Transcriptomic Analysis of Acetaminophen Biodegradation by Penicillium chrysogenum var. halophenolicum and Insights into Energy and Stress Response Pathways
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Microorganisms and Culture Conditions
2.3. Acetaminophen Removal Experiments
2.4. Analytical Methods
2.5. Cell Viability Assays
2.6. Transcriptome Analysis by Next-Generation Sequencing
2.7. Statistical Analysis
3. Results
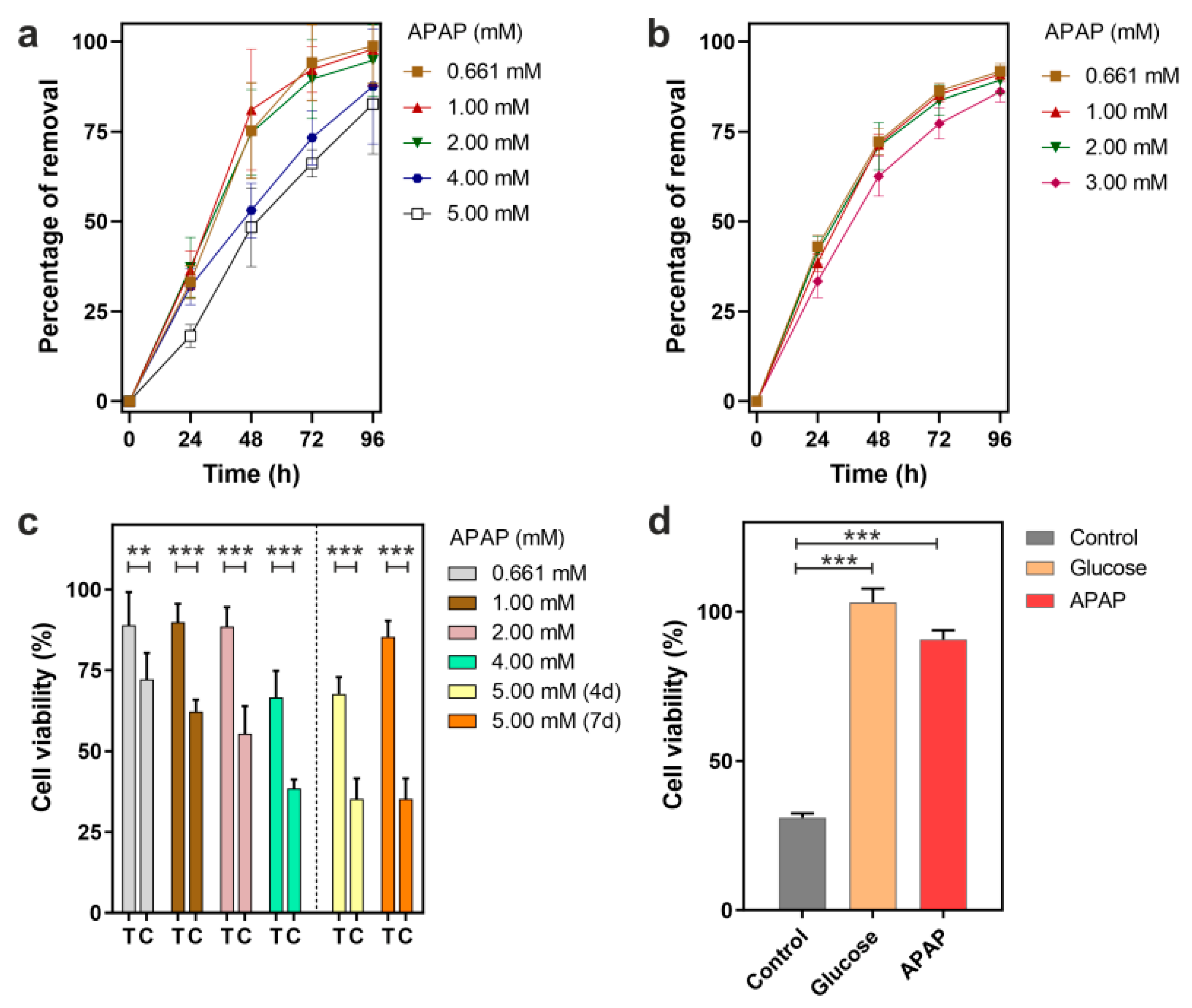
3.1. Removal of APAP by P. chrysogenum var. halophenolicum and Cytotoxic Evaluation
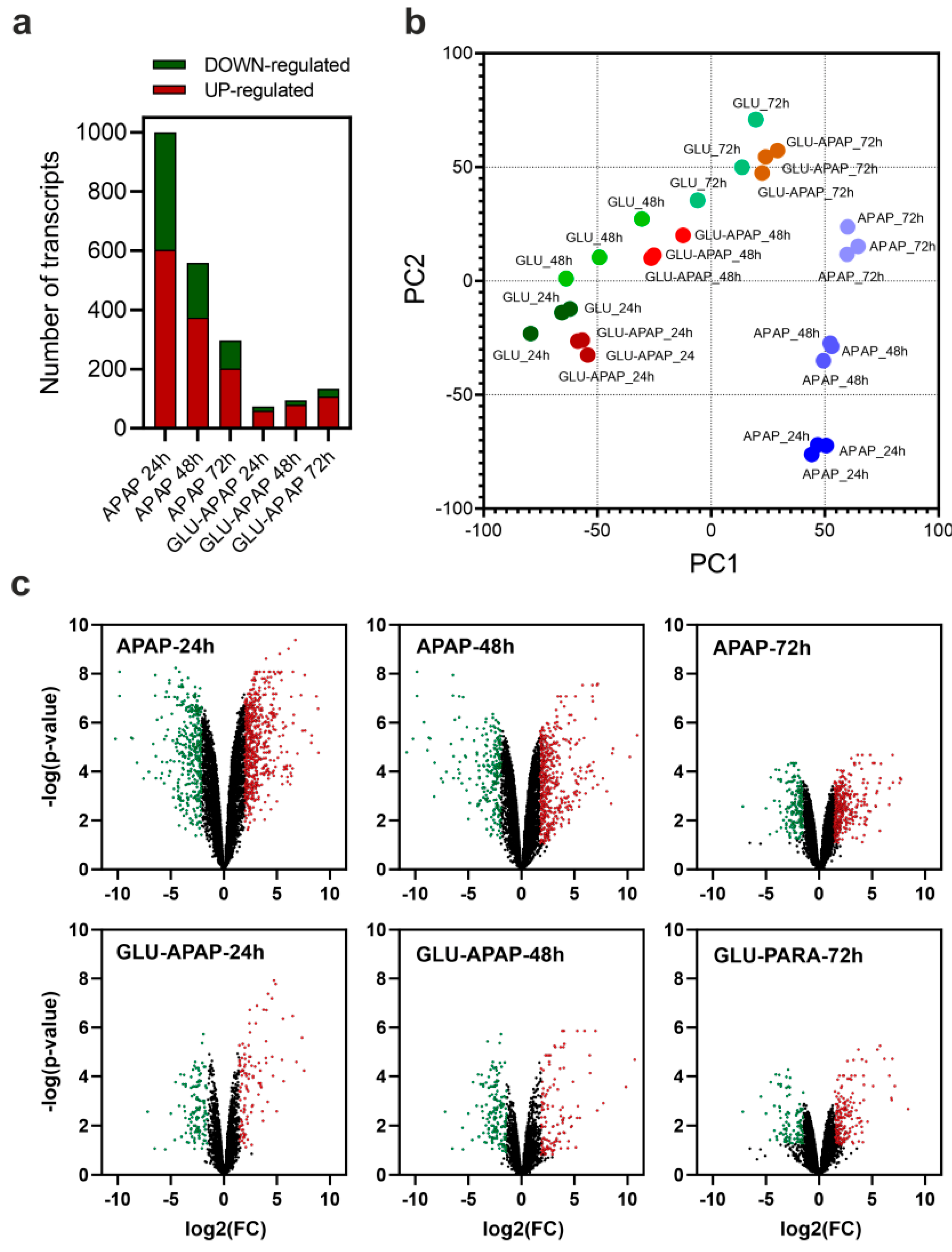
3.2. Transcriptional Analysis of APAP Degradation
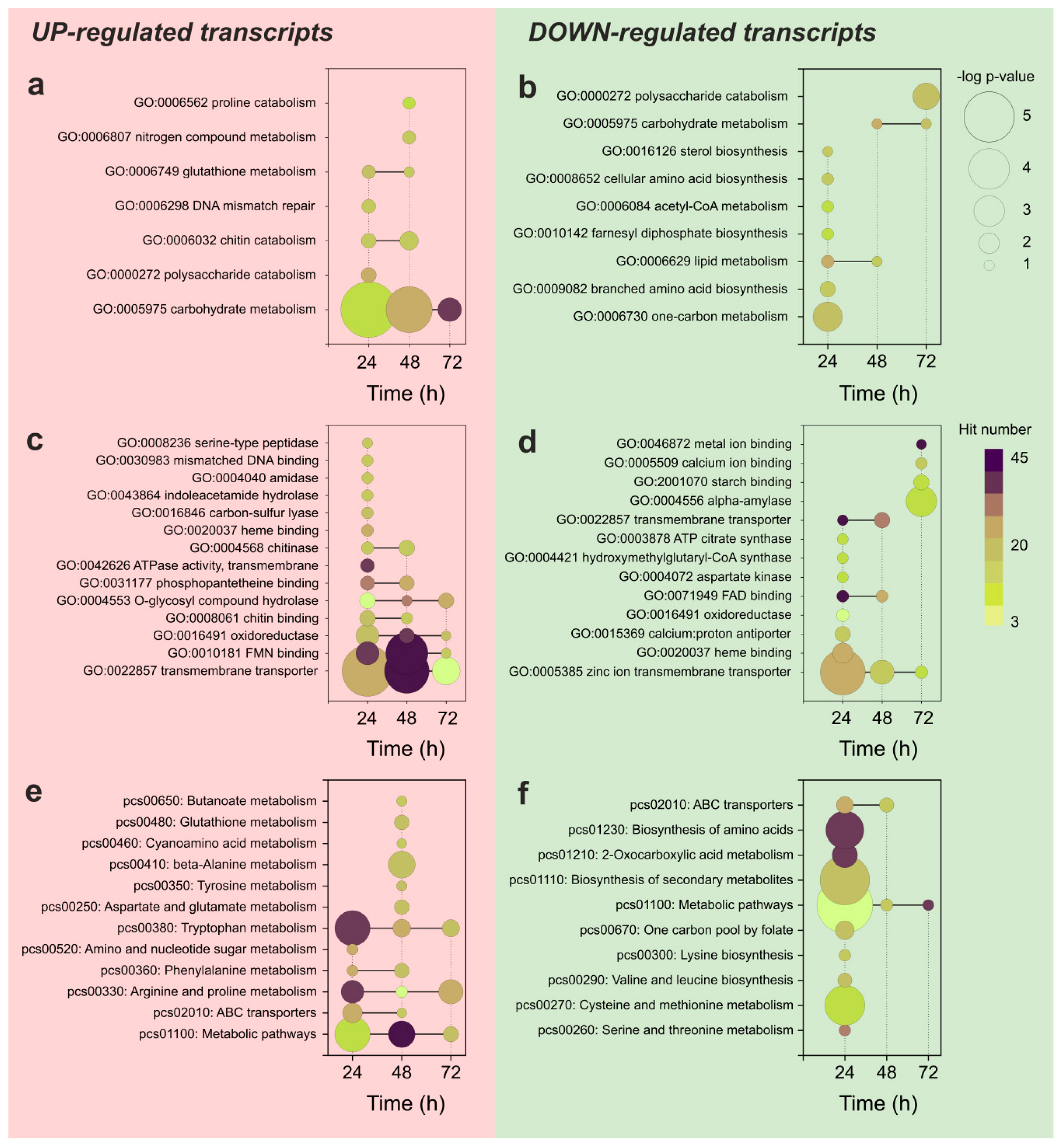
3.3. Functional Analysis of the Differentially Expressed Transcripts during APAP Degradation
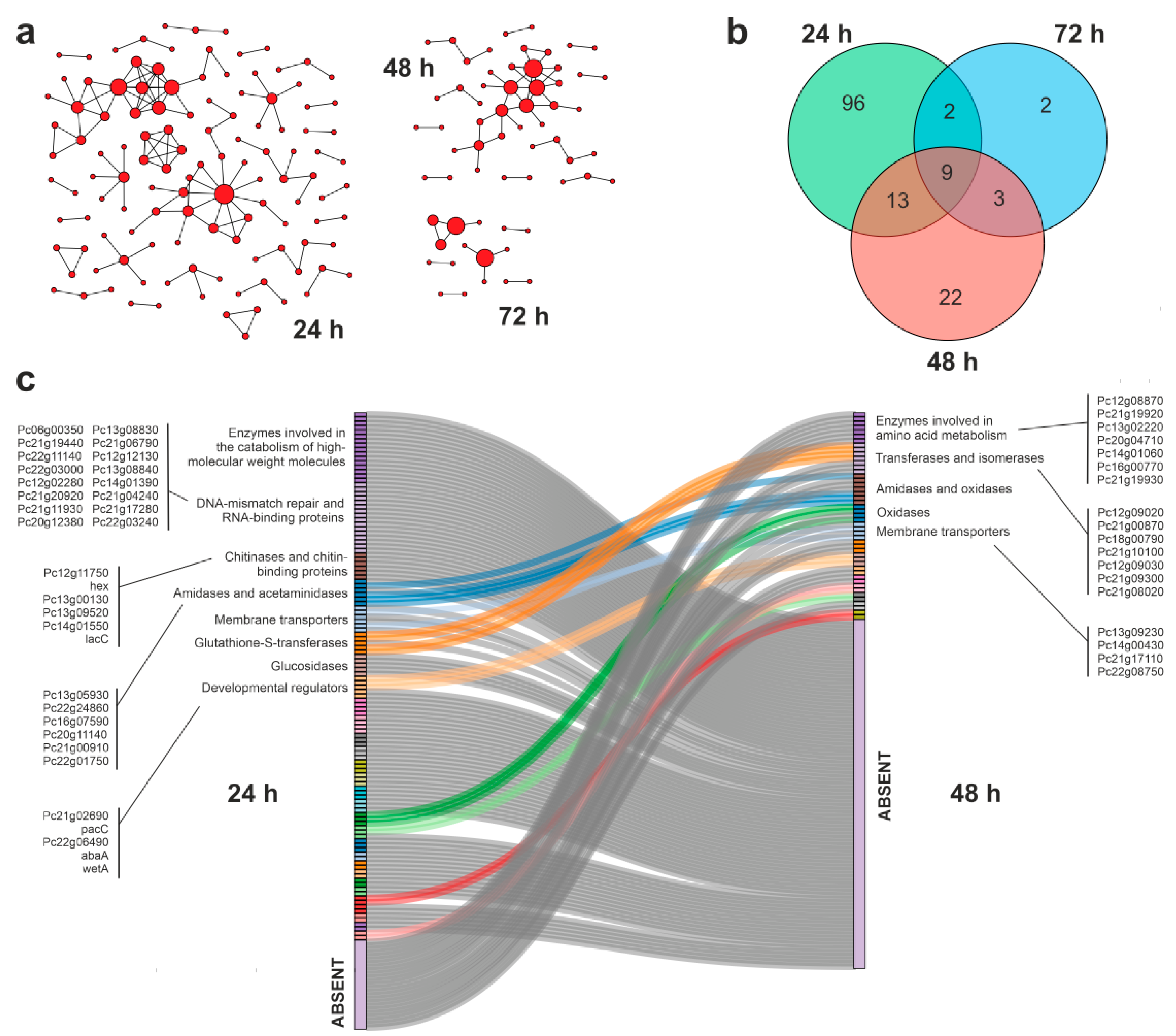
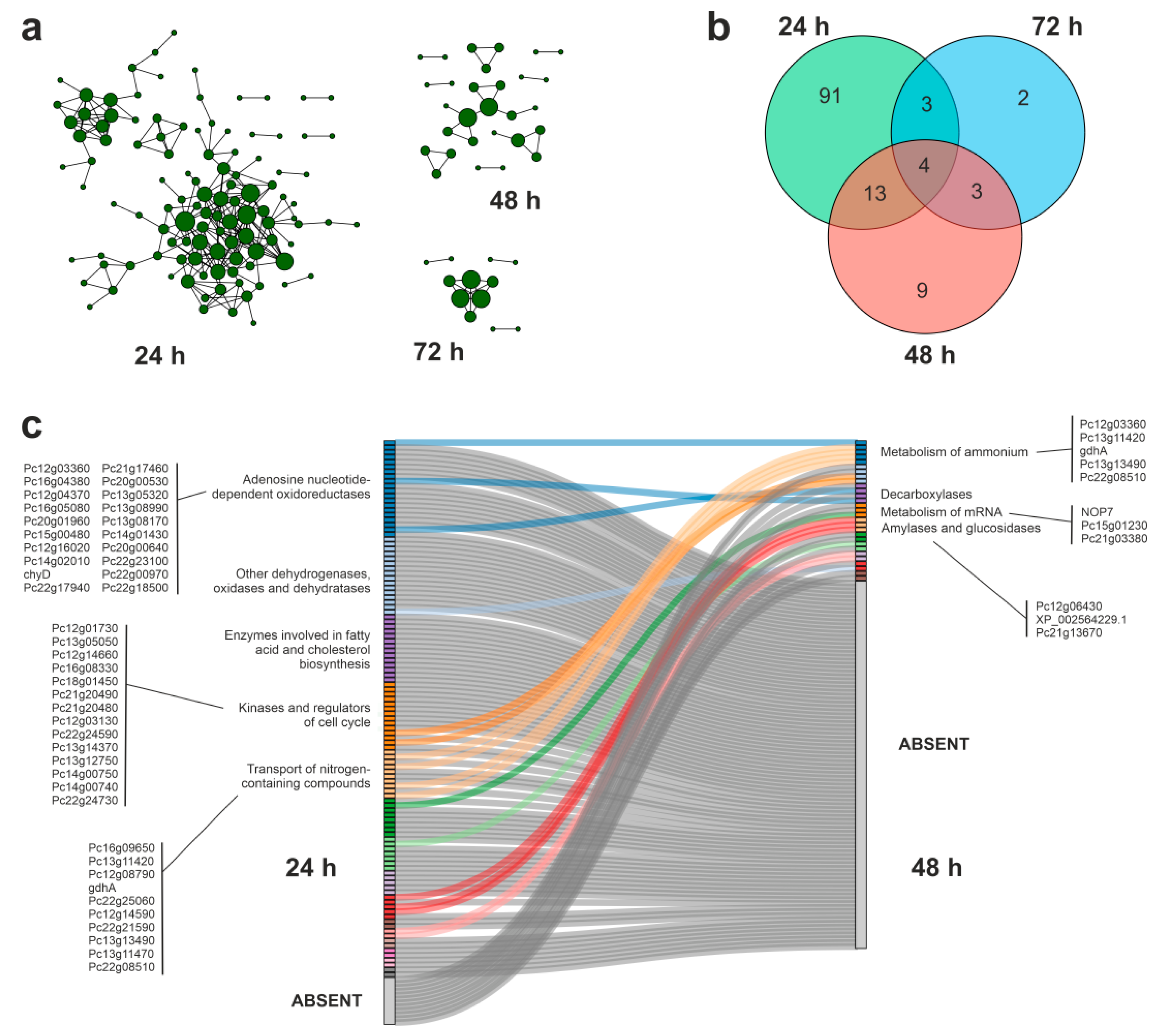
3.4. Protein–Protein Interaction Networks during APAP Degradation
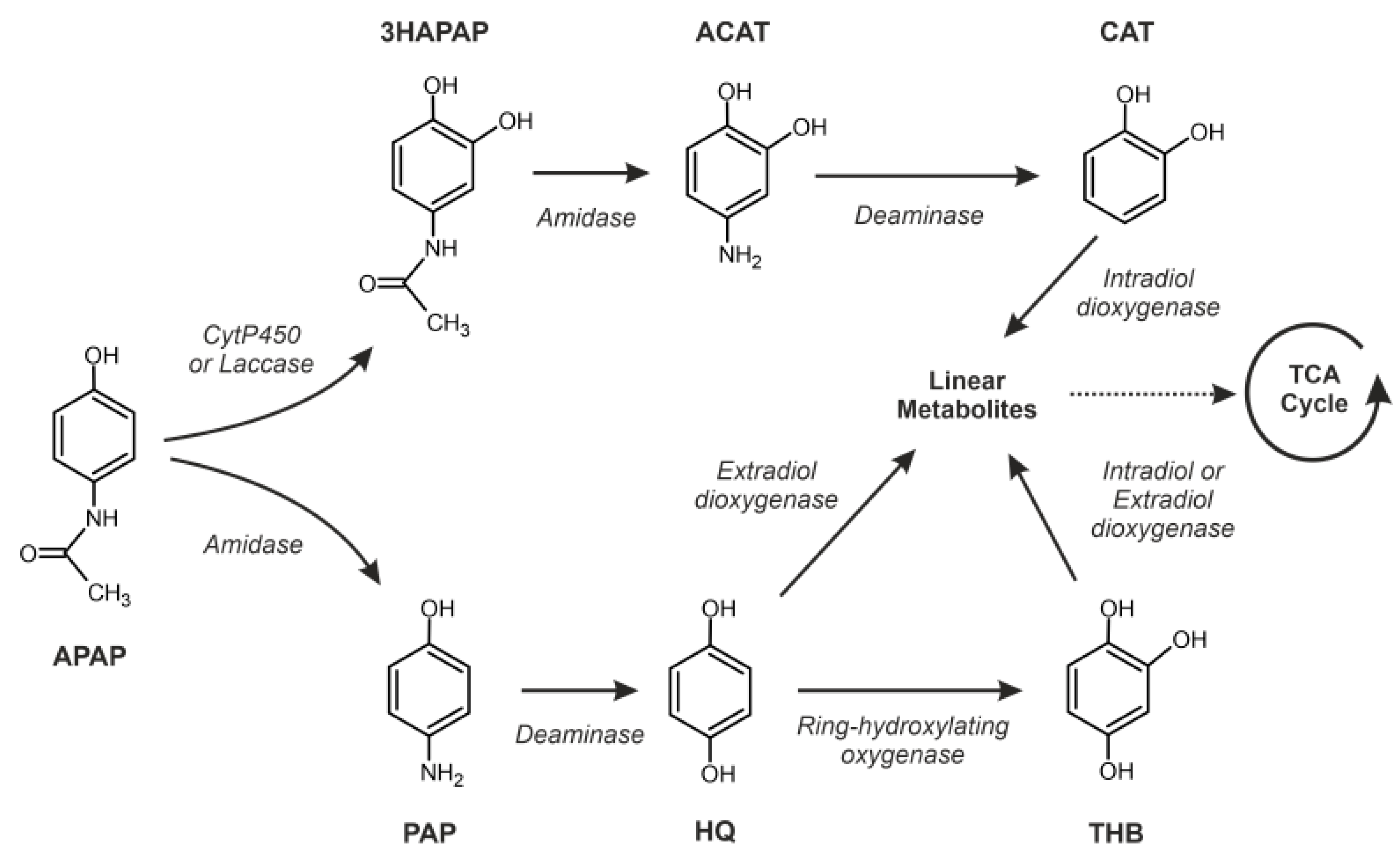
3.5. Putative Enzymes and Coding Genes Involved in APAP Degradation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brodie, B.B.; Axelrod, J. The fate of acetanilide in man. J. Pharmacol. Exp. Ther. 1948, 94, 29–38. [Google Scholar] [PubMed]
- Ramachandran, A.; Jaeschke, H. A mitochondrial journey through acetaminophen hepatotoxicity. Food Chem. Toxicol. 2020, 140, 111282. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Graudins, A. Risk prediction of hepatotoxicity in paracetamol poisoning. Clin Toxicol. 2017, 55, 879–892. [Google Scholar] [CrossRef] [PubMed]
- K’Oreje, K.O.; Vergeynst, L.; Ombaka, D.; De Wispelaere, P.; Okoth, M.; Van Langenhove, H.; Demeestere, K. Occurrence patterns of pharmaceutical residues in wastewater, surface water and groundwater of Nairobi and Kisumu city, Kenya. Chemosphere 2016, 149, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.; Silva, L.J.; Meisel, L.M.; Lino, C.M.; Pena, A. Environmental impact of pharmaceuticals from Portuguese wastewaters: Geographical and seasonal occurrence, removal and risk assessment. Environ. Res. 2015, 136, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef]
