Cutaneous Fungal Infections Caused by Dermatophytes and Non-Dermatophytes: An Updated Comprehensive Review of Epidemiology, Clinical Presentations, and Diagnostic Testing
Abstract
1. Introduction
2. Epidemiology
2.1. Dermatophyte Infections
2.2. Non-Dermatophyte Infections
2.3. Resistant Cutaneous Dermatophytosis
| Disease | Europe | South America | ||||
|---|---|---|---|---|---|---|
| Switzerland [17] (2001–2018) | Ireland [18] (2001–2020) | Slovakia [36] (2014–2016) | Germany [37] (2014–2016) | Brazil [19] (2011–2019) | Argentina [38] (2002–2007) | |
| N = 10,958 | N = 2263 | N = 2103 | N = 1252 | N = 10,396 | N = 1313 | |
| Tinea capitis | n = 830 | n = 100 | n = 44 (including tinea faciei) | n = 28 | n = 435 | n = 269 |
| 1. T. violaceum | 1. T. tonsurans | 1. T. mentagrophytes | 1. T. mentagrophytes | 1. T. tonsurans | 1. M. canis | |
| 2. M. audouinii | 2. M. canis | 2. M. canis | 2. M. canis | 2. M. canis | 2. T. mentagrophytes | |
| 3. T. soudanense | 3. T. rubrum | 3. M. audouinii | 3. T. benhamiae | 3. N. gypsea | 3. N. gypsea | |
| Tinea faciei | n = 283 | n = 10 | - | n = 14 | n = 151 | - |
| 1. T. mentagrophytes | 1. T. tonsurans | 1. T. rubrum | 1. T. rubrum | |||
| 2. T. benhamiae | 2. T. verrucosum | 2. T. benhamiae | 2. N. gypsea | |||
| 3. T. rubrum | - | 3. M. canis | 3. T. interdigitale | |||
| Tinea corporis | n = 1006 | n = 64 | n = 169 | n = 185 | n = 1148 | n = 202 |
| 1. T. mentagrophytes | 1. T. rubrum | 1. T. tonsurans | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. rubrum | 2. M. canis | 2. T. rubrum | 2. T. benhamiae | 2. M. canis | 2. T. mentagrophytes | |
| 3. M. canis | 3. T. tonsurans | 3. T. mentagrophytes | 3. T. interdigitale | 3. T. tonsurans | 3. M. canis | |
| Tinea manuum | n = 169 | n = 19 | n = 100 | n = 48 | n = 231 | n = 26 |
| 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. mentagrophytes | |
| 2. T. mentagrophytes | 2. T. verrucosum | 2. T. mentagrophytes | 2. T. interdigitale | 2. T. interdigitale | 2. T. rubrum | |
| 3. T. benhamiae | - | 3. T. tonsurans | 3. T. benhamiae | 3. T. tonsurans | 3. Trichophyton spp. | |
| Tinea cruris | n = 427 | n = 6 | n = 245 | - | n = 588 | n = 53 |
| 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | ||
| 2. T. mentagrophytes | 2. E. floccosum | 2. T. interdigitale | 2. T. interdigitale | 2. M. canis | ||
| 3. M. canis | - | 3. E. floccosum | 3. T. tonsurans | 3. T. mentagrophytes | ||
| Tinea pedis | n = 2439 | n = 134 | n = 649 | n = 398 | n = 3222 | n = 77 |
| 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. interdigitale | 2. T. mentagrophytes | 2. T. interdigitale | 2. T. interdigitale | 2. T. interdigitale | 2. T. interdigitale | |
| 3. E. floccosum | 3. T. interdigitale | 3. T. mentagrophytes | 3. T. mentagrophytes | 3. E. floccosum | 3. Trichophyton spp. | |
| Onychomycosis | n = 5803 | n = 1617 | n = 896 | n = 579 | n = 4621 | n = 671 |
| 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. interdigitale | 2. T. mentagrophytes | 2. T. interdigitale | 2. T. interdigitale | 2. T. interdigitale | 2. T. interdigitale | |
| 3. T. soudanense | 3. T. interdigitale | 3. T. tonsurans | - | 3. T. mentagrophytes | 3. Trichophyton spp. | |
| Diseases | Asia | |||||
| Iran [39] (2008–2010) | Iran [40] (2010–2014) | India [26] (2014–2015) | China [21] (2004–2014) | Japan [20] (2016) | Thailand [16] (2014–2016) | |
| N = 777 | N = 1535 | N = 66 | N = 588 | N = 1268 | N = 2350 | |
| Tinea capitis | n = 15 | n = 80 | n = 5 | n = 109 | n = 15 | n = 19 |
| 1. M. canis | 1. T. tonsurans | 1. T. tonsurans | 1. M. canis | 1. M. canis | 1. T. rubrum | |
| 2. T. tonsurans | 2. T. mentagrophytes | 2. T. violaceum | 2. T. mentagrophytes | 2. T. rubrum | 2. T. mentagrophytes | |
| 3. T. interdigitale | 3. T. rubrum | - | 3. T. violaceum | 3. T. tonsurans | 3. M. canis | |
| Tinea faciei | n = 9 | - | - | n = 22 | - | n = 50 |
| 1. T. tonsurans | 1. T. mentagrophytes | 1. T. rubrum | ||||
| 2. M. canis | 2. T. rubrum | 2. T. mentagrophytes | ||||
| 3. T. interdigitale | 3. M. canis | 3. M. canis | ||||
| Tinea corporis | n = 131 | n = 242 | n = 20 | n = 61 | n = 188 | n = 276 |
| 1. T. interdigitale | 1. T. tonsurans | 1. T. interdigitale | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. rubrum | 2. T. rubrum | 2. T. tonsurans | 2. M. canis | 2. M. canis | 2. M. canis | |
| 3. M. canis | 3. E. floccosum | 3. M. gypseum | 3. M. gypseum | 3. T. interdigitale | 3. T. mentagrophytes | |
| Tinea manuum | n = 16 | n = 155 | - | n = 20 | n = 19 | n = 54 |
| 1. T. interdigitale | 1. T. tonsurans | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | ||
| 2. T. rubrum | 2. E. floccosum | 2. M. canis | 2. T. interdigitale | 2. T. mentagrophytes | ||
| - | 3. T. verrucosum | - | - | 3. M. canis | ||
| Tinea cruris | n = 171 | n = 457 | n = 35 | n = 72 | n = 90 | n = 198 |
| 1. E. floccosum | 1. E. floccosum | 1. T. interdigitale | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. rubrum | 2. T. rubrum | 2. T. tonsurans | 2. M. gypseum | 2. E. floccosum | 2. T. mentagrophytes | |
