Pneumocystis Exacerbates Inflammation and Mucus Hypersecretion in a Murine, Elastase-Induced-COPD Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
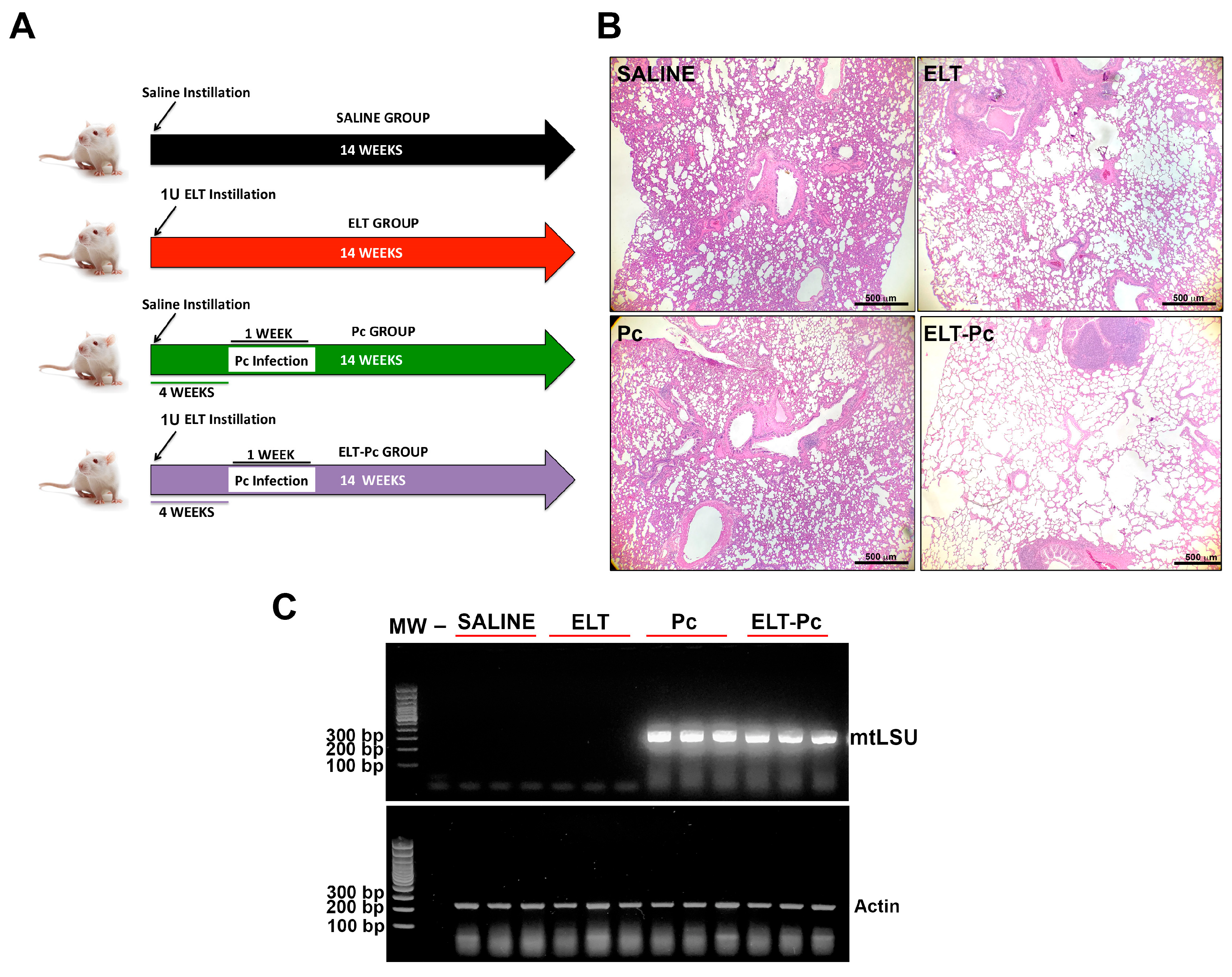
2.2. Animal Model
2.3. Histology
2.4. Detection of Pneumocystis
2.5. Gene Expression Determinations
2.6. Protein Extractions
2.7. Western Blotting Determinations
2.8. Statistics
3. Results
3.1. Pneumocystis Increase COPD-Features in an Elastase-Induced COPD Rat Model
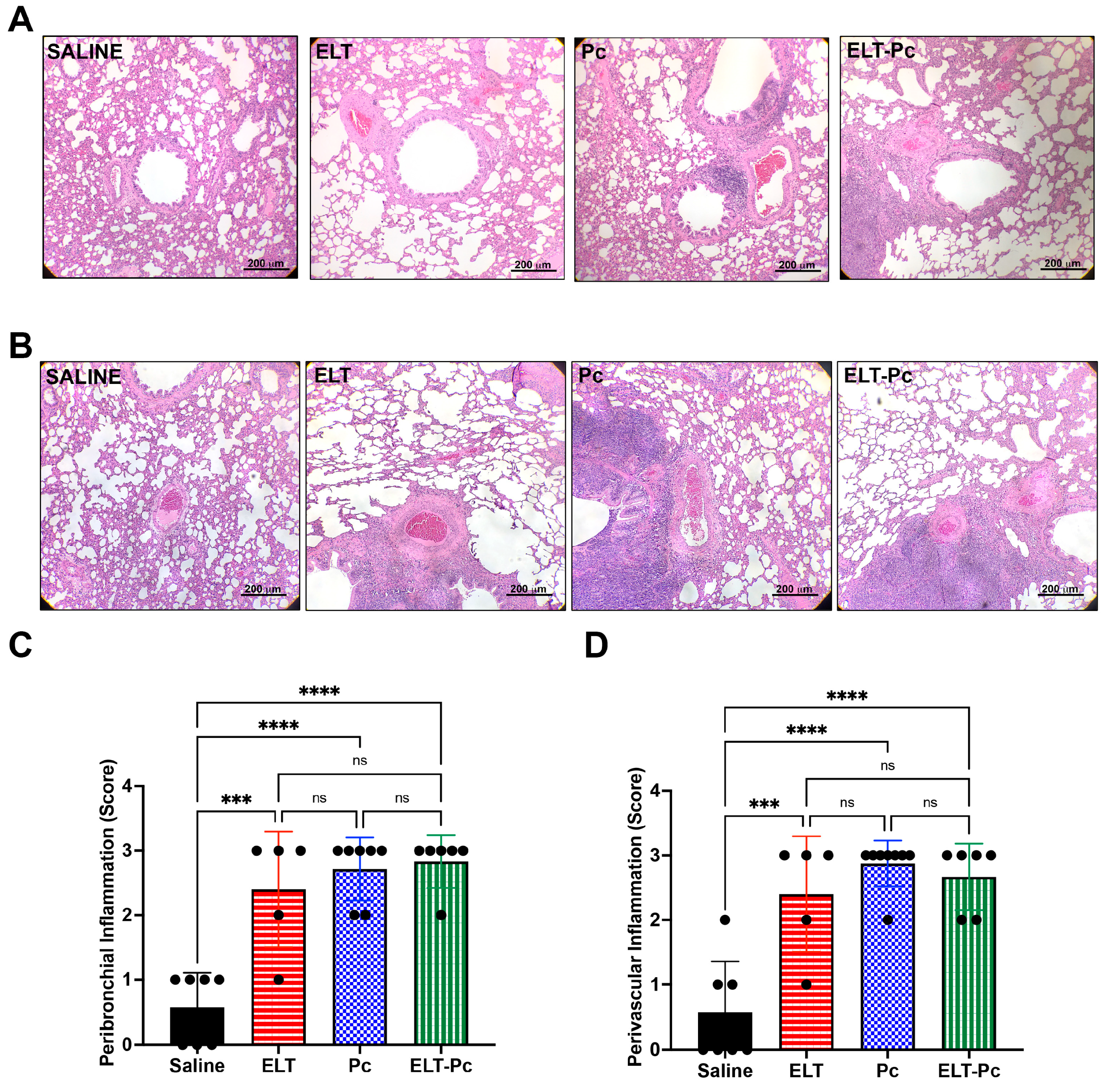
3.2. Pneumocystis and Elastase Increase the Number of Inflammatory Cuffs, without a Noticeable Additive Effect in this Elastase-Induced COPD Rat Model
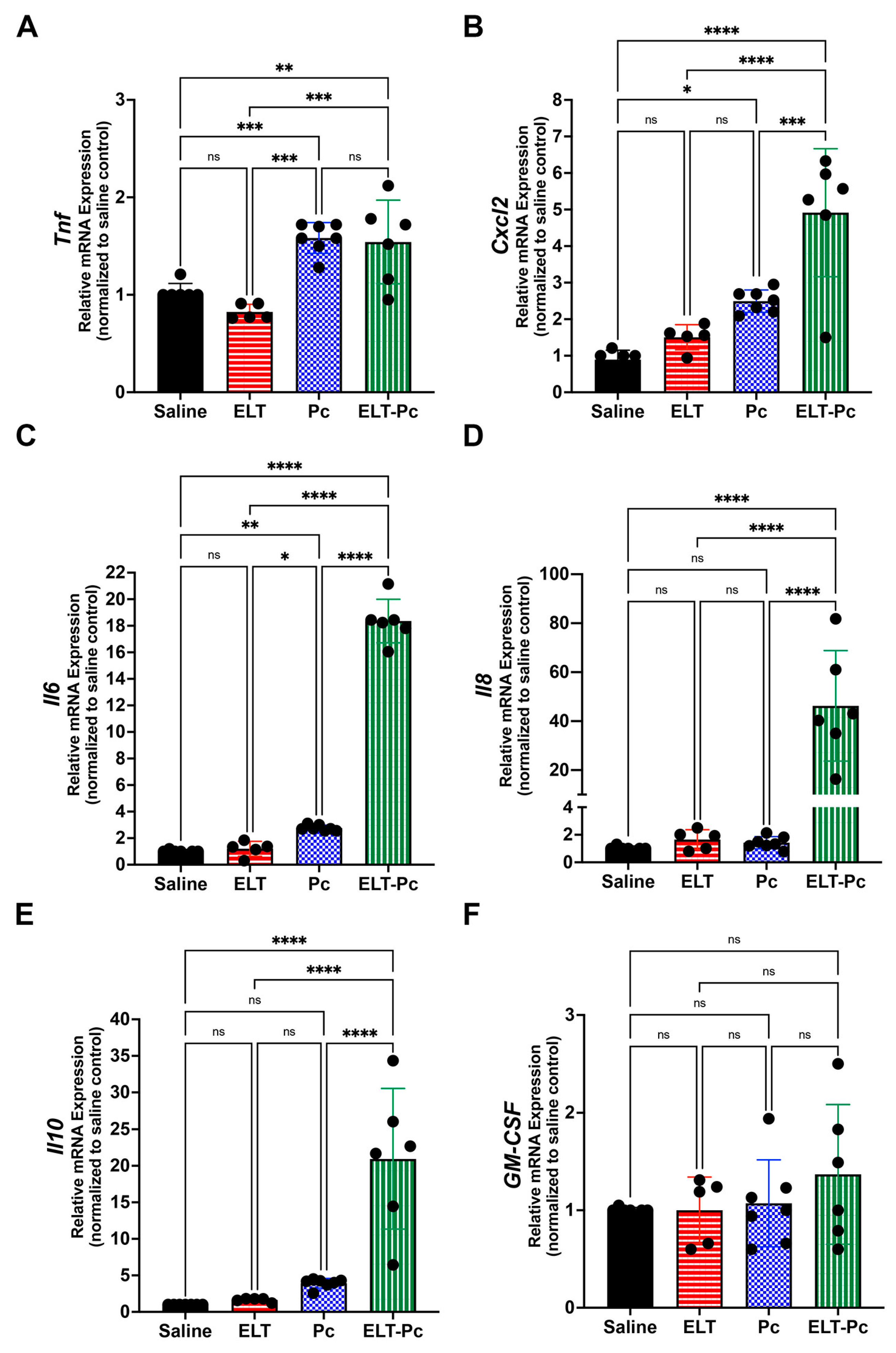
3.3. Pneumocystis Increases Inflammatory Markers in an Elastase-Induced COPD Rat Model
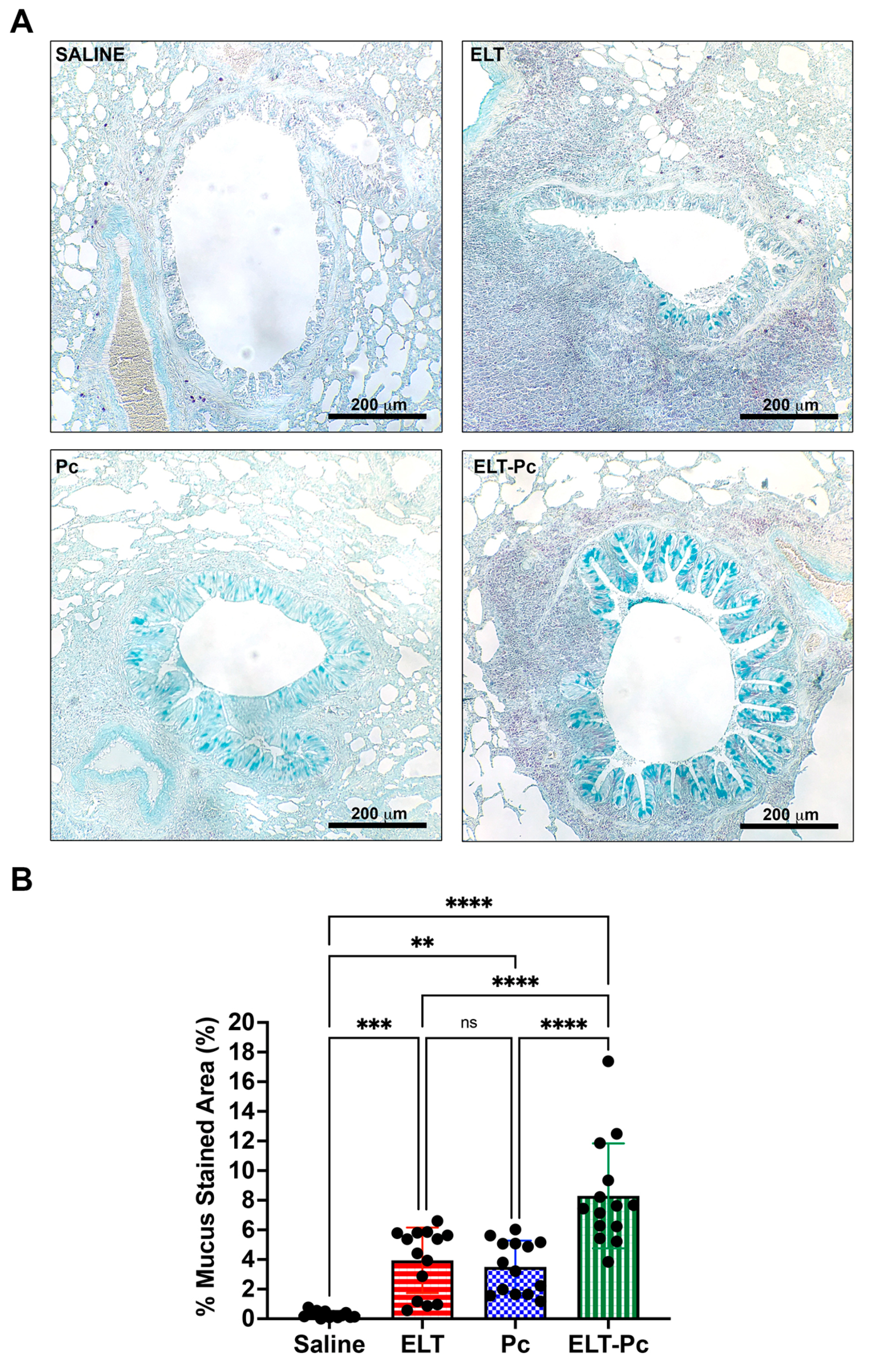
3.4. Pneumocystis Increases Mucus Secretion in the Airway Epithelium of Elastase-Induced COPD Rats
3.5. Pneumocystis Increases Mucin Levels in an Elastase-Induced COPD Rat Model
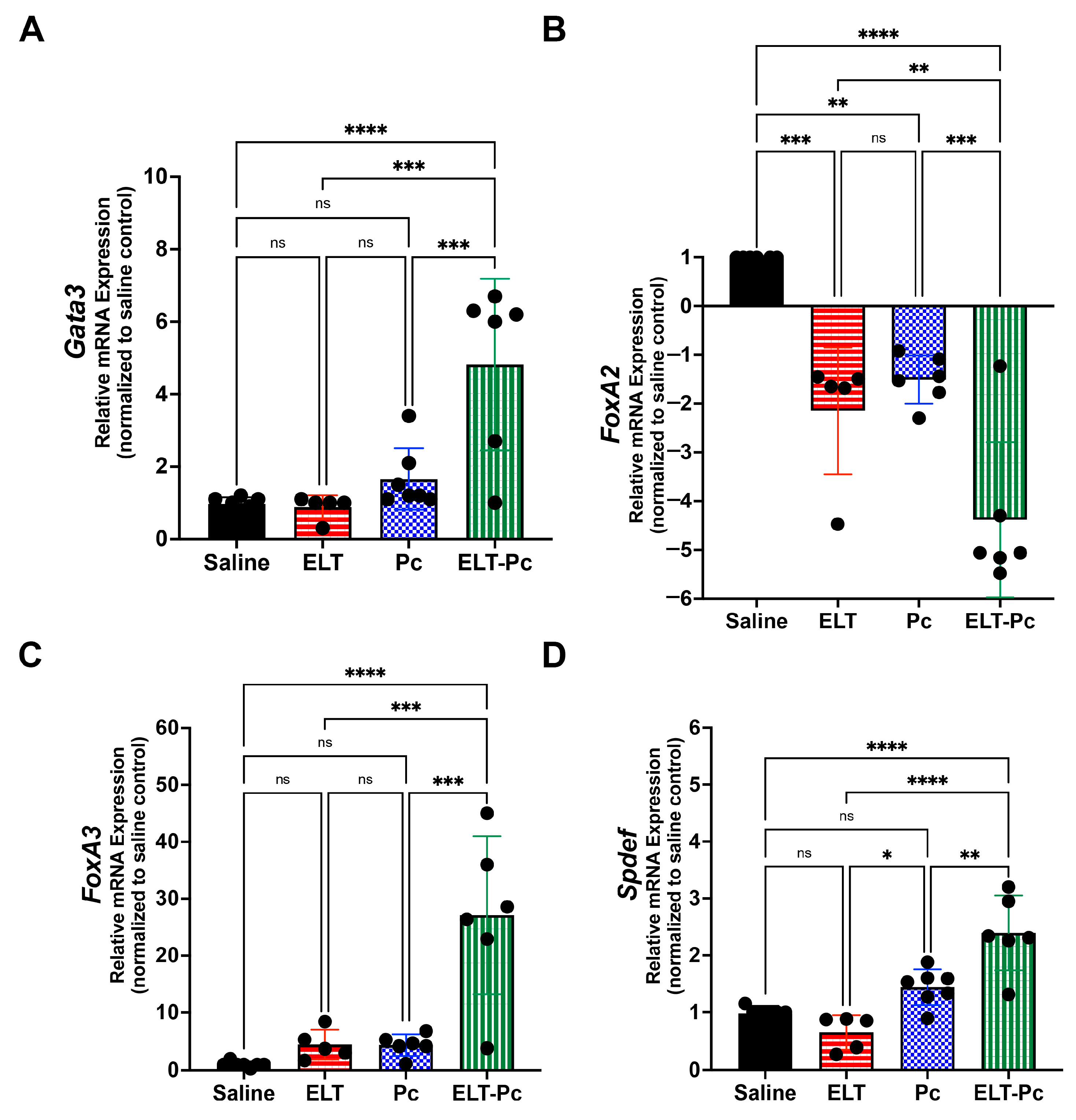
3.6. Pneumocystis Modulates Synergically STAT6-Dependent Transcription Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, V.; Criner, G.J. Chronic Bronchitis and Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 187, 228–237. [Google Scholar] [CrossRef] [PubMed]
- McDonough, J.E.; Yuan, R.; Suzuki, M.; Seyednejad, N.; Elliott, W.M.; Sanchez, P.G.; Wright, A.C.; Gefter, W.B.; Litzky, L.; Coxson, H.O.; et al. Small-Airway Obstruction and Emphysema in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2011, 365, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Mio, T.; Romberger, D.J.; Thompson, A.B.; Robbins, R.A.; Heires, A.; Rennard, S.I. Cigarette Smoke Induces Interleukin-8 Release from Human Bronchial Epithelial Cells. Am. J. Respir. Crit. Care Med. 1997, 155, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Retamales, I.; Elliott, W.M.; Meshi, B.; Coxson, H.O.; Pare, P.D.; Sciurba, F.C.; Rogers, R.M.; Hayashi, S.; Hogg, J.C. Amplification of Inflammation in Emphysema and Its Association with Latent Adenoviral Infection. Am. J. Respir. Crit. Care Med. 2001, 164, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Hellermann, G.R.; Nagy, S.B.; Kong, X.; Lockey, R.F.; Mohapatra, S.S. Mechanism of Cigarette Smoke Condensate-Induced Acute Inflammatory Response in Human Bronchial Epithelial Cells. Respir. Res. 2002, 3, 15. [Google Scholar] [CrossRef]
