Triphenylphosphonium (TPP)-Conjugated Quinolone Analogs Displayed Significantly Enhanced Fungicidal Activity Superior to Its Parent Molecule
Abstract
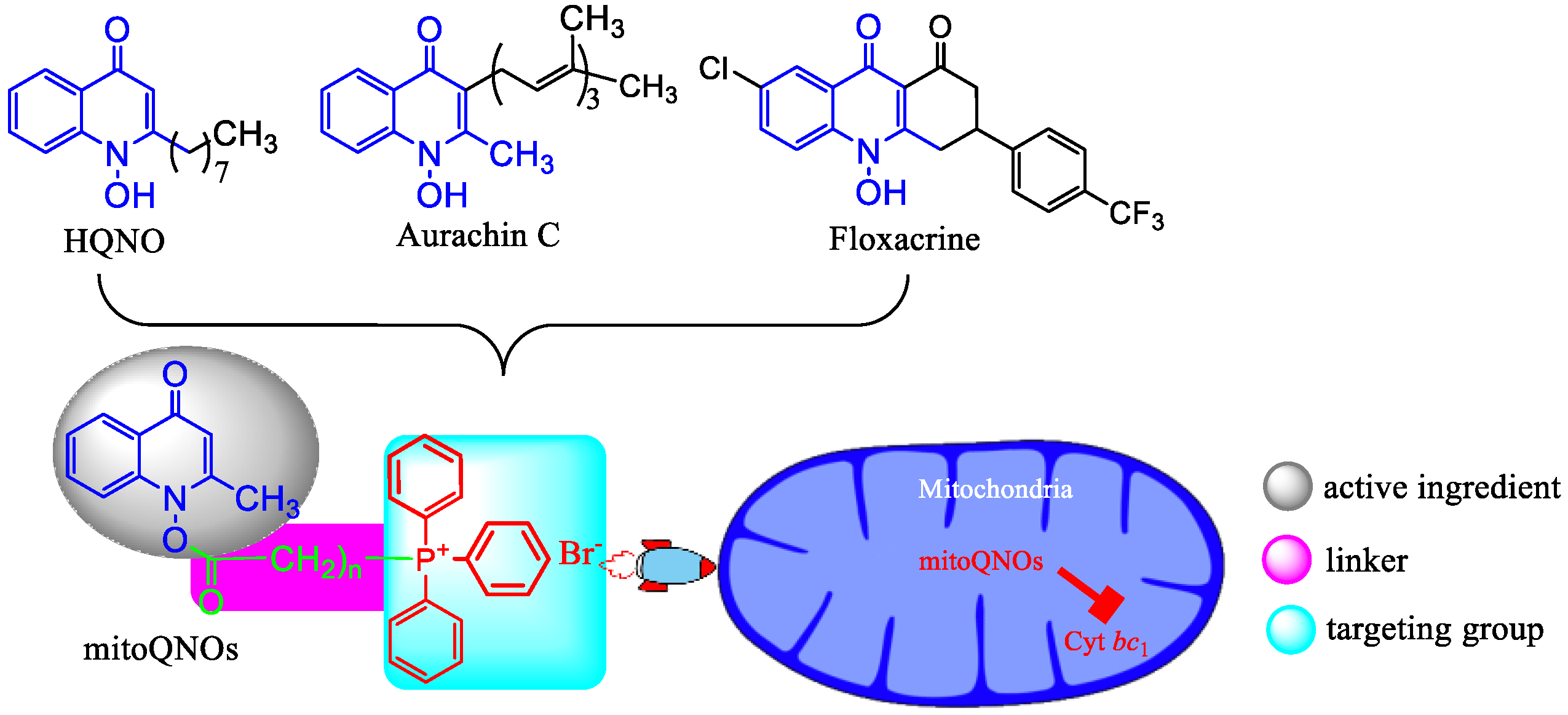
1. Introduction
2. Materials and Methods
3. Results
Subsection
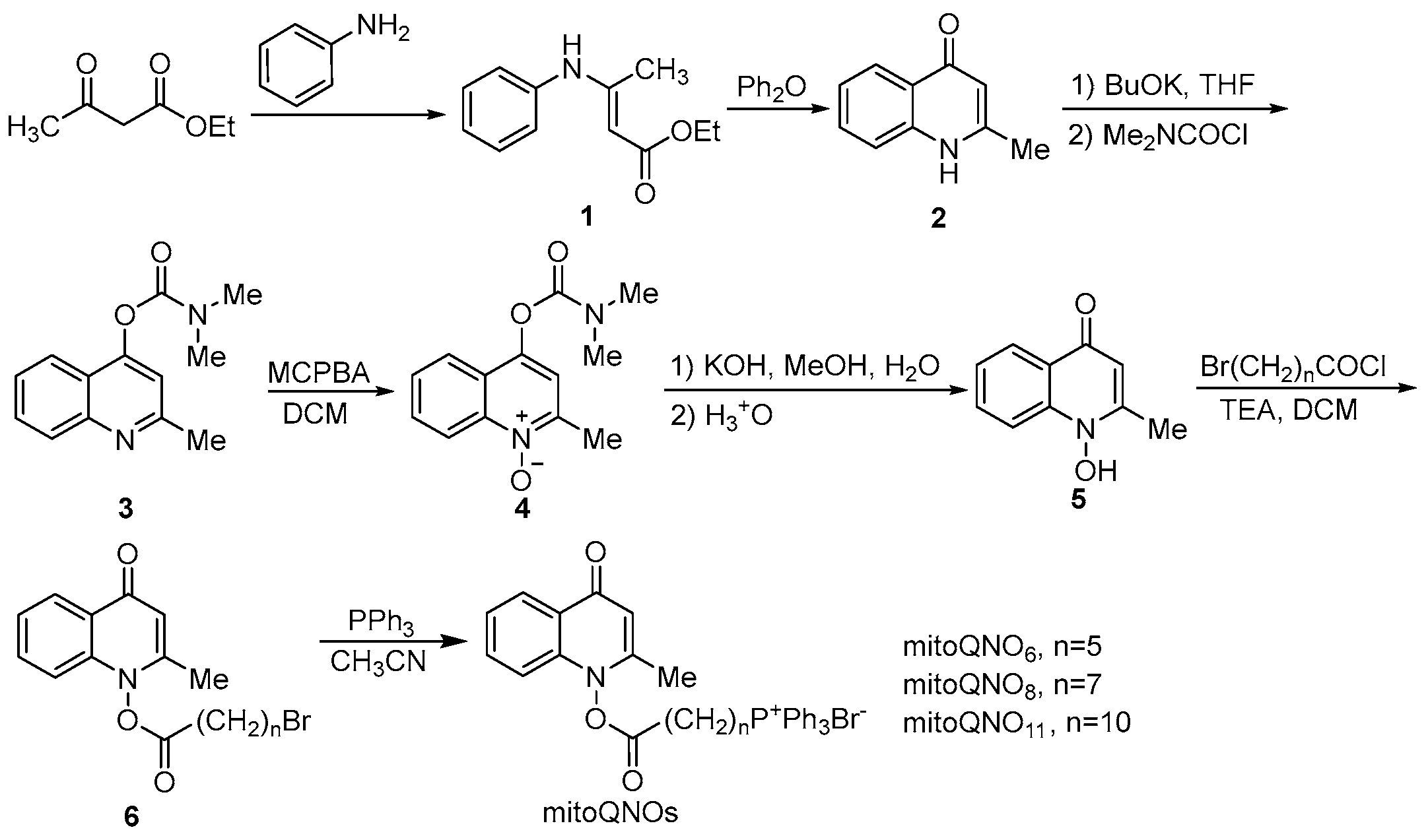
- Chemistry. The key intermediate 2-methyl-1-hydroxy-4(1H)-quinolone (5) was prepared with a similar method described by Cross and Cornforth [20,21]. It was then acylated to give 6, which reacted with triphenylphosphine by a nucleophilic substitution to afford mitoQNOs (Scheme 1)Scheme 1. The synthetic pathway of mitoQNOs.
