Fungemia in Hospitalized Adult Patients with Hematological Malignancies: Epidemiology and Risk Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Scenario
2.2. Study Design
2.3. Definitions
2.4. Statistical Analysis
2.5. Ethical Aspects
3. Results
3.1. Characteristics of the Population
3.2. Neutrophil Count
3.3. Microorganisms Identified and Treatment Received
3.4. Survival Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J. Mycoses Study Group Impact of Treatment Strategy on Outcomes in Patients with Candidemia and Other Forms of Invasive Candidiasis: A Patient-Level Quantitative Review of Randomized Trials. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54, 1110–1122. [Google Scholar] [CrossRef]
- Cornely, O.A.; Gachot, B.; Akan, H.; Bassetti, M.; Uzun, O.; Kibbler, C.; Marchetti, O.; de Burghgraeve, P.; Ramadan, S.; Pylkkanen, L.; et al. Epidemiology and Outcome of Fungemia in a Cancer Cohort of the Infectious Diseases Group (IDG) of the European Organization for Research and Treatment of Cancer (EORTC 65031). Clin. Infect. Dis. 2015, 61, 324–331. [Google Scholar] [CrossRef]
- Colombo, A.L.; Nucci, M.; Park, B.J.; Nouér, S.A.; Arthington-Skaggs, B.; da Matta, D.A.; Warnock, D.; Morgan, J. Epidemiology of Candidemia in Brazil: A Nationwide Sentinel Surveillance of Candidemia in Eleven Medical Centers. J. Clin. Microbiol. 2006, 44, 2816–2823. [Google Scholar] [CrossRef]
- Colombo, A.L.; Cortes, J.A.; Zurita, J.; Guzman-Blanco, M.; Alvarado Matute, T.; de Queiroz Telles, F.; Santolaya, M.E.; Tiraboschi, I.N.; Echevarría, J.; Sifuentes, J.; et al. Recommendations for the Diagnosis of Candidemia in Latin America. Rev. Iberoam. Micol. 2013, 30, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Alvarado-Matute, T.; Tiraboschi, I.N.; Cortes, J.; Zurita, J.; Guzman-Blanco, M.; Santolaya, M.E.; Thompson, L.; Sifuentes-Osornio, J.; et al. Epidemiology of Candidemia in Latin America: A Laboratory-Based Survey. PLoS ONE 2013, 8, e59373. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Queiroz-Telles, F.; Tobón, A.M.; Restrepo, A.; Colombo, A.L. Epidemiology of Opportunistic Fungal Infections in Latin America. Clin. Infect. Dis. 2010, 51, 561–570. [Google Scholar] [CrossRef]
- Cornely, O.A.; Hoenigl, M.; Lass-Flörl, C.; Chen, S.C.-A.; Kontoyiannis, D.P.; Morrissey, C.O.; Thompson, G.R.; for the Mycoses Study Group Education and Research Consortium (MSG-ERC) and the European Confederation of Medical Mycology (ECMM). Defining Breakthrough Invasive Fungal Infection–Position Paper of the Mycoses Study Group Education and Research Consortium and the European Confederation of Medical Mycology. Mycoses 2019, 62, 716–729. [Google Scholar] [CrossRef]
- Freifeld, A.G.; Bow, E.J.; Sepkowitz, K.A.; Boeckh, M.J.; Ito, J.I.; Mullen, C.A.; Raad, I.I.; Rolston, K.V.; Young, J.-A.H.; Wingard, J.R. Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 52, e56–e93. [Google Scholar] [CrossRef]
- Sipsas, N.V.; Lewis, R.E.; Tarrand, J.; Hachem, R.; Rolston, K.V.; Raad, I.I.; Kontoyiannis, D.P. Candidemia in Patients with Hematologic Malignancies in the Era of New Antifungal Agents (2001-2007): Stable Incidence but Changing Epidemiology of a Still Frequently Lethal Infection. Cancer 2009, 115, 4745–4752. [Google Scholar] [CrossRef]