| 3. T. interdigitale | 3. T. mentagrophytes | 3. T. rubrum | 3. T. mentagrophytes | 3. N. gypsea | 3. E. floccosum | |
| Tinea pedis | n = 353 | n = 466 | n = 4 | n = 105 | n = 665 | n = 716 |
| 1. T. interdigitale | 1. T. mentagrophytes | 1. T. interdigitale | 1. T. rubrum | 1. T. rubrum | 1. T. mentagrophytes | |
| 2. T. rubrum | 2. T. rubrum | 2. T. tonsurans | 2. T. mentagrophytes | 2. T. interdigitale | 2. T. rubrum | |
| 3. E. floccosum | 3. E. floccosum | - | - | 3. E. floccosum | 3. E. floccosum | |
| Onychomycosis | n = 82 | n = 135 | n = 2 | n = 199 | n = 290 | n = 1137 |
| 1. T. rubrum | 1. T. rubrum | 1. T. interdigitale | 1. T. rubrum | 1. T. rubrum | 1. T. rubrum | |
| 2. T. interdigitale | 2. T. mentagrophytes | - | 2. T. mentagrophytes | 2. T. interdigitale | 2. T. mentagrophytes | |
| 3. E. floccosum | 3. E. floccosum | - | - | 3. M. canis | 3. T. tonsurans | |
| Organisms | Europe | South America | Africa | Asia | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Switzerland [17] (2001–2018) | Greece [41,42] (2004–2015) (2015–2017) | Serbia [43] (2012–2014) | Guatemala [44] (2008–2011) | French Guiana [45] (2006–2009) | Ethiopia [46] (2015–2019) | Morocco [47] (2006–2010) | Iran [48] (2007–2014) | Israel [49] (2001–2015) | China [50] (2001–2020) | Thailand [51] (2014–2019) | |
| N = 17,175 * | N = 1450 ** | N = 190 ** | N = 4220 ** | N = 205 * | N = 571 * | N = 1335 * | N = 648 * | N = 27,093 * | N = 32,190 * | N = 2740 ** | |
| Acremonium spp. | 1078 *** | 41 | - | 2 | - | 1 | - | 3 | 26 | - | - |
| Alternaria spp. | - | - | - | 1 | - | 3 | - | 3 | 15 | - | - |
| Aspergillus spp. | - | 1 | 2 | 11 | 2 | 28 | 14 | 108 | 53 | 958 | - |
| Cladosporium spp. | - | - | - | 3 | - | 14 | - | 2 | 1 | - | - |
| Fusarium spp. | 1078 *** | 10 | 1 | 1 | 5 | 14 | 6 | 7 | 14 | 106 | 253 |
| Neoscytalidium dimidiatum | - | - | 2 | - | 29 | 11 | 27 | - | - | - | 360 |
| Penicillium spp. | - | - | - | - | 1 | 14 | - | 3 | 2 | 496 | - |
| Scopulariopsis brevicaulis | - | 48 | 2 | 8 | 2 | 4 | - | 8 | 22 | 45 | - |
| Other molds | 6996 | - | 1 | 6 | 5 | - | 20 | 9 | 24 | 522 | - |
| Total | 8074 (47%) | 100 (6.9%) | 8 (4.2%) | 32 (0.8%) | 44 (21.5%) | 89 (15.6%) | 67 (5%) | 143 (22%) | 157 (0.6%) | 2127 (6.6%) | N/A |
3. Body Sites, Dermatophyte Species and Their Geographical Distribution
3.1. Tinea Capitis
3.2. Tinea Faciei
3.3. Tinea Corporis
3.4. Tinea Cruris
3.5. Tinea Manuum
3.6. Tinea Pedis
3.7. Onychomycosis
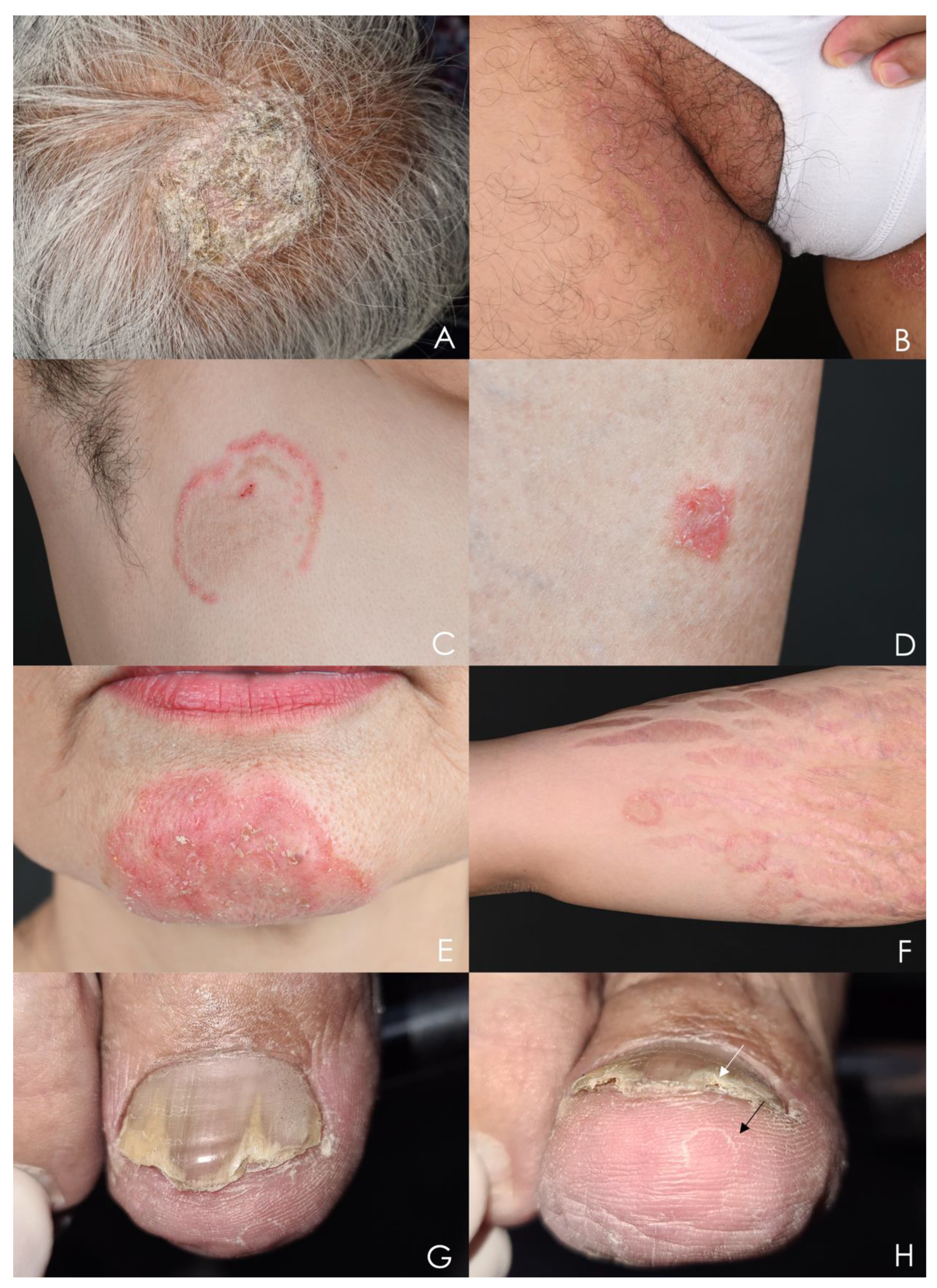
4. Clinical Presentations
4.1. Tinea Capitis
4.2. Tinea Coporis, Tinea Faciei, and Tinea Cruris
4.3. Tinea Manuum, Tinea Pedis
4.4. Onychomycosis
5. Diagnostic Testing
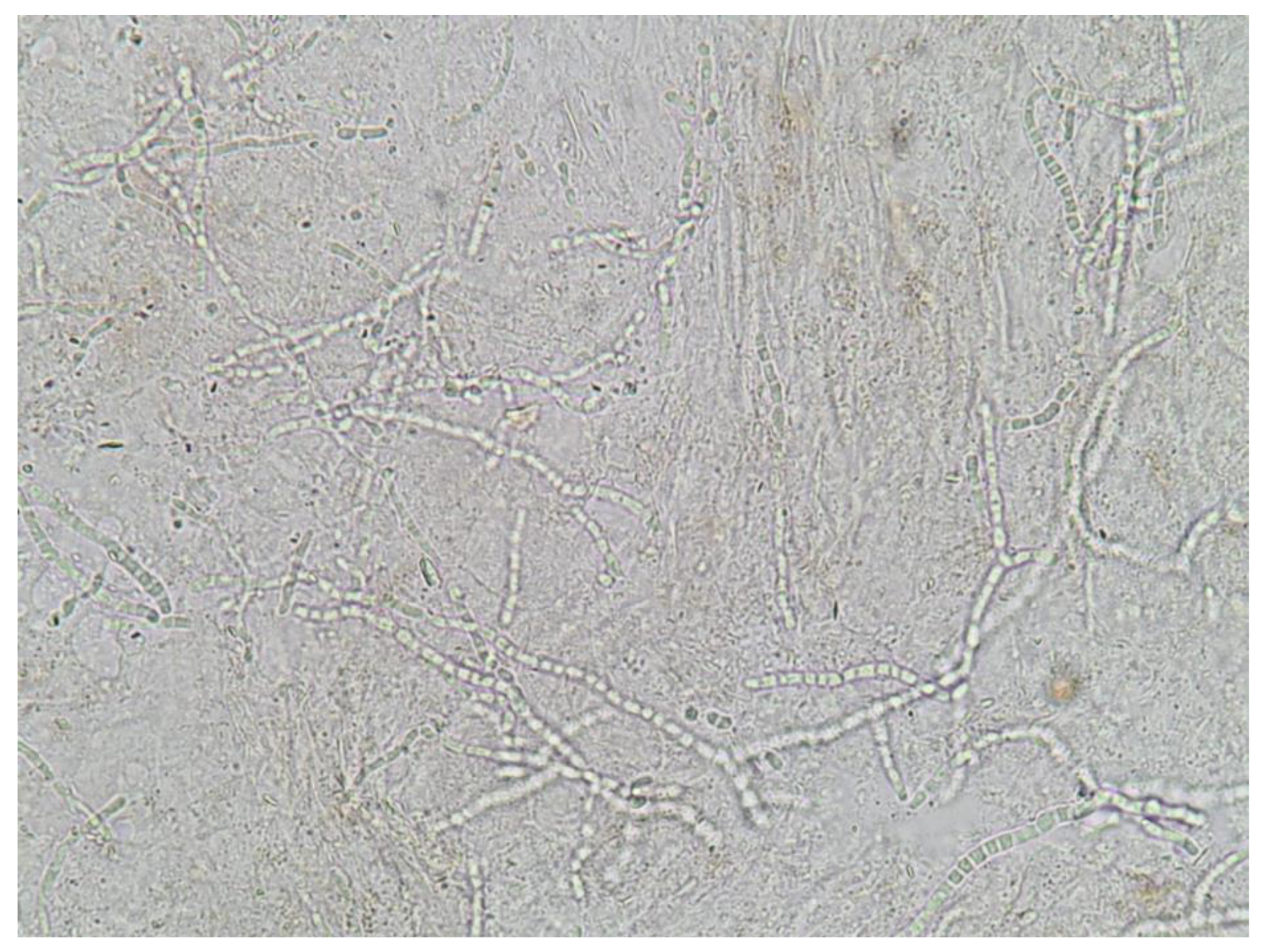
5.1. Direct Microscopic Examination
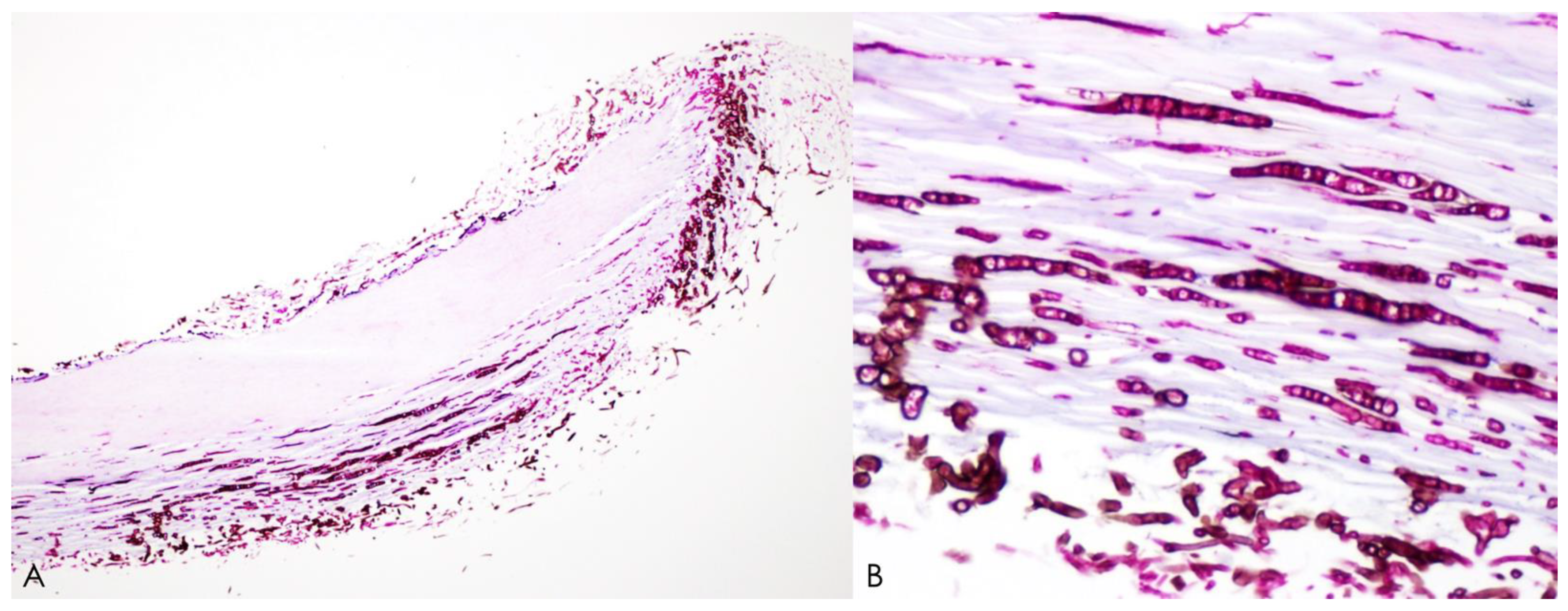
5.2. Histopathology
5.3. Fungal Culture
- identification of fungal hyphae in KOH examination
- isolation of non-dermatophytes in culture
- repeated isolation of the same non-dermatophytes in fungal culture
- growth of the inoculum in at least 5 out of 20 fragments
- absence of dermatophyte isolation in culture
- supporting evidence of onychomycosis from histology
5.4. Molecular Diagnosis
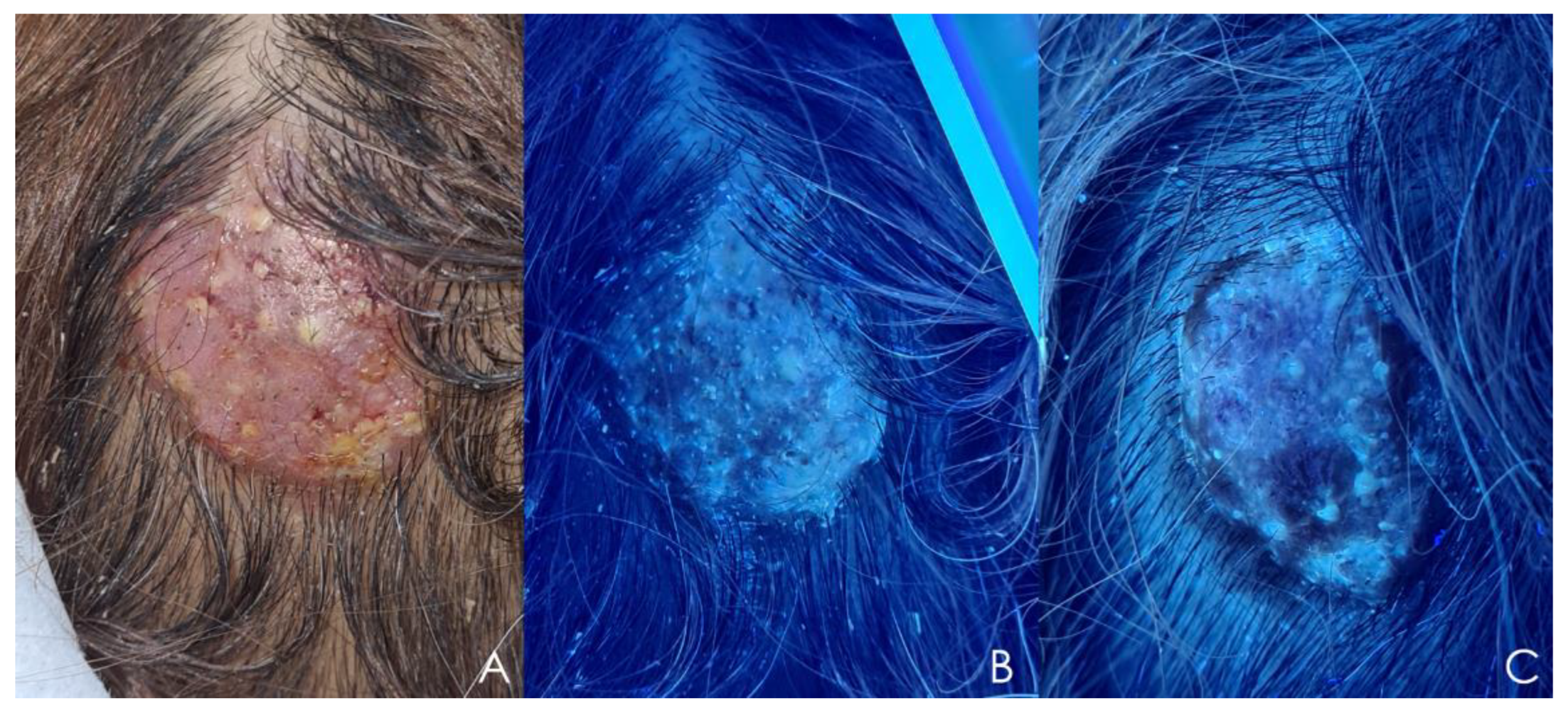
5.5. Dermoscopy
5.6. Wood’s Light Examination
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Havlickova, B.; Czaika, V.A.; Friedrich, M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51 (Suppl. 4), 2–15. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.B.; Panda, S.; Nenoff, P.; Singal, A.; Rudramuruthy, S.M.; Uhrlass, S.; Das, S.; Bisherwal, K.; Shaw, D.; Vasani, R. The unprecedented epidemic-like scenario of dermatophytosis in India: I. Epidemiology, risk factors and clinical features. Indian J. Dermatol. Venereol. Leprol. 2021, 87, 154–175. [Google Scholar] [CrossRef]
- Coulibaly, O.; L’Ollivier, C.; Piarroux, R.; Ranque, S. Epidemiology of human dermatophytoses in Africa. Med. Mycol. 2018, 56, 145–161. [Google Scholar] [CrossRef]
- Emmons, C.W. Dermatophytes: Natural grouping based on the form of the spores and accessory organs. Arch. Derm. Syphilol. 1934, 30, 337–362. [Google Scholar] [CrossRef]
- de Hoog, G.S.; Dukik, K.; Monod, M.; Packeu, A.; Stubbe, D.; Hendrickx, M.; Kupsch, C.; Stielow, J.B.; Freeke, J.; Göker, M.; et al. Toward a novel multilocus phylogenetic taxonomy for the dermatophytes. Mycopathologia 2017, 182, 5–31. [Google Scholar] [CrossRef]
- Uhrlaß, S.; Verma, S.B.; Gräser, Y.; Rezaei-Matehkolaei, A.; Hatami, M.; Schaller, M.; Neoff, P. Trichophyton indotineae-an emerging pathogen causing recalcitrant dermatophytoses in India and worldwide-a multidimensional perspective. J. Fungi 2022, 8, 757. [Google Scholar] [CrossRef]
- Philpot, C.M. Geographical distribution of the dermatophytes: A review. Epidemiol. Infect. 1978, 80, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, C.; Bouchara, J.-P.; Mignon, B. Updates on the epidemiology of dermatophyte infections. Mycopathologia 2008, 166, 335–352. [Google Scholar] [CrossRef] [PubMed]
