Unveiling the Morphostructural Plasticity of Zoonotic Sporotrichosis Fungal Strains: Possible Implications for Sporothrix brasiliensis Virulence and Pathogenicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
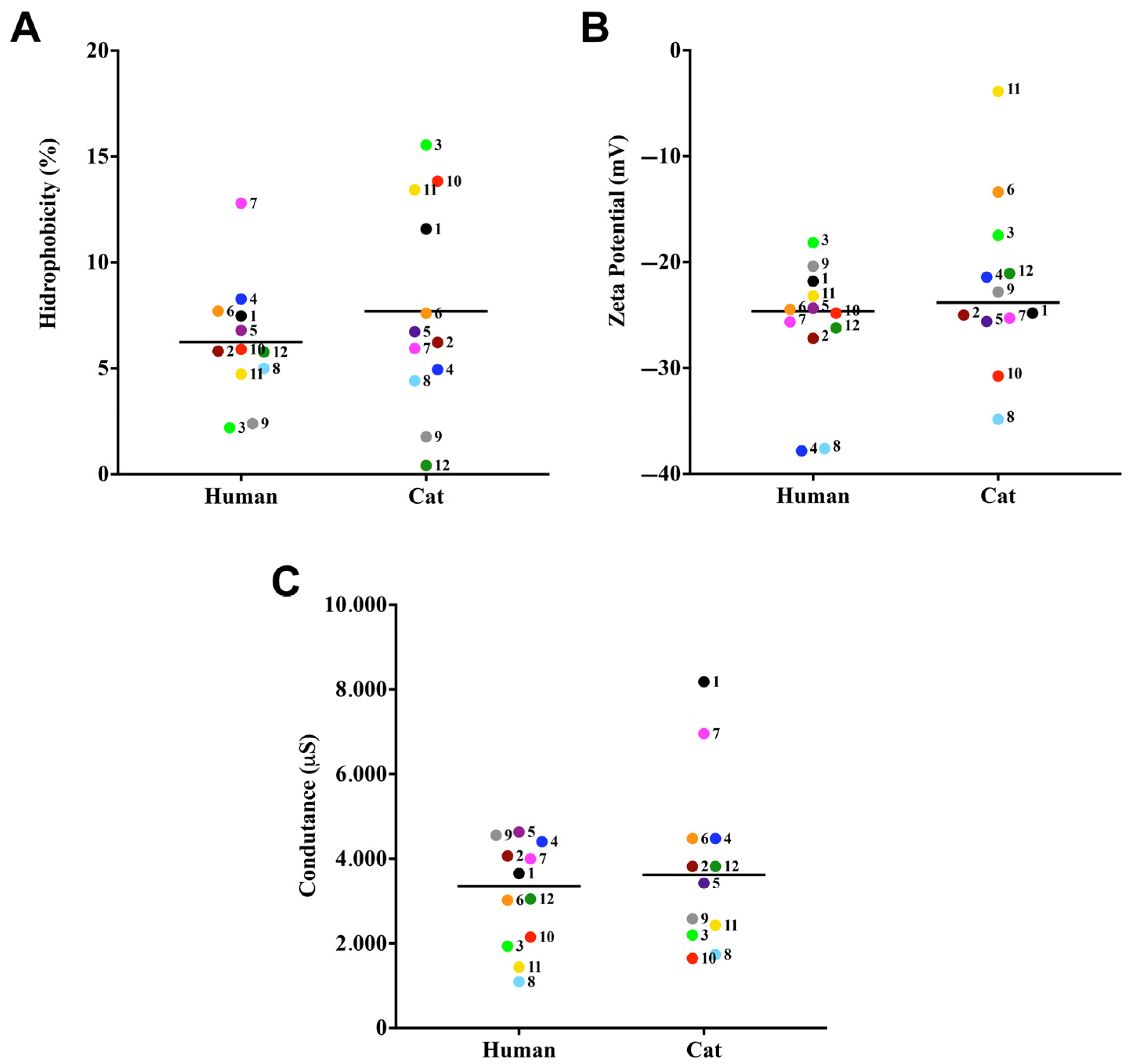
2.2. Evaluation of Cell Surface Hydrophobicity
2.3. Evaluation of Cellular Electronegativity
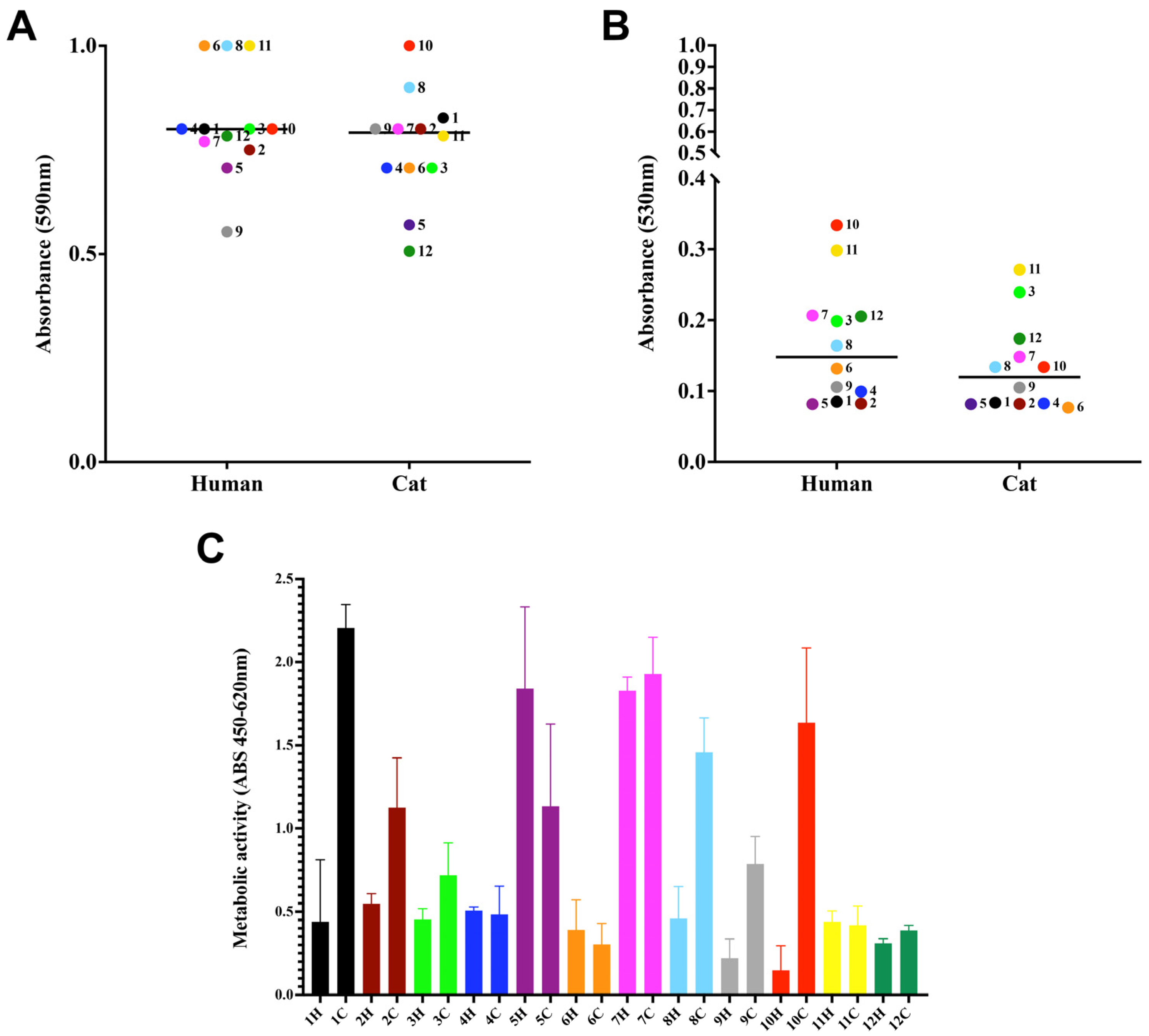
2.4. Biofilm Formation on Polystyrene
2.4.1. Biomass Quantification
2.4.2. Cellular Viability
2.4.3. Characterization of Extracellular Matrix Production
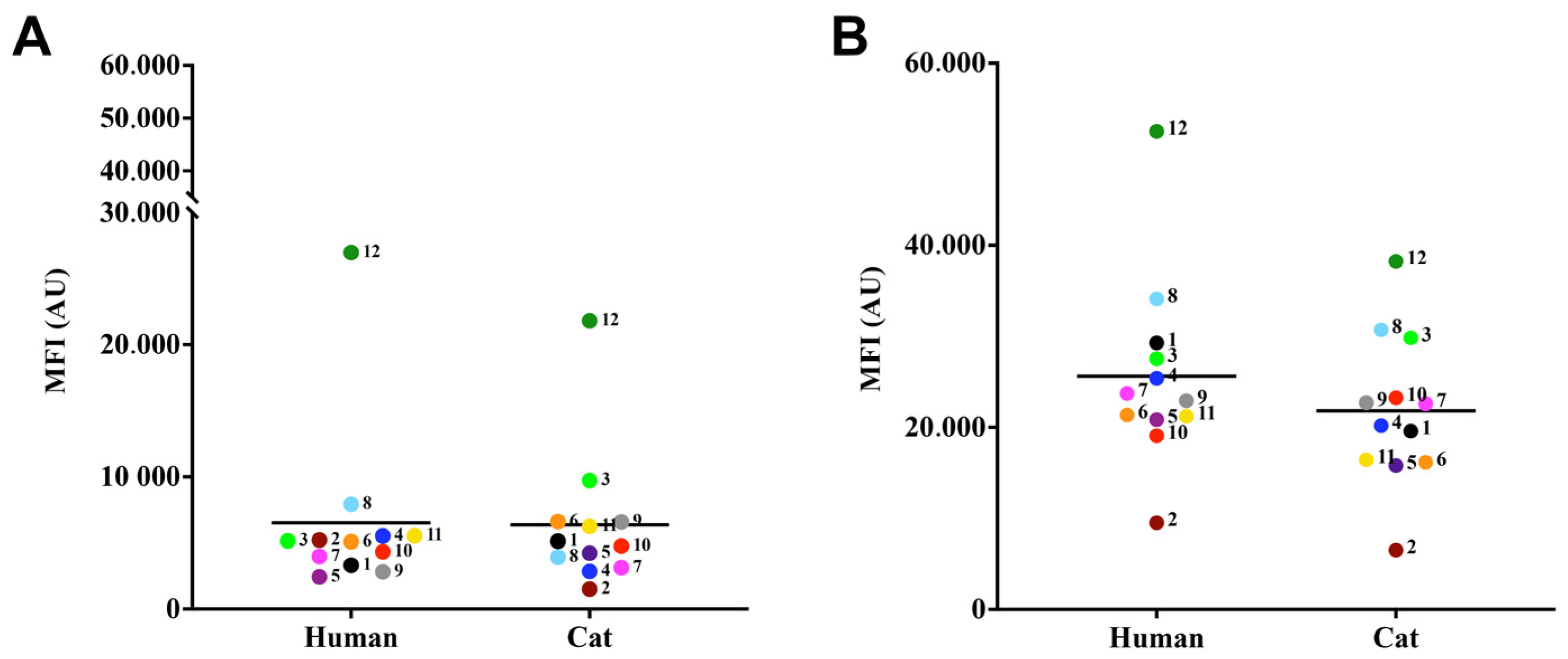
2.5. Detection of Lipid Bodies in the Fungal Cell
