COVID-19 Is an Independent Risk Factor for Detrimental Invasive Fungal Disease in Patients on Veno-Venous Extracorporeal Membrane Oxygenation: A Retrospective Study
Abstract
1. Introduction
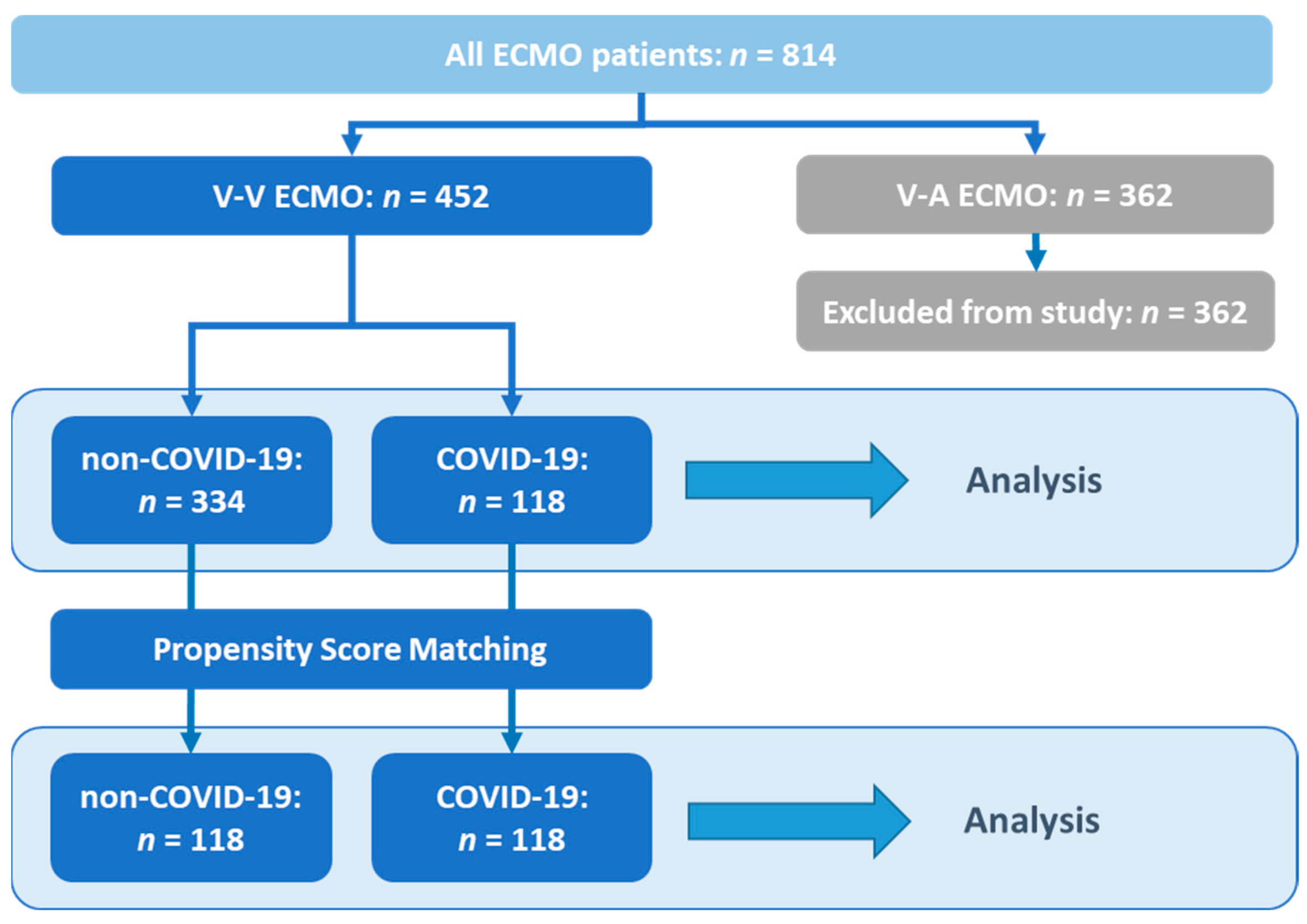
2. Materials and Methods
3. Results
3.1. Study Population and Patient Characteristics
3.2. Overall Outcome
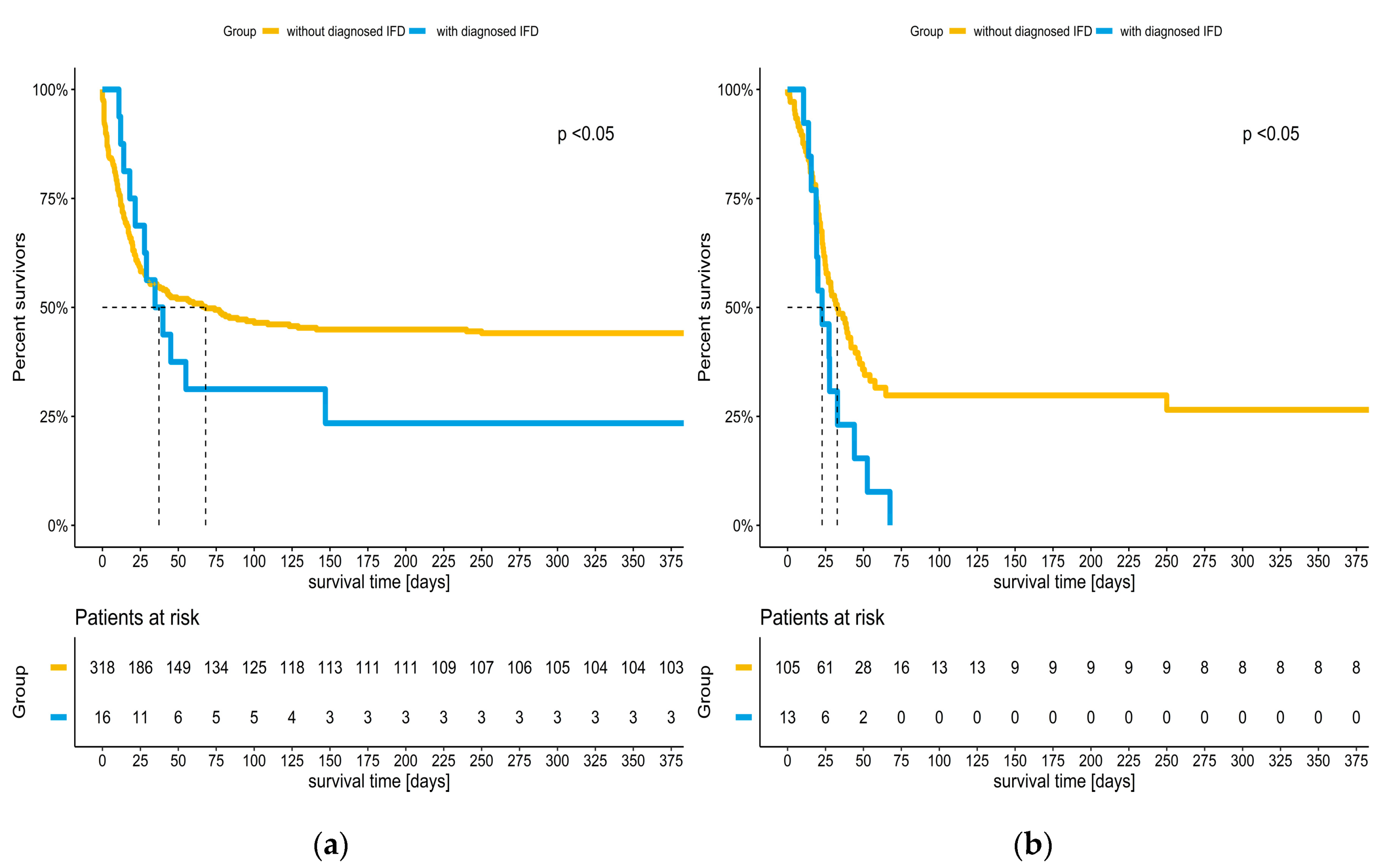
3.3. Incidence of IFD and Impact on Survival of COVID-19 Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cavayas, Y.A.; Yusuff, H.; Porter, R. Fungal Infections in Adult Patients on Extracorporeal Life Support. Crit. Care 2018, 22, 98. [Google Scholar] [CrossRef] [PubMed]
- Poth, J.M.; Schewe, J.-C.; Putensen, C.; Ehrentraut, S.F. Impact of Invasive Fungal Diseases on Survival under Veno-Venous Extracorporeal Membrane Oxygenation for ARDS. JCM 2022, 11, 1940. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive Aspergillosis in Patients Admitted to the Intensive Care Unit with Severe Influenza: A Retrospective Cohort Study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Guervilly, C.; Roch, A.; Ranque, S.; Forel, J.-M.; Hraiech, S.; Xeridat, F.; Adda, M.; Papazian, L. A Strategy Based on Galactomannan Antigen Detection and PCR for Invasive Pulmonary Aspergillosis Following Influenza A (H1N1) Pneumonia. J. Infect. 2012, 65, 470–473. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.F. Invasive Aspergillosis Associated With Severe Influenza Infections. Open Forum Infect. Dis. 2016, 3, ofw171. [Google Scholar] [CrossRef]
- Rodriguez-Goncer, I.; Thomas, S.; Foden, P.; Richardson, M.D.; Ashworth, A.; Barker, J.; Geraghty, C.G.; Muldoon, E.G.; Felton, T.W. Invasive Pulmonary Aspergillosis Is Associated with Adverse Clinical Outcomes in Critically Ill Patients Receiving Veno-Venous Extracorporeal Membrane Oxygenation. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1251–1257. [Google Scholar] [CrossRef]
- Guery, B.P.; Arendrup, M.C.; Auzinger, G.; Azoulay, É.; Borges Sá, M.; Johnson, E.M.; Müller, E.; Putensen, C.; Rotstein, C.; Sganga, G.; et al. Management of Invasive Candidiasis and Candidemia in Adult Non-Neutropenic Intensive Care Unit Patients: Part I. Epidemiology and Diagnosis. Intensive Care Med. 2009, 35, 55–62. [Google Scholar] [CrossRef]
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk Factors for Invasive Candida Infection in Critically Ill Patients. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef]
- Shishido, A.A.; Mathew, M.; Baddley, J.W. Overview of COVID-19-Associated Invasive Fungal Infection. Curr. Fungal Infect. Rep. 2022, 16, 87–97. [Google Scholar] [CrossRef]
