Identification and Functional Analysis of CAP Genes from the Wheat Stripe Rust Fungus Puccinia striiformis f. sp. tritici
Abstract
1. Introduction
2. Materials and Methods
2.1. Bioinformatics Analysis of Pst CAP Genes
2.2. Sequence Polymorphism Analysis of Pst CAP Proteins
2.3. Strains and Plants, Gene Cloning, and Plasmid Construction
2.4. Yeast Signal Peptide Secretion Validation
2.5. RNA Isolation and RT-qPCR Analysis
2.6. BSMV-Mediated Gene Silencing of PsCAP Genes
3. Results
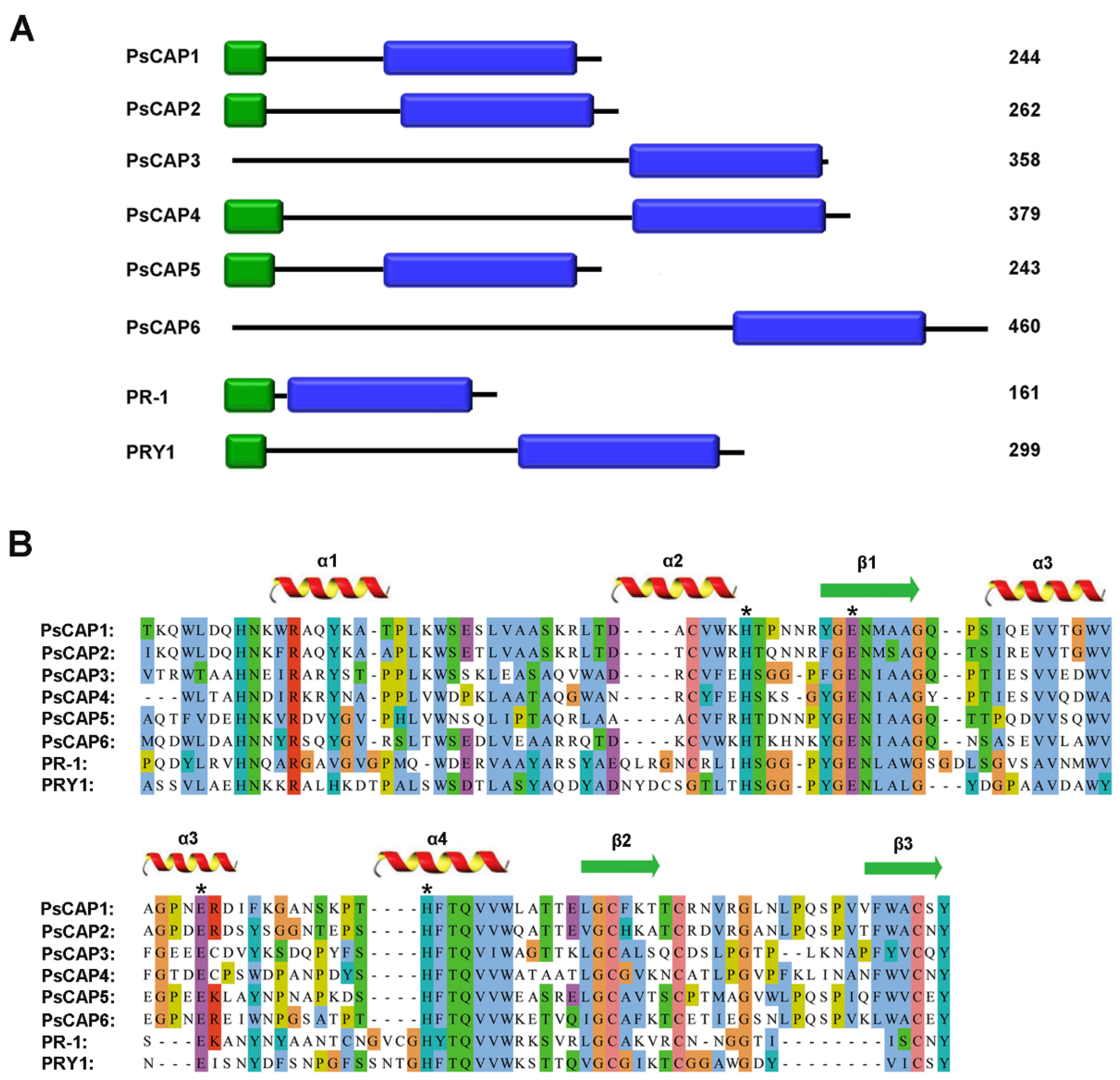
3.1. Pst Contains Six CAP Genes That Are Specifically Expanded in Rust Fungi
3.2. Intraspecific Variation of PsCAP Genes
3.3. Secretion Validation of Predicted Signal Peptides of CAP Proteins
3.4. PsCAP Genes Are Highly Expressed in Pst Parasitic Stages
3.5. Three PsCAP Genes Are Required for Pst Infection on Wheat
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gibbs, G.; Roelants, K.; O’Bryan, M. The CAP superfamily: Cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins-roles in reproduction, cancer, and immune defense. Endocr. Rev. 2008, 29, 865–897. [Google Scholar] [CrossRef] [PubMed]
- Schneiter, R.; Di Pietro, A. The CAP protein superfamily: Function in sterol export and fungal virulence. Biomol. Concepts 2013, 4, 519–525. [Google Scholar] [CrossRef]
- Choudhary, V.; Schneiter, R. Pathogen-Related Yeast (PRY) proteins and members of the CAP superfamily are secreted sterol-binding proteins. Proc. Natl. Acad. Sci. USA. 2012, 109, 16882–16887. [Google Scholar] [CrossRef] [PubMed]
- Prados-Rosales, R.; Roldán-Rodríguez, R.; Serena, C.; López-Berges, M.; Guarro, J.; Martínez-del-Pozo, A.; Di Pietro, A. A PR-1-like Protein of Fusarium oxysporum functions in virulence on mammalian hosts. J. Biol. Chem. 2012, 287, 21970–21979. [Google Scholar] [CrossRef] [PubMed]
- Rohm, M.; Lindemann, E.; Hiller, E.; Ermert, D.; Lemuth, K.; Trkulja, D.; Sogukpinar, O.; Brunner, H.; Rupp, S.; Urban, C.F.; et al. A family of secreted pathogenesis-related proteins in Candida albicans. Mol. Microbiol. 2013, 87, 132–151. [Google Scholar] [CrossRef]
- Teixeira, P.J.; Thomazella, D.P.; Vidal, R.O.; Prado, P.F.; Reis, O.; Baroni, R.M.; Franco, S.F.; Mieczkowski, P.; Pereira, G.A.; Mondego, J.M. The fungal pathogen Moniliophthora perniciosa has genes similar to plant PR-1 that are highly expressed during its interaction with cacao. PLoS ONE 2012, 7, e45929. [Google Scholar] [CrossRef]
- Lu, S.; Edwards, M.C. Molecular characterization and functional analysis of PR-1-like proteins identified from the wheat head blight fungus Fusarium graminearum. Phytopathology 2018, 108, 510–520. [Google Scholar] [CrossRef]
