Tacrolimus (FK506) Exhibits Fungicidal Effects against Candida parapsilosis Sensu Stricto via Inducing Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Drug Susceptibility Testing
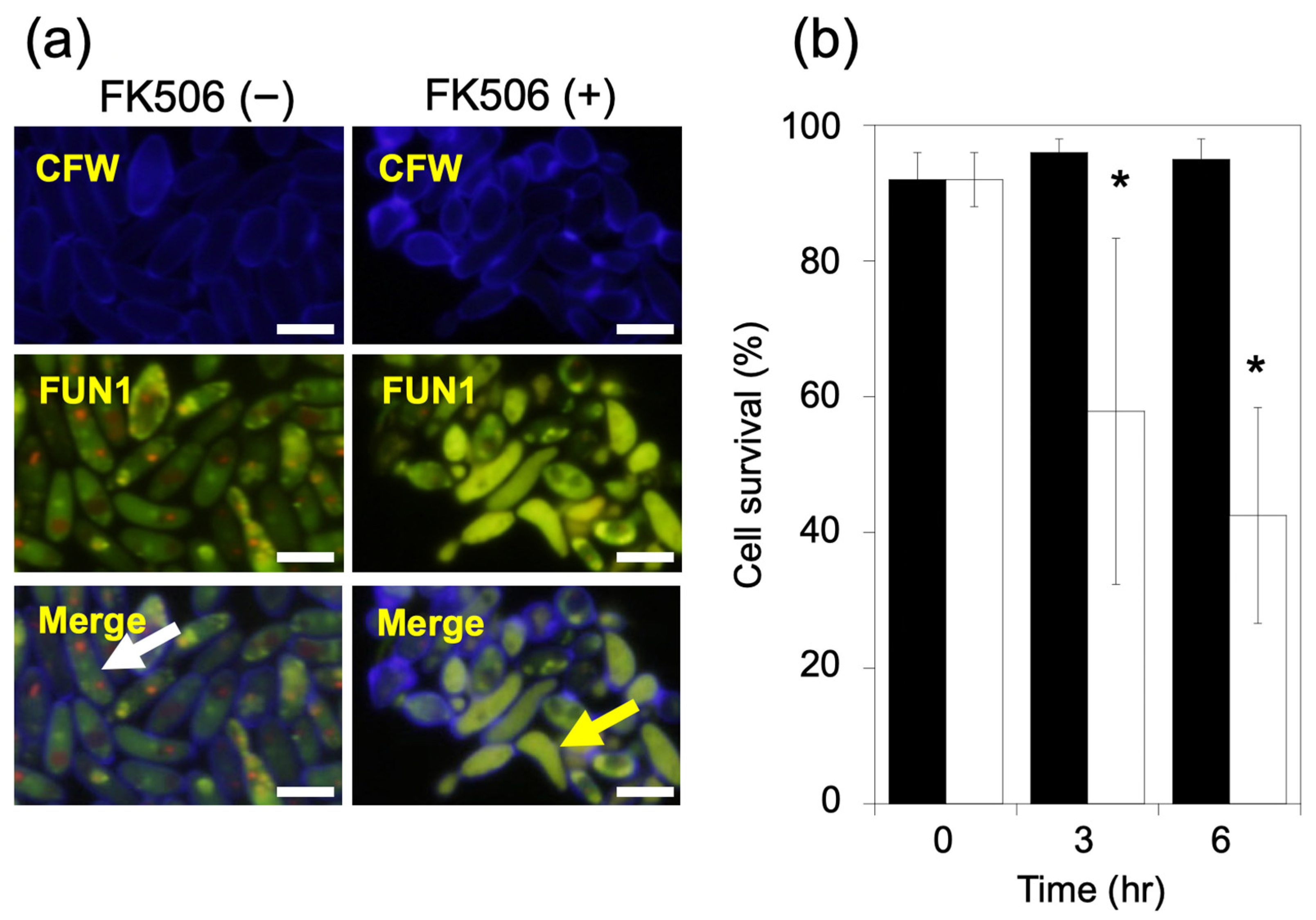
2.2. Yeast Cell Survival Assay Using Fluorescence Microscopy
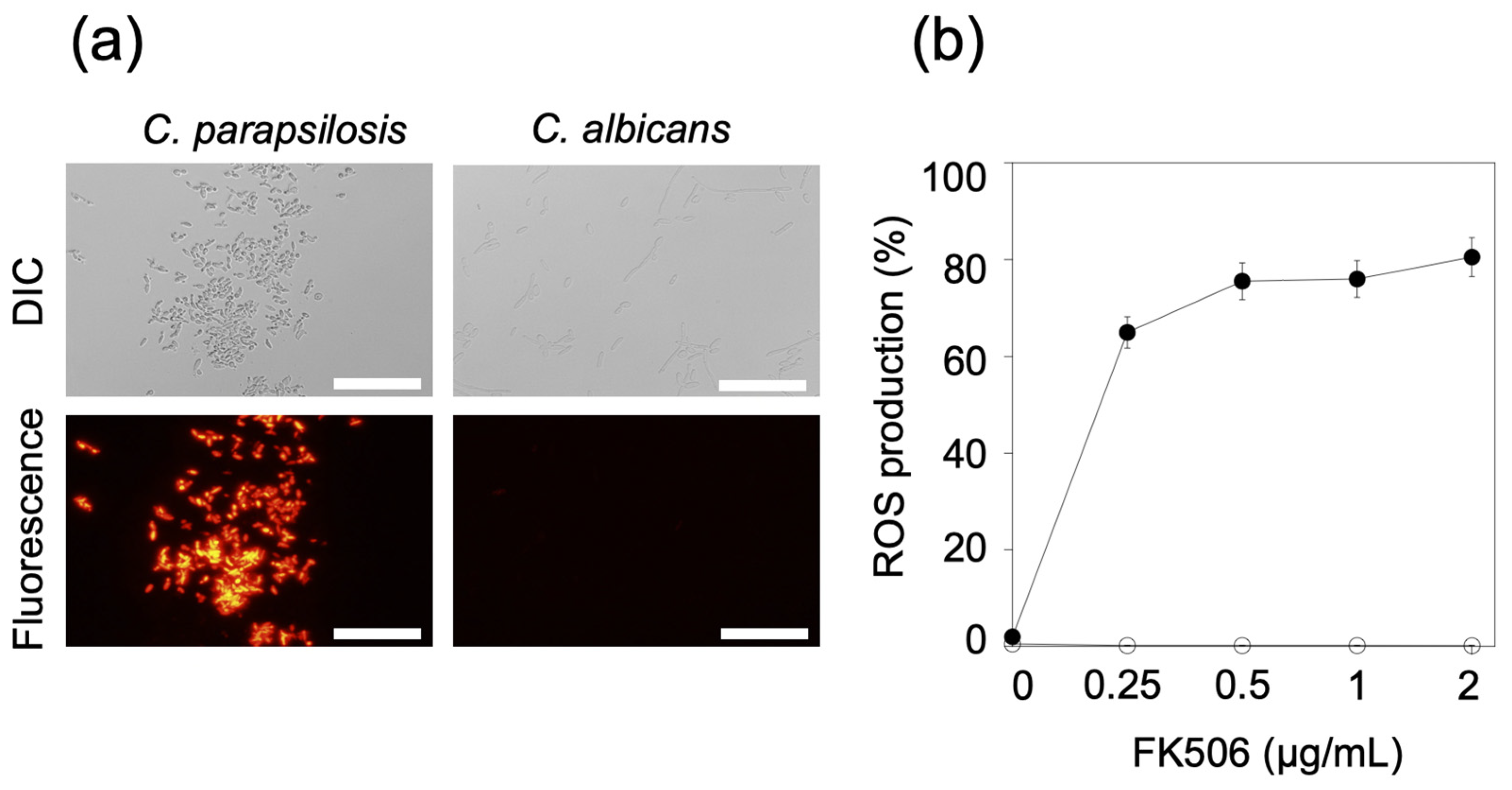
2.3. Measurement of Mitochondrial Reactive Oxygen Species
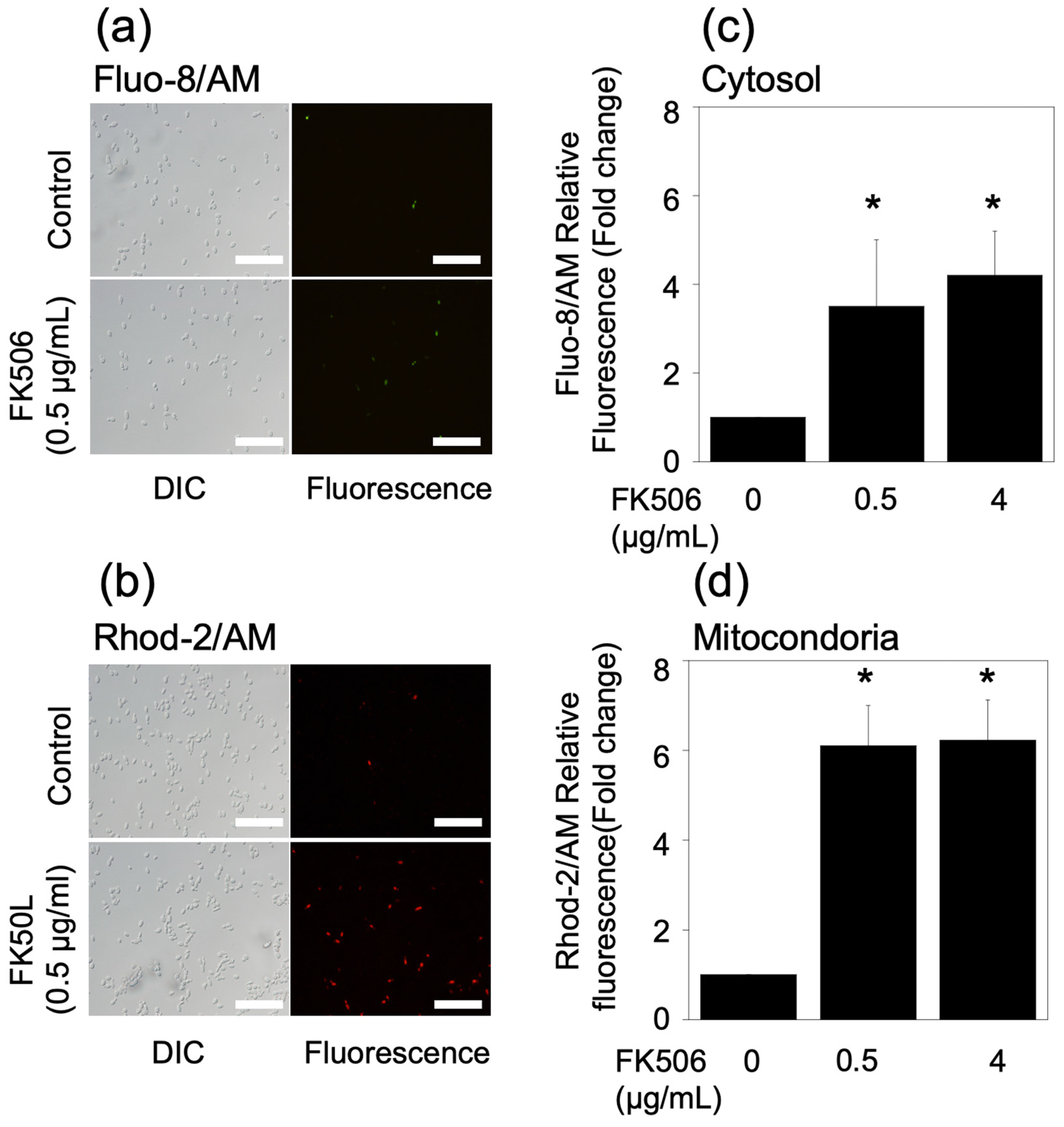
2.4. Measurement of Ca2+ Levels in Cytosol and Mitochondria
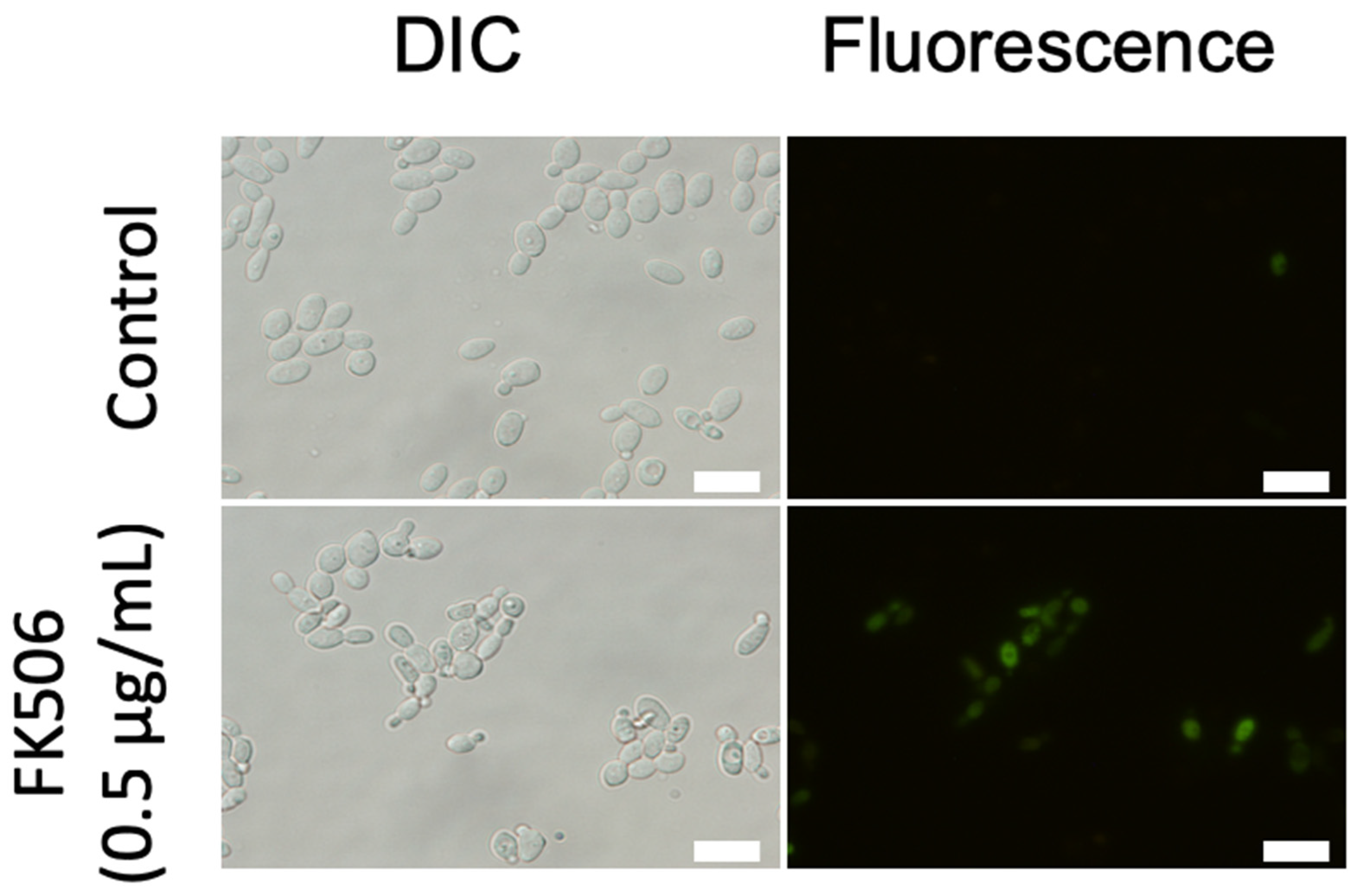
2.5. Detection of Metacaspase Activity
2.6. DNA and Nuclei Damages Assay
2.7. Statistical Analysis
3. Results
3.1. Antifungal Activity of FK506 and CsA
| Species | Number of Strains | FK506 (µg/mL) | CsA (µg/mL) | ||
|---|---|---|---|---|---|
| 27 °C | 37 °C | 27 °C | 37 °C | ||
| Candida parapsilosis sensu stricto | 15 | 0.125–0.5 | 0.125–0.5 | >8 | >8 |
| Candida metapsilosis | 4 | >8 | >8 | >8 | >8 |
| Candida orthopsilosis | 2 | >8 | >8 | >8 | >8 |
| Candida albicans | 6 | >8 | >8 | >8 | >8 |
| Candida auris | 2 | >8 | >8 | >8 | >8 |
| Candida dubliniensis | 5 | >8 | >8 | >8 | >8 |
| Candida glabrata (=Nakaseomyces glabratus) | 7 | >8 | >8 | >8 | >8 |
| Candida guilliermondii (=Meyerozyma guilliermondii) | 6 | >8 | >8 | >8 | >8 |
| Candida kefyr (=Kluyveromyces marxianus) | 4 | >8 | >8 | >8 | >8 |
