Abstract
Eumycetoma is an infectious disease caused by various fungal pathogens. The disease is characterised by black and pale-yellowish grain discharge. In this communication, we report a case of eumycetoma with a pale grain foot-eumycetoma caused by Fusarium falciforme. The patient presented at the outpatient clinic of the Mycetoma Research Centre in Sudan. The causative agent was initially misidentified as Aspergillus nidulans based on its seemingly similar histopathological appearance. However, sequencing the internally transcribed spacer region of the extracted grain confirmed infection with Fusarium falciforme. Although the patient received Itraconazole and underwent surgical excision, the disease was recurrent. To our knowledge, this is the first report on Fusarium falciforme causing eumycetoma in Sudan, indicating the expansion of the geographical distribution of this pathogen. This calls for raising the awareness of healthcare providers and improving the diagnostic and surveillance systems in at-risk areas to improve the case management and reduce the threat of further spread. Considering the potential impacts of F. falciforme infection including threatening the global health, food security, and ecosystem balance, as well as loss of biodiversity and negative socioeconomic changes in endemic countries, we recommend the implementation of an integrated transdisciplinary One Health strategy for the prevention and control of emerging infectious diseases including F. falciforme.
1. Introduction
Mycetoma is a neglected tropical disease of serious public health concern in endemic countries in Asia, Latin America, and Africa including Sudan. This progressive destructive disease can be caused by either bacteria (actinomycetoma) or fungi (eumycetoma) infection [1]. The disease is characterised by painless subcutaneous swelling, multiple sinuses, seropurulent discharge, and the presence of grains. Additionally, the disease usually starts as a small lesion on the affected site, which is usually the foot, after the inoculation of the causative agents. However, inside the body of the host, the pathogen population clusters into granule-like structures called grains of various colours depending to the causative agents [1,2,3,4]. Therefore, the structure and colour of the grains are commonly used by healthcare providers as diagnostic features.
Eumycetoma is the most prevalent type of mycetoma in certain countries around the world including India, Senegal, and Sudan and has serious clinical courses including being challenging to treat [3]. Infection is usually acquired by introducing infectious fungi through broken skin including traumatic inoculation or through contamination of an open wound [1]. Interestingly, eumycetoma can be further subdivided into two groups according to the colour of the grains: pale-grained eumycetoma and black-grained eumycetoma. Among the black-grained eumycetoma causative agents, the most prevalent species is Madurella mycetomatis, followed by Falciformispora senegalensis and Trematosphaeria grisea, while the most prevalent pale-grained eumycetoma agents are Scedosporium boydii, Acremonium falciforme, Neotestudina rosatii, and Fusarium spp. [1,2,3].
There are limited cases of eumycetoma due to Fusarium spp. reported worldwide [5]. However, they are increasingly emerging, and infections are associated with a wide spectrum of localised diseases such as keratitis, onychomycosis, cellulitis, and mycetoma, as well as disseminated infections that are usually fatal [5]. Clinically, eumycetoma caused by Fusarium spp. is indistinguishable from other mycetoma infections [6,7,8,9,10,11,12,13,14,15,16,17,18,19]. Therefore, robust diagnostic techniques are needed for the early detection of this rare infection. To date, eumycetoma caused by Fusarium spp. has never been reported in Sudan.
In this communication, we report the first-ever case of eumycetoma caused by Fusarium falciforme in Sudan.
2. Case Report
A 40-year-old housewife from the White Nile State, central Sudan (Figure 1), was presented to the outpatient clinic at the Mycetoma Research Centre (MRC) in December 2018. The patient was complaining from recurrent painless swelling of the right foot. Her condition started three years prior to presentation with slight, painless swelling of the right foot in the medial aspect overlying the foot arch, measuring 4 cm × 5 cm in diameter. The infection had a gradual onset and progressed to affecting multiple sinuses and causing seropurulent discharge containing yellowish grains. The patient reported that she previously underwent a surgical excision under local anaesthesia elsewhere. Nevertheless, no other treatment was prescribed to the patient. She did not recall any previous history of local trauma or a family history of a similar health condition. The initial clinical examinations, medical history, and socioeconomic investigations were determined not to be related to the recent complaint; however, her geographical area of residency, White Nile State, is endemic to other mycetoma infections (Figure 1).
Figure 1.
Map of Sudan indicates the original location of the first case of Fusarium falciforme eumycetoma in Sudan; White Nile State highlighted in red.