- Han, Z.; Xiong, D.; Xu, Z.; Liu, T.; Tian, C. The Cytospora chrysosperma virulence effector CcCAP1 mainly localizes to the plant nucleus to suppress plant immune responses. mSphere 2021, 6, e00883-e00820. [Google Scholar] [CrossRef]
- Begum, S.; Iqbal, M.; Ahmed, I.; Fayyaz, M.; Shahzad, A.; Ali, G.M. Allelic variation at loci controlling stripe rust resistance in spring wheat. J. Genet. 2014, 93, 579–586. [Google Scholar] [CrossRef]
- Chen, W.; Wellings, C.; Chen, X.; Kang, Z.; Liu, T. Wheat stripe (yellow) rust caused by Puccinia striiformis sp. tritici. Mol. Plant Pathol. 2014, 15, 433–446. [Google Scholar] [CrossRef]
- Wellings, C.R. Global status of stripe rust: A review of historical and current threats. Euphytica 2011, 179, 129–141. [Google Scholar] [CrossRef]
- Chen, X. Pathogens which threaten food security: Puccinia striiformis, the wheat stripe rust pathogen. Food Secur. 2020, 12, 239–251. [Google Scholar] [CrossRef]
- Petersen, R.H. The rust fungus life cycle. Bot. Rev. 1974, 40, 453–513. [Google Scholar] [CrossRef]
- Gan, P.H.; Dodds, P.N.; Hardham, A.R. Plant Infection by biotrophic fungal and oomycete pathogens. In Signaling and Communication in Plant Symbiosis; Perotto, S., Baluška, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 183–212. [Google Scholar]
- Cheng, Y.; Wang, X.; Yao, J.; Voegele, R.T.; Zhang, Y.; Wang, W.; Huang, L.; Kang, Z. Characterization of protein kinase PsSRPKL, a novel pathogenicity factor in the wheat stripe rust fungus. Environ. Microbiol. 2015, 17, 2601–2617. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.; Song, N.; Zhao, M.; Liu, R.; Feng, H.; Wang, X.; Kang, Z. Pst-milR1, an important pathogenicity factor of Puccinia striiformis (Pst), impairs wheat resistance to Pst by suppressing wheat pathogenesis related gene 2. N. Phytol. 2017, 215, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Xu, Q.; Zhao, J.; Yue, M.; Wang, J.; Wang, X.; Kang, Z.; Wang, X. A rust fungus effector directly binds plant pre-mRNA splice site to reprogram alternative splicing and suppress host immunity. Plant Biotechnol. J. 2022, 20, 1167–1181. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.; Clamp, M.; Barton, G.J. Jalview Version 2-multiple sequence alignment editor and analysis workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef]
- Holzberg, S.; Brosio, P.; Gross, C.; Pogue, G.P. Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J. 2002, 30, 315–327. [Google Scholar] [CrossRef]
