Fungal Diversity Associated with Thirty-Eight Lichen Species Revealed a New Genus of Endolichenic Fungi, Intumescentia gen. nov. (Teratosphaeriaceae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Identification of Lichen Samples
2.2. Isolation of Fungi from Lichen Thalli
2.3. DNA Extraction, PCR Amplification, Sequencing, and Preliminary Identification
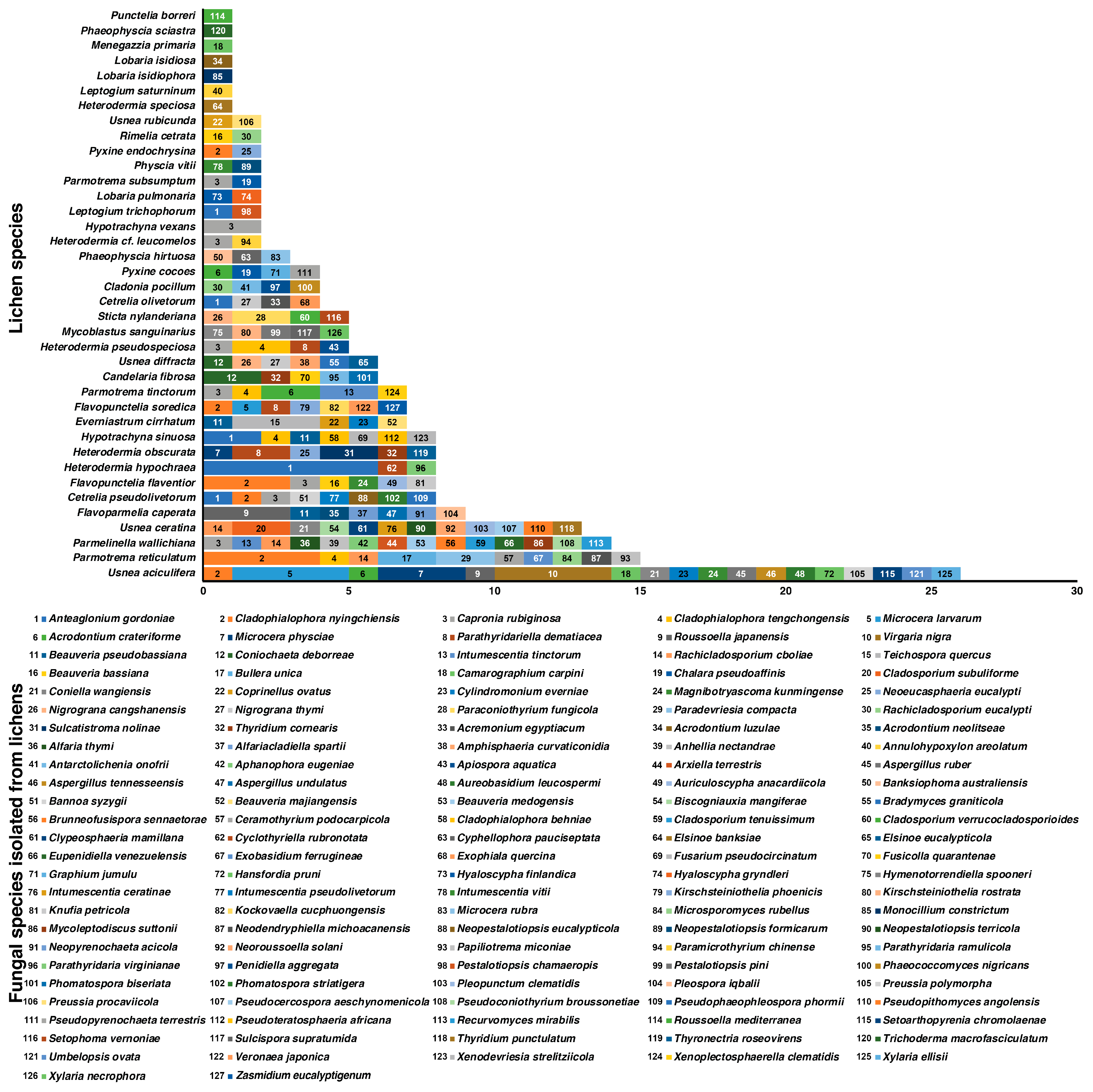
2.4. Guilds of Fungal Species Isolated from Lichens
2.5. Phylogenetic Analyses
2.6. Morphology and Growth Studies
3. Results
3.1. Collection and Identification of the Lichen Samples
3.2. Isolation of the Fungi from Lichen Thalli and Preliminary Identification
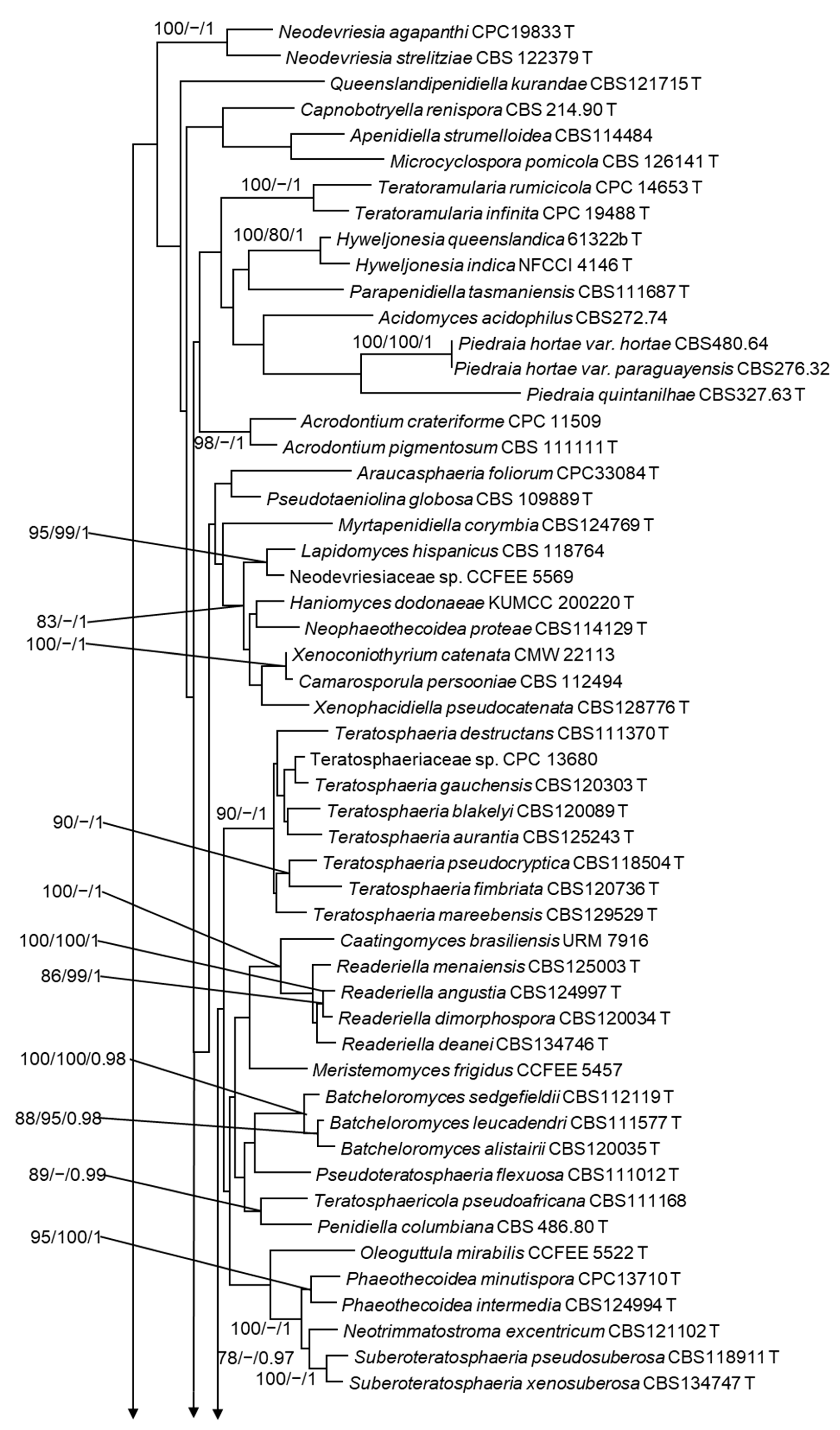
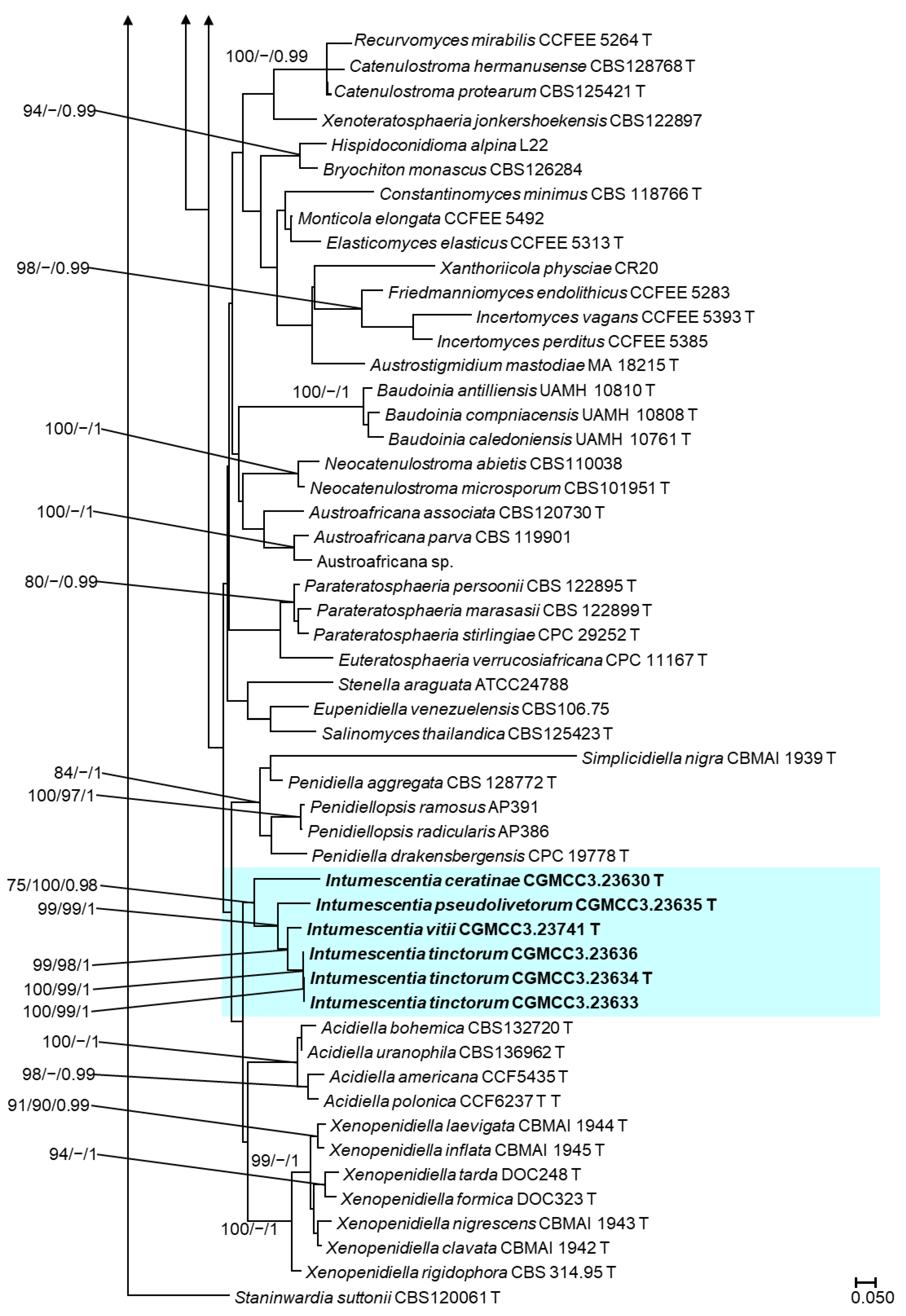
3.3. Phylogenetic Analyses
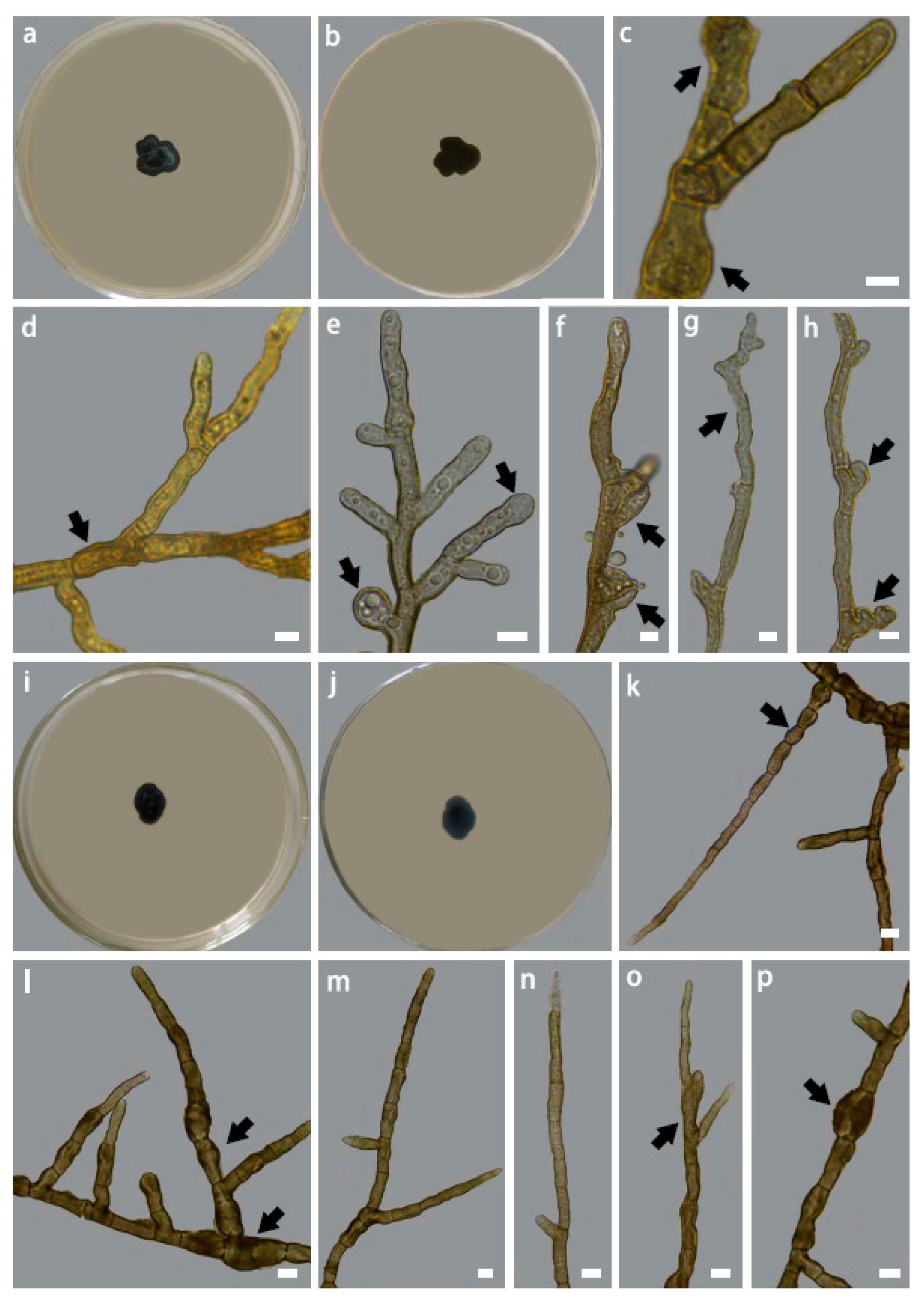
3.4. Taxonomy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crous, P.W.; Braun, U.; Groenewald, J.Z. Mycosphaerella is polyphyletic. Stud. Mycol. 2007, 58, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Wijayawardene, N.N. Outline of fungi and fungus-like taxa. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Wanasinghe, D.N.; Mortimer, P.E.; Xu, J. Insight into the systematics of microfungi colonizing dead woody twigs of Dodonaea viscosa in Honghe (China). J. Fungi 2021, 7, 180. [Google Scholar] [CrossRef] [PubMed]