- MitoQNO11 exhibited significantly enhanced fungicidal activity. Results concerning fungicidal activities of mitoQNOs against 11 plant pathogenic fungi, preliminarily determined at a concentration of 70 μmol, are shown in Table 1. As we previously described for SDH inhibition [15], the activity of mitoQNOs was also enhanced with the increase in chain length of the TPP-conjugated linker. For example, the inhibition rates of mitoQNO6, mitoQNO8, and mitoQNO11 against S. sclerotiorum were 19.86%, 45.44%, and 91.47%, respectively. This is mainly because of the increase in liposolubility, which makes it easier to pass through the membrane (Table 1). Meanwhile, it was also found that mitoQNO11 exhibited excellent fungicidal activity against P. aphanidermatum, P. capsica, and B. cinerea, and the inhibition rates were 75.63%, 87.50%, and 78.88%, respectively. Compared with the lead molecule MQNO, mitoQNO11 showed significantly enhanced fungicidal activity against all tested pathogenic fungi.Table 1. In vitro fungicidal activity of title compounds.
Pathogen 1 Inhibition Rate (70 μmol/L, %) MQNO MitoQNO6 MitoQNO8 MitoQNO11 Boscalid Kresoxim-Methyl PA 15.21 ± 1.06 0 ± 0.59 0 ± 0.51 75.63 ± 0.88 9.79 ± 1.06 81.25 ± 7.14 PC 10.90 ± 0.54 2.70 ± 0.31 29.96 ± 1.66 87.5 ± 1.32 17.11 ± 1.25 30.19 ± 0.54 SS 14.75 ± 1.59 19.86 ± 2.63 45.44 ± 2.65 91.47 ± 2.68 95.31 ± 0.60 40.32 ± 0.60 BC 13.01 ± 7.97 0 ± 0.59 0 ± 0.59 78.88 ± 5.33 92.05 ± 0.59 30.78 ± 0.30 BB 18.10 ± 3.85 0 ± 0.54 0 ± 0.31 41.67 ± 0.59 54.79 ± 1.93 72.08 ± 0.78 ET 2.15 ± 0.33 2.86 ± 0.88 4.73 ± 0.33 24.86 ± 0.57 95.32 ± 0.66 26.5 ± 0.66 CA 2.95 ± 0.55 0 ± 0.63 3.61 ± 1.09 19.23 ± 0.63 94.65 ± 1.09 50.47 ± 1.09 FO 5.18 ± 0.83 5.18 ± 1.14 6.29 ± 0.87 49.58 ± 1.26 38.20 ± 0.32 59.39 ± 0.63 RS 5.42 ± 1.18 0.42 ± 0.59 0.42 ± 0.59 45.00 ± 1.02 87.08 ± 0.59 72.92 ± 0.29 PI 0 ± 0.65 5.55 ± 1.72 7.15 ± 0.15 15.64 ± 3.24 57.36 ± 1.49 63.09 ± 0.32 CL 9.35 ± 2.03 3.12 ± 1.48 6.00 ± 0.68 15.83 ± 1.02 40.53 ± 1.48 1.44 ± 1.02 logP 2 0.49 5.65 6.66 8.16 4.64 4.13 1. PA = Pythium aphanidermatum; PC = Phytophthora capsici; SS = Sclerotinia sclerotiorum; BC = Botrytis cinerea; BB = Botryosphaeria berengeriana; ET = Exserohilum turcicum; CA = Cercospora arachidicola Hori; FO = Fusarium oxysporum f.sp. vasinfectum; RS = Rhizoctonia solani; PI = Phytophthora infestans; CL = Colletotrichum lagenarium. 2. Calculated by Molinspiration Cheminformatics 2019.
- In order to verify the effectiveness of mitoQNO11, we further determined its precision toxicity against four pathogenic fungi (Table 2). Compared with the selected fungicides that act on the mitochondrial respiratory chain, mitoQNO11 showed good inhibitory effects towards the four tested strains. The EC50 values for P. capsici, P. aphanidermatum, B. cinereal, and S. sclerotiorum were 7.42, 51.42, 21.73, and 4.43 μmol/L, respectively. Especially, mitoQNO11 exhibited excellent fungicidal activity against P. capsici, a pathogenic fungus causing phytophthora blight of pepper, which has become increasingly serious in recent years [22].Table 2. Precision toxicity of mitoQNO11 against four pathogenic fungi.