- Barnes, P.J. The Cytokine Network in Asthma and Chronic Obstructive Pulmonary Disease. J. Clin. Investig. 2008, 118, 3546–3556. [Google Scholar] [CrossRef]
- Sommerhoff, C.P.; Nadel, J.A.; Basbaum, C.B.; Caughey, G.H. Neutrophil Elastase and Cathepsin G Stimulate Secretion from Cultured Bovine Airway Gland Serous Cells. J. Clin. Investig. 1990, 85, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Witko-Sarsat, V.; Halbwachs-Mecarelli, L.; Schuster, A.; Nusbaum, P.; Ueki, I.; Canteloup, S.; Lenoir, G.; Descamps-Latscha, B.; Nadel, J.A. Proteinase 3, a Potent Secretagogue in Airways, Is Present in Cystic Fibrosis Sputum. Am. J. Respir. Cell Mol. Biol. 1999, 20, 729–736. [Google Scholar] [CrossRef]
- Barnes, P.J.; Shapiro, S.D.; Pauwels, R.A. Chronic Obstructive Pulmonary Disease: Molecular and Cellularmechanisms. Eur. Respir. J. 2003, 22, 672–688. [Google Scholar] [CrossRef]
- Bucchioni, E.; Kharitonov, S.A.; Allegra, L.; Barnes, P.J. High Levels of Interleukin-6 in the Exhaled Breath Condensate of Patients with COPD. Respir. Med. 2003, 97, 1299–1302. [Google Scholar] [CrossRef]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The Nature of Small-Airway Obstruction in Chronic Obstructive Pulmonary Disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef]
- Gosker, H.R.; Langen, R.C.J.; Bracke, K.R.; Joos, G.F.; Brusselle, G.G.; Steele, C.; Ward, K.A.; Wouters, E.F.M.; Schols, A.M.W.J. Extrapulmonary Manifestations of Chronic Obstructive Pulmonary Disease in a Mouse Model of Chronic Cigarette Smoke Exposure. Am. J. Respir. Cell Mol. Biol. 2009, 40, 710–716. [Google Scholar] [CrossRef]
- Tomoda, K.; Kubo, K.; Nishii, Y.; Yamamoto, Y.; Yoshikawa, M.; Kimura, H. Changes of Ghrelin and Leptin Levels in Plasma by Cigarette Smoke in Rats. J. Toxicol. Sci. 2012, 37, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Hurd, S.; Anzueto, A.; Barnes, P.J.; Buist, S.A.; Calverley, P.; Fukuchi, Y.; Jenkins, C.; Rodriguez-Roisin, R.; van Weel, C.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: GOLD Executive Summary. Am. J. Respir. Crit. Care Med. 2007, 176, 532–555. [Google Scholar] [CrossRef] [PubMed]
- Jobse, B.N.; McCurry, C.A.; Morissette, M.C.; Rhem, R.G.; Stämpfli, M.R.; Labiris, N.R. Impact of Inflammation, Emphysema, and Smoking Cessation on V/Q in Mouse Models of Lung Obstruction. Respir. Res. 2014, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.L.; Cosio, M.; Churg, A. Animal Models of Chronic Obstructive Pulmonary Disease. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2008, 295, L1–L15. [Google Scholar] [CrossRef]