- Al-Odaini, N.A.; Zakaria, M.P.; Yaziz, M.I.; Surif, S. Multi-residue analytical method for human pharmaceuticals and synthetic hormones in river water and sewage effluents by solid-phase extraction and liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2010, 1217, 6791–6806. [Google Scholar] [CrossRef]
- Boleda, M.R.; Galceran, M.T.; Ventura, F. Validation and uncertainty estimation of a multiresidue method for pharmaceuticals in surface and treated waters by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2013, 1286, 146–158. [Google Scholar] [CrossRef]
- da Silva, B.F.; Jelic, A.; Lopez-Serna, R.; Mozeto, A.A.; Petrovic, M.; Barcelo, D. Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin, Spain. Chemosphere 2011, 85, 1331–1339. [Google Scholar] [CrossRef]
- Pereira, A.; Silva, L.J.G.; Laranjeiro, C.S.M.; Meisel, L.M.; Lino, C.M.; Pena, A. Human pharmaceuticals in Portuguese rivers: The impact of water scarcity in the environmental risk. Sci. Total Environ. 2017, 609, 1182–1191. [Google Scholar] [CrossRef]
- Fekadu, S.; Alemayehu, E.; Dewil, R.; Van der Bruggen, B. Pharmaceuticals in freshwater aquatic environments: A comparison of the African and European challenge. Sci. Total Environ. 2019, 654, 324–337. [Google Scholar] [CrossRef]
- de Voogt, P.; Janex-Habibi, M.L.; Sacher, F.; Puijker, L.; Mons, M. Development of a common priority list of pharmaceuticals relevant for the water cycle. Water Sci. Technol. 2009, 59, 39–46. [Google Scholar] [CrossRef]
- Ashfaq, M.; Noor, N.; Saif Ur Rehman, M.; Sun, Q.; Mustafa, G.; Faizan Nazar, M.; Yu, C.P. Determination of commonly used pharmaceuticals in hospital waste of Pakistan and evaluation of their ecological risk assessment. Clean Soil Air Water 2017, 45, 1500392. [Google Scholar] [CrossRef]
- Lindim, C.; de Zwart, D.; Cousins, I.T.; Kutsarova, S.; Kuhne, R.; Schuurmann, G. Exposure and ecotoxicological risk assessment of mixtures of top prescribed pharmaceuticals in Swedish freswaters. Chemosphere 2019, 220, 344–352. [Google Scholar] [CrossRef]