Compound EC50 (μmol/L) P. capsici S. sclerotiorum B. cinerea P. aphanidermatum MQNO >140 >140 >140 >140 mitoQNO11 7.42 ± 0.73 4.43 ± 1.51 21.73 ± 1.71 51.42 ± 3.67 boscalid >140 0.83 ± 0.07 6.79 ± 0.64 >140 kresoxim-methyl >140 71.41 ± 18.88 >140 51.56 ± 18.63 pyrimorph 3.77 ± 0.19 74.33 ± 8.59 >140 >140
- MitoQNO11 effectively inhibited the activity of cytochrome bc1 complex. Most of the 1-hydroxy-4-quinolone derivatives were reported as cytochrome bc1 complex inhibitors, so the effect of mitoQNO11 on bc1 complex was also investigated. As shown in Figure 2A, mitoQNO11 exhibited more effective inhibitory ability against cytochrome bc1 complex compared to pyrimorph (a potent fungicide against P. capsici mainly acting on cell wall syntheses well as on bc1 complex) [23]. The IC50 values of mitoQNO11 and pyrimorph were 3.33 and 6.89 μmol/L, respectively (Table S1). Therefore, mitoQNO11 is a highly potent inhibitor of bc1 complex, even though it might have other action mechanisms.Figure 2. Influences comparison of mitoQNO11 with pyrimorph on the respiration and ATP production of P. capsici. (A) Column chart depicting the inhibition rate of cytochrome bc1 complex of P. capsica. (B) Column chart depicting the respiratory inhibiting rate of P. capsica. (C) Changes of ATP content in hyphae after treatment with mitoQNO11. (D) ATP inhibitory rate of mitoQNO11 and pyrimorph. Data points correspond to the mean of three independent experiments ± standard error of the mean.Figure 2. Influences comparison of mitoQNO11 with pyrimorph on the respiration and ATP production of P. capsici. (A) Column chart depicting the inhibition rate of cytochrome bc1 complex of P. capsica. (B) Column chart depicting the respiratory inhibiting rate of P. capsica. (C) Changes of ATP content in hyphae after treatment with mitoQNO11. (D) ATP inhibitory rate of mitoQNO11 and pyrimorph. Data points correspond to the mean of three independent experiments ± standard error of the mean.
- MitoQNO11 strongly suppressed mycelium respiration. As an important part of ETC, the inhibition of the bc1 complex results in the inhibition of mitochondria respiration. Therefore, the influence of mitoQNO11 on the mycelium respiration of P. capsici was measured. As shown in Figure 2B, at a concentration of 35 μmol/L, the inhibition rate of mitoQNO11 was 4.2 times higher than that of pyrimorph, and the inhibition was dose-dependent.
- MitoQNO11 strongly inhibited ATP generation. Inhibition of respiration implies a significant reduction in ATP synthesis by the pathogen. Therefore, we monitored the changes in cellular ATP of P. capsici after drug treatment by firefly luciferase. After three hours of treatment at a concentration of 35 μmol/L, the ATP level in the mitoQNO11 group was reduced by 50%, whereas in the pyrimorph group, it was reduced by less than 10% (Figure 2C); the inhibition was found to be dose-dependent. Even at 140 μmol/L, the ATP level in the pyrimorph group decreased by less than half, whereas the inhibition rate of mitoQNO11 reached to 85.08% after three hours of treatment at the same concentration (Figure 2D).
- MitoQNO11 strongly reduced mitochondrial membrane potential. The integrity of the ETC function is the critical element in maintaining MMP (∆Ψm). Next, we investigated the impact of mitoQNO11 on ∆Ψm by using a cationic dye JC-1, a potent indicator of MMP changes due to the formation of red fluorescent ‘J-aggregates’.