2.6. Quantification of Chitin in the Cell Wall
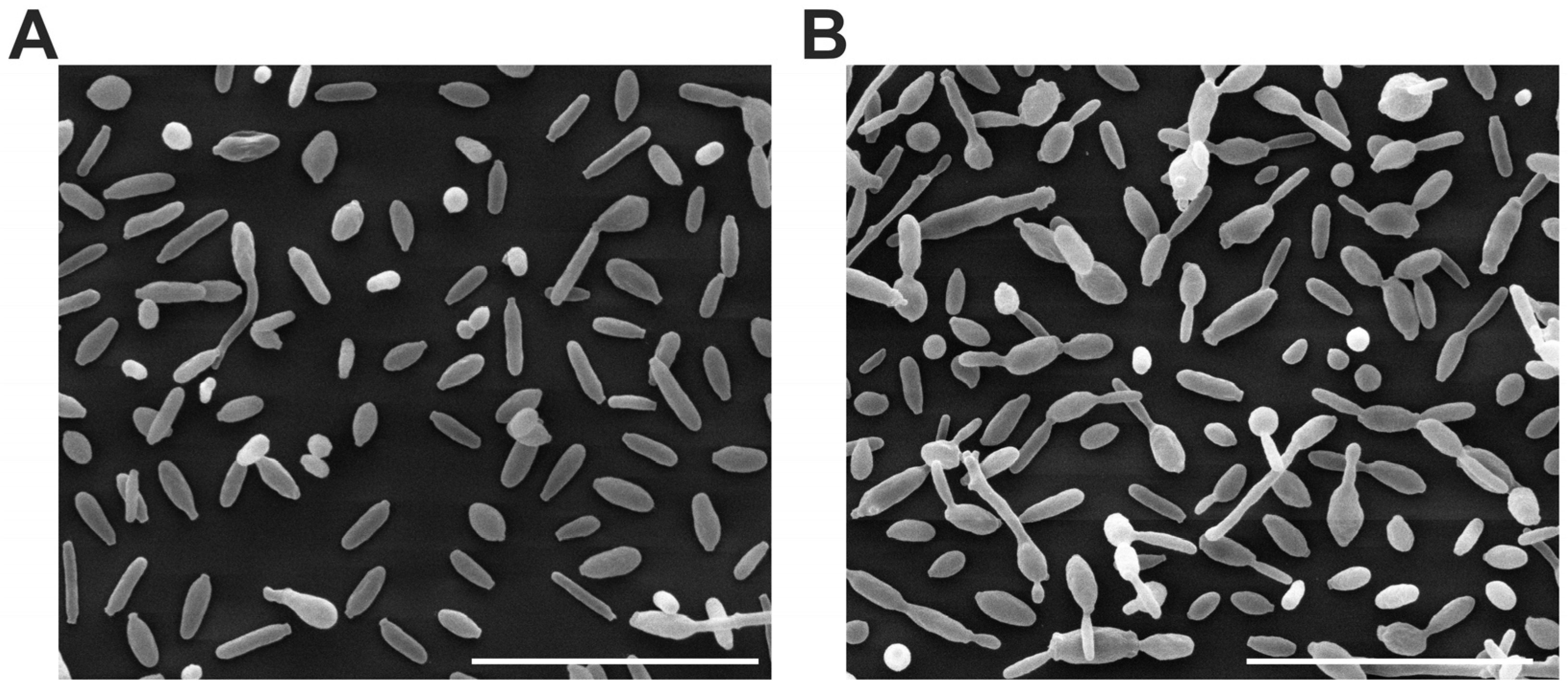
2.7. Ultrastructural Analysis of the Cell Surface
2.8. Statistical Treatment
3. Results
3.1. Cell Surface Properties
3.2. Biofilm Properties
3.3. Lipid Body and Chitin Contents
3.4. Ultrastructural Analysis of the Cell Surface
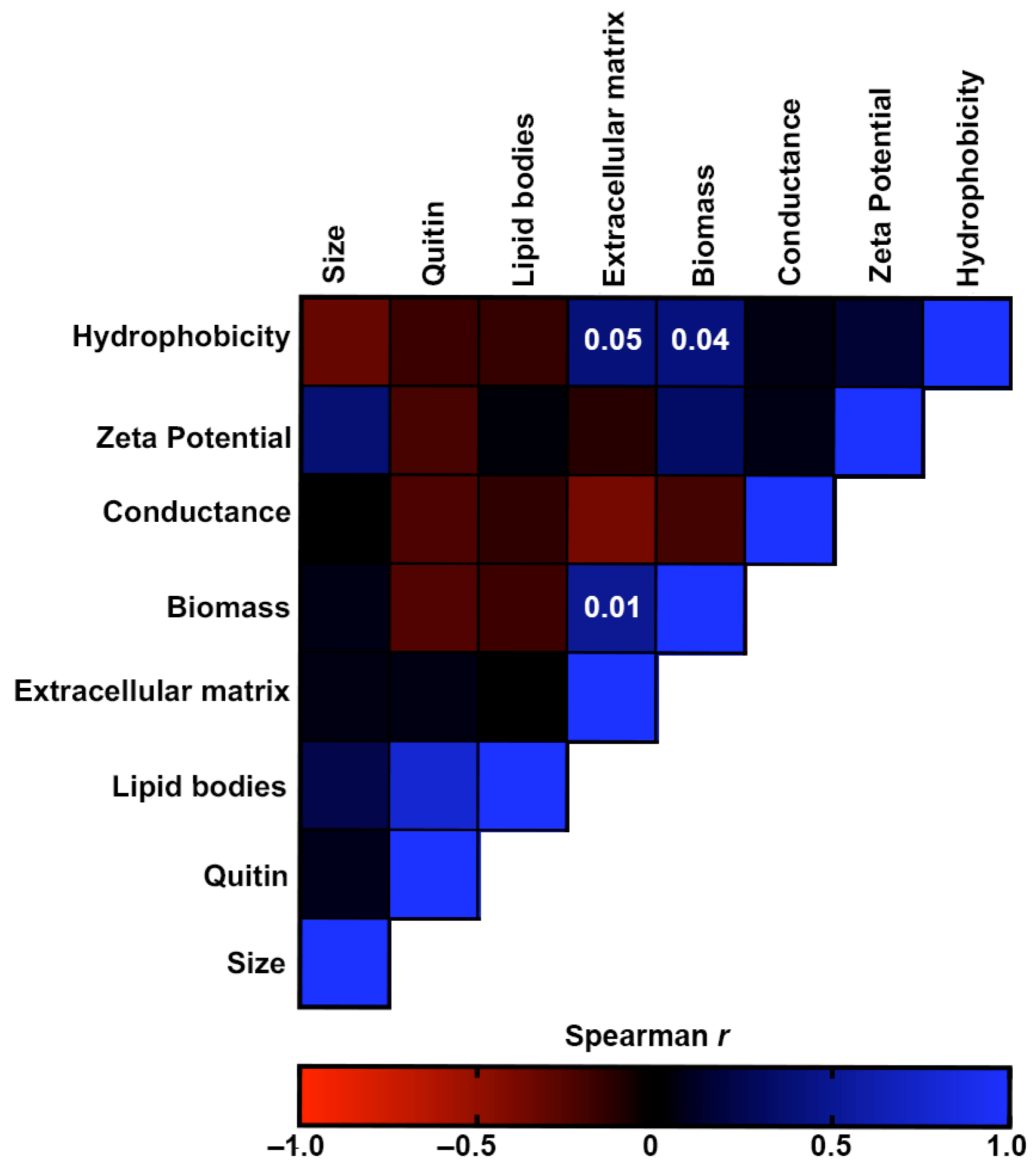
3.5. Comparative Analysis of Morphostructural Properties
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabañes, F.J. Sporotrichosis in Brazil: Animals + humans = one Health. Rev. Iberoam. Micol. 