| Candida krusei (=Pichia kudriavzevii) | 6 | >8 | >8 | >8 | >8 |
| Candida lusitaniae (=Clavispora lusitaniae) | 1 | >8 | >8 | >8 | >8 |
| Candida tropicalis | 7 | >8 | >8 | >8 | >8 |
| Candida viswanathii | 1 | >8 | >8 | >8 | >8 |
| Candida norvegensis (=Pichia norvegensis) | 1 | >8 | >8 | >8 | >8 |
3.2. Fluorescence Staining for Cell Viability
3.3. Mitochondrial ROS Production in FK506-Treated Cells
3.4. Measurement of Ca2+ Levels
3.5. Metacaspase Activity
3.6. Nuclear Fragmentation and DNA Damage
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Steinbach, W.J.; Reedy, J.L.; Cramer, R.A., Jr.; Perfect, J.R.; Heitman, J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 2007, 5, 418–430. [Google Scholar] [CrossRef]
- Juvvadi, P.R.; Lee, S.C.; Heitman, J.; Steinbach, W.J. Calcineurin in fungal virulence and drug resistance: Prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence 2017, 8, 186–197. [Google Scholar] [CrossRef]
- Fox, D.S.; Heitman, J. Good fungi gone bad: The corruption of calcineurin. Bioessays 2002, 24, 894–903. [Google Scholar] [CrossRef]
- Odom, A.; Muir, S.; Lim, E.; Toffaletti, D.L.; Perfect, J.; Heitman, J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997, 16, 2576–2589. [Google Scholar] [CrossRef]