- Quaedvlieg, W.; Binder, M.; Groenewald, J.Z.; Summerell, B.A.; Carnegie, A.J.; Burgess, T.I.; Crous, P.W. Introducing the consolidated species concept to resolve species in the teratosphaeriaceae. Persoonia 2014, 33, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.; Gusmão, L.; Dianese, J. Checklist of ascomycetes recorded on eucalypts in Brazil (1976–2022). Asian J. Mycol. 2022, 5, 107–129. [Google Scholar] [CrossRef]
- Andjic, V.; Carnegie, A.J.; Pegg, G.S.; Hardy, G.E.S.J.; Maxwell, A.; Crous, P.W.; Pérez, C.; Wingfield, M.J.; Burgess, T.I. 23 years of research on Teratosphaeria leaf blight of Eucalyptus. For. Ecol. Manage. 2019, 443, 19–27. [Google Scholar] [CrossRef]
- Ruibal, C.; Selbmann, L.; Avci, S.; Martin-Sanchez, P.M.; Gorbushina, A.A. Roof-Inhabiting cousins of rock-inhabiting fungi: Novel melanized microcolonial fungal species from photocatalytically reactive subaerial surfaces. Life 2018, 8, 30. [Google Scholar] [CrossRef]
- O’Connell, L.M.; Santos, R.; Springer, G.; Burne, R.A.; Nascimento, M.M.; Richards, V.P. Site-specific profiling of the dental mycobiome reveals strong taxonomic shifts during progression of early-childhood caries. Appl. Environ. Microbiol. 2020, 86, e02825-19. [Google Scholar] [CrossRef]
- Coleine, C.; Pombubpa, N.; Zucconi, L.; Onofri, S.; Turchetti, B.; Buzzini, P.; Stajich, J.E.; Selbmann, L. Uncovered microbial diversity in antarctic cryptoendolithic communities sampling three representative locations of the victoria land. Microorganisms 2020, 8, 942. [Google Scholar] [CrossRef]
- Morrison, E.S.; Thomas, P.; Ogram, A.; Kahveci, T.; Turner, B.L.; Chanton, J.P. Characterization of bacterial and fungal communities reveals novel consortia in tropical oligotrophic peatlands. Microb. Ecol. 2021, 82, 188–201. [Google Scholar] [CrossRef]