2020, 37, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Gremião, I.D.F.; Marques, M.; Oliveira, E.; de Miranda, L.H.M.; Freitas, D.F.S.; Pereira, S.A. Geographic Expansion of Sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Rachman, R.; Ligaj, M.; Chinthapalli, S.; Wani, R.S. Zoonotic Acquisition of Cutaneous Sporothrix braziliensis Infection in the UK. BMJ Case Rep. 2022, 15, e248418. [Google Scholar] [CrossRef] [PubMed]
- Underbill, D.M.; Gantner, B. Integration of Toll-like Receptor and Phagocytic Signaling for Tailored Immunity. Microbes Infect. 2004, 6, 1368–1373. [Google Scholar] [CrossRef]
- Guzman-Beltran, S.; Perez-Torres, A.; Coronel-Cruz, C.; Torres-Guerrero, H. Phagocytic Receptors on Macrophages Distinguish between Different Sporothrix schenckii Morphotypes. Microbes Infect. 2012, 14, 1093–1101. [Google Scholar] [CrossRef]
- Tamez-Castrellón, A.; Romeo, O.; García-Carnero, L.; Lozoya-Pérez, N.; Mora-Montes, H. Virulence Factors in Sporothrix schenckii, One of the Causative Agents of Sporotrichosis. Curr. Protein Pept. Sci. 2020, 21, 295–312. [Google Scholar] [CrossRef]
- BRASIL IBGE-Instituto Brasileiro de Geografia e Estatística IBGE-Instituto Brasileiro de Geografia e Estatística. Censo Demogr. 2005, 2010, 1–5.