- Onclinx, C.; De Maertelaer, V.; Gustin, P.; Gevenois, P.A. Elastase-Induced Pulmonary Emphysema in Rats: Comparison of Computed Density and Microscopic Morphometry. Radiology 2006, 241, 763–770. [Google Scholar] [CrossRef]
- Furuya, N.; Takenaga, M.; Ohta, Y.; Tokura, Y.; Hamaguchi, A.; Sakamaki, A.; Kida, H.; Handa, H.; Nishine, H.; Mineshita, M.; et al. Cell Therapy with Adipose Tissue-Derived Stem/Stromal Cells for Elastase-Induced Pulmonary Emphysema in Rats. Regen. Med. 2012, 7, 503–512. [Google Scholar] [CrossRef]
- Bianchi, A.; Tibiletti, M.; Kjørstad, Å.; Birk, G.; Schad, L.R.; Stierstorfer, B.; Rasche, V.; Stiller, D. Three-Dimensional Accurate Detection of Lung Emphysema in Rats Using Ultra-Short and Zero Echo Time MRI: 3D Ute and Zte Mri to Investigate Emphysema. NMR Biomed. 2015, 28, 1471–1479. [Google Scholar] [CrossRef]
- Lüthje, L.; Raupach, T.; Michels, H.; Unsöld, B.; Hasenfuss, G.; Kögler, H.; Andreas, S. Exercise Intolerance and Systemic Manifestations of Pulmonary Emphysema in a Mouse Model. Respir. Res. 2009, 10, 7. [Google Scholar] [CrossRef]
- D’Anna, S.E.; Maniscalco, M.; Cappello, F.; Carone, M.; Motta, A.; Balbi, B.; Ricciardolo, F.L.M.; Caramori, G.; Di Stefano, A. Bacterial and Viral Infections and Related Inflammatory Responses in Chronic Obstructive Pulmonary Disease. Ann. Med. 2021, 53, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Love, M.E.; Proud, D. Respiratory Viral and Bacterial Exacerbations of COPD—The Role of the Airway Epithelium. Cells 2022, 11, 1416. [Google Scholar] [CrossRef]
- Fleischman, J.K.; Greenberg, H.; Web, A. Small Airways Dysfunction in Patients with AIDS and Pneumocystis Carinii Pneumonia. AIDS Patient Care STDs 1996, 10, 16–20. [Google Scholar] [CrossRef]
- Ma, L.; Cissé, O.H.; Kovacs, J.A. A Molecular Window into the Biology and Epidemiology of Pneumocystis spp. Clin. Microbiol. Rev. 2018, 31, e00009-18. [Google Scholar] [CrossRef] [PubMed]
- Vargas, S.L.; Ponce, C.A.; Gallo, M.; Pérez, F.; Astorga, J.-F.; Bustamante, R.; Chabé, M.; Durand-Joly, I.; Iturra, P.; Miller, R.F.; et al. Near-Universal Prevalence of Pneumocystis and Associated Increase in Mucus in the Lungs of Infants with Sudden Unexpected Death. Clin. Infect. Dis. 2013, 56, 171–179. [Google Scholar] [CrossRef]