- Hoenigl, M. Invasive Fungal Disease Complicating Coronavirus Disease 2019: When It Rains, It Spores. Clin. Infect. Dis. 2021, 73, e1645–e1648. [Google Scholar] [CrossRef]
- Baddley, J.W.; Thompson, G.R.; Chen, S.C.-A.; White, P.L.; Johnson, M.D.; Nguyen, M.H.; Schwartz, I.S.; Spec, A.; Ostrosky-Zeichner, L.; Jackson, B.R.; et al. Coronavirus Disease 2019-Associated Invasive Fungal Infection. Open Forum Infect Dis 2021, 8, ofab510. [Google Scholar] [CrossRef]
- Nuh, A.; Ramadan, N.; Nwankwo, L.; Donovan, J.; Patel, B.; Shah, A.; Desai, S.R.; Armstrong-James, D. COVID-19 Associated Pulmonary Aspergillosis in Patients on Extracorporeal Membrane Oxygenation Treatment—A Retrospective Study. J. Fungi 2023, 9, 398. [Google Scholar] [CrossRef]
- Alessandri, F.; Ceccarelli, G.; Migliara, G.; Baccolini, V.; Russo, A.; Marzuillo, C.; Ceparano, M.; Giordano, G.; Tozzi, P.; Galardo, G.; et al. High Incidence of Candidemia in Critically Ill COVID-19 Patients Supported by Veno-Venous Extracorporeal Membrane Oxygenation: A Retrospective Study. J. Fungi 2023, 9, 119. [Google Scholar] [CrossRef]
- Schmidt, M.; Bailey, M.; Sheldrake, J.; Hodgson, C.; Aubron, C.; Rycus, P.T.; Scheinkestel, C.; Cooper, D.J.; Brodie, D.; Pellegrino, V.; et al. Predicting Survival after Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Failure. The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) Score. Am. J. Respir. Crit. Care Med. 2014, 189, 1374–1382. [Google Scholar] [CrossRef]
- Le Gall, J.R.; Lemeshow, S.; Saulnier, F. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA 1993, 270, 2957–2963. [Google Scholar] [CrossRef]
- Guenther, U.; Koegl, F.; Theuerkauf, N.; Maylahn, J.; Andorfer, U.; Weykam, J.; Muders, T.; Putensen, C. Nursing workload indices TISS-10, TISS-28, and NEMS: Higher workload with agitation and delirium is not reflected. Med. Klin. Intensiv. Intensivmed. Notfmed. 2016, 111, 57–64. [Google Scholar] [CrossRef]
- Rilinger, J.; Krötzsch, K.; Bemtgen, X.; Jäckel, M.; Zotzmann, V.; Lang, C.N.; Kaier, K.; Duerschmied, D.; Supady, A.; Bode, C.; et al. Long-Term Survival and Health-Related Quality of Life in Patients with Severe Acute Respiratory Distress Syndrome and Veno-Venous Extracorporeal Membrane Oxygenation Support. Crit. Care 2021, 25, 410. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID Task Force on Practical Management of Invasive Candidiasis in Critically Ill Patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef]
- Vandewoude, K.H.; Blot, S.I.; Depuydt, P.; Benoit, D.; Temmerman, W.; Colardyn, F.; Vogelaers, D. Clinical Relevance of Aspergillus Isolation from Respiratory Tract Samples in Critically Ill Patients. Crit. Care 2006, 10, R31. [Google Scholar] [CrossRef]