She had a normal pulse rate (76/min), respiratory rate (17/min), blood pressure (122/80), and temperature (37 °C). Systemic examinations including cardiovascular (CVS), respiratory, central nervous system (CNS), endocrine, and gastrointestinal (GIT) were all within the normal range. Local examination of the affected limb revealed a firm, painless, non-compressible, and non-pulsatile mass of less than 10 cm in size fixed to the deep structures and skin; moreover, the skin was normal and no hypo or hyperpigmentation was noticed. There were multiple active and healed sinuses and discharge. Nonetheless, no regional lymphadenopathy was detected.
Examining the patient liver functions showed a serum bilirubin level of 0.4 mg/dL, total protein of 7.6 g/d/L, and serum albumin level of 4.9 g/dL. Moreover, the tests revealed an alkaline phosphatase level of 91 U/L, aspartate aminotransferase (AST) level of 17 U/L, and alanine aminotransferase (ALT) level of 21 U/L. Testing renal functions of the patient revealed normal blood urea of 23 mg/dL and serum creatinine of 0.43 mg/dL. Her random blood glucose was 123 mg/dL. Furthermore, additional complete blood count examinations reported a normal total white blood cell count (WBCs) of 9.0 × 103, haemoglobin count of 14.1 g/dL, and platelet count of 355 × 103. Viral screening produced negative results for human immunodeficiency virus (HIV) as well as hepatitis B and C.
Moreover, ultrasound examination showed multiple superficial cavities containing fluid and echogenic thick-walled grains, which aligns with eumycetoma (Figure 2A). An X-ray of the infection site confirmed the existence of soft tissue swelling with no bone involvement (Figure 2B).
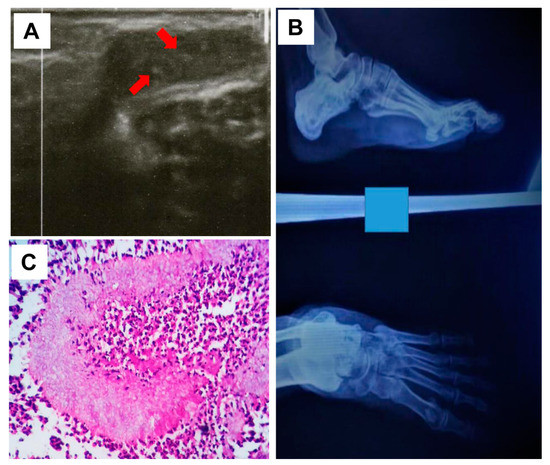
Figure 2.
Ultrasound imaging of the infection site with multiple cavities and sharp echoes in line with mycetoma grains pointed by the red arrows (A), and X-ray showing the affected (left) foot confirming the lack of bone involvement (B). (C) Grains of Fusarium falciforme stained a pale colour with H&E and surrounded by neutrophils and epithelioid cells (H and E; 40X).
A lesional deep surgical excisional biopsy (SEB) was performed to collect a sample that was taken and split into three parts; two parts were immersed in a sterile normal saline solution to be reserved for molecular diagnosis and mycology characterization. The third part of the sample was preserved in 10% neutral buffer formalin and sent to a histopathology laboratory for further processing and examinations.
The report from the histopathology and macroscopical examination showed a normal epidermis. The lesion had ill-defined margins, with pale structures appearing at the centre of each pocket. A formalin-fixed, paraffin-embedded (FFPE) tissue block was prepared from the surgical biopsy. The tissue block was cut using a rotary microtome (Leica, Wetzlar, Germany) and 3 µm sections were subsequently obtained. The sections were stained using haematoxylin and eosin (H and E) staining. A microscopic examination performed using a light microscope on the stained sections of the sample revealed the presence of a pale grain surrounded by a zone of inflammatory cells. It also showed the existence of predominantly polymorphonuclear cells, lymphocytes, plasma cells, macrophages, and epitheloid cells (Figure 2C). However, due to this presentation, it was initially misidentified as Aspergillus nidulans.
Nevertheless, we attempted to culture the extracted grains to isolate the pathogen for further microbiological characterization, using the samples that were preserved in sterile normal saline. The grains were extracted from the tissue using sterile blades and washed three times in sterile normal saline. Then, the grains were cultivated using selective and non-selective media including blood agar (BA) and Sabaroud dextrose agar (SDA) supplemented with chloramphenicol (0.05 g/L). After that, the grains were incubated at a general culturing condition of 37 °C. The culture was monitored for growth every three days; however, no growth was observed after a two-month incubation period. Therefore, the grains were properly disposed of along with the culture media according to our laboratory protocol. Therefore, unfortunately, no growth was obtained. This in turn, limited our ability to evaluate and test the susceptibility of the pathogens to Itraconazole, the currently recommended treatment for eumycetoma infection in Sudan.