- Monod, M.; Jaccoud, S.; Zaugg, C.; Léchenne, B.; Baudraz, F.; Panizzon, R. Survey of dermatophyte infections in the Lausanne area Switzerland. Dermatology 2002, 205, 201–203. [Google Scholar] [CrossRef]
- Weitzman, I.; Chin, N.-X.; Kunjukunju, N.; Della-Latta, P. A survey of dermatophytes isolated from human patients in the United States from 1993 to 1995. J. Am. Acad. Dermatol. 1998, 39, 255–261. [Google Scholar] [CrossRef]
- Imwidthaya, S.; Thianprasit, M.; Omcharoen, V. Prevalence of dermatophytosis in Siriraj Hospital. J. Med. Assoc. Thai 1987, 70, 331–334. [Google Scholar] [PubMed]
- Chadeganipour, M.; Shadzi, S.; Dehghan, P.; Movahed, M. Prevalence and aetiology of dermatophytoses in Isfahan, Iran. Mycoses 1997, 40, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Ellabib, M.S.; Khalifa, Z.; Kavanagh, K. Dermatophytes and other fungi associated with skin mycoses in Tripoli, Libya. Mycoses 2002, 45, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Liu, W. The changing face of dermatophytic infections worldwide. Mycopathologia 2017, 182, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Kim, S.L.; Jang, Y.H.; Lee, S.J.; Kim, D.W.; Bang, Y.J.; Jun, J.B. Increasing prevalence of trichophyton rubrum identified through an analysis of 115,846 cases over the last 37 years. J. Korean Med. Sci. 2015, 30, 639–643. [Google Scholar] [CrossRef]
- Bunyaratavej, S.; Limphoka, P.; Kiratiwongwan, R.; Leeyaphan, C. Survey of skin and nail fungal infections by subject age among Thai adults and the etiological organisms. Southeast Asian J. Trop. Med. Public Health 2019, 50, 1132–1138. [Google Scholar]
- Bontems, O.; Fratti, M.; Salamin, K.; Guenova, E.; Monod, M. Epidemiology of dermatophytoses in Switzerland according to a survey of dermatophytes isolated in Lausanne between 2001 and 2018. J. Fungi. 2020, 6, 95. [Google Scholar] [CrossRef]
- Powell, J.; Porter, E.; Field, S.; O’Connell, N.H.; Carty, K.; Dunne, C.P. Epidemiology of dermatomycoses and onychomycoses in Ireland (2001–2020): A single-institution review. Mycoses 2022, 65, 770–779. [Google Scholar] [CrossRef]
- de Oliveira Pereira, F.; Gomes, S.M.; Lima da Silva, S.; Paula de Castro Teixeira, A.; Lima, I.O. The prevalence of dermatophytoses in Brazil: A systematic review. J. Med. Microbiol. 2021, 70, 001321. [Google Scholar] [CrossRef]
- Shimoyama, H.; Sei, Y. Epidemiological survey of dermatomycoses in Japan. Med. Mycol. J. 2019, 60, 75–82. [Google Scholar] [CrossRef]
- Cai, W.; Lu, C.; Li, X.; Zhang, J.; Zhan, P.; Xi, L.; Sun, J.; Yu, X. Epidemiology of superficial fungal infections in Guangdong, Southern China: A retrospective study from 2004 to 2014. Mycopathologia 2016, 181, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Sacheli, R.; Cuypers, L.; Seidel, L.; Darfouf, R.; Adjetey, C.; Lagrou, K.; Hayette, M.P. Epidemiology of dermatophytes in Belgium: A 5 years’ survey. Mycopathologia 2021, 186, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Summerbell, R.C.; Kane, J.; Krajden, S. Onychomycosis, tinea pedis and tinea manuum caused by non-dermatophytic filamentous fungi. Mycoses 1989, 32, 609–619. [Google Scholar] [CrossRef] [PubMed]
- Schechtman, R.C. Nondermatophytic filamentous fungi infection in South America--reality or misdiagnosis? Dermatol. Clin. 2008, 26, 271–283. [Google Scholar] [CrossRef]
- Gupta, A.K.; Summerbell, R.C.; Venkataraman, M.; Quinlan, E.M. Nondermatophyte mould onychomycosis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1628–1641. [Google Scholar] [CrossRef]
- Dabas, Y.; Xess, I.; Singh, G.; Pandey, M.; Meena, S. Molecular identification and antifungal susceptibility patterns of clinical dermatophytes following CLSI and EUCAST Guidelines. J. Fungi 2017, 3, 17. [Google Scholar] [CrossRef]
- Nenoff, P.; Verma, S.B.; Vasani, R.; Burmester, A.; Hipler, U.C.; Wittig, F.; Krüger, C.; Nenoff, K.; Wiegand, C.; Saraswat, A.; et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes—A molecular study. Mycoses 2019, 62, 336–356. [Google Scholar] [CrossRef]
- Kano, R.; Kimura, U.; Kakurai, M.; Hiruma, J.; Kamata, H.; Suga, Y.; Harada, K. Trichophyton indotineae sp. nov.: A new highly terbinafine-resistant anthropophilic dermatophyte species. Mycopathologia 2020, 185, 947–958. [Google Scholar] [CrossRef]
- Ebert, A.; Monod, M.; Salamin, K.; Burmester, A.; Uhrlaß, S.; Wiegand, C.; Hipler, U.C.; Krüger, C.; Koch, D.; Wittig, F.; et al. Alarming India-wide phenomenon of antifungal resistance in dermatophytes: A multicentre study. Mycoses 2020, 63, 717–728. [Google Scholar] [CrossRef]
- Kong, X.; Tang, C.; Singh, A.; Ahmed, S.A.; Al-Hatmi, A.M.S.; Chowdhary, A.; Nenoff, P.; Gräser, Y.; Hainsworth, S.; Zhan, P.; et al. Antifungal susceptibility and mutations in the squalene epoxidase gene in dermatophytes of the trichophyton mentagrophytes species complex. Antimicrob. Agents Chemother. 2021, 65, e0005621. [Google Scholar] [CrossRef]