- Blot, S.I.; Taccone, F.S.; Van den Abeele, A.-M.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.A.; Misset, B.; Rello, J.; et al. A Clinical Algorithm to Diagnose Invasive Pulmonary Aspergillosis in Critically Ill Patients. Am. J. Respir. Crit. Care Med. 2012, 186, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zwiener, I.; Blettner, M.; Hommel, G. Survival Analysis. Dtsch. Ärzteblatt Int. 2011, 108, 163–169. [Google Scholar] [CrossRef]
- Karagiannidis, C.; Strassmann, S.; Merten, M.; Bein, T.; Windisch, W.; Meybohm, P.; Weber-Carstens, S. High In-Hospital Mortality Rate in Patients with COVID-19 Receiving Extracorporeal Membrane Oxygenation in Germany: A Critical Analysis. Am. J. Respir. Crit. Care Med. 2021, 204, 991–994. [Google Scholar] [CrossRef] [PubMed]
- Karagiannidis, C.; Slutsky, A.S.; Bein, T.; Windisch, W.; Weber-Carstens, S.; Brodie, D. Complete Countrywide Mortality in COVID Patients Receiving ECMO in Germany throughout the First Three Waves of the Pandemic. Crit. Care 2021, 25, 413. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Lebreton, G.; Pineton de Chambrun, M.; Hékimian, G.; Chommeloux, J.; Bréchot, N.; Luyt, C.-E.; Leprince, P.; Combes, A.; Schmidt, M. Outcomes of Patients Denied Extracorporeal Membrane Oxygenation during the COVID-19 Pandemic in Greater Paris, France. Am. J. Respir. Crit. Care Med. 2021, 204, 994–997. [Google Scholar] [CrossRef]
- Lebreton, G.; Schmidt, M.; Ponnaiah, M.; Folliguet, T.; Para, M.; Guihaire, J.; Lansac, E.; Sage, E.; Cholley, B.; Mégarbane, B.; et al. Extracorporeal Membrane Oxygenation Network Organisation and Clinical Outcomes during the COVID-19 Pandemic in Greater Paris, France: A Multicentre Cohort Study. Lancet Respir. Med. 2021, 9, 851–862. [Google Scholar] [CrossRef]
- Monk, E.J.M.; Rautemaa-Richardson, R.; Felton, T.; Montalti, A.; Parkes, M.; Templeton, R.; Ashworth, A.; Garcia, M.; Barker, J.; Thomas, S. Incidence of Candidaemia in Prolonged Venovenous Extracorporeal Membrane Oxygenation. J. Hosp. Infect. 2021, 119, 49–53. [Google Scholar] [CrossRef]
- Aubron, C.; Cheng, A.C.; Pilcher, D.; Leong, T.; Magrin, G.; Cooper, D.J.; Scheinkestel, C.; Pellegrino, V. Infections Acquired by Adults Who Receive Extracorporeal Membrane Oxygenation: Risk Factors and Outcome. Infect. Control Hosp. Epidemiol. 2013, 34, 24–30. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, S.; Ko, H.H.; Ha, S.O.; Lee, S.H.; Kim, Y.K. Different Characteristics of Bloodstream Infection during Venoarterial and Venovenous Extracorporeal Membrane Oxygenation in Adult Patients. Sci. Rep. 2021, 11, 9498. [Google Scholar] [CrossRef]