- Eddens, T.; Campfield, B.T.; Serody, K.; Manni, M.L.; Horne, W.; Elsegeiny, W.; McHugh, K.J.; Pociask, D.; Chen, K.; Zheng, M.; et al. A Novel CD4 + T Cell–Dependent Murine Model of Pneumocystis -Driven Asthma-like Pathology. Am. J. Respir. Crit. Care Med. 2016, 194, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Iturra, P.A.; Rojas, D.A.; Pérez, F.J.; Méndez, A.; Ponce, C.A.; Bonilla, P.; Bustamante, R.; Rodríguez, H.; Beltrán, C.J.; Vargas, S.L. Progression of Type 2 Helper T Cell–Type Inflammation and Airway Remodeling in a Rodent Model of Naturally Acquired Subclinical Primary Pneumocystis Infection. Am. J. Pathol. 2018, 188, 417–431. [Google Scholar] [CrossRef]
- Méndez, A.; Rojas, D.A.; Ponce, C.A.; Bustamante, R.; Beltrán, C.J.; Toledo, J.; García-Angulo, V.A.; Henriquez, M.; Vargas, S.L. Primary Infection by Pneumocystis Induces Notch-Independent Clara Cell Mucin Production in Rat Distal Airways. PLoS ONE 2019, 14, e0217684. [Google Scholar] [CrossRef] [PubMed]
- Rojas, D.A.; Iturra, P.A.; Méndez, A.; Ponce, C.A.; Bustamante, R.; Gallo, M.; Bórquez, P.; Vargas, S.L. Increase in Secreted Airway Mucins and Partial Muc5b STAT6/FoxA2 Regulation during Pneumocystis Primary Infection. Sci. Rep. 2019, 9, 2078. [Google Scholar] [CrossRef] [PubMed]
- Perez-Nazario, N.; Rangel-Moreno, J.; O’Reilly, M.A.; Pasparakis, M.; Gigliotti, F.; Wright, T.W. Selective Ablation of Lung Epithelial IKK2 Impairs Pulmonary Th17 Responses and Delays the Clearance of Pneumocystis. J. Immunol. 2013, 191, 4720–4730. [Google Scholar] [CrossRef]
- Morris, A.; Sciurba, F.C.; Lebedeva, I.P.; Githaiga, A.; Elliott, W.M.; Hogg, J.C.; Huang, L.; Norris, K.A. Association of Chronic Obstructive Pulmonary Disease Severity and Pneumocystis Colonization. Am. J. Respir. Crit. Care Med. 2004, 170, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Sivam, S.; Sciurba, F.C.; Lucht, L.A.; Zhang, Y.; Duncan, S.R.; Norris, K.A.; Morris, A. Distribution of Pneumocystis Jirovecii in Lungs from Colonized COPD Patients. Diagn. Microbiol. Infect. Dis. 2011, 71, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Khodavaisy, S.; Mortaz, E.; Mohammadi, F.; Aliyali, M.; Fakhim, H.; Badali, H. Pneumocystis Jirovecii Colonization in Chronic Obstructive Pulmonary Disease (COPD). Curr. Med. Mycol. 2015, 1, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Shipley, T.W.; Kling, H.M.; Morris, A.; Patil, S.; Kristoff, J.; Guyach, S.E.; Murphy, J.E.; Shao, X.; Sciurba, F.C.; Rogers, R.M.; et al. Persistent Pneumocystis Colonization Leads to the Development of Chronic Obstructive Pulmonary Disease in a Nonhuman Primate Model of AIDS. J. Infect. Dis. 2010, 202, 302–312. [Google Scholar] [CrossRef]