- Cruz, M.C.; Del Poeta, M.; Wang, P.; Wenger, R.; Zenke, G.; Quesniaux, V.F.J.; Movva, N.R.; Perfect, J.R.; Cardenas, M.E.; Heitman, J. Immunosuppressive and No nimmunosuppressive Cyclosporine Analogs Are Toxic to the Opportunistic Fungal Pathogen Cryptococcus neoformans via Cyclophilin-Dependent Inhibition of Calcineurin. Antimicrob. Agents Chemother. 2000, 44, 143–149. [Google Scholar] [CrossRef]
- Singh, N.; Alexander, B.D.; Lortholary, O.; Dromer, F.; Gupta, K.L.; John, G.T.; del Busto, R.; Klintmalm, G.B.; Somani, J.; Lyon, G.M.; et al. Cryptococcus neoformans in Organ Transplant Recipients: Impact of Calcineurin-Inhibitor Agents on Mortality. J. Infect. Dis. 2007, 195, 756–764. [Google Scholar] [CrossRef] [PubMed]
- High, K.P.; Washburn, R.G. Invasive Aspergillosis in Mice Immunosuppressed with Cyclosporin A, Tacrolimus (FK506), or Sirolimus (Rapamycin). J. Infect. Dis. 1997, 175, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Negri, M.; Henriques, M.; Oliveira, R.; Williams, D.W.; Azeredo, J. Candida glabrata, Candida parapsilosis and Candida tropicalis: Biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol. Rev. 2012, 36, 288–305. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef]
- Nett, J.E.; Andes, D.R. Contributions of the Biofilm Matrix to Candida Pathogenesis. J. Fungi 2020, 6, 21. [Google Scholar] [CrossRef]