- León, C.; Alvarez-Lerma, F.; Ruiz-Santana, S.; León, M.A.; Nolla, J.; Jordá, R.; Saavedra, P.; Palomar, M. EPCAN Study Group Fungal Colonization and/or Infection in Non-Neutropenic Critically Ill Patients: Results of the EPCAN Observational Study. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 233–242. [Google Scholar] [CrossRef]
- Macauley, P.; Epelbaum, O. Epidemiology and Mycology of Candidaemia in Non-Oncological Medical Intensive Care Unit Patients in a Tertiary Center in the United States: Overall Analysis and Comparison between Non-COVID-19 and COVID-19 Cases. Mycoses 2021, 64, 634–640. [Google Scholar] [CrossRef]
- Agrifoglio, A.; Cachafeiro, L.; Figueira, J.C.; Añón, J.M.; García de Lorenzo, A. Critically Ill Patients with COVID-19 and Candidaemia: We Must Keep This in Mind. J. Mycol. Med. 2020, 30, 101012. [Google Scholar] [CrossRef]
- Mastrangelo, A.; Germinario, B.N.; Ferrante, M.; Frangi, C.; Li Voti, R.; Muccini, C.; Ripa, M. COVID-BioB Study Group Candidemia in Coronavirus Disease 2019 (COVID-19) Patients: Incidence and Characteristics in a Prospective Cohort Compared With Historical Non-COVID-19 Controls. Clin. Infect. Dis. 2021, 73, e2838–e2839. [Google Scholar] [CrossRef]
- Segrelles-Calvo, G.; de S Araújo, G.R.; Llopis-Pastor, E.; Carrillo, J.; Hernández-Hernández, M.; Rey, L.; Melean, N.R.; Escribano, I.; Antón, E.; Zamarro, C.; et al. Candida Spp. Co-Infection in COVID-19 Patients with Severe Pneumonia: Prevalence Study and Associated Risk Factors. Respir. Med. 2021, 188, 106619. [Google Scholar] [CrossRef]
- White, P.L.; Dhillon, R.; Cordey, A.; Hughes, H.; Faggian, F.; Soni, S.; Pandey, M.; Whitaker, H.; May, A.; Morgan, M.; et al. A National Strategy to Diagnose Coronavirus Disease 2019–Associated Invasive Fungal Disease in the Intensive Care Unit. Clin. Infect. Dis. 2021, 73, e1634–e1644. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef]
- Gangneux, J.-P.; Dannaoui, E.; Fekkar, A.; Luyt, C.-E.; Botterel, F.; De Prost, N.; Tadié, J.-M.; Reizine, F.; Houzé, S.; Timsit, J.-F.; et al. Fungal Infections in Mechanically Ventilated Patients with COVID-19 during the First Wave: The French Multicentre MYCOVID Study. Lancet Respir. Med. 2022, 10, 180–190. [Google Scholar] [CrossRef]
- Evert, K.; Dienemann, T.; Brochhausen, C.; Lunz, D.; Lubnow, M.; Ritzka, M.; Keil, F.; Trummer, M.; Scheiter, A.; Salzberger, B.; et al. Autopsy Findings after Long-Term Treatment of COVID-19 Patients with Microbiological Correlation. Virchows Arch. 2021, 479, 97–108. [Google Scholar] [CrossRef]
- Kula, B.E.; Clancy, C.J.; Hong Nguyen, M.; Schwartz, I.S. Invasive Mould Disease in Fatal COVID-19: A Systematic Review of Autopsies. Lancet Microbe 2021, 2, e405–e414. [Google Scholar] [CrossRef]