- Calderon, E.J.; Rivero, L.; Respaldiza, N.; Morilla, R.; Montes-Cano, M.A.; Friaza, V.; Munoz-Lobato, F.; Varela, J.M.; Medrano, F.J.; de la Horra, C. Systemic Inflammation in Patients with Chronic Obstructive Pulmonary Disease Who Are Colonized with Pneumocystis Jiroveci. Clin. Infect. Dis. 2007, 45, e17–e19. [Google Scholar] [CrossRef]
- Christensen, P.J.; Preston, A.M.; Ling, T.; Du, M.; Fields, W.B.; Curtis, J.L.; Beck, J.M. Pneumocystis Murina Infection and Cigarette Smoke Exposure Interact To Cause Increased Organism Burden, Development of Airspace Enlargement, and Pulmonary Inflammation in Mice. Infect. Immun. 2008, 76, 3481–3490. [Google Scholar] [CrossRef]
- Rabacal, W.; Rayens, E.; Norris, K. Pneumocystis as a Co-Factor in Pulmonary Diseases. OBM Genet. 2018, 2, 1–18. [Google Scholar] [CrossRef]
- Lugade, A.A.; Bogner, P.N.; Thanavala, Y. Murine Model of Chronic Respiratory Inflammation. In Crossroads between Innate and Adaptive Immunity III; Pulendran, B., Katsikis, P.D., Schoenberger, S.P., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2011; Volume 780, pp. 125–141. ISBN 978-1-4419-5631-6. [Google Scholar]
- Vargas, S.L.; Hughes, W.T.; Wakefield, A.E.; Oz, H.S. Limited Persistence in and Subsequent Elimination of Pneumocystis Carinii from the Lungs after P. Carinii Pneumonia. J. Infect. Dis. 1995, 172, 506–510. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Swain, S.D.; Meissner, N.N.; Siemsen, D.W.; McInnerney, K.; Harmsen, A.G. Pneumocystis Elicits a STAT6-Dependent, Strain-Specific Innate Immune Response and Airway Hyperresponsiveness. Am. J. Respir. Cell Mol. Biol. 2012, 46, 290–298. [Google Scholar] [CrossRef]
- Fitzpatrick, M.E.; Tedrow, J.R.; Hillenbrand, M.E.; Lucht, L.; Richards, T.; Norris, K.A.; Zhang, Y.; Sciurba, F.C.; Kaminski, N.; Morris, A. Pneumocystis Jirovecii Colonization Is Associated with Enhanced Th1 Inflammatory Gene Expression in Lungs of Humans with Chronic Obstructive Pulmonary Disease: Pneumocystis Colonization and Human COPD. Microbiol. Immunol. 2014, 58, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; An, C. Role of Pneumocystis Jirovecii Infection in Chronic Obstructive Pulmonary Disease Progression in an Immunosuppressed Rat Pneumocystis Pneumonia Model. Exp. Ther. Med. 2020, 19, 3133–3142. [Google Scholar] [CrossRef] [PubMed]