- Rizk, S.M.; Magdy, M.; Leo, F.; Werner, O.; Rashed, M.A.; Ros, R.M.; Urzi, C. A new extremotolerant ecotype of the fungus Pseudotaeniolina globosa isolated from Djoser Pyramid, Memphis Necropolis, Egypt. J. Fungi 2021, 7, 104. [Google Scholar] [CrossRef]
- Pérez-Ortega, S.; Garrido-Benavent, I.; De Los Ríos, A. Austrostigmidium, a new austral genus of lichenicolous fungi close to rock-inhabiting meristematic fungi in Teratosphaeriaceae. Lichenologist 2015, 47, 143–156. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L.; Feng, X.; Gong, X. Core taxa and photobiont-microbial interaction within the lichen Heterodermia obscurata (Physciaceae, Heterodermia). Symbiosis 2022, 86, 187–204. [Google Scholar] [CrossRef]
- Nash, T.H. III Lichen Biology; Cambridge University Press: Cambridge, UK, 2008; pp. 27–39. [Google Scholar] [CrossRef]
- Nelsen, M.P.; Lucking, R.; Boyce, C.K.; Lumbsch, H.T.; Ree, R.H. No support for the emergence of lichens prior to the evolution of vascular plants. Geobiology 2020, 18, 3–13. [Google Scholar] [CrossRef]
- Muggia, L.; Grube, M. Fungal diversity in lichens: From extremotolerance to interactions with algae. Life 2018, 8, 15. [Google Scholar] [CrossRef]
- Elkhateeb, W.; Daba, G. Fungi over fungi, endophytic fungi associated with mushroom fruiting bodies and lichens. J. Pharm. Pharm. Res. 2021, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Moreno, L.F.; Vicente, V.A.; de Hoog, S. Black yeasts in the omics era: Achievements and challenges. Med. Mycol. 