- Branco, J.; Miranda, I.M.; Rodrigues, A.G. Candida parapsilosis Virulence and Antifungal Resistance Mechanisms: A Comprehensive Review of Key Determinants. J. Fungi 2023, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Onyewu, C.; Blankenship, J.R.; Del Poeta, M.; Heitman, J. Ergosterol Biosynthesis Inhibitors Become Fungicidal when Combined with Calcineurin Inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 2003, 47, 956–964. [Google Scholar] [CrossRef]
- Sun, S.; Li, Y.; Guo, Q.; Shi, C.; Yu, J.; Ma, L. In Vitro Interactions between Tacrolimus and Azoles against Candida albicans Determined by Different Methods. Antimicrob. Agents Chemother. 2008, 52, 409–417. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Zhang, C.; Gao, Y.; Zhang, X.; Sun, S. Resistance reversal induced by a combination of fluconazole and tacrolimus (FK506) in Candida glabrata. J. Med. Microbiol. 2015, 64, 44–52. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; M27-A3; CLSI: Wayne, PA, USA, 2008. [Google Scholar]
- Zhou, L.D.; Lu, Y.N.; Liu, Q.; Shi, X.M.; Tian, J. Modulation of TRPV1 function by Citrus reticulata (tangerine) fruit extract for the treatment of sensitive skin. J. Cosmet. Dermatol. 2022, 22, 1369–1376. [Google Scholar] [CrossRef]
- Yun, D.G.; Lee, D.G. Silibinin triggers yeast apoptosis related to mitochondrial Ca2+ influx in Candida albicans. Int. J. Biochem. Cell Biol. 2016, 80, 1–9. [Google Scholar] [CrossRef]