- The mycelium of P. capsici was treated with mitoQNO11, pyrimorph, or FCCP (a well-known uncoupler that can easily cause a reduction in mitochondrial membrane potential), respectively, at 20 μmol/L for 3 h at 25 °C, followed by staining with JC-1. As shown in Figure 3A, in the control group the granular mitochondria in hyphae emitted red fluorescence, whereas no obvious green fluorescence was observed, indicating high ∆Ψm. In the mitoQNO11 group, we observed the obvious disappearance of red fluorescence and an enhancement of green fluorescence, indicating the decrease in ∆Ψm. By analyzing its mean fluorescence intensity (Figure 3B), it was found that the intensity of red fluorescence decreased by half compared to the control group, whereas the intensity of green fluorescence was three times higher than that of the control group (Figure 3C). However, there was no decrease in red fluorescence in pyrimorph treatment group compared with the control group, and similar results were obtained by analyzing the mean fluorescence intensity. As a positive control, after treatment with FCCP, the obvious disappearance of red fluorescence and the enhancement of green fluorescence were also observed (Figure 3A,B). These results indicated that the MMP of P. capsici was strongly reduced by mitoQNO11.Figure 3. Effect of mitoQNO11 on mitochondrial membrane potential as monitored by JC-1 staining. JC-1 aggregates accumulate in the mitochondrial matrix with high ∆Ψm, emitting orange-red fluorescence; upon depolarization of the mitochondrial membrane, JC-1 aggregates dissipate into the cytoplasm as monomers emitting green fluorescence. (A) JC-1 staining was observed by fluorescence microscopy after treatment with mitoQNO11, pyrimorph, and FCCP at 20 μmol/L. Scale bar 50 μm. (B) Change in mean fluorescence intensity (MFI) of red fluorescence (JC-1 aggregates) after treatment with mitoQNO11, pyrimorph, and FCCP, respectively, at 20 μmol/L. (C) Change in mean fluorescence intensity of green fluorescence (JC-1 monomer) after treatment with mitoQNO11, pyrimorph, and FCCP at 20 μmol/L. Data points correspond to the mean of three independent experiments ± standard error of the mean.Figure 3. Effect of mitoQNO11 on mitochondrial membrane potential as monitored by JC-1 staining. JC-1 aggregates accumulate in the mitochondrial matrix with high ∆Ψm, emitting orange-red fluorescence; upon depolarization of the mitochondrial membrane, JC-1 aggregates dissipate into the cytoplasm as monomers emitting green fluorescence. (A) JC-1 staining was observed by fluorescence microscopy after treatment with mitoQNO11, pyrimorph, and FCCP at 20 μmol/L. Scale bar 50 μm. (B) Change in mean fluorescence intensity (MFI) of red fluorescence (JC-1 aggregates) after treatment with mitoQNO11, pyrimorph, and FCCP, respectively, at 20 μmol/L. (C) Change in mean fluorescence intensity of green fluorescence (JC-1 monomer) after treatment with mitoQNO11, pyrimorph, and FCCP at 20 μmol/L. Data points correspond to the mean of three independent experiments ± standard error of the mean.
- MitoQNO11 strongly induced ROS production. Mitochondrial dysfunction is closely related to the excessive reactive oxygen species (ROS) and the respiratory chain is the main source of ROS in eukaryote. The inhibition of the respiratory chain can lead to the disruption of oxidative stress balance. Then we examined the changes of intracellular ROS level using an oxidation-sensitive probe, 2′,7′-dichlorofluorescin diacetate (DCFH-DA).
- As shown in the control group, no fluorescence signal was observed (Figure 4). However, obvious green fluorescence was observed in the mitoQNO11 group, and we could see bright green fluorescence distributed in dots on the hyphae. In stark contrast, there was no obvious green fluorescence in the pyrimorph group. By analyzing mean fluorescence intensity, it was found that the green fluorescence intensity doubled after mitoQNO11 treatment. These observations suggested that mitoQNO11 has a strong capability to induce ROS generation in phytopathogens. Considering that the cytochrome bc1 complex is one of the main sources of mitochondrial ROS and that 1-hydroxy-4-quinolone derivatives mainly target the Qi site [24,25,26], we propose that the primary mechanism of action of mitoQNO11 is that it accumulates in mitochondria and binds to the Qi site of cytochrome bc1 complex, thus leading to the leakage of electrons differentiated from the Qo site, resulting in the production of large amounts of ROS (Figure 5).