- Etchecopaz, A.; Toscanini, M.A.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.A.; Bendezú, K.; Nusblat, A.D.; Iachini, R.; Cuestas, M.L. Sporothrix brasiliensis: A Review of an Emerging South American Fungal Pathogen, Its Related Disease, Presentation and Spread in Argentina. J. Fungi 2021, 7, 170. [Google Scholar] [CrossRef]
- Arrillaga-Moncrieff, I.; Capilla, J.; Mayayo, E.; Marimon, R.; Mariné, M.; Gené, J.; Cano, J.; Guarro, J. Different Virulence Levels of the Species of Sporothrix in a Murine Model. Clin. Microbiol. Infect. 2009, 15, 651–655. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; Figueiredo-Carvalho, M.H.G.; Brito-Santos, F.; Almeida-Silva, F.; Oliveira, M.M.E.; Zancopé-Oliveira, R.M. Melanins Protect Sporothrix brasiliensis and Sporothrix schenckii from the Antifungal Effects of Terbinafine. PLoS ONE 2016, 11, e0152796. [Google Scholar] [CrossRef]
- Casadevall, A. The Pathogenic Potential of a Microbe. Msphere 2017, 2. [Google Scholar] [CrossRef]
- Read, S.I.; Sperling, L.C. Feline Sporotrichosis: Transmission to Man. Arch. Dermatol. 1982, 118, 429–431. [Google Scholar] [CrossRef]
- Latgé, J.P. Tasting the Fungal Cell Wall. Cell Microbiol. 2010, 12, 863–872. [Google Scholar] [CrossRef]
- Arana, D.M.; Prieto, D.; Román, E.; Nombela, C.; Alonso-Monge, R.; Pla, J.; Alonso-Monge, R.; Pla, J. The Role of the Cell Wall in Fungal Pathogenesis. Microb. Biotechnol. 2009, 2, 308. [Google Scholar]
- Villalobos-Duno, H.L.; Barreto, L.A.; Alvarez-Aular, Á.; Mora-Montes, H.M.; Lozoya-Pérez, N.E.; Franco, B.; Lopes-Bezerra, L.M.; Niño-Vega, G.A. Comparison of Cell Wall Polysaccharide Composition and Structure Between Strains of Sporothrix schenckii and Sporothrix brasiliensis. Front. Microbiol. 2021, 12, 2686. [Google Scholar] [CrossRef]
- Lopes-Bezerra, L.M.; Walker, L.A.; Niño-Vega, G.; Mora-Montes, H.M.; Neves, G.W.P.P.; Villalobos-Duno, H.; Barreto, L.; Garcia, K.; Franco, B.; Martínez-Álvarez, J.A.; et al. Cell Walls of the Dimorphic Fungal Pathogens Sporothrix schenckii and Sporothrix brasiliensis Exhibit Bilaminate Structures and Sloughing of Extensive and Intact Layers. PLoS Negl. Trop. Dis. 2018, 12, e0006169. [Google Scholar] [CrossRef]
- Lozoya-Pérez, N.E.; García-Carnero, L.C.; Martínez-Álvarez, J.A.; Martínez-Duncker, I.; Mora-Montes, H.M. Tenebrio molitor as an Alternative Model to Analyze the Sporothrix Species Virulence. Infect. Drug Resist. 2021, 14, 2059–2072. [Google Scholar] [CrossRef]
- Hounsa, C.-G.; Brandt, E.V.; Thevelein, J.; Hohmann, S.; Prior, B.A. Role of Trehalose in Survival of Saccharomyces Cerevisiae under Osmotic Stress. Microbiology 1998, 144, 671–680. [Google Scholar] [CrossRef]
- Martínez-Álvarez, J.A.; Pérez-García, L.A.; Mellado-Mojica, E.; López, M.G.; Martínez-Duncker, I.; Lópes-Bezerra, L.M.; Mora-Montes, H.M. Sporothrix schenckii Sensu Stricto and Sporothrix brasiliensis Are Differentially Recognized by Human Peripheral Blood Mononuclear Cells. Front. Microbiol. 2017, 8, 843. [Google Scholar] [CrossRef]
- Silva-Bailão, M.G.; Lima, P.d.S.; Oliveira, M.M.E.; Oliveira, L.C.; Almeida-Paes, R.; Borges, C.L.; Bailão, A.M.; Coelho, A.S.G.; Soares, C.M.d.A.; Zancopé-Oliveira, R.M. Comparative Proteomics in the Three Major Human Pathogenic Species of the Genus Sporothrix. Microbes Infect. 2021, 23, 104762. [Google Scholar] [CrossRef]
- Jones, D.S.; Tobler, D.J.; Schaperdoth, I.; Mainiero, M.; Macalady, J.L. Community Structure of Subsurface Biofilms in the Thermal Sulfidic Caves of Acquasanta Terme, Italy. Appl. Environ. Microbiol. 2010, 76, 5902–5910. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Emanuel, C.; Cutting, K.F.; Williams, D.W. Microbiology of the Skin and the Role of Biofilms in Infection. Int. Wound J. 2012, 9, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C.; Ramage, G.; Rajendran, R.; Sherry, L.; Williams, C. Fungal Biofilm Resistance. Int. J. Microbiol. 2012, 2012, 528521. [Google Scholar] [CrossRef]