- Madeo, F.; Herker, E.; Maldener, C.; Wissing, S.; Lächelt, S.; Herlan, M.; Fehr, M.; Lauber, K.; Sigrist, S.J.; Wesselborg, S.; et al. A Caspase-Related Protease Regulates Apoptosis in Yeast. Mol. Cell 2002, 9, 911–917. [Google Scholar] [CrossRef]
- Daniel, B.; De Coster, M.A. Quantification of sPLA2-induced early and late apoptosis changes in neuronal cell cultures using combined TUNEL and DAPI staining. Brain Res. Brain Res. Protoc. 2004, 13, 144–150. [Google Scholar] [CrossRef]
- Farrugia, G.; Balzan, R. Oxidative Stress and Programmed Cell Death in Yeast. Front. Oncol. 2012, 2, 64. [Google Scholar] [CrossRef]
- Makovitzki, A.; Avrahami, D.; Shai, Y. Ultrashort antibacterial and antifungal lipopeptides. Proc. Natl. Acad. Sci. USA 2006, 103, 15997–16002. [Google Scholar] [CrossRef]
- Lee, H.; Woo, E.R.; Lee, D.G. (-)-Nortrachelogenin from Partrinia scabiosaefolia elicits an apoptotic response in Candida albicans. FEMS Yeast Res. 2016, 16, fow013. [Google Scholar] [CrossRef] [PubMed]
- Munoz, A.J.; Wanichthanarak, K.; Meza, E.; Petranovic, D. Systems biology of yeast cell death. FEMS Yeast Res. 2012, 12, 249–265. [Google Scholar] [CrossRef]
- Carraro, M.; Bernardi, P. Calcium and reactive oxygen species in regulation of the mitochondrial permeability transition and of programmed cell death in yeast. Cell Calcium 2016, 60, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.G.; Tan, S.