To confirm the causative agent, we extracted the total DNA from the grains that were obtained by SEB using the ZR Fungal/Bacterial DNA MiniPrep ™ kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s guidelines as described previously [6,7]. Amplification and sequencing of the rDNA Internal Transcribed Spacer (ITS) region were performed according to the manufacturer’s instructions [8]. To identify the causative agent at the species level, the PCR product generated with the pan-fungal ITS primers was sequenced with the BigDye Terminator v3.1 Cycle Sequencing kit on an ABI 3130 Genetic Analyzer capillary sequencer according to the manufacturer’s instructions. The sequences were analysed using Chromas software (Technelysium Pty Ltd., South Brisbane, Australia), and a Blast search was performed on the publicly available database (https://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed date 13 March 2023) to identify the causative agent according to the similarity score.
The ITS sequence was 100% identical to the Fusarium falciforme strain CFE-139 with the accession number MN653259 and 99.81% identical to the F. falciforme-type strain CBS 475.67 with the accession number MG189935. Our sequences were deposited to the GenBank database under the accession number OQ200549.
Accordingly, the patient was given 200 mg of Itraconazole twice daily and 5 mg of folic acid daily. Per the treatment standard protocol at the MRC, the patient was followed-up every six weeks in the outpatient clinic to monitor the treatment progress, development of side effects, and clinical outcome. In December 2021, the patient underwent lesional wide surgical excision and was discharged with an uneventful postoperative recovery on the medical treatment. In May 2022, during a follow-up visit, the patient underwent a lesional ultrasound examination that showed no evidence of mycetoma recurrence. A second ultrasound examination was performed three months later; unfortunately, disease recurrence was confirmed. The patient was advised to continue on 200 mg Itraconazole twice daily and 5 mg folic acid daily, and they are currently undergoing clinical monitoring. However, in response to expert feedback during the revision of this report, we are currently exploring the possibility of updating the treatment plan by prescribing Voriconazole to the patient, though this depends on our ability to secure the drug, which is currently unavailable in the country.
3. Literature Review to Improve Our Case Management
Our extensive literature review revealed that different antifungal regimens were used to treat fusariosis. Available details about previously reported cases of Fusarium eumycetoma are summarised in Table 1.

Table 1.
Geographical distribution, medical characteristics, treatment, and clinical outcome of previously reported cases of fusariosis.
However, azoles were still the most commonly used drugs. Itraconazole was used in 31.4% of the reported cases, followed by ketoconazole (11.4%) and amphotericin B (8.6%). Furthermore, the treatment outcome differed from patient to patient. Some patients were cured, while others developed postoperative disease recurrence. Moreover, for all the reported patients, combined antifungal therapy and surgical excision were implemented (Table 1). Nevertheless, this variation in the treatment plan could be attributed to the limitations in drug availability in the country, similar to our current situation. This underscores the urgent need for novel drugs for eumycetoma that do not require invasive surgery to reduce the potential complications that sometimes lead to disability.
4. Discussion
In this communication, we report the first case of a rare eumycetoma caused by Fusarium falciforme in Sudan. F. falciforme is a member of the Nectriaceae family and hypocreales order. Fungi species causing eumycetoma belong to eight different orders, with Diaporthales, Eurotiales, Hypocreales, Microascales, and Onygenales able to produce pale grains [1]. Interestingly, in Sudan, eumycetoma is predominantly caused by the fungus Madurella mycetomatis, followed by Falciformispora senegalensis, and both of them are characterised by the production of black grains [3].
The initial misdiagnosis of this case highlights the limitations in the current diagnostic tools for mycetoma, particularly in limited-resource settings such as Sudan. Additionally, the lack of growth in our microbial culture limited our capacity to evaluate the susceptibility of the causative agent to the locally available treatment regimes.
The identification of causative organisms at the species level is vital not only for treatment but also for understanding the disease epidemiology, the development of effective prevention and control measures, and monitoring of the effectiveness of interventions [8]. Sequencing the ITS region is currently considered the gold standard for identifying fungal species [8]. In this report, the sequence analysis presented 100% similarity with Fusarium falciforme. Recent advances in molecular techniques, especially those based on sequencing, show promise for the accurate and rapid diagnosis and characterization of mycetoma causative agents at the species level. Sequencing the ITS region is particularly useful when traditional techniques such as culture-based and histopathology techniques fail in identifying the causative agents at the species level [1,2,3].
Worldwide, F. falciforme is rarely reported as a causative agent for eumycetoma. Therefore, little is known about the epidemiology and risk factors associated with F. falciforme infection and transmission. Similarly, limited reports are available about other Fusarium species, such as F. solani, F. oxysporum, F. subglutinans, F. moniliforme, F. keratoplasticum, and F. pseudensiforme, as causative agents of eumycetoma [41,42,43,44,45].