- Brilhante, R.S.N.; Correia, E.E.M.; Guedes, G.M.d.M.; de Oliveira, J.S.; Castelo-Branco, D.d.S.C.M.; Cordeiro, R.d.A.; Pinheiro, A.d.Q.; Chaves, L.J.Q.; Pereira Neto, W.d.A.; Sidrim, J.J.C.; et al. In Vitro Activity of Azole Derivatives and Griseofulvin against Planktonic and Biofilm Growth of Clinical Isolates of Dermatophytes. Mycoses 2018, 61, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; Fernandes, M.R.; Pereira, V.S.; Costa, A.d.C.; de Oliveira, J.S.; de Aguiar, L.; Rodrigues, A.M.; de Camargo, Z.P.; Pereira-Neto, W.A.; Sidrim, J.J.C.; et al. Biofilm Formation on Cat Claws by Sporothrix Species: An Ex Vivo Model. Microb. Pathog. 2021, 150, 104670. [Google Scholar]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Molecular Diagnosis of Pathogenic Sporothrix Species. PLoS Negl. Trop. Dis. 2015, 9, e0004190. [Google Scholar] [CrossRef]
- Reis, R.S.; Almeida-Paes, R.; Muniz, M.d.M.; Tavares, P.M.e.S.; Monteiro, P.C.F.; Schubach, T.M.P.; Gutierrez-Galhardo, M.C.; Zancopé-Oliveira, R.M.; Santos Reis, R.; Almeida-Paes, R.; et al. Molecular Characterisation of Sporothrix schenckii Isolates from Humans and Cats Involved in the Sporotrichosis Epidemic in Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 2009, 104, 769–774. [Google Scholar] [CrossRef]
- Hazen, K.C.; Plotkin, B.J.; Klimas, D.M. Influence of Growth Conditions on Cell Surface Hydrophobicity of Candida Albicans and Candida Glabrata. Infect. Immun. 1986, 54, 269–271. [Google Scholar] [CrossRef]
- Ramos, L.S.; Oliveira, S.S.C.; Silva, L.N.; Granato, M.Q.; Gonçalves, D.S.; Frases, S.; Seabra, S.H.; Macedo, A.J.; Kneipp, L.F.; Branquinha, M.H.; et al. Surface, Adhesiveness and Virulence Aspects of Candida Haemulonii Species Complex. Med. Mycol. 2020, 58, 973–986. [Google Scholar]
- Sze, A.; Erickson, D.; Ren, L.; Li, D. Zeta-Potential Measurement Using the Smoluchowski Equation and the Slope of the Current-Time Relationship in Electroosmotic Flow. J. Colloid Interface Sci. 2003, 261, 402–410. [Google Scholar] [CrossRef]
- Mello, T.P.; Oliveira, S.S.C.; Frasés, S.; Branquinha, M.H.; Santos, A.L.S. Surface Properties, Adhesion and Biofilm Formation on Different Surfaces by Scedosporium Spp. and Lomentospora Prolificans. Biofouling 2018, 34, 800–814. [Google Scholar] [CrossRef]
- Mowat, E.; Butcher, J.; Lang, S.; Williams, C.; Ramage, G. Development of a Simple Model for Studying the Effects of Antifungal Agents on Multicellular Communities of Aspergillus Fumigatus. J. Med. Microbiol. 2007, 56, 1205–1212. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of Biofilm by Candida and Non-Candida Spp. Isolates Causing Fungemia: Comparison of Biomass Production and Metabolic Activity and Development of Cut-off Points. Int. J. Med. Microbiol. 2014, 304, 1192–1198. [Google Scholar] [CrossRef]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of Multiple Methods for Quantification of Microbial Biofilms Grown in Microtiter Plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar]
- Dos Santos, G.M.P.; Borba-Santos, L.P.; Vila, T.; Gremião, I.D.F.; Pereira, S.A.; De Souza, W.; Rozental, S. Sporothrix Spp. Biofilms Impact in the Zoonotic Transmission Route: Feline Claws Associated Biofilms, Itraconazole Tolerance, and Potential Repurposing for Miltefosine. Pathogens 2022, 11, 206. [Google Scholar] [CrossRef]
- Choi, N.-Y.; Kang, S.-Y.; Kim, K.-J. Artemisia Princeps Inhibits Biofilm Formation and Virulence-Factor Expression of Antibiotic-Resistant Bacteria. BioMed Res. Int. 2015, 2015, 239519. [Google Scholar] [CrossRef]