- Andreozzi, R.; Caprio, V.; Marotta, R.; Vogna, D. Paracetamol oxidation from aqueous solutions by means of ozonation and H2O2/UV system. Water Res. 2003, 37, 993–1004. [Google Scholar] [CrossRef]
- Brillas, E.; Sires, I.; Arias, C.; Cabot, P.L.; Centellas, F.; Rodriguez, R.M.; Garrido, J.A. Mineralization of paracetamol in aqueous medium by anodic oxidation with a boron-doped diamond electrode. Chemosphere 2005, 58, 399–406. [Google Scholar] [CrossRef]
- Manu, B.; Mahamood, S. Enhanced degradation of paracetamol by UV-C supported photo-Fenton process over Fenton oxidation. Water Sci. Technol. 2011, 64, 2433–2438. [Google Scholar] [CrossRef]
- Yang, L.; Yu, L.E.; Ray, M.B. Degradation of paracetamol in aqueous solutions by TiO2 photocatalysis. Water Res. 2008, 42, 3480–3488. [Google Scholar] [CrossRef]
- Audino, F.; Campanya, G.; Perez-Moya, M.; Espuna, A.; Graells, M. Systematic optimization approach for the efficient management of the photo-Fenton treatment process. Sci. Total Environ. 2019, 646, 902–913. [Google Scholar] [CrossRef]
- Stucchi, M.; Elfiad, A.; Rigamonti, M.; Khan, H.; Boffito, D.C. Water treatment: Mn-TiO2 synthesized by ultrasound with increased aromatics adsorption. Ultrason SonoChem. 2018, 44, 272–279. [Google Scholar] [CrossRef]
- Xu, B.; Zhan, G.; Du, H.; Luo, H.; Wang, T.; Zhan, C.; Yang, Y. Degradation of acetaminophen in aqueous solution by UV and UV-activated sludge processes. Water Sci. Technol. 2018, 78, 2088–2095. [Google Scholar] [CrossRef] [PubMed]
- Perelo, L.W. Review: In situ and bioremediation of organic pollutants in aquatic sediments. J. Hazard. Mater. 2010, 177, 81–89. [Google Scholar] [CrossRef] [PubMed]