- Khurana, A.; Agarwal, A.; Agrawal, D.; Panesar, S.; Ghadlinge, M.; Sardana, K.; Sethia, K.; Malhotra, S.; Chauhan, A.; Mehta, N. Effect of different itraconazole dosing regimens on cure rates, treatment duration, safety, and relapse rates in adult patients with tinea corporis/cruris: A randomized clinical trial. JAMA. Dermatol. 2022, 158, 1269–1278. [Google Scholar] [CrossRef]
- Posso-De Los Rios, C.J.; Tadros, E.; Summerbell, R.C.; Scott, J.A. Terbinafine resistant Trichophyton indotineae isolated in patients with superficial dermatophyte infection in canadian patients. J. Cutan. Med. Surg. 2022, 26, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Yaguchi, T.; Maeda, M.; Alshahni, M.M.; Salamin, K.; Guenova, E.; Feuermann, M.; Monod, M. Gene amplification of CYP51B: A new mechanism of resistance to azole compounds in Trichophyton indotineae. Antimicrob. Agents Chemother. 2022, 66, e0005922. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Kimura, U.; Noguchi, H.; Hiruma, M. Clinical isolate of a multi-antifungal-resistant Trichophyton rubrum. Antimicrob. Agents Chemother. 2022, 66, e0239321. [Google Scholar] [CrossRef]
- Leeyaphan, C.; Makimura, K.; Yamanishi, C.; Bunyaratavej, S.; Hau, C.; Tada, Y.; Suthammarak, W.; Kaewsutthi, S.; Phaitoonwattanakij, S.; Watanab, S. PCR-Based diagnosis of Neoscytalidium dimidiatum infection using internal transcribed spacer 1 region of ribosomal DNA primers. Siriraj Med. J. 2018, 70, 28–35. [Google Scholar]
- Baranová, Z.; Kampe, T.; Dorko, E.; Rimárová, K. Epidemiological and clinical aspects of dermatophytoses in Eastern Slovakia: A retrospective three-year study. Cent. Eur. J. Public Health 2018, 26, S72–S75. [Google Scholar] [CrossRef]
- Kromer, C.; Celis, D.; Hipler, U.C.; Zampeli, V.A.; Mößner, R.; Lippert, U. Dermatophyte infections in children compared to adults in Germany: A retrospective multicenter study in Germany. J. Dtsch. Dermatol. Ges. 2021, 19, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.; Refojo, N.; Davel, G.; Lima, N.; Dias, N.; Passos da Silva, C.M.F.; Canteros, C.E. Epidemiology of dermatophytoses in 31 municipalities of the province of Buenos Aires, Argentina: A 6-year study. Rev. Iberoam. Micol. 2018, 35, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Rezaei-Matehkolaei, A.; Makimura, K.; de Hoog, S.; Shidfar, M.R.; Zaini, F.; Eshraghian, M.; Naghan, P.A.; Mirhendi, H. Molecular epidemiology of dermatophytosis in Tehran, Iran, a clinical and microbial survey. Med. Mycol. 2013, 51, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Zamani, S.; Sadeghi, G.; Yazdinia, F.; Moosa, H.; Pazooki, A.; Ghafarinia, Z.; Abbasi, M.; Shams-Ghahfarokhi, M.; Razzaghi-Abyaneh, M. Epidemiological trends of dermatophytosis in Tehran, Iran: A five-year retrospective study. J. Mycol. Med. 2016, 26, 351–358. [Google Scholar] [CrossRef]
- Maraki, S.; Mavromanolaki, V.E. Epidemiology of onychomycosis in Crete, Greece: A 12-year study. Mycoses 2016, 59, 798–802. [Google Scholar] [CrossRef]
- Gregoriou, S.; Mpali, N.; Vrioni, G.; Hatzidimitriou, E.; Chryssou, S.-E.; Rigopoulos, D. Epidemiology of onychomycosis in an academic nail unit in South Greece during a three-year period. Ski. Appendage Disord. 2019, 6, 102–107. [Google Scholar] [CrossRef]
- Dubljanin, E.; Džamić, A.; Vujčić, I.; Grujičić, S.; Arsenijević, V.A.; Mitrović, S.; Čalovski, I.C. Epidemiology of onychomycosis in Serbia: A laboratory-based survey and risk factor identification. Mycoses 2017, 60, 25–32. [Google Scholar] [CrossRef]
- Martínez-Herrera, E.O.; Arroyo-Camarena, S.; Tejada-García, D.L.; Porras-López, C.F.; Arenas, R. Onychomycosis due to opportunistic molds. An. Bras. Dermatol. 2015, 90, 334–337. [Google Scholar] [CrossRef]
- Simonnet, C.; Berger, F.; Gantier, J.C. Epidemiology of superficial fungal diseases in French Guiana: A three-year retrospective analysis. Med. Mycol. 2011, 49, 608–611. [Google Scholar] [CrossRef]
- Araya, S.; Abuye, M.; Negesso, A.E. Epidemiological characterization of dermatomycosis in Ethiopia. Clin. Cosmet. Investig. Dermatol. 2021, 14, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Halim, I.; El Kadioui, F.; Soussi Abdallaoui, M. Onychomycosis in Casablanca (Morocco). J. Mycol. Med. 2013, 23, 9–14. [Google Scholar] [CrossRef]
- Chadeganipour, M.; Mohammadi, R. Causative agents of onychomycosis: A 7-year study. J. Clin. Lab. Anal. 2016, 30, 1013–1020. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.; Shemer, A.; Hochberg, M.; Keness, Y.; Shvarzman, R.; Mandelblat, M.; Frenkel, M.; Segal, E. Onychomycosis in Israel: Epidemiological aspects. Mycoses 2015, 58, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Zhang, M.; Liu, W.; Liang, G. Epidemiology of Onychomycosis in Chinese Mainland: A 30-year Retrospective Study. Mycopathologia 2022, 187, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Phaitoonwattanakij, S.; Leeyaphan, C.; Lertrujiwanit, K.; Bunyaratavej, S. Predisposing factors, clinical features and treatment outcomes of Fusarium onychomycosis and comparison of its characteristics with Neoscytalidium onychomycosis. J. Mycol. Med. 2021, 31, 101165. [Google Scholar] [CrossRef] [PubMed]