2018, 56, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Chakarwarti, J. The diversity of endolichenic fungi—A review. Asian J. Mycol. 2020, 3, 490–511. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Arnold, A.E. Community analysis reveals close affinities between endophytic and endolichenic fungi in mosses and lichens. Microb. Ecol. 2010, 60, 340–353. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Govindarajulu, M.B.; Rajamani, T.; Tripathi, M.; Joshi, Y. Endolichenic fungi in lichens of Champawat district, Uttarakhand, northern India. Mycol. Prog. 2017, 16, 205–211. [Google Scholar] [CrossRef]
- Yu, N.H.; Park, S.Y.; Kim, J.A.; Park, C.H.; Jeong, M.H.; Oh, S.O.; Hong, S.G.; Talavera, M.; Divakar, P.K.; Hur, J.S. Endophytic and endolichenic fungal diversity in maritime Antarctica based on cultured material and their evolutionary position among Dikarya. Fungal Syst. Evol. 2018, 2, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wei, X.L.; Wei, Y.Z.; Liu, H.Y.; Yu, L.Y. Diversity and distribution of cultured endolichenic fungi in the Ny-Alesund Region, Svalbard (high arctic). Extremophiles 2016, 20, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Cao, W.; Wang, Y.; Li, S.; Li, X.; Bose, T.; Si, H.L. Melanodevriesia, a new genus of endolichenic oleaginous black yeast recovered from the Inner Mongolia Region of China. Fungal Syst. Evol. 2022, 9, 1–9. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Guo, S.; Guo, L. Endophytic fungi associated with lichens in Baihua mountain of Beijing, China. Fungal Divers. 2007, 25, 69–80. [Google Scholar]