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.-J. Research progress of quinolone compounds. Liaoning Chem. Ind. 2019, 48, 792–795, 799. [Google Scholar]
- Mohammed, H.H.; Abuo-Rahma, G.E.-D.A.; Abbas, S.; Abdelhafez, E.-S.M. Current Trends and Future Directions of Fluoroquinolones. Curr. Med. Chem. 2019, 26, 3132–3149. [Google Scholar] [CrossRef]
- Chu, X.-M.; Wang, C.; Liu, W.; Liang, L.-L.; Gong, K.-K.; Zhao, C.-Y.; Sun, K.-L. Quinoline and quinolone dimers and their biological activities: An overview. Eur. J. Med. Chem. 2019, 161, 101–117. [Google Scholar] [CrossRef]
- Refojo, P.N.; Teixeira, M.; Pereira, M.M. The alternative complex III of Rhodothermus marinus and its structural and functional association with caa3 oxygen reductase. Biochim. Biophys. Acta 2010, 1797, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Hoefle, G.; Kunze, B. Biosynthesis of Aurachins A-L in Stigmatella aurantiaca: A Feeding Study. J. Nat. Prod. 2008, 71, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Hoefnagel, M.H.N.; Wiskich, J.T.; Madgwick, S.A.; Patterson, Z.; Oettmeier, W.; Rich, P.R. New inhibitors of the ubiquinol oxidase of higher plant mitochondria. Eur. J. Biochem. 1995, 233, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Yap, L.L.; Lin, M.T.; Ouyang, H.; Samoilova, R.I.; Dikanov, S.A.; Gennis, R.B. The quinone-binding sites of the cytochrome bo3 ubiquinol oxidase from Escherichia coli. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1924–1932. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M.P.; Gut, J.; Rodrigues, T.; Ribeiro, M.H.; Lopes, F.; Rosenthal, P.J.; Moreira, R.; dos Santos, D.J.V.A. Exploring the Molecular Basis of Qo bc1 Complex Inhibitors Activity to Find Novel Antimalarials Hits. Mol. Inform. 2013, 32, 659–670. [Google Scholar] [CrossRef]
- Romero, A.H. Role of Trifluoromethyl Substitution in Design of Antimalarial Quinolones: A Comprehensive Review. Top. Curr. Chem. 2019, 377, 9. [Google Scholar] [CrossRef]
- Liu, X.D.; Wang, D.W.; Xie, L. Correlation between quinolone uptakes by BCECs in vitro and brain-to-plasma concentration ratios in rats. Eur. J. Drug Metab. Pharmacokinet. 2005, 30, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Ogbolu, D.O.; Daini, O.A.; Alli, A.O.T.; Webber, M.A. Reduced intracellular drug accumulation augments fluoroquinolone and β-lactam drugs resistance in clinical Gram negative bacteria from Nigeria. Afr. J. Microbiol. Res. 2015, 9, 1082–1088. [Google Scholar]
- Ndi, M.; Marin-Buera, L.; Salvatori, R.; Singh, A.P.; Ott, M. Biogenesis of the bc1 Complex of the Mitochondrial Respiratory Chain. J. Mol. Biol. 2018, 430, 3892–3905. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yan, Q.; Li, X.; Zhang, M. Current status of resistance and development of strobilurin fungicide. Agrochemicals. 2011, 50, 547–551, 572. [Google Scholar]
- Wang, J.; Li, J.; Xiao, Y.; Fu, B.; Qin, Z. TPP-based mitocans: A potent strategy for anticancer drug design. RSC Med. Chem. 2020, 11, 858–875. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Zhang, X.; Du, S.; Han, X.; Li, J.-Q.; Xiao, Y.; Xu, Z.; Wu, Q.; Xu, L.; et al. Fungicidal Action of the Triphenylphosphonium-Driven Succinate Dehydrogenase Inhibitors Is Mediated by Reactive Oxygen Species and Suggests an Effective Resistance Management Strategy. J. Agric. Food Chem. 2022, 70, 111–123. [Google Scholar] [CrossRef]
- Hughes, J.K.; Hodge, A.; Fitter, A.H.; Atkin, O.K. Mycorrhizal respiration: Implications for global scaling relationships. Trends Plant Sci. 2008, 13, 583–588. [Google Scholar] [CrossRef]