- Dogra, S.; Ramam, M. Difficult dermatophytosis. JAMA Dermatol. 2022, 158, 1243–1244. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Foley, K.A. Evidence for biofilms in onychomycosis. G. Ital. Dermatol. Venereol. 2018, 154, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, A.R.; Shokri, H.; Vahedi, G. Factors in etiology and predisposition of adult tinea capitis and review of published literature. Mycopathologia 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Rodríguez-Cerdeira, C.; Martínez-Herrera, E.; Szepietowski, J.C.; Pinto-Almazán, R.; Frías-De-León, M.G.; Espinosa-Hernández, V.M.; Chávez-Gutiérrez, E.; García-Salazar, E.; Vega-Sánchez, D.C.; Arenas, R.; et al. A systematic review of worldwide data on tinea capitis: Analysis of the last 20 years. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 844–883. [Google Scholar] [CrossRef]
- Osman, M.; Kasir, D.; Rafei, R.; Kassem, I.I.; Ismail, M.B.; El Omari, K.; Dabboussi, F.; Cazer, C.; Papon, N.; Bouchara, J.P.; et al. Trends in the epidemiology of dermatophytosis in the middle east and north Africa region. Int. J. Dermatol. 2022, 61, 935–968. [Google Scholar] [CrossRef]
- Udomphan, P.; Bunyaratavej, S.; Leeyaphan, C.; Matthapan, L.; Lertrujiwanit, K.; Pattanaprichakul, P. Review of adult tinea capitis cases presenting to Siriraj hospital, Bangkok, Thailand. Southeast Asian J. Trop. Med. Public Health 2019, 50, 905–911. [Google Scholar]
- Song, G.; Zhang, M.; Liu, W.; Liang, G. Changing face of epidemiology of dermatophytoses in Chinese Mainland: A 30 years nationwide retrospective study from 1991 to 2020. Mycoses 2022, 65, 440–448. [Google Scholar] [CrossRef]
- Carrascal-Correa, D.F.; Zuluaga, A.; González, A. Species distribution of the main aetiologic agents causing skin dermatophytosis in Colombian patients: A 23-year experience at a mycological reference center. Mycoses 2020, 63, 494–499. [Google Scholar] [CrossRef]
- Borges, A.; Brasileiro, A.; Galhardas, C.; Apetato, M. Tinea faciei in a central Portuguese hospital: A 9-year survey. Mycoses 2018, 61, 283–285. [Google Scholar] [CrossRef]
- Atzori, L.; Aste, N.; Pau, M. Tinea faciei due to Microsporum Canis in children: A survey of 46 cases in the district of Cagliari (Italy). Pediatr. Dermatol. 2012, 29, 409–413. [Google Scholar] [CrossRef]
- Maraki, S.; Mavromanolaki, V.E. Epidemiology of dermatophytoses in Crete, Greece. Med. Mycol. J. 2016, 57, e69–e75. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Zarrinfar, H.; Naseri, A.; Najafzadeh, M.J.; Fata, A.; Parian, M.; Khorsand, I.; Babič, M.N. Epidemiology of dermatophytosis in northeastern Iran; A subtropical region. Curr. Med. Mycol. 2019, 5, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, R.; Asahina, Y.; Yaguchii, T.; Sato, T. Tinea barbae due to Trichophyton rubrum successfully treated using oral fosravuconazole l-lysine ethanolate. J. Dermatol. 2020, 47, e254–e255. [Google Scholar] [CrossRef]
- Kirsten, H.; Haiduk, J.; Nenoff, P.; Uhrlaß, S.; Ziemer, M.; Simon, J.C. Tinea barbae profunda due to Trichophyton mentagrophytes: Case report and review. Hautarzt 2019, 70, 601–611. [Google Scholar] [CrossRef]
- Müller, V.L.; Kappa-Markovi, K.; Hyun, J.; Georgas, D.; Silberfarb, G.; Paasch, U.; Uhrlaß, S.; Nenoff, P.; Schaller, J. Tinea capitis et barbae caused by Trichophyton tonsurans: A retrospective cohort study of an infection chain after shavings in barber shops. Mycoses 2021, 64, 428–436. [Google Scholar] [CrossRef]
- Baeza-Hernández, G.; de la Soledad Vallejo-Ruiz, M.; Rubio-Aguilera, R.F.; Romero-Maté, A.; Martínez-Morán, C. An unexpected dermatophyte? Two remarkable cases of tinea barbae by Trichophyton benhamiae. Dermatol. Pract. Concept. 2023, 13, e2023037. [Google Scholar] [CrossRef]
- Saxena, V.; Shenoy, M.M.; Devrari, J.C.; Pai, V.; Agrawal, V. A mycological study of tinea corporis: A changing epidemiological trend from Trichophyton rubrum to Trichophyton mentagrophytes in India. Indian. J. Dermatol. Venereol. Leprol. 2020, 86, 607. [Google Scholar]
- Castellanos, J.; Guillén-Flórez, A.; Valencia-Herrera, A.; Toledo-Bahena, M.; Ramírez-Cortés, E.; Toussaint-Caire, S.; Mena-Cedillos, C.; Salazar-García, M.; Bonifaz, A. Unusual inflammatory tinea infections: Majocchi’s granuloma and deep/systemic dermatophytosis. J. Fungi 2021, 7, 929. [Google Scholar] [CrossRef] [PubMed]
- Zalewski, A.; Goldust, M.; Szepietowski, J.C. Tinea gladiatorum: Epidemiology, clinical aspects, and management. J. Clin. Med. 2022, 11, 4066. [Google Scholar] [CrossRef] [PubMed]
- Hiruma, J.; Ogawa, Y.; Hiruma, M. Trichophyton tonsurans infection in Japan: Epidemiology, clinical features, diagnosis and infection control. J. Dermatol. 2015, 42, 245–249. [Google Scholar] [CrossRef]