- Horn, D.L.; Neofytos, D.; Anaissie, E.J.; Fishman, J.A.; Steinbach, W.J.; Olyaei, A.J.; Marr, K.A.; Pfaller, M.A.; Chang, C.-H.; Webster, K.M. Epidemiology and Outcomes of Candidemia in 2019 Patients: Data from the Prospective Antifungal Therapy Alliance Registry. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 48, 1695–1703. [Google Scholar] [CrossRef]
- Zirkel, J.; Klinker, H.; Kuhn, A.; Abele-Horn, M.; Tappe, D.; Turnwald, D.; Einsele, H.; Heinz, W.J. Epidemiology of Candida Blood Stream Infections in Patients with Hematological Malignancies or Solid Tumors. Med. Mycol. 2012, 50, 50–55. [Google Scholar] [CrossRef]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picardi, M.; Bonini, A.; Chierichini, A.; et al. The Epidemiology of Fungal Infections in Patients with Hematologic Malignancies: The SEIFEM-2004 Study. Haematologica 2006, 91, 1068–1075. [Google Scholar] [PubMed]
- Chen, C.-Y.; Huang, S.-Y.; Tsay, W.; Yao, M.; Tang, J.-L.; Ko, B.-S.; Chou, W.-C.; Tien, H.-F.; Hsueh, P.-R. Clinical Characteristics of Candidaemia in Adults with Haematological Malignancy, and Antimicrobial Susceptibilities of the Isolates at a Medical Centre in Taiwan, 2001-2010. Int. J. Antimicrob. Agents 2012, 40, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, M.; Marchesi, F.; Candoni, A.; Cattaneo, C.; Nosari, A.; Veggia, B.; Verga, L.; Fracchiolla, N.; Vianelli, N.; Del Principe, M.I.; et al. Fungaemia in Haematological Malignancies: SEIFEM-2015 Survey. Eur. J. Clin. Investig. 2019, 49, e13083. [Google Scholar] [CrossRef]
- Gamaletsou, M.N.; Walsh, T.J.; Zaoutis, T.; Pagoni, M.; Kotsopoulou, M.; Voulgarelis, M.; Panayiotidis, P.; Vassilakopoulos, T.; Angelopoulou, M.K.; Marangos, M.; et al. A Prospective, Cohort, Multicentre Study of Candidaemia in Hospitalized Adult Patients with Haematological Malignancies. Clin. Microbiol. Infect. 2014, 20, O50–O57. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Guinea, J. Global Trends in the Distribution of Candida Species Causing Candidemia. Clin. Microbiol. Infect. 2014, 20, 5–10. [Google Scholar] [CrossRef]
- Toda, M.; Williams, S.R.; Berkow, E.L.; Farley, M.M.; Harrison, L.H.; Bonner, L.; Marceaux, K.M.; Hollick, R.; Zhang, A.Y.; Schaffner, W.; et al. Population-Based Active Surveillance for Culture-Confirmed Candidemia—Four Sites, United States, 2012–2016. MMWR Surveill. Summ. 2019, 68, 1–15. [Google Scholar] [CrossRef]
- Seagle, E.E.; Williams, S.L.; Chiller, T.M. Recent Trends in the Epidemiology of Fungal Infections. Infect. Dis. Clin. N. Am. 2021, 35, 237–260. [Google Scholar] [CrossRef]
- Ricotta, E.E.; Lai, Y.L.; Babiker, A.; Strich, J.R.; Kadri, S.S.; Lionakis, M.S.; Prevots, D.R.; Adjemian, J. Invasive Candidiasis Species Distribution and Trends, United States, 2009-2017. J. Infect. Dis. 2021, 223, 1295–1302. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Iqbal, N.; Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Bolden, C.B.; Baughman, W.; Stein, B.; Hollick, R.; Park, B.J.; et al. Species Identification and Antifungal Susceptibility Testing of Candida Bloodstream Isolates from Population-Based Surveillance Studies in Two U.S. Cities from 2008 to 2011. J. Clin. Microbiol. 2012, 50, 3435–3442. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging Opportunistic Yeast Infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Andes, D.R.; Diekema, D.J.; Horn, D.L.; Reboli, A.C.; Rotstein, C.; Franks, B.; Azie, N.E. Epidemiology and Outcomes of Invasive Candidiasis Due to Non-Albicans Species of Candida in 2,496 Patients: Data from the Prospective Antifungal Therapy (PATH) Registry 2004–2008. PLoS ONE 2014, 9, e101510. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; Pappas, P.G. Invasive Candidiasis. Infect. Dis. Clin. N. Am. 2016, 30, 103–124. [Google Scholar] [CrossRef]