- Fekkar, A.; Lampros, A.; Mayaux, J.; Poignon, C.; Demeret, S.; Constantin, J.-M.; Marcelin, A.-G.; Monsel, A.; Luyt, C.-E.; Blaize, M. Occurrence of Invasive Pulmonary Fungal Infections in Patients with Severe COVID-19 Admitted to the ICU. Am. J. Respir. Crit. Care Med. 2021, 203, 307–317. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.A.; Bassetti, M.; Calandra, T. ESCMID* Guideline for the Diagnosis and Management of Candida Diseases 2012: Non-Neutropenic Adult Patients. Clin. Microbiol. Infect. 2012, 18, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.; Griesel, M.; Mikolajewska, A.; Mueller, A.; Nothacker, M.; Kley, K.; Metzendorf, M.-I.; Fischer, A.-L.; Kopp, M.; Stegemann, M.; et al. Systemic Corticosteroids for the Treatment of COVID-19. Cochrane Database Syst. Rev. 2021, 2021, CD014963. [Google Scholar] [CrossRef]
- Kayaaslan, B.; Eser, F.; Kaya Kalem, A.; Bilgic, Z.; Asilturk, D.; Hasanoglu, I.; Ayhan, M.; Tezer Tekce, Y.; Erdem, D.; Turan, S.; et al. Characteristics of Candidemia in COVID-19 Patients; Increased Incidence, Earlier Occurrence and Higher Mortality Rates Compared to Non-COVID-19 Patients. Mycoses 2021, 64, 1083–1091. [Google Scholar] [CrossRef]
- Kayaaslan, B.; Kaya Kalem, A.; Asilturk, D.; Kaplan, B.; Dönertas, G.; Hasanoglu, I.; Eser, F.; Korkmazer, R.; Oktay, Z.; Ozkocak Turan, I.; et al. Incidence and Risk Factors for COVID-19 Associated Candidemia (CAC) in ICU Patients. Mycoses 2022, 65, 508–516. [Google Scholar] [CrossRef]
- The RECOVERY Collaborative Group Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [CrossRef]
- Schilling, J.; Buda, S.; Tolksdorf, K. Zweite Aktualisierung der Retrospektiven Phaseneinteilung der COVID-19- Pandemie in Deutschland. Epidemiol. Bull. 2022, 10, 3–5. [Google Scholar] [CrossRef]
- Karagiannis, F.; Peukert, K.; Surace, L.; Michla, M.; Nikolka, F.; Fox, M.; Weiss, P.; Feuerborn, C.; Maier, P.; Schulz, S.; et al. Impaired Ketogenesis Ties Metabolism to T Cell Dysfunction in COVID-19. Nature 2022, 609, 801–807. [Google Scholar] [CrossRef]
- Guillamet, C.V.; Vazquez, R.; Micek, S.T.; Ursu, O.; Kollef, M. Development and Validation of a Clinical Prediction Rule for Candidemia in Hospitalized Patients with Severe Sepsis and Septic Shock. J. Crit. Care 2015, 30, 715–720. [Google Scholar] [CrossRef]
- Michalopoulos, A.S.; Geroulanos, S.; Mentzelopoulos, S.D. Determinants of Candidemia and Candidemia-Related Death in Cardiothoracic ICU Patients. Chest 2003, 124, 2244–2255. [Google Scholar] [CrossRef]