- Er, Y.X.; Lee, S.C.; Than, L.T.; Muslim, A.; Leong, K.F.; Kwan, Z.; Sayed, I.M.; Lim, Y.A.L. Tinea imbricata among the indigenous communities: Current global epidemiology and research gaps associated with host genetics and skin microbiota. J. Fungi 2022, 8, 202. [Google Scholar] [CrossRef]
- Polunin, I. Tinea imbricata in Malaya. Br. J. Dermatol. 1952, 64, 378–384. [Google Scholar] [CrossRef]
- Budimulja, U. Tinea imbricata in central Kalimantan. Mal. J. Dermatol. 1995, 8, 17–21. [Google Scholar]
- Shenoy, M.M.; Rengasamy, M.; Dogra, S.; Kaur, T.; Asokan, N.; Sarveswari, K.N.; Poojary, S.; Aror, D.; Patil, S.; Das, A.; et al. A multicentric clinical and epidemiological study of chronic and recurrent dermatophytosis in India. Mycoses 2022, 65, 13–23. [Google Scholar] [CrossRef]
- Cruz, R.; Carvajal, L. Frequency of Epidermophyton floccosum in isolated dermatophytea laboratory from the region of Valparaiso, Chile. Time frame: 1990–2010. Rev. Chilena. Infectol. 2018, 35, 262–265. [Google Scholar] [CrossRef]
- Dias, M.F.R.G.; Quaresma-Santos, M.V.P.; Bernardes-Filho, F.; Amorim, A.G.D.F.; Schechtman, R.C.; Azulay, D.R. Update on therapy for superficial mycoses: Review article part I. An. Bras. Dermatol. 2013, 88, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Tsuchihashi, H.; Hiruma, M.; Kano, R.; Ikeda, S. Tinea corporis due to Trichophyton erinacei probably transmitted from a hedgehog the second case report from Japan. Med. Mycol. J. 2018, 59, E77–E79. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.; Huang, J.; Chew, K.L.; Jaffar, H.; Tan, C. Pustular tinea manuum from trichophyton erinacei infection. JAAD. Case Rep. 2018, 4, 18–20. [Google Scholar] [CrossRef]
- Weishaupt, J.; Kolb-Mäurer, A.; Lempert, S.; Nenoff, P.; Uhrlaß, S.; Hamm, H.; Goebeler, M. A different kind of hedgehog pathway: Tinea manus due to Trichophyton erinacei transmitted by an African pygmy hedgehog (Atelerix albiventris). Mycoses 2014, 57, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Perrier, P.; Monod, M. Tinea manuum caused by Trichophyton erinacei: First report in Switzerland. Int. J. Dermatol. 2015, 54, 959–960. [Google Scholar] [CrossRef] [PubMed]
- Phaitoonwattanakij, S.; Leeyaphan, C.; Bunyaratavej, S.; Chinhiran, K. Trichophyton erinacei onychomycosis: The first to evidence a proximal subungual onychomycosis pattern. Case Rep. Dermatol. 2019, 11, 198–203. [Google Scholar] [CrossRef]
- Moseley, I.; Ragi, S.D.; Ouellette, S.; Rao, B. Tinea pedis in underrepresented groups: All of us database analysis. Mycoses 2023, 66, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Kiraz, N.; Metintas, S.; Oz, Y.; Koc, F.; Koku Aksu, E.A.; Kalyoncu, C.; Kasifoglu, N.; Cetin, E.; Arikan, I. The prevalence of tinea pedis and tinea manuum in adults in rural areas in Turkey. Int. J. Environ. Health Res. 2010, 20, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Ilkit, M.; Durdu, M. Tinea pedis: The etiology and global epidemiology of a common fungal infection. Crit. Rev. Microbiol. 2015, 41, 374–388. [Google Scholar] [CrossRef]
- Ongsri, P.; Bunyaratavej, S.; Leeyaphan, C.; Pattanaprichakul, P.; Ongmahutmongkol, P.; Komoltri, C.; Kulthanan, K. Prevalence and clinical correlation of superficial fungal foot infection in Thai naval rating cadets. Mil. Med. 2018, 183, e633–e637. [Google Scholar] [CrossRef]
- Toukabri, N.; Dhieb, C.; El Euch, D.; Rouissi, M.; Mokni, M.; Sadfi-Zouaoui, N. Prevalence, etiology, and risk factors of tinea pedis and tinea unguium in Tunisia. Can. J. Infect. Dis. Med. Microbiol. 2017, 2017, 6835725. [Google Scholar] [CrossRef]
- Ungpakorn, R.; Lohaprathan, S.; Reangchainam, S. Prevalence of foot diseases in outpatients attending the institute of dermatology, Bangkok, Thailand. Clin. Exp. Dermatol. 2004, 29, 87–90. [Google Scholar] [CrossRef]
- Assadamongkol, R.; Lertwattanarak, R.; Wannachalee, T.; Bunyaratavej, S.; Leeyaphan, C.; Matthapan, L. Prevalence, risk factors, and type of organism in fungal foot infection and toenail onychomycosis in Thai diabetic patients. J. Med. Assoc. Thai. 2016, 99, 659–664. [Google Scholar]
- Ingordo, V.; Naldi, L.; Fracchiolla, S.; Colecchia, B. Prevalence and risk factors for superficial fungal infections among Italian navy cadets. Dermatology 2004, 209, 190–196. [Google Scholar] [CrossRef]
- Leeyaphan, C.; Bunyarata, S.; Chadchavalpanichaya, N.; Rujitharanawong, C.; Phaitoonwattanakij, S.; Matthapan, L. Clinical and laboratory findings in trauma-induced nail dystrophy versus onychomycosis. Siriraj Med. J. 2018, 70, 490–495. [Google Scholar]
- Lipner, S.R.; Scher, R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Dermatol. 2019, 80, 835–851. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Hajjeh, R.A.; Scher, R.; Konnikov, N.; Gupta, A.K.; Summerbell, R.; Sullivan, S.; Daniel, R.; Krusinski, P.; Fleckman, P.; et al. A large-scale North American study of fungal isolates from nails: The frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J. Am. Acad. Dermatol. 2000, 43, 641–648. [Google Scholar] [CrossRef]