- Colombo, A.L.; Garnica, M.; Aranha Camargo, L.F.; Da Cunha, C.A.; Bandeira, A.C.; Borghi, D.; Campos, T.; Senna, A.L.; Valias Didier, M.E.; Dias, V.C.; et al. Candida Glabrata: An Emerging Pathogen in Brazilian Tertiary Care Hospitals. Med. Mycol. 2013, 51, 38–44. [Google Scholar] [CrossRef]
- Moretti, M.L.; Trabasso, P.; Lyra, L.; Fagnani, R.; Resende, M.R.; de Oliveira Cardoso, L.G.; Schreiber, A.Z. Is the Incidence of Candidemia Caused by Candida Glabrata Increasing in Brazil? Five-Year Surveillance of Candida Bloodstream Infection in a University Reference Hospital in Southeast Brazil. Med. Mycol. 2013, 51, 225–230. [Google Scholar] [CrossRef]
- Cuervo-Maldonado, S.I.; Bermúdez, C.D.; Enciso, L.; Gómez-Rincón, J.C.; Castillo, J.S.; Sánchez, R.; Ballesteros, M.P.; Buitrago, G.; Gamboa, Ó.A.; Acosta, S.; et al. Guía de práctica clínica para el diagnóstico y el tratamiento de las infecciones bacterianas y micóticas en pacientes oncológicos mayores de 15 años con neutropenia febril posquimioterapia de alto riesgo. Rev. Colomb. Cancerol. 2014, 18, 186–196. [Google Scholar] [CrossRef]
- Cortés, J.A.; Reyes, P.; Gómez, C.H.; Cuervo, S.I.; Rivas, P.; Casas, C.A.; Sánchez, R. Clinical and Epidemiological Characteristics and Risk Factors for Mortality in Patients with Candidemia in Hospitals from Bogotá, Colombia. Braz. J. Infect. Dis. 2014, 18, 631–637. [Google Scholar] [CrossRef]
- Wingard, J.R. Importance of Candida Species Other than C. Albicans as Pathogens in Oncology Patients. Clin. Infect. Dis. 1995, 20, 115–125. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Vaziri, I.; Hanna, H.A.; Boktour, M.; Thornby, J.; Hachem, R.; Bodey, G.P.; Raad, I.I. Risk Factors for Candida Tropicalis Fungemia in Patients with Cancer. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 33, 1676–1681. [Google Scholar] [CrossRef]
- Krcmery, V.; Barnes, A.J. Non-Albicans Candida Spp. Causing Fungaemia: Pathogenicity and Antifungal Resistance. J. Hosp. Infect. 2002, 50, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, P.; Giannella, M.; Fanciulli, C.; Guinea, J.; Valerio, M.; Rojas, L.; Rodríguez-Créixems, M.; Bouza, E. Candida Tropicalis Fungaemia: Incidence, Risk Factors and Mortality in a General Hospital. Clin. Microbiol. Infect. 2011, 17, 1538–1545. [Google Scholar] [CrossRef] [PubMed]
- Lortholary, O.; Renaudat, C.; Sitbon, K.; Desnos-Ollivier, M.; Bretagne, S.; Dromer, F.; The French Mycoses Study Group. The Risk and Clinical Outcome of Candidemia Depending on Underlying Malignancy. Intensive Care Med. 2017, 43, 652–662. [Google Scholar] [CrossRef]