- De Gusseme, B.; Vanhaecke, L.; Verstraete, W.; Boon, N. Degradation of acetaminophen by Delftia tsuruhatensis and Pseudomonas aeruginosa in a membrane bioreactor. Water Res. 2011, 45, 1829–1837. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, J.; Zhu, R.; Zhou, Q.; Chen, J. Degradation of paracetamol by pure bacterial cultures and their microbial consortium. Appl. MicroBiol. BioTechnol. 2013, 97, 3687–3698. [Google Scholar] [CrossRef]
- Zur, J.; Pinski, A.; Marchlewicz, A.; Hupert-Kocurek, K.; Wojcieszynska, D.; Guzik, U. Organic micropollutants paracetamol and ibuprofen-toxicity, biodegradation, and genetic background of their utilization by bacteria. Environ. Sci. Pollut. Res. Int. 2018, 25, 21498–21524. [Google Scholar] [CrossRef]
- Rios-Miguel, A.B.; Smith, G.J.; Cremers, G.; van Alen, T.; Jetten, M.S.M.; Op den Camp, H.J.M.; Welte, C.U. Microbial paracetamol degradation involves a high diversity of novel amidase enzyme candidates. Water Res. X 2022, 16, 100152. [Google Scholar] [CrossRef]
- Leitao, A.L. Potential of Penicillium species in the bioremediation field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef]
- Cruz-Morato, C.; Lucas, D.; Llorca, M.; Rodriguez-Mozaz, S.; Gorga, M.; Petrovic, M.; Barcelo, D.; Vicent, T.; Sarra, M.; Marco-Urrea, E. Hospital wastewater treatment by fungal bioreactor: Removal efficiency for pharmaceuticals and endocrine disruptor compounds. Sci. Total Environ. 2014, 493, 365–376. [Google Scholar] [CrossRef]
- Esterhuizen-Londt, M.; Schwartz, K.; Pflugmacher, S. Using aquatic fungi for pharmaceutical bioremediation: Uptake of acetaminophen by Mucor hiemalis does not result in an enzymatic oxidative stress response. Fungal Biol. 2016, 120, 1249–1257. [Google Scholar] [CrossRef]
- Hart, A.; Orr, D.L. The degradation of paracetamol (4-hydroxyacetanilide) and other substituted acetanilides by a Penicillium species. Antonie Van Leeuwenhoek 1975, 41, 239–247. [Google Scholar] [CrossRef]
- Mbokou, S.F.; Pontie, M.; Razafimandimby, B.; Bouchara, J.P.; Njanja, E.; Tonle Kenfack, I. Evaluation of the degradation of acetaminophen by the filamentous fungus Scedosporium dehoogii using carbon-based modified electrodes. Anal. Bioanal. Chem. 2016, 408, 5895–5903. [Google Scholar] [CrossRef]