The clinical presentation of this case was similar to other eumycetoma patients. Therefore, the patient was treated with the standard mycetoma treatment protocol, which combines medical treatment with lesional wide local surgical excision.
Resistance to the currently available antifungal agents is widely reported worldwide among the different Fusarium spp. including F. falciforme [46,47]. More than 90% of the isolates tested for Itraconazole susceptibility showed minimal inhibitory concentrations (MICs) above 64 µg/mL. F. falciforme was reported to be more susceptible to terbinafine, as 50% of the F. falciforme isolates had MICs of ≤4 µg/mL [46,47]. However, as terbinafine is unavailable in Sudan, patients have been advised to continue on Itraconazole.
Due to the globally limited investment in developing novel treatments for mycetoma, limited treatment options are currently available and the disease treatment protocols are developed mainly for the locally most common infection, which is M. mycetomatis in Sudan. It is currently not fully known whether eumycetoma caused by other fungal species would clinically respond to the same standard treatment. It is thus important to document the treatment outcome of patients affected by such rare causative agents. Therefore, due to its limited susceptibility to most antifungal agents, the European guidelines for fusariosis recommended using amphotericin B followed by Voriconazole to treat fusariosis. Interestingly, our reported case demonstrated a case of subcutaneous Fusarium infection treated with Itraconazole per MRC guidelines, but the patient encountered a relapse, indicating that this treatment plan is inappropriate medical therapy. Nonetheless, although an international expert has recently recommended the use of Voriconazole to treat our current patient, we could not implement this recommendation due to the unavailability of the drug in Sudan. However, considering the very dire risk of the further spread of fusariosis in the country and emergence of other similar cases in the future, we are currently working with the relevant authorities to consider the introduction of Voriconazole in the country. Nevertheless, we will also need to investigate how such an effective yet expensive drug could be made affordable for the mostly poor patients with mycetoma.
Several risk factors including climate change, globalization, and the spread of drug resistance are driving the rapid emergence and spread of novel emerging pathogens in new areas [48]. In our current study, we have identified the first-ever infection of F. falciforme in Sudan in the White Nile State, which has an open international border with South Sudan and hosts thousands of refugees from the neighbouring country (Figure 1). This suggests the role of high cross-country movements of forcibly displaced populations and the humanitarian respondents in the spread of infectious diseases [49,50,51]. Therefore, robust diagnostic tools including sequencing should be incorporated within the integrated disease surveillance and response systems for the early detection and accurate characterization of pathogens [8]. This will substantially improve case management as well as prevention and control strategies. Infections with F. falciforme have been reported among forest trees (Acacia mangium), industrial and medicinally important plants such as industrial hemp (Cannabis sativa) [52], and other economically important plants such as tobacco (Nicotiana tabacum L.) [53]. Additionally, F. falciforme infections have also been reported among edible crops including chickpea [54], onion (Allium cepa) [55], Phaseolus vulgaris [56], and soybean (Glycine max L.) [57]. Furthermore, infections with F. falciforme have been reported among some endangered animal species such as sea turtles [58]. These worldwide growing reports indicate that the devastating impacts of the increasingly emerging and spreading infections of F. falciforme are not limited to the negative health and socioeconomic effects on endemic countries in Africa, Asia, and the Americas. Additional negative impacts of the spread of F. falciforme include threats to food security and a loss of biodiversity, as well as endangering forest and ecosystem stability. Therefore, successful prevention and control of F. falciforme require the implementation of a transdisciplinary One Health strategy including the integration of disease surveillance and response systems [59,60,61,62]. Furthermore, such a strategy needs to be supported by improved diagnosis, cross-country and multisectoral coordinated surveillance systems, comprehensive documentation, and immediate public sharing of data, particularly urgently needed information such as new emergences of novel pathogens and drug resistance [59]. Moreover, more investment is urgently needed for developing novel drugs and innovative prevention and control measures [1].
5. Conclusions
This case report is alarming as it indicates an expansion of the geographical distribution of F. falciforme through novel emergence into new areas, particularly in resource-poor settings that are already challenged by limited diagnostic capacities and treatment options. Therefore, countries at risk are urgently encouraged to invest in improving their diagnostic capacity, surveillance systems, and case management as well as implementing a transdisciplinary One Health strategy for the prevention and control of emerging infectious diseases including F. falciforme.