- Jacobs, K.A.; Collins-Racie, L.A.; Colbert, M.; Duckett, M.K.; Golden-Fleet, M.; Kelleher, K.; Kriz, R.; LaVallie, E.; Merberg, D.; Spaulding, V.; et al. A genetic selection for isolating cDNAs encoding secreted proteins. Gene 1997, 198, 289–296. [Google Scholar] [CrossRef]
- Oh, S.-K.; Young, C.; Lee, M.; Oliva, R.; Bozkurt, T.; Cano, L.; Win, J.; Bos, J.; Liu, H.; Damme, M.; et al. In planta expression screens of Phytophthora infestans RXLR effectors reveal diverse phenotypes, including activation of the Solanum bulbocastanum disease resistance protein Rpi-blb2. Plant Cell Online 2009, 21, 2928–2947. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Schiestl, R.H.; Willems, A.R.; Woods, R.A. Studies on the transformation of intact yeast cells by the liac/ss-dna/peg procedure. Yeast 1995, 11, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Kale, S.D.; Wang, Q.; Wang, D.; Pan, Q.; Cao, H.; Meng, Y.; Kang, Z.; Tyler, B.; Shan, W. Rust secreted protein Ps87 is conserved in diverse fungal pathogens and contains a RXLR-like motif sufficient for translocation into plant cells. PLoS ONE 2011, 6, e27217. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Huang, L.; Buchenauer, H. Ultrastructural changes and localization of lignin and callose in compa and tible and incompatible interactions between wheat and Puccinia striiformis. J. Plant Dis. Prot. 2002, 109, 25–37. [Google Scholar]
- Zhang, Y.; Qu, Z.; Zheng, W.; Liu, B.; Wang, X.; Xue, X.; Xu, L.; Huang, L.; Han, Q.; Zhao, J.; et al. Stage-specific gene expression during urediniospore germination in Puccinia striiformis f. sp. tritici. BMC Genom. 2008, 9, 203. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, L.; Wang, Z.; Chen, X.; Zhang, H.; Yao, J.; Zhan, G.; Chen, W.; Huang, L.; Kang, Z. Identification of eighteen berberis species as alternate hosts of Puccinia striiformis f. sp tritici and virulence variation in the pathogen isolates from natural infection of barberry plants in China. Phytopathology 2013, 103, 927–934. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Breen, S.; Williams, S.J.; Outram, M.; Kobe, B.; Solomon, P.S. Emerging insights into the functions of pathogenesis-related protein 1. Trends Plant Sci. 2017, 22, 871–879. [Google Scholar] [CrossRef]
- Niderman, T.; Genetet, I.; Bruyere, T.; Gees, R.; Stintzi, A.; Legrand, M.; Mosinger, E. Pathogenesis-related PR-1 proteins are antifungal. Isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans. Plant Physiol. 1995, 108, 17–27. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Kammen, A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. ‘Samsun’ and ‘Samsun NN’: II. Changes in protein constitution after infection with tobacco mosaic virus. Virology 1970, 40, 199–211. [Google Scholar] [CrossRef]