- Leitão, A.L.; Garcia-Estrada, C.; Ullan, R.V.; Guedes, S.F.; Martin-Jimenez, P.; Mendes, B.; Martin, J.F. Penicillium chrysogenum var. halophenolicum, a new halotolerant strain with potential in the remediation of aromatic compounds in high salt environments. MicroBiol. Res. 2012, 167, 79–89. [Google Scholar] [CrossRef]
- Houbraken, J.; Frisvad, J.C.; Samson, R.A. Fleming’s penicillin producing strain is not Penicillium chrysogenum but P. rubens. IMA Fungus 2011, 2, 87–95. [Google Scholar] [CrossRef]
- Ferreira-Guedes, S.; Leitao, A.L. Simultaneous removal of dihydroxybenzenes and toxicity reduction by Penicillium chrysogenum var. halophenolicum under saline conditions. EcoToxicol. Environ. Saf. 2018, 150, 240–250. [Google Scholar] [CrossRef]
- Pereira, P.; Enguita, F.J.; Ferreira, J.; Leitao, A.L. DNA damage induced by hydroquinone can be prevented by fungal detoxification. Toxicol. Rep. 2014, 1, 1096–1105. [Google Scholar] [CrossRef]
- Leitão, A.L.; Duarte, M.P.; Oliveira, J.S. Degradation of phenol by a halotolerant strain of Penicillium chrysogenum. Int. Biodeterior. Biodegrad. 2007, 59, 220–225. [Google Scholar] [CrossRef]
- Fierro, F.; Montenegro, E.; Gutierrez, S.; Martin, J.F. Mutants blocked in penicillin biosynthesis show a deletion of the entire penicillin gene cluster at a specific site within a conserved hexanucleotide sequence. Appl. MicroBiol. BioTechnol. 1996, 44, 597–604. [Google Scholar] [CrossRef]
- Kapron, B.; Luszczki, J.; Paneth, A.; Wujec, M.; Siwek, A.; Karcz, T.; Mordyl, B.; Gluch-Lutwin, M.; Grybos, A.; Nowak, G.; et al. Molecular mechanism of action and safety of 5-(3-chlorophenyl)-4-hexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione—A novel anticonvulsant drug candidate. Int. J. Med. Sci. 2017, 14, 741–749. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Haering, M.; Habermann, B.H. RNfuzzyApp: An R shiny RNA-seq data analysis app for visualisation, differential expression analysis, time-series clustering and enrichment analysis. F1000Res 2021, 10, 654. [Google Scholar] [CrossRef] [PubMed]