Author Contributions
Conceptualization, E.E.S., A.A., H.F.E., W.W.J.v.d.S. and A.H.F.; writing—original draft preparation, E.E.S., A.A., H.F.E., W.W.J.v.d.S., S.M.B. and A.H.F.; writing—review and editing, E.E.S., A.A., H.F.E., W.W.J.v.d.S. and A.H.F.; investigation and data curation, E.E.S., A.A., H.F.E., W.W.J.v.d.S. and A.H.F.; visualization, E.E.S. and A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
The patient provided written consent.
Data Availability Statement
All data collected during this study are included in this report.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siddig, E.E.; Ahmed, A.; Ali, Y.; Bakhiet, S.M.; Mohamed, N.S.; Ahmed, E.S.; Fahal, A.H. Eumycetoma Medical Treatment: Past, Current Practice, Latest Advances and Perspectives. Microbiol. Res. 2021, 12, 899–906. [Google Scholar] [CrossRef]
- Siddig, E.E.; Mhmoud, N.A.; Bakhiet, S.M.; Abdallah, O.B.; Mekki, S.O.; El Dawi, N.I.; Van de Sande, W.; Fahal, A.H. The Accuracy of Histopathological and Cytopathological Techniques in the Identification of the Mycetoma Causative Agents. PLoS Negl. Trop. Dis. 2019, 13, e0007056. [Google Scholar] [CrossRef] [PubMed]
- van de Sande, W.W. Global burden of human mycetoma: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2013, 7, e2550. [Google Scholar] [CrossRef] [PubMed]
- Siddig, E.E.; Ahmed, A.; Hassan, O.B.; Bakhiet, S.M.; Verbon, A.; Fahal, A.H.; van de Sande, W.W.J. Using a Madurella mycetomatis-specific PCR on grains obtained via non-invasive fine-needle aspirated material is more accurate than cytology. Mycoses 2023, 66, 477–482. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Cutaneous infection by Fusarium species in healthy and immunocompromised hosts: Implications for diagnosis and management. Clin. Infect. Dis. 2002, 35, 909–920. [Google Scholar] [CrossRef]
- Venugopal, P.V.; Venugopal, T.V. Pale grain eumycetomas in Madras. Australas. J. Dermatol. 1995, 36, 149–151. [Google Scholar] [CrossRef]
- Lim, W.; Siddig, E.; Eadie, K.; Nyuykonge, B.; Ahmed, S.; Fahal, A.; Verbon, A.; Smit, S.; Van De Sande, W.W. The development of a novel diagnostic PCR for Madurella mycetomatis using a comparative genome approach. PLoS Negl. Trop. Dis. 2020, 14, e0008897. [Google Scholar] [CrossRef]
- Siddig, E.E.; Verbon, A.; Bakhiet, S.; Fahal, A.H.; van de Sande, W.W. The developed molecular biological identification tools for mycetoma causative agents: An update. Acta Trop. 2022, 225, 106205. [Google Scholar] [CrossRef]
- Mariat, F. Sur la distribution ge´ographique et la re´partition des agents de myce´tomes. Bull. Soc. Pathol. Exot. 1963, 56, 35–45. [Google Scholar]
- Gamet, A.; Brottes, H.; Essomba, R. Nouveaux cas de mycétomes dépistés au Cameroun [New cases of mycetoma detected in Cameroun]. Bull. Soc. Pathol. Exot. Filiales. 1964, 57, 1191–1195. [Google Scholar]
- Peloux, Y.; Segretain, G. Myce´tomes a` Fusarium. Bull. Soc. Fr. Mycol. Med. 1966, 1, 31–32. [Google Scholar]
- Destombes, P.; Mariat, F.; Rosati, L.; Segretain, G. Les mycétomes en Somalie—Conclusions d′une enquête menée de 1959 à 1964 [Mycetoma in Somalia—Results of a survey done from 1959 to 1964]. Acta Trop. 1977, 34, 355–373. [Google Scholar] [PubMed]
- Hay, R.J.; Mackenzie, D.W. The histopathological features of pale grain eumycetoma. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Mackenzie, D.W. Mycetoma (madura foot) in the United Kingdom—A survey of forty-four cases. Clin. Exp. Dermatol. 1983, 8, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Thianprasit, M.; Sivayathorn, A. Black dot mycetoma. Mykosen 1984, 27, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Ajello, L.; Padhye, A.; Chandler, F.; McGinnis, M.; Morganti, L.; Alberici, F. Fusarium moniliforme, a new mycetoma agent. Restudy of a European case. Eur. J. Epidemiol. 1985, 1, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Baudraz-Rosselet, F.; Monod, M.; Borradori, L.; Ginalsky, J.; Vion, B.; Boccard, C.; Frenk, E. Mycetoma of the foot due to Fusarium sp. treated with oral ketoconazole. Dermatology 1992, 184, 303–305. [Google Scholar] [CrossRef]