- Uknes, S.; Mauch-Mani, B.; Moyer, M.; Potter, S.; Williams, S.; Dincher, S.; Chandler, D.; Slusarenko, A.; Ward, E.; Ryals, J. Acquired resistance in Arabidopsis. Plant Cell 1992, 4, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Gojobori, T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol. Biol. Evol. 1986, 3, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.; Gibbs, G.M.; Merriner, D.J.; Kerr, J.B.; O’Bryan, M.K. Cysteine-rich secretory proteins are not exclusively expressed in the male reproductive tract. Dev. Dyn. 2008, 237, 3313–3323. [Google Scholar] [CrossRef] [PubMed]
- Loon, L.C.V.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Nowara, D.; Gay, A.; Lacomme, C.; Shaw, J.; Ridout, C.; Douchkov, D.; Hensel, G.; Kumlehn, J.; Schweizer, P. HIGS: Host-induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell 2010, 22, 3130. [Google Scholar] [CrossRef] [PubMed]
- Asojo, O.A.; Goud, G.; Dhar, K.; Loukas, A.; Zhan, B.; Deumic, V.; Liu, S.; Borgstahl, G.; Hotez, P. X-ray structure of Na-ASP-2, a pathogenesis-related-1 protein from the nematode parasite, Necator americanus, and a vaccine antigen for human hookworm infection. J. Mol. Biol. 2005, 346, 801–814. [Google Scholar] [CrossRef]
- Serrano, R.L.; Kuhn, A.; Hendricks, A.; Helms, J.B.; Sinning, I.; Groves, M.R. Structural analysis of the human Golgi-associated plant pathogenesis related protein GAPR-1 implicates dimerization as a regulatory mechanism. J. Mol. Biol. 2004, 339, 173–183. [Google Scholar] [CrossRef]
- Szyperski, T.; Fernandez, C.; Mumenthaler, C.; Wüthrich, S.K. Structure comparison of human glioma pathogenesis-related protein GliPR and the plant pathogenesis-related protein P14a indicates a functional link between the human immune system and a plant defense system. Proc. Natl. Acad. Sci. USA 1998, 95, 2262–2266. [Google Scholar] [CrossRef]
- Eberle, H.B.; Serrano, R.L.; Füllekrug, J.; Schlosser, A.; Lehmann, W.D.; Lottspeich, F.; Kaloyanova, D.; Wieland, F.T.; Helms, J.B. Identification and characterization of a novel human plant pathogenesis-related protein that localizes to lipid-enriched microdomains in the Golgi complex. J. Cell Sci. 2002, 115, 827–838. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, K.; Yao, J.; Li, S.; Wang, X.; Huang, L.; Kang, Z. PSTha5a23, a candidate effector from the obligate biotrophic pathogen Puccinia striiformis f. sp. tritici, is involved in plant defense suppression and rust pathogenicity. Environ. Microbiol. 2017, 19, 1717–1729. [Google Scholar] [CrossRef]