- Chen, W.; Wang, P.; Lu, Y.; Jin, T.; Lei, X.; Liu, M.; Zhuang, P.; Liao, J.; Lin, Z.; Li, B.; et al. Decreased expression of mitochondrial miR-5787 contributes to chemoresistance by reprogramming glucose metabolism and inhibiting MT-CO3 translation. Theranostics 2019, 9, 5739–5754. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.; Wang, J.; Zhang, Z.; Zhai, Y.; Yang, G.-Y.; Sun, X. Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochem. Biophys. Res. Commun. 2014, 444, 182–188. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Jiang, Z.-Y.; Zhu, Q.; Zhong, G.-H. Discovery of β-Carboline Oxadiazole Derivatives as Fungicidal Agents against Rice Sheath Blight. J. Agric. Food Chem. 2018, 66, 9598–9607. [Google Scholar] [CrossRef]
- Cornforth, J.W.; James, A.T. Structure of naturally occurring antagonist of dihydrostreptomycin. Biochem. J. 1956, 63, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Cross, R.M.; Manetsch, R. Divergent Route to Access Structurally Diverse 4-Quinolones via Mono or Sequential Cross-Couplings. J. Org. Chem. 2010, 75, 8654–8657. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-J.; Long, X.-H.; Li, E.-Z. Evaluation of antifungal phenolics from Helianthus tuberosus L. leaves against Phytophthora capsici Leonian by chemometric analysis. Molecules 2019, 24, 4300. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-M.; Esser, L.; Zhou, F.; Li, C.; Zhou, Y.-H.; Yu, C.-A.; Qin, Z.-H.; Xia, D. Studies on inhibition of respiratory cytochrome bc1 complex by the fungicide pyrimorph suggest a novel inhibitory mechanism. PLoS ONE 2014, 9, e93765. [Google Scholar] [CrossRef]
- Cooley, J.W.; Ohnishi, T.; Daldal, F. Binding dynamics at the quinone reduction (Qi) site influence the equilibrium interactions of the iron sulfur protein and hydroquinone oxidation (Qo) site of the cytochrome bc1 complex. Biochemistry 2005, 44, 10520–10532. [Google Scholar] [CrossRef]
- David Hong, W.; Leung, S.C.; Amporndanai, K.; Davies, J.; Priestley, R.S.; Nixon, G.L.; Berry, N.G.; Samar Hasnain, S.; Antonyuk, S.; Ward, S.A.; et al. Potent Antimalarial 2-Pyrazolyl Quinolone bc1 (Qi) Inhibitors with Improved Drug-Like Properties. ACS Med. Chem. Lett. 2018, 9, 1205–1210. [Google Scholar] [CrossRef]
- Thierbach, S.; Birmes, F.S.; Letzel, M.C.; Hennecke, U.; Fetzner, S. Chemical Modification and Detoxification of the Pseudomonas aeruginosa Toxin 2-Heptyl-4-hydroxyquinoline N-Oxide by Environmental and Pathogenic Bacteria. ACS Chem. Biol. 2017, 12, 2305–2312. [Google Scholar] [CrossRef]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radical Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Liu, X.; Yin, F.; Xu, Y.; Fu, B.; Li, J.; Qin, Z. Triphenylphosphonium (TPP)-Conjugated Quinolone Analogs Displayed Significantly Enhanced Fungicidal Activity Superior to Its Parent Molecule. J. Fungi 2023, 9, 685. https://doi.org/10.3390/jof9060685
Wang J, Liu X, Yin F, Xu Y, Fu B, Li J, Qin Z. Triphenylphosphonium (TPP)-Conjugated Quinolone Analogs Displayed Significantly Enhanced Fungicidal Activity Superior to Its Parent Molecule. Journal of Fungi. 2023; 9(6):685. https://doi.org/10.3390/jof9060685
Chicago/Turabian StyleWang, Jiayao, Xuelian Liu, Fahong Yin, Yanjun Xu, Bin Fu, Jiaqi Li, and Zhaohai Qin. 2023. "Triphenylphosphonium (TPP)-Conjugated Quinolone Analogs Displayed Significantly Enhanced Fungicidal Activity Superior to Its Parent Molecule" Journal of Fungi 9, no. 6: 685. https://doi.org/10.3390/jof9060685
APA StyleWang, J., Liu, X., Yin, F., Xu, Y., Fu, B., Li, J., & Qin, Z. (2023). Triphenylphosphonium (TPP)-Conjugated Quinolone Analogs Displayed Significantly Enhanced Fungicidal Activity Superior to Its Parent Molecule. Journal of Fungi, 9(6), 685. https://doi.org/10.3390/jof9060685