- Gold, J.A.W.; Wu, K.; Jackson, B.R.; Benedict, K. Opportunities to improve guideline adherence for the diagnosis and treatment of onychomycosis: Analysis of commercial insurance claims data, United States. J. Am. Acad. Dermatol. 2023, 88, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Sigurgeirsson, B.; Baran, R. The prevalence of onychomycosis in the global population: A literature study. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- de Berker, D. Clinical practice. Fungal nail disease. N. Engl. J. Med. 2009, 360, 2108–2116. [Google Scholar] [CrossRef]
- Limphoka, P.; Bunyaratavej, S.; Leeyaphan, C. Fingernail onychomycosis caused by Microsporum canis in a teenager. Pediatr. Dermatol. 2021, 38, 524–525. [Google Scholar] [CrossRef]
- Bunyaratavej, S.; Prasertworonun, N.; Leeyaphan, C.; Chaiwanon, O.; Muanprasat, C.; Matthapan, L. Distinct characteristics of scytalidium dimidiatum and non-dermatophyte onychomycosis as compared with dermatophyte onychomycosis. J. Dermatol. 2015, 42, 258–262. [Google Scholar] [CrossRef]
- Cursi, I.B.; Freitas, L.B.; Neves Mde, L.; Silva, I.C. Onycomychosis due to Scytalidium spp.: A clinical and epidemiologic study at a University Hospital in Rio de Janeiro, Brazil. An. Bras. Dermatol. 2011, 86, 689–693. [Google Scholar] [CrossRef]
- Ebihara, M.; Makimura, K.; Sato, K.; Abe, S.; Tsuboi, R. Molecular detection of dermatophytes and nondermatophytes in onychomycosis by nested polymerase chain reaction based on 28S ribosomal RNA gene sequences. Br. J. Dermatol. 2009, 161, 1038–1044. [Google Scholar] [CrossRef]
- Ranawaka, R.R.; Nagahawatte, A.; Gunasekara, T.A.; Weerakoon, H.S.; de Silva, S.H. Randomized, double-blind, comparative study on efficacy and safety of itraconazole pulse therapy and terbinafine pulse therapy on nondermatophyte mold onychomycosis: A study with 90 patients. J. Dermatolog. Treat. 2016, 27, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Drummond-Main, C.; Cooper, E.A.; Brintnell, W.; Piraccini, B.M.; Tosti, A. Systematic review of nondermatophyte mold onychomycosis: Diagnosis, clinical types, epidemiology, and treatment. J. Am. Acad. Dermatol. 2012, 66, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Piraccini, B.M.; Lorenzi, S. Onychomycosis caused by nondermatophytic molds: Clinical features and response to treatment of 59 cases. J. Am. Acad. Dermatol. 2000, 42, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Guilhermetti, E.; Takahachi, G.; Shinobu, C.S.; Svidzinski, T.I. Fusarium spp. as agents of onychomycosis in immunocompetent hosts. Int. J. Dermatol. 2007, 46, 822–826. [Google Scholar] [CrossRef]
- Hay, R.J. Tinea capitis: Current status. Mycopathologia 2017, 182, 87–93. [Google Scholar] [CrossRef]
- Bunyaratavej, S.; Leeyaphan, C.; Rujitharanawong, C.; Muanprasat, C.; Matthapan, L. Clinical and laboratory characteristics of a tinea capitis outbreak among novice Buddhist monks. Pediatr. Dermatol. 2017, 34, 371–373. [Google Scholar] [CrossRef]
- Degreef, H. Clinical forms of dermatophytosis (ringworm infection). Mycopathologia 2008, 166, 257–265. [Google Scholar] [CrossRef]
- Bunyaratavej, S.; Kiratiwongwan, R.; Komoltri, C.; Lertrujiwanit, K.; Leeyaphan, C. Predictive equation to identify infection due to anthropophilic or zoophilic dermatophytes based on clinical features and risk factors: A ten-year retrospective study. Indian J. Dermatol. Venereol. Leprol. 2022, 88, 416–419. [Google Scholar] [CrossRef]
- Romano, C.; Maritati, E.; Gianni, C. Tinea incognito in Italy: A 15-year survey. Mycoses 2006, 49, 383–387. [Google Scholar] [CrossRef]
- Chamorro, M.J.; House, S.A. Tinea Manuum; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mizumoto, J. Two feet-one hand syndrome. Cureus 2021, 13, e20758. [Google Scholar] [CrossRef]
- Gelotar, P.; Vachhani, S.; Patel, B.; Makwana, N. The prevalence of fungi in fingernail onychomycosis. J. Clin. Diagnostic Res. 2013, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Bunyaratavej, S.; Pattanaprichakul, P.; Sitthinamsuwan, P.; Pongkittilar, B.; Prasertsook, S.; Wongdama, S.; Yan, C.; Leeyaphan, C. Clinical clues to differentiate between dermatophyte onychomycosis (DP-OM) and dermatophytoma-like traumatic onychodystrophy (DP-TO). Biomed. Res. Int. 2022, 2022, 8519376. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Versteeg, S.G.; Shear, N.H. Confirmatory testing prior to initiating onychomycosis therapy is cost-effective. J. Cutan. Med. Surg. 2018, 22, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Bunyaratavej, S.; Pattanaprichakul, P.; Srisuma, S.; Leeyaphan, C. Experiences and factors that influence potassium hydroxide examination by microscopists. Med. Mycol. J. 2016, 57, E29–E34. [Google Scholar] [CrossRef]
- Meireles, T.E.F.; Rocha, M.F.G.; Brilhante, R.S.N.; Cordeiro, R.D.A.; Sidrim, J.J.C. Successive mycological nail tests for onychomycosis: A strategy to improve diagnosis efficiency. Braz. J. Infect. Dis. 2008, 12, 333–337. [Google Scholar] [CrossRef]