- Si, H.L.; Su, Y.M.; Zheng, X.X.; Ding, M.Y.; Bose, T.; Chang, R.L. Phylogenetic and morphological analyses of Coniochaeta isolates recovered from Inner Mongolia and Yunnan revealed three new endolichenic fungal species. MycoKeys 2021, 83, 105–121. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Yang, J.; Zou, Y.; Zhao, X.Q. Diversity of endophytic bacterial and fungal microbiota associated with the medicinal lichen Usnea longissima at high altitudes. Front. Microbiol. 2022, 13, 958917. [Google Scholar] [CrossRef]
- Si, H.L.; Zheng, X.X.; Lin, X.; Su, Y.M.; Bose, T.; Chang, R.L. Dlhawksworthia flavoparmeliae sp. nov., a new endolichenic fungus in Phaeosphaeriaceae. Phytotaxa 2021, 525, 51–58. [Google Scholar] [CrossRef]
- Liu, J.; Hu, Y.; Luo, X.; Castañeda-Ruíz, R.F.; Xia, J.; Xu, Z.; Cui, R.; Shi, X.; Zhang, L.; Ma, J. Molecular phylogeny and morphology reveal four novel species of Corynespora and Kirschsteiniothelia (Dothideomycetes, Ascomycota) from China: A checklist for Corynespora reported worldwide. J. Fungi 2023, 9, 107. [Google Scholar] [CrossRef]
- Wang, L. Lichens of Yunnan in China; Shanghai Science and Technology Press: Shanghai, China, 2012. [Google Scholar]
- Wang, L.; Qian, Z. Illustrated Medicinal Lichens of China; Yunnan Science and Technology Press: Yunnan, China, 2012. [Google Scholar]