- Haider, S.; Ballester, B.; Smedley, D.; Zhang, J.; Rice, P.; Kasprzyk, A. BioMart Central Portal--unified access to biological data. Nucleic Acids Res. 2009, 37, W23–W27. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–221. [Google Scholar] [CrossRef] [PubMed]
- Supek, F.; Bosnjak, M.; Skunca, N.; Smuc, T. REVIGO summarizes and visualizes long lists of gene ontology terms. PLoS ONE 2011, 6, e21800. [Google Scholar] [CrossRef]
- Fey, V.; Jambulingam, D.; Sara, H.; Heron, S.; Sipeky, C.; Schleutker, J. BioCPR–A Tool for Correlation Plots. Data 2021, 6, 97. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Nagpal, S.; Baksi, K.D.; Kuntal, B.K.; Mande, S.S. NetConfer: A web application for comparative analysis of multiple biological networks. BMC Biol. 2020, 18, 53. [Google Scholar] [CrossRef]
- Behrends, V.; Giskeodegard, G.F.; Bravo-Santano, N.; Letek, M.; Keun, H.C. Acetaminophen cytotoxicity in HepG2 cells is associated with a decoupling of glycolysis from the TCA cycle, loss of NADPH production, and suppression of anabolism. Arch. Toxicol. 2019, 93, 341–353. [Google Scholar] [CrossRef]
- Zur, J.; Wojcieszynska, D.; Hupert-Kocurek, K.; Marchlewicz, A.; Guzik, U. Paracetamol—Toxicity and microbial utilization. Pseudomonas moorei KB4 as a case study for exploring degradation pathway. Chemosphere 2018, 206, 192–202. [Google Scholar] [CrossRef]
- Ruiz-Villafan, B.; Cruz-Bautista, R.; Manzo-Ruiz, M.; Passari, A.K.; Villarreal-Gomez, K.; Rodriguez-Sanoja, R.; Sanchez, S. Carbon catabolite regulation of secondary metabolite formation, an old but not well-established regulatory system. Microb. BioTechnol. 2022, 15, 1058–1072. [Google Scholar] [CrossRef]
- Gluck-Thaler, E.; Haridas, S.; Binder, M.; Grigoriev, I.V.; Crous, P.W.; Spatafora, J.W.; Bushley, K.; Slot, J.C. The Architecture of Metabolism Maximizes Biosynthetic Diversity in the Largest Class of Fungi. Mol. Biol. Evol. 2020, 37, 2838–2856. [Google Scholar] [CrossRef]
