franzot, S.P.; Montella, L.; Casadevall, A. Phenotypic Switching in the Human Pathogenic Fungus Cryptococcus neoformans Is Associated with Changes in Virulence and Pulmonary Inflammatory Response in Rodents. Proc. Natl. Acad. Sci. USA 1998, 95, 14967–14972. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Cleare, W.; Feldmesser, M.; Glatman-Freedman, A.; Goldman, D.L.; Kozel, T.R.; Lendvai, N.; Mukherjee, J.; Pirofski, L.A.; Rivera, J.; et al. Characterization of a Murine Monoclonal Antibody to Cryptococcus neoformans Polysaccharide That Is a Candidate for Human Therapeutic Studies. Antimicrob. Agents Chemother. 1998, 42, 1437–1446. [Google Scholar] [CrossRef]
- Smith, M.C.; Crist, R.M.; Clogston, J.D.; McNeil, S.E. Zeta Potential: A Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem. 2017, 409, 5779–5787. [Google Scholar] [CrossRef]
- Falke, S.; Betzel, C. Dynamic Light Scattering (DLS). In Radiation in Bioanalysis: Spectroscopic Techniques and Theoretical Methods; Pereira, A.S., Tavares, P., Limão-Vieira, P., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 173–193. [Google Scholar] [CrossRef]
- Goldburg, W.I. Dynamic Light Scaterring. Am. J. Phys. 1999, 67, 1093. [Google Scholar] [CrossRef]
- Xia, Q.; Xiao, H.; Pan, Y.; Wang, L. Microrheology, Advances in Methods and Insights. Adv. Colloid Interface Sci. 2018, 257, 71–85. [Google Scholar] [CrossRef]
- Bhat, S.; Jun, D.; Paul, B.C.; Dahms, T.E.S. Viscoelasticity in biological systems: A special focus on microbes. In Viscoelasticity—From Theory to Biological Applications; de Vicente, J., Ed.; InTech Open Science: London, UK; Rijeka, Croatia, 2012; Volume I, pp. 123–156. [Google Scholar]
- De Araújo, G.R.; Viana, N.B.; Pontes, B.; Frases, S. Rheological Properties of Cryptococcal Polysaccharide Change with Fiber Size, Antibody Binding and Temperature. Future Microbiol. 2019, 14, 867–884. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Zaragoza, O. Multiple Disguises for the Same Party: The Concepts of Morphogenesis and Phenotypic Variations in Cryptococcus neoformans. Front. Microbiol. 2011, 2, 181. [Google Scholar] [CrossRef] [PubMed]
- Frases, S.; Nimrichter, L.; Viana, N.B.; Nakouzi, A.; Casadevall, A. Cryptococcus neoformans Capsular Polysaccharide and Exopolysaccharide Fractions Manifest Physical, Chemical, and Antigenic Differences. Eukaryot. Cell 2008, 7, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Araújo, G.R.d.S.; Fontes, G.N.; Leão, D.; Rocha, G.M.; Pontes, B.; Sant’Anna, C.; de Souza, W.; Frases, S. Cryptococcus neoformans Capsular Polysaccharides Form Branched and Complex Filamentous Networks Viewed by High-Resolution Microscopy. J. Struct. Biol. 2016, 193, 75–82. [Google Scholar] [CrossRef]
- Zamvil, S.S.; Steinman, L. The T Lymphocyte in Experimental Allergic Encephalomyelitis. Annu. Rev. Immunol. 1990, 8, 579–621. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, D.; Webb, C.; Bree, M.; Meshorer, A.; Arnon, R.; Sela, M. Suppression of Experimental Allergic Encephalomyelitis in Rhesus Monkeys by a Synthetic Basic Copolymer. Clin. Immunol. Immunopathol. 1974, 3, 256–262. [Google Scholar] [CrossRef]