- Gholibeigianet, M. CTAB-Extraction method in Plant tissue. Majid Gholibeigian’s Lab. 2021. Available online: https://www.researchgate.net/publication/348693878. (accessed on 23 January 2021).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several species of Cryptococcus. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerase II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Groenewald, J.Z.; Nakashima, C.; Nishikawa, J.; Shin, H.D.; Park, J.H.; Jama, A.N.; Groenewald, M.; Braun, U.; Crous, P.W. Species concepts in Cercospora: Spotting the weeds among the roses. Stud. Mycol. 2013, 75, 115–170. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Quaedvlieg, W.; Groenewald, J.Z.; de Jesús Yáñez-Morales, M.; Crous, P.W. DNA barcoding of Mycosphaerella species of quarantine importance to Europe. Persoonia 2012, 29, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990. [CrossRef] [PubMed]
- Põlme, S.; Abarenkov, K.; Nilsson, R.H.; Lindahl, B.D.; Clemmensen, K.E.; Kauserud, H.; Nguyen, N.; Kjøller, R.; Bates, S.T.; Baldrian, P.; et al. FungalTraits: A user-friendly traits database of fungi and fungus-like stramenopiles. Fungal Divers. 2020, 105, 1–16. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Sudhir, K.; Glen, S.; Koichiro, T. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. Creating the CIPRES science gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, USA, 14 November 2010; pp. 1–8. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef]
- Rambaut, A. FigTree v1. 4.0. A Graphical Viewer of Phylogenetic Trees. 2012. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 5 December 2012).