-X.; Dawes, I.W. Reactive oxygen species and yeast apoptosis. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2008, 1783, 1354–1368. [Google Scholar] [CrossRef] [PubMed]
- Kondratskyi, A.; Kondratska, K.; Skryma, R.; Prevarskaya, N. Ion channels in the regulation of apoptosis. Biochim. Biophys. Acta 2015, 1848, 2532–2546. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Hamann, A.; Brust, D.; Osiewacz, H.D. Apoptosis pathways in fungal growth, development and ageing. Trends Microbiol. 2008, 16, 276–283. [Google Scholar] [CrossRef]
- Ramsdale, M. Programmed cell death in pathogenic fungi. Biochim. Biophys. Acta 2008, 1783, 1369–1380. [Google Scholar] [CrossRef]
- Carmona-Gutierrez, D.; Eisenberg, T.; Büttner, S.; Meisinger, C.; Kroemer, G.; Madeo, F. Apoptosis in yeast: Triggers, pathways, subroutines. Cell Death Differ. 2010, 17, 763–773. [Google Scholar] [CrossRef]
- Mousavi, S.A.A.; Robson, G.D. Oxidative and amphotericin B-mediated cell death in the opportunistic pathogen Aspergillus fumigatus is associated with an apoptotic-like phenotype. Microbiology 2004, 150, 1937–1945. [Google Scholar] [CrossRef]