- Buiting, A.G.; Visser, L.G.; Barge, R.M.; van’t Wout, J.W. Mycetoom van de voet; een ziekte uit de tropen [Mycetoma of the foot; a disease from the tropics]. Ned. Tijdschr. Geneeskd. 1993, 137, 1513–1515. [Google Scholar]
- de Hoog, G.S.; Buiting, A.; Tan, C.S.; Stroebel, A.B.; Ketterings, C.; de Boer, E.J.; Naafs, B.; Brimicombe, R.; Nohlmans-Paulssen, M.K.; Fabius, G.T.; et al. Diagnostic problems with imported cases of mycetoma in The Netherlands. Mycoses 1993, 36, 81–87. [Google Scholar] [CrossRef]
- Tomimori-Yamashita, J.; Ogawa, M.M.; Hirata, S.H.; Fischman, O.; Michalany, N.S.; Yamashita, H.K.; Alchorne, M.M. Mycetoma caused by Fusarium solani with osteolytic lesions on the hand: Case report. Mycopathologia 2002, 153, 11–14. [Google Scholar] [CrossRef]
- Yera, H.; Bougnoux, M.E.; Jeanrot, C.; Baixench, M.T.; De Pinieux, G.; Dupouy-Camet, J. Mycetoma of the foot caused by Fusarium solani: Identification of the etiologic agent by DNA sequencing. J. Clin. Microbiol. 2003, 41, 1805–1808. [Google Scholar] [CrossRef] [PubMed]
- Palmore, T.N.; Shea, Y.R.; Childs, R.W.; Sherry, R.M.; Walsh, T.J. Fusarium proliferatum soft tissue infection at the site of a puncture by a plant: Recovery, isolation, and direct molecular identification. J. Clin. Microbiol. 2010, 48, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Premkumar, A.; Chakrabarti, A.; Shah, V.N.; Behera, A.; De, D.; Rudramurthy, S.M.; Bhansali, A. Fusarium falciforme infection of foot in a patient with type 2 diabetes mellitus: A case report and review of the literature. Mycopathologia 2013, 176, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.; Bonifaz, A.; Tirado-Sánchez, A.; Meis, J.F.; de Hoog, G.S.; Ahmed, S.A. Fusarium species causing eumycetoma: Report of two cases and comprehensive review of the literature. Mycoses 2017, 60, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Katkar, V.J.; Tankhiwale, S.S.; Kurhade, A. Fusarium soloni mycetoma. Indian. J. Dermatol. 2011, 56, 315–317. [Google Scholar] [PubMed]
- Campos-Macías, P.; Arenas-Guzmán, R.; Hernández-Hernández, F. Fusarium subglutinans: A new eumycetoma agent. Med. Mycol. Case Rep. 2013, 2, 128–131. [Google Scholar] [CrossRef]
- Nakar, C.; Livny, G.; Levy, I.; Samra, Z.; Linder, N.; Ashkenazi, S.; Livne, P.; Sirota, L.; Tikva, P. Mycetoma of the renal pelvis caused by Fusarium species. Pediatr. Infect. Dis. J. 2001, 20, 1182–1183. [Google Scholar] [CrossRef] [PubMed]
- Klokke, A.H. Mycetoma of the foot, a disease from the tropics. Ned. Tijdschr. Geneeskd. 1993, 137, 2056–2057. [Google Scholar]
- Prinja, A.; Roberts, C.; Doherty, T.; Oddy, M.J. An unusual cause of an ankle mass. BMJ Case Rep. 2014, 2014, bcr2014204253. [Google Scholar] [CrossRef]
- Xiujiao, X.; Hong, S.; Ai-e, X. Eumycetoma due to Acremonium falciforme acquired in China. Mycoses 2012, 55, e4–e7. [Google Scholar] [CrossRef]
- Van Etta, L.L.; Peterson, L.R.; Gerding, D.N. Acremonium falciforme (Cephalosporium falciforme) mycetoma in a renal transplant patient. Arch. Dermatol. 1983, 119, 707–708. [Google Scholar] [CrossRef] [PubMed]
- Kudur, M.H.; Prakash, P.; Savitha, M. Fusarium solani causing quasi-invasive infection of the foot in an immunocompetent middle-aged man from South India. Indian. J. Dermatol. 2013, 58, 241. [Google Scholar] [CrossRef] [PubMed]