- O’Neill, J.M.; Schutze, G.E.; Heulitt, M.J.; Simpson, P.M.; Taylor, B.J. Nosocomial Infections during Extracorporeal Membrane Oxygenation. Intensive Care Med. 2001, 27, 1247–1253. [Google Scholar] [CrossRef]
- Biffi, S.; Di Bella, S.; Scaravilli, V.; Peri, A.M.; Grasselli, G.; Alagna, L.; Pesenti, A.; Gori, A. Infections during Extracorporeal Membrane Oxygenation: Epidemiology, Risk Factors, Pathogenesis and Prevention. Int. J. Antimicrob. Agents 2017, 50, 9–16. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Logan, C.; Martin-Loeches, I.; Bicanic, T. Invasive Candidiasis in Critical Care: Challenges and Future Directions. Intensive Care Med. 2020, 46, 2001–2014. [Google Scholar] [CrossRef]
- Paphitou, N.I.; Ostrosky-Zeichner, L.; Rex, J.H. Rules for Identifying Patients at Increased Risk for Candidal Infections in the Surgical Intensive Care Unit: Approach to Developing Practical Criteria for Systematic Use in Antifungal Prophylaxis Trials. Med. Mycol. 2005, 43, 235–243. [Google Scholar] [CrossRef]
- Ostrosky-Zeichner, L.; Sable, C.; Sobel, J.; Alexander, B.D.; Donowitz, G.; Kan, V.; Kauffman, C.A.; Kett, D.; Larsen, R.A.; Morrison, V.; et al. Multicenter Retrospective Development and Validation of a Clinical Prediction Rule for Nosocomial Invasive Candidiasis in the Intensive Care Setting. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 271–276. [Google Scholar] [CrossRef]
- Blumberg, H.M.; Jarvis, W.R.; Soucie, J.M.; Edwards, J.E.; Patterson, J.E.; Pfaller, M.A.; Rangel-Frausto, M.S.; Rinaldi, M.G.; Saiman, L.; Wiblin, R.T.; et al. Risk Factors for Candidal Bloodstream Infections in Surgical Intensive Care Unit Patients: The NEMIS Prospective Multicenter Study. Clin. Infect. Dis. 2001, 33, 177–186. [Google Scholar] [CrossRef]
- Eggimann, P.; Barberini, L.; Calandra, T.; Marchetti, O. Invasive Candida Infections in the ICU: Candidiasis in the ICU. Mycoses 2012, 55, 65–72. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.S.; Castiñeiras, A.C.; Nouér, S.A. Increased Incidence of Candidemia in a Tertiary Care Hospital with the COVID-19 Pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef]

| COVID-19 Status (n) | Total (452) | Non-COVID-19 (334) | COVID-19 (118) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) upon ICU admission (median, [IQR]) | 55.9 [47.0; 64.0] | 54.9 [45.7; 64.5] | 57.4 [49.8; 62.2] | 0.519 |
| Height (cm (median [IQR])) | 175.0 [168; 180.0] | 175.0 [167.0; 180.0] | 175.0 [170.0; 180.0] | 0.192 |
| Weight (kg (median [IQR])) | 90.0 [80.0; 110.0] | 90.0 [76.2; 110.0] | 90.0 [85.0; 104.2] | 0.064 |
| BMI (kg/m2 (median [IQR])) | 29.2 [26.1; 35.1] | 29.0 [25.0; 34.8] | 29.4 [27.6; 35.1] | 0.051 |
| Gender (female (%)) | 150 (33) | 115 (34) | 35 (30) | 0.344 |
| Ventilation | ||||