- Christman, J.W.; Sadikot, R.T.; Blackwell, T.S. The Role of Nuclear Factor-κ B in Pulmonary Diseases. Chest 2000, 117, 1482–1487. [Google Scholar] [CrossRef] [PubMed]
- Szulakowski, P.; Crowther, A.J.L.; Jiménez, L.A.; Donaldson, K.; Mayer, R.; Leonard, T.B.; MacNee, W.; Drost, E.M. The Effect of Smoking on the Transcriptional Regulation of Lung Inflammation in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2006, 174, 41–50. [Google Scholar] [CrossRef]
- Koch, A.; Giembycz, M.; Stirling, R.G.; Lim, S.; Adcock, I.; Waßermann, K.; Erdmann, E.; Chung, K.F. Effect of Smoking on MAP Kinase-Induced Modulation of IL-8 in Human Alveolar Macrophages. Eur. Respir. J. 2004, 23, 805–812. [Google Scholar] [CrossRef]
- Culpitt, S.V.; Rogers, D.F.; Shah, P.; De Matos, C.; Russell, R.E.K.; Donnelly, L.E.; Barnes, P.J. Impaired Inhibition by Dexamethasone of Cytokine Release by Alveolar Macrophages from Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2003, 167, 24–31. [Google Scholar] [CrossRef]
- Vlahos, R.; Bozinovski, S.; Chan, S.P.J.; Ivanov, S.; Lindén, A.; Hamilton, J.A.; Anderson, G.P. Neutralizing Granulocyte/Macrophage Colony–Stimulating Factor Inhibits Cigarette Smoke–Induced Lung Inflammation. Am. J. Respir. Crit. Care Med. 2010, 182, 34–40. [Google Scholar] [CrossRef]
- Basilico, P.; Cremona, T.P.; Oevermann, A.; Piersigilli, A.; Benarafa, C. Increased Myeloid Cell Production and Lung Bacterial Clearance in Mice Exposed to Cigarette Smoke. Am. J. Respir. Cell Mol. Biol. 2016, 54, 424–435. [Google Scholar] [CrossRef]
- Shibata, S.; Miyake, K.; Tateishi, T.; Yoshikawa, S.; Yamanishi, Y.; Miyazaki, Y.; Inase, N.; Karasuyama, H. Basophils Trigger Emphysema Development in a Murine Model of COPD through IL-4–Mediated Generation of MMP-12–Producing Macrophages. Proc. Natl. Acad. Sci. USA 2018, 115, 13057–13062. [Google Scholar] [CrossRef]
- Oliveira, M.V.; Abreu, S.C.; Padilha, G.A.; Rocha, N.N.; Maia, L.A.; Takiya, C.M.; Xisto, D.G.; Suki, B.; Silva, P.L.; Rocco, P.R.M. Characterization of a Mouse Model of Emphysema Induced by Multiple Instillations of Low-Dose Elastase. Front. Physiol. 2016, 7, 457. [Google Scholar] [CrossRef]