- Abi-chacra, É.A.; Souza, L.O.P.; Cruz, L.P.; Braga-Silva, L.A.; Gonçalves, D.S.; Sodré, C.L.; Ribeiro, M.D.; Seabra, S.H.; Figueiredo-Carvalho, M.H.G.; Barbedo, L.S.; et al. Phenotypical Properties Associated with Virulence from Clinical Isolates Belonging to the Candida Parapsilosis Complex. FEMS Yeast Res. 2013, 13, 831–848. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-Enabled Heat Mapping for All. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Rossow, J.A.; Queiroz-Telles, F.; Caceres, D.H.; Beer, K.D.; Jackson, B.R.; Pereira, J.G.; Gremião, I.D.F.; Pereira, S.A. A One Health Approach to Combatting Sporothrix brasiliensis: Narrative Review of an Emerging Zoonotic Fungal Pathogen in South America. J. Fungi 2020, 6, 247. [Google Scholar] [CrossRef]
- Araújo, G.R.D.S.; De Souza, W.; Frases, S. The Hidden Pathogenic Potential of Environmental Fungi. Future Microbiol. 2017, 12, 1533–1540. [Google Scholar] [CrossRef]
- Rediguieri, B.C.; da Cruz Bahiense, I.; de Carvalho, J.A.; Leite, G.R.; Falqueto, A.; Rodrigues, A.M.; Gonçalves, S.S. Clinical, Epidemiological, and Epizootic Features of Sporothrix brasiliensis in Espírito Santo, Brazil. Ecohealth 2022, 19, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.; González, C.; Blank, O.; Ramírez, V.; del Río, C.; Santibáñez, S.; Pena, P. Sporotrichosis Outbreak Due to Sporothrix brasiliensis in Domestic Cats in Magallanes, Chile: A One-Health-Approach Study. J. Fungi 2023, 9, 226. [Google Scholar] [CrossRef]
- Orofino-Costa, R.; Freitas, D.F.S.; Bernardes-Engemann, A.R.; Rodrigues, A.M.; Talhari, C.; Ferraz, C.E.; Veasey, J.V.; Quintella, L.; de Sousa, M.S.L.A.; Vettorato, R.; et al. Human Sporotrichosis: Recommendations from the Brazilian Society of Dermatology for the Clinical, Diagnostic and Therapeutic Management. An. Bras. Dermatol. 2022, 97, 757–777. [Google Scholar] [CrossRef]
- Ortiz-Ramírez, J.A.; Cuéllar-Cruz, M.; López-Romero, E. Cell Compensatory Responses of Fungi to Damage of the Cell Wall Induced by Calcofluor White and Congo Red with Emphasis on Sporothrix schenckii and Sporothrix globosa. A Review. Front. Cell. Infect. Microbiol. 2022, 12, 976924. [Google Scholar] [CrossRef]
- Lopes-Bezerra, L.M.; Mora-Montes, H.M.; Zhang, Y.; Nino-Vega, G.; Rodrigues, A.M.; De Camargo, Z.P.; De Hoog, S. Sporotrichosis between 1898 and 2017: The Evolution of Knowledge on a Changeable Disease and on Emerging Etiological Agents. Med. Mycol. 2018, 56, S126–S143. [Google Scholar]
- de Groot, P.W.J.; Bader, O.; de Boer, A.D.; Weig, M.; Chauhan, N. Adhesins in Human Fungal Pathogens: Glue with Plenty of Stick. Eukaryot. Cell 2013, 12, 470. [Google Scholar] [CrossRef]
- Lipke, P.N. What We Do Not Know about Fungal Cell Adhesion Molecules. J. Fungi 2018, 4, 59. [Google Scholar] [CrossRef]
- Araújo, G.R.d.S.; Viana, N.B.; Gómez, F.; Pontes, B.; Frases, S. The Mechanical Properties of Microbial Surfaces and Biofilms. Cell Surf. 2019, 5, 100028. [Google Scholar] [CrossRef]
- Ramage, G.; Mowat, E.; Jones, B.; Williams, C.; Lopez-Ribot, J. Our Current Understanding of Fungal Biofilms. Crit. Rev. Microbiol. 2009, 35, 340–355. [Google Scholar] [CrossRef]
- Raut, J.; Rathod, V.; Karuppayil, S.M. Cell Surface Hydrophobicity and Adhesion: A Study on Fifty Clinical Isolates of Candida Albicans. Nihon. Ishinkin Gakkai Zasshi 2010, 51, 131–136. [Google Scholar] [CrossRef]
- De Araújo, G.R.; Viana, N.B.; Pontes, B.; Frases, S. Rheological Properties of Cryptococcal Polysaccharide Change with Fiber Size, Antibody Binding and Temperature. Future Microbiol. 2019, 14, 867–884. [Google Scholar] [CrossRef]
- Miyake, Y.; Tsunoda, T.; Minagi, S.; Akagawa, Y.; Tsuru, H.; Suginaka, H. Antifungal Drugs Effect Adherence of Candida Albicans to Acrylic Surfaces by Changing the Zeta-Potential of Fungal Cells. FEMS Microbiol. Lett. 1990, 69, 211–214. [Google Scholar] [CrossRef]