| Days of IMV prior to ECMO (median [IQR]) | 2.0 [0.9; 6.0] | 2.0 [0.4; 5.0] | 3.0 [1.0; 8.8] | 0.005 |
| Total days on IMV (median [IQR]) | 28.9 [15.0; 49.0] | 27.4 [13.0; 49.0] | 31.8 [22.8; 48.4] | 0.022 |
| Tracheotomy at any time (yes (%)) | 201 (45) | 148 (44) | 53 (45) | 0.873 |
| Proning prior to ECMO (yes (%)) | 183 (40) | 99 (30) | 84 (71) | <0.001 |
| Proning on ECMO (yes (%)) | 265 (59) | 154 (46) | 111 (94) | <0.001 |
| Other Organ Dysfunction | ||||
| SAPS-II 24 h after ECMO initiation (median [IQR]) | 45.5 [38.0; 54.2] | 47.0 [38.0; 55.0] | 42.0 [38.0; 49.0] | 0.007 |
| SAPS-II upon discharge (median [IQR]) | 46.5 [31.0; 60.0] | 45.0 [30.0; 59.5] | 48.0 [35.0; 60.5] | 0.267 |
| TISS-10 24 h after ECMO initiation (median [IQR]) | 27.0 [22.2; 33.0] | 28.0 [23.0; 34.0] | 27.0 [22.0; 31.0] | 0.009 |
| TISS-10 upon discharge (median [IQR]) | 23.5 [10.0; 31.0] | 22.0 [10.0; 30.0] | 27.0 [15.0; 33.0] | 0.009 |
| SOFA score (median [IQR]) | 9.0 [7.0; 10.0] | 9.0 [7.0; 11.0] | 8.0 [6.0; 9.0] | <0.001 |
| CKRT prior to ECMO (yes (%)) | 125 (28) | 107 (32) | 18 (15) | <0.001 |
| CPR prior to ECMO (yes (%)) | 45 (10) | 42 (13) | 3 (3) | 0.002 |
| CCI (mean (SD)) | 1.6 (1.9) | 1.9 (2.0) | 0.8 (1.3) | <0.001 |
| Etiology of Respiratory Failure | ||||
| Not specified | 1 (0.2) | 1 (0.3) | ||
| Aspiration pneumonitis (n (%)) | 39 (9) | 39 (12) | ||
| Asthma (n (%)) | 4 (1) | 4 (1) | ||
| Bacterial pneumonia (n (%)) | 72 (16) | 72 (22) | ||
| Non-respiratory and chronic respiratory diagnoses (n (%)) | 25 (6) | 25 (7) | ||
| Other acute respiratory diagnosis (n (%)) | 125 (28) | 125 (37) | ||
| Trauma/burn (n (%)) | 8 (2) | 8 (2) | ||
| Viral pneumonia (n (%)) | 178 (39) | 60 (18) | 118 (100) |
| COVID-19 Status (n) | Total (452) | Non-COVID-19 (334) | COVID-19 (118) | p |
|---|---|---|---|---|
| RESP score (median [IQR]) | −1.0 [−3.0; 2.0] | −1.0 [−3.0; 2.0] | 1.0 [−1.0; 2.0] | <0.001 |
| Time on ECMO (days (median [IQR])) | 12.0 [7.6; 20.0] | 10.0 [6.0; 16.6] | 20.3 [13.8; 28.5] | <0.001 |
| ECMO weaning successful (yes (%)) | 234 (52) | 191 (57) | 43 (36) | <0.001 |
| Survival to discharge (yes (%)) | 185 (41) | 149 (45) | 36 (31) | 0.007 |
| Days from ECMO initiation to death in non-survivors (median [IQR]) | 34.0 [13.9; 382.8] | 40.7 [11.9; 551.5] | 28.1 [18.8; 50.3] | 0.029 |
| ICU: length of stay (days (median [IQR])) | 25.9 [14.0; 47.9] | 24.9 [12.9; 48.4] | 28.0 [18.4; 46.5] | 0.278 |
| Hospital: length of stay (days (median [IQR])) | 28.1 [15.0; 53.5] | 28.1 [14.1; 57.3] | 28.0 [18.4; 46.5] | 0.849 |
| COVID-19 Status (n) | Total (452) | Non-COVID-19 (334) | COVID-19 (118) | p |
|---|---|---|---|---|