- Wanat, K.A.; Dominguez, A.R.; Carter, Z.; Legua, P.; Bustamante, B.; Micheletti, R.G. Bedside diagnostics in dermatology: Viral, bacterial, and fungal infections. J. Am. Acad. Dermatol. 2017, 77, 197–218. [Google Scholar] [CrossRef]
- Verrier, J.; Monod, M. Diagnosis of dermatophytosis using molecular biology. Mycopathologia 2017, 182, 193–202. [Google Scholar] [CrossRef]
- Salakshna, N.; Bunyaratavej, S.; Matthapan, L.; Lertrujiwanit, K.; Leeyaphan, C. A cohort study of risk factors, clinical presentations, and outcomes for dermatophyte, nondermatophyte, and mixed toenail infections. J. Am. Acad. Dermatol. 2018, 79, 1145–1146. [Google Scholar] [CrossRef]
- Begum, J.; Mir, N.A.; Lingaraju, M.C.; Buyamayum, B.; Dev, K. Recent advances in the diagnosis of dermatophytosis. J. Basic. Microbiol. 2020, 60, 293–303. [Google Scholar] [CrossRef]
- Petrucelli, M.F.; Abreu, M.H.; Cantelli, B.A.M.; Segura, G.G.; Nishimura, F.G.; Bitencourt, T.A.; Marins, M.; Fachin, A.L. Epidemiology and diagnostic perspectives of dermatophytoses. J. Fungi 2020, 6, 310. [Google Scholar] [CrossRef]
- Su, H.; Packeu, A.; Ahmed, S.A.; Al-Hatmi, A.M.S.; Blechert, O.; İlkit, M.; Hagen, F.; Gräser, Y.; Liu, W.; Deng, S.; et al. Species distinction in the Trichophyton rubrum complex. J. Clin. Microbiol. 2019, 57, e00352-19. [Google Scholar] [CrossRef]
- Koo, S.H.; Teoh, Y.L.; Koh, W.L.; Ochi, H.; Tan, S.K.; Sim, D.M.F.; Jiang, B.; Tan, A.L.; Tan, T.Y.; Lim, S.P.R. Development and validation of a real-time multiplex PCR assay for the detection of dermatophytes and Fusarium spp. J. Med. Microbiol. 2019, 68, 1641–1648. [Google Scholar] [CrossRef] [PubMed]
- Leeyaphan, C.; Suphatsathienkul, P.; Limphoka, P.; Kiratiwongwan, R.; Bunyaratavej, S. Sulphur nuggets: A distinct dermoscopic feature of onychomycosis. Med. Mycol. J. 2021, 62, 63–65. [Google Scholar] [CrossRef]
- Litaiem, N.; Nakouri, I.; Bouhlel, S.; Mansour, Y.; Bouchakoua, M.; Zegaloui, F. Dermoscopic features of toenail onychomycosis. J. Am. Podiatr. Med. Assoc. 2020, 110, Article_4. [Google Scholar] [CrossRef] [PubMed]
- Veasey, J.V.; Miguel, B.; Bedrikow, R.B.; Mota, R.D.C., Jr.; Buarque, V. Wood’s lamp in dermatology: Applications in the daily practice. Surg. Cosmet. Dermatol. 2017, 9, 324–326. [Google Scholar] [CrossRef]
| Habitat | Anthropophilic Dermatophytes | Zoophilic Dermatophytes | Geophilic Dermatophytes |
|---|---|---|---|
| Dermatophyte species | Trichophyton concentricum | Trichophyton benhamiae | Nannizzia aenygmaticum |
| Trichophyton indotineae | Trichophyton bullosum | Nannizzia corniculata | |
| Trichophyton interdigitale | Trichophyton equinum | Nannizzia fulva | |
| Trichophyton rubrum | Trichophyton eriotrephon | Nannizzia gypsea | |
| Trichophyton schoenleinii | Trichophyton erinacei | Nannizzia incurvata | |
| Trichophyton soudanense | Trichophyton mentagrophytes | Nannizzia praecox | |
| Trichophyton tonsurans | Trichophyton quinckeanum | Paraphyton cookei | |
| Trichophyton violaceum | Trichophyton simii | Paraphyton cookiellum | |
| Epidermophyton floccosum | Trichophyton verrucosum | Arthroderma ciferrii | |
| Microsporum andouinii | Nannizzia nana | Arthroderma cuniculi | |
| Microsporum ferrugineum | Nannizzia persicolor | Arthroderma curreyi | |
| Arthroderma onychocola | Paraphyton mirabile | Arthroderma eboreum | |
| Lophophyton gallinae | Arthroderma gertleri | ||
| Microsporum canis | Arthroderma gloriae | ||
| Arthroderma amazonicum | Arthroderma insingulare | ||
| Arthroderma flavescens | Arthroderma lenticulare | ||
| Arthroderma redellii | Arthroderma melis | ||
| Arthroderma vespertilii | Arthroderma multifidum | ||
| Arthroderma phaseoliforme | |||
| Arthroderma quadrifidum | |||
| Arthroderma thuringiensis | |||
| Arthroderma tuberculatum | |||
| Arthroderma uncinatum |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanyachailert, P.; Leeyaphan, C.; Bunyaratavej, S. Cutaneous Fungal Infections Caused by Dermatophytes and Non-Dermatophytes: An Updated Comprehensive Review of Epidemiology, Clinical Presentations, and Diagnostic Testing. J. Fungi 2023, 9, 669. https://doi.org/10.3390/jof9060669
Chanyachailert P, Leeyaphan C, Bunyaratavej S. Cutaneous Fungal Infections Caused by Dermatophytes and Non-Dermatophytes: An Updated Comprehensive Review of Epidemiology, Clinical Presentations, and Diagnostic Testing. Journal of Fungi. 2023; 9(6):669. https://doi.org/10.3390/jof9060669
Chicago/Turabian StyleChanyachailert, Pattriya, Charussri Leeyaphan, and Sumanas Bunyaratavej. 2023. "Cutaneous Fungal Infections Caused by Dermatophytes and Non-Dermatophytes: An Updated Comprehensive Review of Epidemiology, Clinical Presentations, and Diagnostic Testing" Journal of Fungi 9, no. 6: 669. https://doi.org/10.3390/jof9060669
APA StyleChanyachailert, P., Leeyaphan, C., & Bunyaratavej, S. (2023). Cutaneous Fungal Infections Caused by Dermatophytes and Non-Dermatophytes: An Updated Comprehensive Review of Epidemiology, Clinical Presentations, and Diagnostic Testing. Journal of Fungi, 9(6), 669. https://doi.org/10.3390/jof9060669