- Wei, J.; Wang, X.; Hu, Z.; Wang, X.; Wang, J.; Wang, J.; Huang, X.; Kang, Z.; Tang, C. Puccinia striiformis effector Hasp98 facilitates pathogenicity by blocking the kinase activity of wheat TaMAPK4. J. Integr. Plant Biol. 2023, 65, 249–264. [Google Scholar] [CrossRef]
- Braun, B.R.; Head, W.S.; Wang, M.X.; Johnson, A.D. Identification and characterization of TUP1-regulated genes in Candida albicans. Genetics 2000, 156, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yin, Z.; Nie, J.; Lin, Y.; Huang, L. Identification and virulence analysis of CAP superfamily genes in Valsa mali. Sci. Agric. Sin. 2021, 54, 3440–3450. [Google Scholar]
- Gamir, J.; Darwiche, R.; van’t, P.; Choudhary, V.; Stumpe, M.; Schneiter, R.; Mauch, F. The sterol-binding activity of PATHOGENESIS-RELATED PROTEIN 1 reveals the mode of action of an antimicrobial protein. Plant J. 2017, 89, 502–509. [Google Scholar] [CrossRef] [PubMed]
| Single Nucleotide Polymorphisms (SNPs) a | Compute Overall Mean Distance Estimation e | |||||
|---|---|---|---|---|---|---|
| Gene Name | NS b | NNS c | Insertion /Deletin d | dN | dS | dN/dS |
| PsCAP1 | 3 | 1 | — | 0.0005 | 0.0033 | 0.15 |
| PsCAP2 | 6 | 3 | — | 0.0014 | 0.0045 | 0.31 |
| PsCAP3 | 12 | 2 | — | 0.0007 | 0.0185 | 0.04 |
| PsCAP4 | 3 | 1 | 15 | 0.0006 | 0.0029 | 0.21 |
| PsCAP5 | 10 | 3 | — | 0.0019 | 0.0168 | 0.11 |
| PsCAP6 | 9 | 2 | — | 0.0009 | 0.0092 | 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Zhang, Y.; Guo, H.; Gan, P.; Cai, M.; Kang, Z.; Cheng, Y. Identification and Functional Analysis of CAP Genes from the Wheat Stripe Rust Fungus Puccinia striiformis f. sp. tritici. J. Fungi 2023, 9, 734. https://doi.org/10.3390/jof9070734
Zhao M, Zhang Y, Guo H, Gan P, Cai M, Kang Z, Cheng Y. Identification and Functional Analysis of CAP Genes from the Wheat Stripe Rust Fungus Puccinia striiformis f. sp. tritici. Journal of Fungi. 2023; 9(7):734. https://doi.org/10.3390/jof9070734
Chicago/Turabian StyleZhao, Mengxin, Yanhui Zhang, Hualong Guo, Pengfei Gan, Mengmeng Cai, Zhensheng Kang, and Yulin Cheng. 2023. "Identification and Functional Analysis of CAP Genes from the Wheat Stripe Rust Fungus Puccinia striiformis f. sp. tritici" Journal of Fungi 9, no. 7: 734. https://doi.org/10.3390/jof9070734
APA StyleZhao, M., Zhang, Y., Guo, H., Gan, P., Cai, M., Kang, Z., & Cheng, Y. (2023). Identification and Functional Analysis of CAP Genes from the Wheat Stripe Rust Fungus Puccinia striiformis f. sp. tritici. Journal of Fungi, 9(7), 734. https://doi.org/10.3390/jof9070734