- Hashimoto, S.; Kobayashi, A.; Kooguchi, K.; Kitamura, Y.; Onodera, H.; Nakajima, H. Upregulation of Two Death Pathways of Perforin/Granzyme and FasL/Fas in Septic Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2000, 161, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Eddens, T.; Elsegeiny, W.; Nelson, M.P.; Horne, W.; Campfield, B.T.; Steele, C.; Kolls, J.K. Eosinophils Contribute to Early Clearance of Pneumocystis Murina Infection. J. Immunol. 2015, 195, 185–193. [Google Scholar] [CrossRef] [PubMed]
- van den Berge, M.; ten Hacken, N.H.T.; Cohen, J.; Douma, W.R.; Postma, D.S. Small Airway Disease in Asthma and COPD. Chest 2011, 139, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Radicioni, G.; Ceppe, A.; Ford, A.A.; Alexis, N.E.; Barr, R.G.; Bleecker, E.R.; Christenson, S.A.; Cooper, C.B.; Han, M.K.; Hansel, N.N.; et al. Airway Mucin MUC5AC and MUC5B Concentrations and the Initiation and Progression of Chronic Obstructive Pulmonary Disease: An Analysis of the SPIROMICS Cohort. Lancet Respir. Med. 2021, 9, 1241–1254. [Google Scholar] [CrossRef]
- Singanayagam, A.; Footitt, J.; Marczynski, M.; Radicioni, G.; Cross, M.T.; Finney, L.J.; Trujillo-Torralbo, M.-B.; Calderazzo, M.A.; Zhu, J.; Aniscenko, J.; et al. Airway Mucins Promote Immunopathology in Virus-Exacerbated Chronic Obstructive Pulmonary Disease. J. Clin. Investig. 2022, 132, e120901. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Choe, S.; Lau, G.W. Inactivation of FOXA2 by Respiratory Bacterial Pathogens and Dysregulation of Pulmonary Mucus Homeostasis. Front. Immunol. 2020, 11, 515. [Google Scholar] [CrossRef]
- Chen, G.; Korfhagen, T.R.; Karp, C.L.; Impey, S.; Xu, Y.; Randell, S.H.; Kitzmiller, J.; Maeda, Y.; Haitchi, H.M.; Sridharan, A.; et al. Foxa3 Induces Goblet Cell Metaplasia and Inhibits Innate Antiviral Immunity. Am. J. Respir. Crit. Care Med. 2014, 189, 301–313. [Google Scholar] [CrossRef]
- Rajavelu, P.; Chen, G.; Xu, Y.; Kitzmiller, J.A.; Korfhagen, T.R.; Whitsett, J.A. Airway Epithelial SPDEF Integrates Goblet Cell Differentiation and Pulmonary Th2 Inflammation. J. Clin. Investig. 2015, 125, 2021–2031. [Google Scholar] [CrossRef]
- Huang, X.; Guan, W.; Xiang, B.; Wang, W.; Xie, Y.; Zheng, J. MUC5B Regulates Goblet Cell Differentiation and Reduces Inflammation in a Murine COPD Model. Respir. Res. 2022, 23, 11. [Google Scholar] [CrossRef]
- Miller, R.F.; Daly, K.R.; Walzer, P.D.; Ulloa, A.V.; Ponce, C.A.; Vargas, S.L. Sero-Epidemiology of Pneumocystis Infection among Infants, Children, and Adults in Chile. J. Fungi 2022, 8, 136. [Google Scholar] [CrossRef]

| Gene | Sequence (5′–3′) | Size (bp) | |
|---|---|---|---|