| IFD during ECMO (yes (% of all patients)) | 29 (6.4) # | 16 (4.8) | 13 (11.0) | 0.018 |
| Type of IFD (n (% of IFDs in cohort)) | ||||
| Candidemia | 27 (93.1) | 16 (100.0) | 11 (84.6) | |
| C. albicans | 19 (65.5) # | 13 (81.3) | 6 (46.2) # | |
| C. glabrata | 3 (10.3) | 1 (6.25) | 2 (15.4) | |
| C. krusei | 2 (6.9) | 1 (6.25) | 1 (7.7) | |
| C. parapsilosis | 2 (6.9) | 1 (6.25) | 1 (7.7) | |
| C. kefyr | 1 (3.4) | 1 (7.7) | ||
| C. dubliniensis | 1 (3.4) # | 1 (7.7) # | ||
| Invasive aspergillosis | 2 (0.4) | 2 (15.4) | ||
| Asp. fumigatus | 1 (0.2) | 1 (7.7) | ||
| Asp. terreus | 1 (0.2) | 1 (7.7) | ||
| Time on IMV before IFD (days (median [IQR])) | 17.0 [9.0; 27.0] | 14.5 [4.8; 22.8] | 22.0 [17.0; 28.0] | 0.04 |
| Time on ECMO before IFD (days (median [IQR])) | 12.0 [0.0; 18.0] | 8.5 [0.0; 14.8] | 13.0 [6.0; 23.0] | 0.084 |
| Clearance of IFD (yes (% of all IFD)) | 18 (62.1) | 13 (81.3) | 5 (38.5) | 0.027 |
| Time to clearance (days (median [IQR])) | 3.5 [2.0; 5.0] | 4.0 [3.0; 5.0] | 2 [2.0; 2.0] | |
| Survival to discharge (yes (% of patients with IFD)) | 4 (13.8) | 4 (25.0) | 0 (0.0) | 0.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poth, J.M.; Schewe, J.-C.; Lehmann, F.; Weller, J.; Schmandt, M.W.; Kreyer, S.; Muenster, S.; Putensen, C.; Ehrentraut, S.F. COVID-19 Is an Independent Risk Factor for Detrimental Invasive Fungal Disease in Patients on Veno-Venous Extracorporeal Membrane Oxygenation: A Retrospective Study. J. Fungi 2023, 9, 751. https://doi.org/10.3390/jof9070751
Poth JM, Schewe J-C, Lehmann F, Weller J, Schmandt MW, Kreyer S, Muenster S, Putensen C, Ehrentraut SF. COVID-19 Is an Independent Risk Factor for Detrimental Invasive Fungal Disease in Patients on Veno-Venous Extracorporeal Membrane Oxygenation: A Retrospective Study. Journal of Fungi. 2023; 9(7):751. https://doi.org/10.3390/jof9070751
Chicago/Turabian StylePoth, Jens Martin, Jens-Christian Schewe, Felix Lehmann, Johannes Weller, Mathias Willem Schmandt, Stefan Kreyer, Stefan Muenster, Christian Putensen, and Stefan Felix Ehrentraut. 2023. "COVID-19 Is an Independent Risk Factor for Detrimental Invasive Fungal Disease in Patients on Veno-Venous Extracorporeal Membrane Oxygenation: A Retrospective Study" Journal of Fungi 9, no. 7: 751. https://doi.org/10.3390/jof9070751
APA StylePoth, J. M., Schewe, J.-C., Lehmann, F., Weller, J., Schmandt, M. W., Kreyer, S., Muenster, S., Putensen, C., & Ehrentraut, S. F. (2023). COVID-19 Is an Independent Risk Factor for Detrimental Invasive Fungal Disease in Patients on Veno-Venous Extracorporeal Membrane Oxygenation: A Retrospective Study. Journal of Fungi, 9(7), 751. https://doi.org/10.3390/jof9070751