- Frases, S.; Nimrichter, L.; Viana, N.B.; Nakouzi, A.; Casadevall, A. Cryptococcus Neoformans Capsular Polysaccharide and Exopolysaccharide Fractions Manifest Physical, Chemical, and Antigenic Differences. Eukaryot. Cell 2008, 7, 319–327. [Google Scholar] [CrossRef]
- Pihet, M.; Vandeputte, P.; Tronchin, G.; Renier, G.; Saulnier, P.; Georgeault, S.; Mallet, R.; Chabasse, D.; Symoens, F.; Bouchara, J.P. Melanin Is an Essential Component for the Integrity of the Cell Wall of Aspergillus Fumigatus Conidia. BMC Microbiol. 2009, 9, 177. [Google Scholar] [CrossRef]
- Wargenau, A.; Fleißner, A.; Bolten, C.J.; Rohde, M.; Kampen, I.; Kwade, A. On the Origin of the Electrostatic Surface Potential of Aspergillus Niger Spores in Acidic Environments. Res. Microbiol. 2011, 162, 1011–1017. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; Frases, S.; de Sousa Araújo, G.; Evangelista de Oliveira, M.M.; Gerfen, G.J.; Nosanchuk, J.D.; Zancopé-Oliveira, R.M. Biosynthesis and Functions of a Melanoid Pigment Produced by Species of the Sporothrix Complex in the Presence of L-Tyrosine. Appl. Environ. Microbiol. 2012, 78, 8623–8630. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential-What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Mayer, F.L.; Wilson, D.; Hube, B. Candida Albicans Pathogenicity Mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef]
- Hernández-Chávez, M.J.; Pérez-García, L.A.; Niño-Vega, G.A.; Mora-Montes, H.M. Fungal Strategies to Evade the Host Immune Recognition. J. Fungi 2017, 3, 51. [Google Scholar] [CrossRef]
- Dyląg, M.; Colon-Reyes, R.J.; Kozubowski, L. Titan Cell Formation Is Unique to Cryptococcus Species Complex. Virulence 2020, 11, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Dylag, M.; Colón-Reyes, R.J.; Loperena-álvarez, Y.; Kozubowski, L. Establishing Minimal Conditions Sufficient for the Development of Titan-like Cells in Cryptococcus Neoformans/Gattii Species Complex. Pathogens 2022, 11, 768. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corrêa-Junior, D.; Bastos de Andrade, I.; Alves, V.; Avellar-Moura, I.; Brito de Souza Rabello, V.; Valdez, A.F.; Nimrichter, L.; Zancopé-Oliveira, R.M.; Ribeiro de Sousa Araújo, G.; Almeida-Paes, R.; et al. Unveiling the Morphostructural Plasticity of Zoonotic Sporotrichosis Fungal Strains: Possible Implications for Sporothrix brasiliensis Virulence and Pathogenicity. J. Fungi 2023, 9, 701. https://doi.org/10.3390/jof9070701
Corrêa-Junior D, Bastos de Andrade I, Alves V, Avellar-Moura I, Brito de Souza Rabello V, Valdez AF, Nimrichter L, Zancopé-Oliveira RM, Ribeiro de Sousa Araújo G, Almeida-Paes R, et al. Unveiling the Morphostructural Plasticity of Zoonotic Sporotrichosis Fungal Strains: Possible Implications for Sporothrix brasiliensis Virulence and Pathogenicity. Journal of Fungi. 2023; 9(7):701. https://doi.org/10.3390/jof9070701
Chicago/Turabian StyleCorrêa-Junior, Dario, Iara Bastos de Andrade, Vinicius Alves, Igor Avellar-Moura, Vanessa Brito de Souza Rabello, Alessandro Fernandes Valdez, Leonardo Nimrichter, Rosely Maria Zancopé-Oliveira, Glauber Ribeiro de Sousa Araújo, Rodrigo Almeida-Paes, and et al. 2023. "Unveiling the Morphostructural Plasticity of Zoonotic Sporotrichosis Fungal Strains: Possible Implications for Sporothrix brasiliensis Virulence and Pathogenicity" Journal of Fungi 9, no. 7: 701. https://doi.org/10.3390/jof9070701
APA StyleCorrêa-Junior, D., Bastos de Andrade, I., Alves, V., Avellar-Moura, I., Brito de Souza Rabello, V., Valdez, A. F., Nimrichter, L., Zancopé-Oliveira, R. M., Ribeiro de Sousa Araújo, G., Almeida-Paes, R., & Frases, S. (2023). Unveiling the Morphostructural Plasticity of Zoonotic Sporotrichosis Fungal Strains: Possible Implications for Sporothrix brasiliensis Virulence and Pathogenicity. Journal of Fungi, 9(7), 701. https://doi.org/10.3390/jof9070701