- Mercuţ, D.; Tiţa, C.; Ianoşi, G.; Ianoşi, S.; Tiţa, M. Piciorul de Madura (micetomul) [Madura’s foot (mycetoma)]. Chirurgia 2003, 98, 261–264. [Google Scholar] [PubMed]
- Resnik, B.I.; Burdick, A.E. Improvement of eumycetoma with itraconazole. J. Am. Acad. Dermatol. 1995, 33 Pt 2, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Luque, A.G.; Mujica, M.T.; D’anna, M.L.; Alvarez, D.P. Micetoma podal por fusarium solani (Mart.) Appel & Wollenweber. Boletín Micológico 1991, 6, 1–2. [Google Scholar]
- Destombes, P.; Mariat, F.; Rosati, L.; Segretain, G. Les mycétomes en République de Somalie [Mycetomas in the Republic of Somalia]. C. R. Acad. Hebd. Seances Acad. Sci. D 1966, 263, 2062–2064. [Google Scholar]
- Callebaut, G.; Hooghe, L.; Dratwa, M. Madura′s foot in a renal transplant patient: Report of a case. NDT Plus 2011, 4, 397–398. [Google Scholar] [CrossRef]
- Salas-Coronas, J.; Cabezas-Fernández, T.; Martínez-Lage, M.J.; Villarejo-Ordóñez, A. Micetoma por Fusarium solani [Mycetoma caused by Fusarium solani]. Rev. Clin. Esp. 2011, 211, e16–e18. [Google Scholar] [CrossRef]
- Bonifaz, A.; Saldaña, M.; Araiza, J.; Mercadillo, P.; Tirado-Sánchez, A. Two simultaneous mycetomas caused by Fusarium verticillioides and Madurella mycetomatis. Rev. Inst. Med. Trop. Sao Paulo 2017, 59, e55. [Google Scholar] [CrossRef]
- Ahmed, A.; Mohamed, N.S.; Siddig, E.E.; Algaily, T.; Sulaiman, S.; Ali, Y. The impacts of climate change on displaced populations: A call for action. J. Clim. Chang. Health 2021, 3, 100057. [Google Scholar] [CrossRef]
- Das, L.; Dahiya, D.; Gupta, K.; Prakash, M.; Malhotra, B.; Rastogi, A.; Choudhary, H.; Rudramurthy, S.M.; Dutta, P. Eumycetoma of the Foot due to Fusarium solani in a Person with Diabetes Mellitus: Report of a Case and Review of Literature. Mycopathologia 2021, 186, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Batista, B.G.; De Chaves, M.A.; Reginatto, P.; Saraiva, O.J.; Fuentefria, A.M. Human fusariosis: An emerging infection that is difficult to treat. Rev. Soc. Bras. Med. Trop. 2020, 53, e20200013. [Google Scholar] [CrossRef] [PubMed]
- Martínez López, D.; Pérez Blasco, A.; García Ferrer, L.; Camarena, J.J.; González, R.; Rodrigo Perez, J.L. Eumicetomas por Fusarium oxysporum y Madurella mycetomatis. Descripción de dos casos y revisión de la bibliografía [Eumycetomas by Fusarium oxysporum and Madurella mycetomatis. Description of two cases and literature review]. Rev. Esp. Quimioter. 2022, 35, 566–569. [Google Scholar] [PubMed]
- Hashizume, H.; Taga, S.; Sakata, M.K.; Taha, M.H.M.; Siddig, E.E.; Minamoto, T.; Fahal, A.H.; Kaneko, S. Detection of multiple mycetoma pathogens using fungal metabarcoding analysis of soil DNA in an endemic area of Sudan. PLoS Negl. Trop. Dis. 2022, 16, e0010274. [Google Scholar] [CrossRef]
- Correia, C.; Ferreira, J.; Soares-de-Almeida, L.; Filipe, P. An Unusual Cause of Eumycetoma—Fusarium Solani Keratoplasticum. Actas Dermosifiliogr. 2022, 113, 899. [Google Scholar] [CrossRef] [PubMed]
- Al-Hatmi, A.M.S.; Curfs-Breuker, I.; De Hoog, G.S.; Meis, J.F.; Verweij, P.E. Antifungal Susceptibility Testing of Fusarium: A Practical Approach. J. Fungi 2017, 3, 19. [Google Scholar] [CrossRef]
- Da Rosa, P.D.; Ramirez-Castrillon, M.; Borges, R.; Aquino, V.; Fuentefria, A.M.; Goldani, L.Z. Epidemiological aspects and characterization of the resistance profile of Fusarium spp. in patients with invasive fusariosis. J. Med. Microbiol. 2019, 68, 1489–1496. [Google Scholar] [CrossRef]
- Ahmed, A.; Ali, Y.; Siddig, E.E.; Hamed, J.; Mohamed, N.S.; Khairy, A.; Zinsstag, J. Hepatitis E Virus Outbreak among Tigray War Refugees from Ethiopia, Sudan. Emerg. Infect. Dis. 2022, 28, 1722. [Google Scholar] [CrossRef]