- Nirenberg, H.I. Untersuchungen über die morphologische und biologische Differenzierung in der Fusaium-Sektion Liseola. Mitt. Biol. Bundesanst. Land-u. Forstwirtsch. Berlin Dahlem 1976, 69, 1247. [Google Scholar] [CrossRef]
- Duarte, A.P.M.; Attili-Angelis, D.; Baron, N.C.; Groenewald, J.Z.; Crous, P.W.; Pagnocca, F.C. Riding with the ants. Persoonia 2017, 38, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.H.; Olmo-Ruiz, M.d.; U’Ren, J.M.; Garcia, K.; Pignatta, D.; Wespe, N.; Sandberg, D.C.; Huang, Y.-L.; Hoffman, M.T.; Arnold, A.E. Coniochaeta endophytica sp. nov., a foliar endophyte associated with healthy photosynthetic tissue of Platycladus orientalis (cupressaceae). Plant Fungal Syst. 2019, 64, 65–79. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to imageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Hujslová, M.; Kubátová, A.; Kostovčík, M.; Kolařík, M. Acidiella bohemica gen. et sp. nov. and Acidomyces spp. (Teratosphaeriaceae), the indigenous inhabitants of extremely acidic soils in Europe. Fungal Divers. 2012, 58, 33–45. [Google Scholar] [CrossRef]
- Crous, P.W.; Wingfield, M.J.; Burgess, T.I.; Hardy, G.; Gene, J.; Guarro, J.; Baseia, I.G.; Garcia, D.; Gusmao, L.F.P.; Souza-Motta, C.M.; et al. Fungal planet description sheets: 716–784. Persoonia 2018, 40, 240–393. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Kim, K.M.; Elvebakk, A.; Kim, O.-S.; Jeong, G.; Hong, S.G. Algal and fungal diversity in antarctic lichens. J. Eukaryot. Microbiol. 2015, 62, 196–205. [Google Scholar] [CrossRef]
- Muggia, L.; Fleischhacker, A.; Kopun, T.; Grube, M. Extremotolerant fungi from alpine rock lichens and their phylogenetic relationships. Fungal Divers. 2016, 76, 119–142. [Google Scholar] [CrossRef]
- Rajulu, M.B.G.; Thirunavukkarasu, N.; Kumar, S.S.; Kaur, T.; Reddy, M.S.; Suryanarayanan, T.S. Endolichenic fungal diversity associated with some lichens of the Western Ghats. Planta Med. 2019, 86, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wei, X.L.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Diversity and distribution of lichen-associated fungi in the Ny-Alesund region (Svalbard, High Arctic) as revealed by 454 pyrosequencing. Sci. Rep. 2015, 5, 14850. [Google Scholar] [CrossRef]
- Petrini, O.; Hake, U.; Dreyfuss, M.M. An analysis of fungal communities isolated from fruticose lichens. Mycologia 1990, 82, 444–451. [Google Scholar] [CrossRef]
- Diederich, P.; Lawrey, J.D.; Ertz, D. The 2018 classification and checklist of lichenicolous fungi, with 2000 non-lichenized, obligately lichenicolous taxa. Bryologist 2018, 121, 340–425. [Google Scholar] [CrossRef]
- Kannangara, B.T.; Rajapaksha, R.S.; Paranagama, P.A. Nature and bioactivities of endolichenic fungi in Pseudocyphellaria sp., Parmotrema sp. and Usnea sp. at Hakgala montane forest in Sri Lanka. Lett. Appl. Microbiol. 2009, 48, 203–209. [Google Scholar] [CrossRef]
- Chen, C.-H.; Hsieh, S.-Y.; Yeh, Y.-H.; Kirschner, R. Cladocillium musae, a new genus and species of cercosporoid fungi (Mycosphaerellaceae) on wild banana in Taiwan. Mycol. Prog. 2020, 19, 837–843. [Google Scholar] [CrossRef]
- Flakus, A.; Etayo, J.; Perez-Ortega, S.; Kukwa, M.; Palice, Z.; Rodriguez-Flakus, P. A new genus, Zhurbenkoa, and a novel nutritional mode revealed in the family Malmideaceae (Lecanoromycetes, Ascomycota). Mycologia 2019, 111, 593–611. [Google Scholar] [CrossRef] [PubMed]
- Meswaet, Y.; Mangelsdorff, R.; Yorou, N.S.; Piepenbring, M. Unravelling unexplored diversity of cercosporoid fungi (mycosphaerellaceae, mycosphaerellales, ascomycota) in tropical Africa. MycoKeys 2021, 81, 69–138. [Google Scholar] [CrossRef] [PubMed]
- Baldrian, P.; Větrovský, T.; Lepinay, C.; Kohout, P. High-throughput sequencing view on the magnitude of global fungal diversity. Fungal Divers. 2021, 114, 539–547. [Google Scholar] [CrossRef]
- Thines, M.; Crous, P.W.; Aime, M.C.; Aoki, T.; Cai, L.; Hyde, K.D.; Miller, A.N.; Zhang, N.; Stadler, M. Ten reasons why a sequence-based nomenclature is not useful for fungi anytime soon. IMA Fungus 2018, 9, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Legeay, J.; Husson, C.; Boudier, B.; Louisanna, E.; Baraloto, C.; Schimann, H.; Marcais, B.; Buee, M. Surprising low diversity of the plant pathogen Phytophthora in Amazonian forests. Environ. Microbiol. 2020, 22, 5019–5032. [Google Scholar] [CrossRef] [PubMed]