| pAZ102E (mtSLU) | Forward | GATGGCTGTTTCCAAGCCCA | 227 |
| pAZ102H (mtSLU) | Reverse | GTGTACGTTGCAAAGTACTC | |
| Dhfr11 | Forward | GTTGCACTTACAACTTCTTATGG | 223 |
| Dhfr12 | Reverse | TAGATCCAGAGATTCATTTCGAG | |
| Actin | Forward | CTTGCAGCTCCTCCGTCGCC | 228 |
| Reverse | CTTGCTCTGGGCCTCGTCGC | ||
| Tnfa | Forward | CAGCCGATTTGCCACTTCATA | 71 |
| Reverse | TCCTTAGGGCAAGGGCTCTT | ||
| Cxcl2 | Forward | AACCATCAGGGTACAGGGGT | 187 |
| Reverse | GGGCTTCAGGGTTGAGACAA | ||
| IL6 | Forward | CCCAACTTCCAATGCTCTCCTAATG | 141 |
| Reverse | GCACACTAGGTTTGCCGAGTAGACC | ||
| IL8 | Forward | GAAGATAGATTGCACCGA | 366 |
| Reverse | CATAGCCTCTCACACATTTC | ||
| IL10 | Forward | GGCTCAGCACTGCTATGTTGCC | 116 |
| Reverse | AGCATGTGGGTCTGGCTGACTG | ||
| GM-CSF | Forward | GCCATTGAGTTTGGTGAGGT | 152 |
| Reverse | TCCTAAATGACATGCGTGCT | ||
| Muc5ac | Forward | ACCACGGATATCAGAACCAGC | 199 |
| Reverse | TGTCAAGCCACTTGGTCCAG | ||
| Muc5b | Forward | CCTGAAGTCTTCCCCAGCAG | 202 |
| Reverse | GCATAGAATTGGCAGCCAGC | ||
| Gata3 | Forward | CCATTACCACCTATCCGCCCTA | 163 |
| Reverse | GTAGAGGTTGCCCCGCAGTT | ||
| FoxA2 | Forward | GAAGATGGAAGGGCACGAG | 184 |
| Reverse | TGACATGTTCATGGAGCCTG | ||
| FoxA3 | Forward | AGTGGAGCTACTACCCGGAG | 116 |
| Reverse | GGGAGGGTAGGGAGAGCTAA | ||
| Spdef | Forward | CTTCACTACTGCGCCTCCAC | 193 |
| Reverse | CCACCTGTGCGGAATCTTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas, D.A.; Ponce, C.A.; Bustos, A.; Cortés, V.; Olivares, D.; Vargas, S.L. Pneumocystis Exacerbates Inflammation and Mucus Hypersecretion in a Murine, Elastase-Induced-COPD Model. J. Fungi 2023, 9, 452. https://doi.org/10.3390/jof9040452
Rojas DA, Ponce CA, Bustos A, Cortés V, Olivares D, Vargas SL. Pneumocystis Exacerbates Inflammation and Mucus Hypersecretion in a Murine, Elastase-Induced-COPD Model. Journal of Fungi. 2023; 9(4):452. https://doi.org/10.3390/jof9040452
Chicago/Turabian StyleRojas, Diego A., Carolina A. Ponce, Adriel Bustos, Vicente Cortés, Daniela Olivares, and Sergio L. Vargas. 2023. "Pneumocystis Exacerbates Inflammation and Mucus Hypersecretion in a Murine, Elastase-Induced-COPD Model" Journal of Fungi 9, no. 4: 452. https://doi.org/10.3390/jof9040452
APA StyleRojas, D. A., Ponce, C. A., Bustos, A., Cortés, V., Olivares, D., & Vargas, S. L. (2023). Pneumocystis Exacerbates Inflammation and Mucus Hypersecretion in a Murine, Elastase-Induced-COPD Model. Journal of Fungi, 9(4), 452. https://doi.org/10.3390/jof9040452