- Lewis, R.E.; Cahyame-Zuniga, L.; Leventakos, K.; Chamilos, G.; Ben-Ami, R.; Tamboli, P.; Tarrand, J.; Bodey, G.P.; Luna, M.; Kontoyiannis, D.P. Epidemiology and Sites of Involvement of Invasive Fungal Infections in Patients with Haematological Malignancies: A 20-Year Autopsy Study. Mycoses 2013, 56, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Luna, M.; Lewis, R.E.; Bodey, G.P.; Chemaly, R.; Tarrand, J.J.; Safdar, A.; Raad, I.I.; Kontoyiannis, D.P. Invasive Fungal Infections in Patients with Hematologic Malignancies in a Tertiary Care Cancer Center: An Autopsy Study over a 15-Year Period (1989–2003). Haematologica 2006, 91, 986–989. [Google Scholar] [PubMed]
- Nucci, M.; Marr, K.A.; Queiroz-Telles, F.; Martins, C.A.; Trabasso, P.; Costa, S.; Voltarelli, J.C.; Colombo, A.L.; Imhof, A.; Pasquini, R.; et al. Fusarium Infection in Hematopoietic Stem Cell Transplant Recipients. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2004, 38, 1237–1242. [Google Scholar] [CrossRef]
- Campo, M.; Lewis, R.E.; Kontoyiannis, D.P. Invasive Fusariosis in Patients with Hematologic Malignancies at a Cancer Center: 1998–2009. J. Infect. 2010, 60, 331–337. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Akova, M.; Herbrecht, R.; Viscoli, C.; Arendrup, M.C.; Arikan-Akdagli, S.; Bassetti, M.; Bille, J.; Calandra, T.; Castagnola, E.; et al. ESCMID Guideline for the Diagnosis and Management of Candida Diseases 2012: Adults with Haematological Malignancies and after Haematopoietic Stem Cell Transplantation (HCT). Clin. Microbiol. Infect. 2012, 18, 53–67. [Google Scholar] [CrossRef]
- Liu, C.Y.; Huang, L.J.; Wang, W.S.; Chen, T.L.; Yen, C.C.; Yang, M.H.; Hsiao, L.T.; Liu, C.Y.; Chen, P.M.; Chiou, T.J. Candidemia in Cancer Patients: Impact of Early Removal of Non-Tunneled Central Venous Catheters on Outcome. J. Infect. 2009, 58, 154–160. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E.; Betts, R.F.; Dupont, B.F.; Wu, C.; Buell, D.N.; Kovanda, L.; Lortholary, O. Early Removal of Central Venous Catheter in Patients with Candidemia Does Not Improve Outcome: Analysis of 842 Patients from 2 Randomized Clinical Trials. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 51, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Horn, D.L.; Ostrosky-Zeichner, L.; Morris, M.I.; Ullmann, A.J.; Wu, C.; Buell, D.N.; Kovanda, L.L.; Cornely, O.A. Factors Related to Survival and Treatment Success in Invasive Candidiasis or Candidemia: A Pooled Analysis of Two Large, Prospective, Micafungin Trials. Eur. J. Clin. Microbiol. Infect. Dis. 2010, 29, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Janum, S.; Afshari, A. Central Venous Catheter (CVC) Removal for Patients of All Ages with Candidaemia. Cochrane Database Syst. Rev. 2016, 7, CD011195. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.; Micek, S.; Hampton, N.; Doherty, J.A.; Kumar, A. Septic Shock Attributed to Candida Infection: Importance of Empiric Therapy and Source Control. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Fraser, V.J.; Jones, M.; Dunkel, J.; Storfer, S.; Medoff, G.; Dunagan, W.C. Candidemia in a Tertiary Care Hospital: Epidemiology, Risk Factors, and Predictors of Mortality. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 1992, 15, 414–421. [Google Scholar] [CrossRef]
- Morrell, M.; Fraser, V.J.; Kollef, M.H. Delaying the Empiric Treatment of Candida Bloodstream Infection until Positive Blood Culture Results Are Obtained: A Potential Risk Factor for Hospital Mortality. Antimicrob. Agents Chemother. 2005, 49, 3640–3645. [Google Scholar] [CrossRef]