- Guedes, S.F.; Mendes, B.; Leitao, A.L. Resorcinol degradation by a Penicillium chrysogenum strain under osmotic stress: Mono and binary substrate matrices with phenol. Biodegradation 2011, 22, 409–419. [Google Scholar] [CrossRef]
- Olicón-Hernández, D.R.; Ortúzar, M.; Pozo, C.; González-López, J.; Aranda, E. Metabolic capability of Penicillium oxalicum to transform high concentrations of anti-inflammatory and analgesic drugs. Appl. Sci. 2020, 10, 2479. [Google Scholar] [CrossRef]
- van Bodegom, P. Microbial maintenance: A critical review on its quantification. Microb. Ecol 2007, 53, 513–523. [Google Scholar] [CrossRef]
- Kundu, K.; Marozava, S.; Ehrl, B.; Merl-Pham, J.; Griebler, C.; Elsner, M. Defining lower limits of biodegradation: Atrazine degradation regulated by mass transfer and maintenance demand in Arthrobacter aurescens TC1. ISME J. 2019, 13, 2236–2251. [Google Scholar] [CrossRef]
- Soulas, G.; Lagacherie, B. Modelling of microbial processes that govern degradation of organic substrates in soil, with special reference to pesticides. Philos. Trans. R Soc. Lond. B Biol. Sci. 1990, 329, 369–373. [Google Scholar] [CrossRef]
- McGill, M.R.; Sharpe, M.R.; Williams, C.D.; Taha, M.; Curry, S.C.; Jaeschke, H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Investig. 2012, 122, 1574–1583. [Google Scholar] [CrossRef]
- Wiger, R.; Finstad, H.S.; Hongslo, J.K.; Haug, K.; Holme, J.A. Paracetamol inhibits cell cycling and induces apoptosis in HL-60 cells. Pharmacol. Toxicol. 1997, 81, 285–293. [Google Scholar]
- Wu, S.; Zhang, L.; Chen, J. Paracetamol in the environment and its degradation by microorganisms. Appl. MicroBiol. BioTechnol. 2012, 96, 875–884. [Google Scholar] [CrossRef]
- Sharabi, N.E.; Bordeleau, L.M. Biochemical decomposition of the herbicide N-(3,4-dichlorophenyl)-2-methylpentanamide and related compounds. Appl. MicroBiol. 1969, 18, 369–375. [Google Scholar] [CrossRef]