- Bose, T.; Wingfield, M.J.; Roux, J.; Vivas, M.; Burgess, T.I. Community composition and distribution of Phytophthora species across adjacent native and non-native forests of South Africa. Fungal Ecol. 2018, 36, 17–25. [Google Scholar] [CrossRef]


| Intumescentia tinctorum | Intumescentia vitii | Intumescentia pseudolivetorum | Intumescentia ceratinae | |
|---|---|---|---|---|
| No. of isolates | 3 | 1 | 1 | 1 |
| Conidia | ||||
| Characteristics | - | - | Thick walled, bi- or tri-celled, septa transverse, multi-guttulate | Thick-walled, dark brown in color, multi-guttulate |
| Shapes | - | - | Fusioid to columnar | Columnar to doliiform |
| Mean size (μm) | - | - | 6.65 × 4.4 | 7.49 × 3.29 |
| Range (μm) | - | - | 2.94–15.7 × 3.03–7.07 | 5.12–11.59 × 1.35–3.9 |
| Chain of conidia | ||||
| Characteristics | - | - | Catenulate, two to eight in a chain, apical in position, caducous, | Catenulate, three to four in a chain, lateral or terminal in position, caducous |
| Mean size (μm) | - | - | 24.50 × 5.99 | 31.61 × 3.50 |
| Range (μm) | - | - | 13.46–45.41 × 4.21–11.12 | 7.16–75.41 × 2.09–4.93 |
| Sexual system | Sterile | Sterile | Sterile | Sterile |
| Hypha | ||||
| Characteristics | Hyphae smooth, septate, multi-guttulate, branched, compartments variable in size and often distorted | Hyphae smooth, branched, septate, septa constricted, compartment often peanut-shaped, multi-guttulate, guttles small in size, compartment variable in size often distorted | Hyphae smooth, septate, septa slightly constricted, multi-guttulate, compartments columnar, variable in size | Hyphae asperulous, brown in color, septate, multi-guttulate, branched, compartments variable in size, usually with hyphal swelling that are apical or intercalary in position, lateral branching usually arising from swollen compartments. |
| Color | Light brown | Dark brown | Brown | Blackish brown |
| Mean size (μm) | 3.23 | 4.09 | 2.95 | 3.4 |
| Range (μm) | 2.01–5.05 | 2.64–7.31 | 2.13–4.37 | 1.54–5.03 |
| Hyphal swellings | Globose, sub-globose | Globose, irregular | Sub-globose, irregular | Globose, sub-globose, irregular |
| Colony morphology on PDA | Black-brown, compact, greenish gray in color, tomentose, margin entire, irregularly lobed | Blackish brown, compact, superficial, short tomentose, margin entire, irregularly lobed | Blackish brown, compact, superficial, tomentose, margin, entire, irregularly lobed | Blackish brown in colour, compact, superficial, slightly raised in the left and villose, margin finely serrated, irregularly lobed. |
| Growth temperatures | ||||
| Minimum (°C) | 5 | 5 | 5 | 5 |
| Maximum (°C) | 30 | 30 | 30 | 30 |
| Optimum (°C) | 20 | 25 | 25 | 25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, H.; Wang, Y.; Liu, Y.; Li, S.; Bose, T.; Chang, R. Fungal Diversity Associated with Thirty-Eight Lichen Species Revealed a New Genus of Endolichenic Fungi, Intumescentia gen. nov. (Teratosphaeriaceae). J. Fungi 2023, 9, 423. https://doi.org/10.3390/jof9040423
Si H, Wang Y, Liu Y, Li S, Bose T, Chang R. Fungal Diversity Associated with Thirty-Eight Lichen Species Revealed a New Genus of Endolichenic Fungi, Intumescentia gen. nov. (Teratosphaeriaceae). Journal of Fungi. 2023; 9(4):423. https://doi.org/10.3390/jof9040423
Chicago/Turabian StyleSi, Hongli, Yichen Wang, Yanyu Liu, Shiguo Li, Tanay Bose, and Runlei Chang. 2023. "Fungal Diversity Associated with Thirty-Eight Lichen Species Revealed a New Genus of Endolichenic Fungi, Intumescentia gen. nov. (Teratosphaeriaceae)" Journal of Fungi 9, no. 4: 423. https://doi.org/10.3390/jof9040423
APA StyleSi, H., Wang, Y., Liu, Y., Li, S., Bose, T., & Chang, R. (2023). Fungal Diversity Associated with Thirty-Eight Lichen Species Revealed a New Genus of Endolichenic Fungi, Intumescentia gen. nov. (Teratosphaeriaceae). Journal of Fungi, 9(4), 423. https://doi.org/10.3390/jof9040423