- Collins, J.A.; Schandl, C.A.; Young, K.K.; Vesely, J.; Willingham, M.C. Major DNA Fragmentation Is a Late Event in Apoptosis. J. Histochem. Cytochem. 1997, 45, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Tavanti, A.; Davidson, A.D.; Gow, N.A.R.; Maiden, M.C.J.; Odds, F.C. Candida orthopsilosis and Candida metapsilosis spp. nov. To Replace Candida parapsilosis Groups II and III. J. Clin. Microbiol. 2005, 43, 284–292. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, O.; Takada, S.; Odaka, T.; Futamura, S.; Kurakado, S.; Sugita, T. Tacrolimus (FK506) Exhibits Fungicidal Effects against Candida parapsilosis Sensu Stricto via Inducing Apoptosis. J. Fungi 2023, 9, 778. https://doi.org/10.3390/jof9070778
Cho O, Takada S, Odaka T, Futamura S, Kurakado S, Sugita T. Tacrolimus (FK506) Exhibits Fungicidal Effects against Candida parapsilosis Sensu Stricto via Inducing Apoptosis. Journal of Fungi. 2023; 9(7):778. https://doi.org/10.3390/jof9070778
Chicago/Turabian StyleCho, Otomi, Shintaro Takada, Takahiro Odaka, Satoshi Futamura, Sanae Kurakado, and Takashi Sugita. 2023. "Tacrolimus (FK506) Exhibits Fungicidal Effects against Candida parapsilosis Sensu Stricto via Inducing Apoptosis" Journal of Fungi 9, no. 7: 778. https://doi.org/10.3390/jof9070778
APA StyleCho, O., Takada, S., Odaka, T., Futamura, S., Kurakado, S., & Sugita, T. (2023). Tacrolimus (FK506) Exhibits Fungicidal Effects against Candida parapsilosis Sensu Stricto via Inducing Apoptosis. Journal of Fungi, 9(7), 778. https://doi.org/10.3390/jof9070778