- Shin, J.; Kim, J.E.; Lee, Y.W.; Son, H. Fungal Cytochrome P450s and the P450 Complement (CYPome) of Fusarium graminearum. Toxins 2018, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Fraser, J.A.; Davis, M.A.; Hynes, M.J. The genes gmdA, encoding an amidase, and bzuA, encoding a cytochrome P450, are required for benzamide utilization in Aspergillus nidulans. Fungal Genet. Biol. 2002, 35, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Zerva, A.; Pentari, C.; Termentzi, A.; America, A.H.P.; Zouraris, D.; Bhattacharya, S.K.; Karantonis, A.; Zervakis, G.I.; Topakas, E. Discovery of two novel laccase-like multicopper oxidases from Pleurotus citrinopileatus and their application in phenolic oligomer synthesis. BioTechnol. Biofuels 2021, 14, 83. [Google Scholar] [CrossRef] [PubMed]
- Enguita, F.J.; Leitao, A.L. Hydroquinone: Environmental pollution, toxicity, and microbial answers. BioMed. Res. Int. 2013, 2013, 542168. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enguita, F.J.; Pereira, S.; Leitão, A.L. Transcriptomic Analysis of Acetaminophen Biodegradation by Penicillium chrysogenum var. halophenolicum and Insights into Energy and Stress Response Pathways. J. Fungi 2023, 9, 408. https://doi.org/10.3390/jof9040408
Enguita FJ, Pereira S, Leitão AL. Transcriptomic Analysis of Acetaminophen Biodegradation by Penicillium chrysogenum var. halophenolicum and Insights into Energy and Stress Response Pathways. Journal of Fungi. 2023; 9(4):408. https://doi.org/10.3390/jof9040408
Chicago/Turabian StyleEnguita, Francisco J., Sofia Pereira, and Ana Lúcia Leitão. 2023. "Transcriptomic Analysis of Acetaminophen Biodegradation by Penicillium chrysogenum var. halophenolicum and Insights into Energy and Stress Response Pathways" Journal of Fungi 9, no. 4: 408. https://doi.org/10.3390/jof9040408
APA StyleEnguita, F. J., Pereira, S., & Leitão, A. L. (2023). Transcriptomic Analysis of Acetaminophen Biodegradation by Penicillium chrysogenum var. halophenolicum and Insights into Energy and Stress Response Pathways. Journal of Fungi, 9(4), 408. https://doi.org/10.3390/jof9040408