- Ahmed, A.; Ali, Y.; Elmagboul, B.; Mohamed, O.; Elduma, A.; Bashab, H.; Mahamoud, A.; Khogali, H.; Elaagip, A.; Higazi, T. Dengue fever in the Darfur area, Western Sudan. Emerg. Infect. Dis. 2019, 25, 2125. [Google Scholar] [CrossRef]
- Ahmed, A.; Mahmoud, I.; Eldigail, M.; Elhassan, R.M.; Weaver, S.C. The emergence of Rift Valley Fever in gedaref state urges the need for a cross-border one health strategy and enforcement of the international health regulations. Pathogens 2021, 10, 885. [Google Scholar] [CrossRef]
- Paugh, K.R.; Del Castillo Múnera, J.; Swett, C.L. First Report of Fusarium falciforme (FSSC 3 + 4) Causing Rot of Industrial Hemp (Cannabis sativa) in California. Plant. Dis. 2022, 106, 1753. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Li, X.; Li, C.; Li, C.; Xue, C.; Fang, W.; Zhang, Y.; Song, R.; Xu, M.; He, L.; et al. First Report of Tobacco Root Rot Caused by Fusarium falciforme in China. Plant. Dis. 2022, 107, 960. [Google Scholar] [CrossRef] [PubMed]
- Velarde Felix, S.; Valenzuela, V.; Ortega, P.; Fierros, G.; Rojas, P.; López Orona, C.A.; Retes Manjarrez, J.E. First report of Fusarium falciforme (FSSC 3+4) causing root rot on chickpea in Mexico. Plant. Dis. 2021, 106, 329. [Google Scholar] [CrossRef]
- Sarwadnya, K.; Bhat, G.; Bangi, S.; Jeevitha, D.; Shivakumar, G.; Madalageri, B.B.; Noojibail, P.; Anandalakshmi, R. First report of Fusarium falciforme causing basal rot of onion (Allium cepa) in India. Plant. Dis. 2023, 107, 228. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Nájera, J.F.; Ayvar-Serna, S.; Mena-Bahena, A.; Baranda-Cruz, E.; Vargas-Hernández, M.; Alvarado-Gómez, O.G.; Fuentes-Aragón, D. First report of Fusarium falciforme (FSSC 3+4) causing wilt disease of Phaseolus vulgaris in Mexico. Plant. Dis. 2020, 105, 710. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Feng, C.; Liu, L.; Shi, R.; Han, S.; Song, Y.; Wang, J.; Han, Z.; Zhang, J.; Li, Y.; et al. First report of Fusarium falciforme causing root rot of soybean (Glycine max L.) in Henan, China. Plant Dis. 2022. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Ramirez, J.M.; Abella-Perez, E.; Phillott, A.D.; Sim, J.; Van West, P.; Martin, M.P.; Marco, A.; Dieguez-Uribeondo, J. Global distribution of two fungal pathogens threatening endangered sea turtles. PLoS ONE 2014, 9, e85853. [Google Scholar] [CrossRef] [PubMed]
- Ali, Y.; Ahmed, A.; Siddig, E.E.; Mohamed, N.S. The role of integrated programs in the prevention of COVID-19 in a humanitarian setting. Trans. R. Soc. Trop. Med. Hyg. 2022, 116, 193–196. [Google Scholar] [CrossRef]
- Zinsstag, J.; Hediger, K.; Osman, Y.M.; Abukhattab, S.; Crump, L.; Kaiser-Grolimund, A.; Mauti, S.; Ahmed, A.; Hattendorf, J.; Bonfoh, B.; et al. The Promotion and Development of One Health at Swiss TPH and Its Greater Potential. Diseases 2022, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Hemaida, M.A.; Hagelnur, A.A.; Eltigani, H.F.; Siddig, E.E. Sudden emergence and spread of cutaneous larva migrans in Sudan: A case series calls for urgent actions. IDCases 2023, 32, e01789. [Google Scholar] [CrossRef]
- Ahmed, A.; Hagelnur, A.A.; Eltigani, H.F.; Siddig, E.E. Cutaneous tuberculosis of the foot clinically mimicking mycetoma: A case report. Clin. Case Rep. 2023, 11, e7295. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; El-Amin, R.; Musa, A.M.; Elsayed, M.A.; Fahal, L.A.; Ahmed, E.S.; Ali, Y.; Nebie, I.E.; Mohamed, N.S.; Zinsstag, J.; et al. Guillain-Barre syndrome associated with COVID-19 infection: A case series. Clin. Case Rep. 2023, 11, e6988. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).