- Webb, C.; Teitelbaum, D.; Arnon, R.; Sela, M. In vivo and in vitro Immunological Cross-reactions between Basic Encephalitogen and Synthetic Basic Polypeptides Capable of Suppressing Experimental Allergic Encephalomyelitis. Eur. J. Immunol. 1973, 3, 279–286. [Google Scholar] [CrossRef]
- Iyer, K.R.; Revie, N.M.; Fu, C.; Robbins, N.; Cowen, L.E. Treatment Strategies for Cryptococcal Infection: Challenges, Advances and Future Outlook. Nat. Rev. Microbiol. 2021, 19, 454–466. [Google Scholar] [CrossRef]
- Lewis, J.S.; Graybill, J.R. Fungicidal versus Fungistatic: What’s in a Word? Expert Opin. Pharmacother. 2008, 9, 927–935. [Google Scholar] [CrossRef] [PubMed]
- Thi, M.T.T.; Wibowo, D.; Rehm, B.H.A. Pseudomonas aeruginosa Biofilms. Int. J. Mol. Sci. 2020, 21, 8671. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Casadevall, A. Specific Antibody Can Prevent Fungal Biofilm Formation and This Effect Correlates with Protective Efficacy. Infect. Immun. 2005, 73, 6350–6362. [Google Scholar] [CrossRef] [PubMed]
- Araújo, G.R.d.S.; Alcantara, C.d.L.; Rodrigues, N.; de Souza, W.; Pontes, B.; Frases, S. Ultrastructural Study of Cryptococcus neoformans Surface during Budding Events. Front. Microbiol. 2021, 12, 609244. [Google Scholar] [CrossRef]
- Zaragoza, O.; Rodrigues, M.L.; De Jesus, M.; Frases, S.; Dadachova, E.; Casadevall, A. Chapter 4 The Capsule of the Fungal Pathogen Cryptococcus neoformans, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 68, ISBN 9780123748034. [Google Scholar]
- McFadden, D.; Zaragoza, O.; Casadevall, A. The Capsular Dynamics of Cryptococcus neoformans. Trends Microbiol. 2006, 14, 497–505. [Google Scholar] [CrossRef]
- Martinez, L.R.; Bryan, R.A.; Apostolidis, C.; Morgenstern, A.; Casadevall, A.; Dadachova, E. Antibody-Guided Alpha Radiation Effectively Damages Fungal Biofilms. Antimicrob. Agents Chemother. 2006, 50, 2132–2136. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, V.; Martins, P.H.; Miranda, B.; de Andrade, I.B.; Pereira, L.; Maeda, C.T.; de Sousa Araújo, G.R.; Frases, S. Assessing the In Vitro Potential of Glatiramer Acetate (Copaxone®) as a Chemotherapeutic Candidate for the Treatment of Cryptococcus neoformans Infection. J. Fungi 2023, 9, 783. https://doi.org/10.3390/jof9080783
Alves V, Martins PH, Miranda B, de Andrade IB, Pereira L, Maeda CT, de Sousa Araújo GR, Frases S. Assessing the In Vitro Potential of Glatiramer Acetate (Copaxone®) as a Chemotherapeutic Candidate for the Treatment of Cryptococcus neoformans Infection. Journal of Fungi. 2023; 9(8):783. https://doi.org/10.3390/jof9080783
Chicago/Turabian StyleAlves, Vinicius, Pedro Henrique Martins, Bruna Miranda, Iara Bastos de Andrade, Luiza Pereira, Christina Takiya Maeda, Glauber Ribeiro de Sousa Araújo, and Susana Frases. 2023. "Assessing the In Vitro Potential of Glatiramer Acetate (Copaxone®) as a Chemotherapeutic Candidate for the Treatment of Cryptococcus neoformans Infection" Journal of Fungi 9, no. 8: 783. https://doi.org/10.3390/jof9080783
APA StyleAlves, V., Martins, P. H., Miranda, B., de Andrade, I. B., Pereira, L., Maeda, C. T., de Sousa Araújo, G. R., & Frases, S. (2023). Assessing the In Vitro Potential of Glatiramer Acetate (Copaxone®) as a Chemotherapeutic Candidate for the Treatment of Cryptococcus neoformans Infection. Journal of Fungi, 9(8), 783. https://doi.org/10.3390/jof9080783