- Garey, K.W.; Rege, M.; Pai, M.P.; Mingo, D.E.; Suda, K.J.; Turpin, R.S.; Bearden, D.T. Time to Initiation of Fluconazole Therapy Impacts Mortality in Patients with Candidemia: A Multi-Institutional Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2006, 43, 25–31. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Reddy, B.T.; Hanna, H.; Bodey, G.P.; Tarrand, J.; Raad, I.I. Breakthrough Candidemia in Patients with Cancer Differs from De Novo Candidemia in Host Factors and Candida Species But Not Intensity. Infect. Control Hosp. Epidemiol. 2002, 23, 542–545. [Google Scholar] [CrossRef]
- Mann, P.A.; McNicholas, P.M.; Chau, A.S.; Patel, R.; Mendrick, C.; Ullmann, A.J.; Cornely, O.A.; Patino, H.; Black, T.A. Impact of Antifungal Prophylaxis on Colonization and Azole Susceptibility of Candida Species. Antimicrob. Agents Chemother. 2009, 53, 5026–5034. [Google Scholar] [CrossRef]
- Cornely, O.A.; Vazquez, J.; De Waele, J.; Betts, R.; Rotstein, C.; Nucci, M.; Pappas, P.G.; Ullmann, A.J. Efficacy of Micafungin in Invasive Candidiasis Caused by Common Candida Species with Special Emphasis on Non- Albicans Candida Species. Mycoses 2014, 57, 79–89. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Ebbers, J.; Geurtz, L.; Stefanik, D.; Major, Y.; Edmond, M.B.; Wenzel, R.P.; Seifert, H. Nosocomial Bloodstream Infections Due to Candida Spp. in the USA: Species Distribution, Clinical Features and Antifungal Susceptibilities. Int. J. Antimicrob. Agents 2014, 43, 78–81. [Google Scholar] [CrossRef]
- Jensen, R.H.; Johansen, H.K.; Søes, L.M.; Lemming, L.E.; Rosenvinge, F.S.; Nielsen, L.; Olesen, B.; Kristensen, L.; Dzajic, E.; Astvad, K.M.T.; et al. Posttreatment Antifungal Resistance among Colonizing Candida Isolates in Candidemia Patients: Results from a Systematic Multicenter Study. Antimicrob. Agents Chemother. 2016, 60, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Borst, A.; Raimer, M.T.; Warnock, D.W.; Morrison, C.J.; Arthington-Skaggs, B.A. Rapid Acquisition of Stable Azole Resistance by Candida Glabrata Isolates Obtained before the Clinical Introduction of Fluconazole. Antimicrob. Agents Chemother. 2005, 49, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Chapeland-Leclerc, F.; Hennequin, C.; Papon, N.; Noël, T.; Girard, A.; Socié, G.; Ribaud, P.; Lacroix, C. Acquisition of Flucytosine, Azole, and Caspofungin Resistance in Candida Glabrata Bloodstream Isolates Serially Obtained from a Hematopoietic Stem Cell Transplant Recipient. Antimicrob. Agents Chemother. 2010, 54, 1360–1362. [Google Scholar] [CrossRef] [PubMed]
- Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Stein, B.; Hollick, R.; Lockhart, S.R.; Magill, S.S.; Derado, G.; Park, B.J.; Chiller, T.M. Changes in Incidence and Antifungal Drug Resistance in Candidemia: Results From Population-Based Laboratory Surveillance in Atlanta and Baltimore, 2008–2011. Clin. Infect. Dis. 2012, 55, 1352–1361. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the Epidemiological Landscape of Invasive Candidiasis. J. Antimicrob. Chemother. 2018, 73, i4–i13. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Cleveland, A.A.; Farley, M.M.; Harrison, L.H.; Schaffner, W.; Beldavs, Z.G.; Derado, G.; Pham, C.D.; Lockhart, S.R.; Smith, R.M. Epidemiology and Risk Factors for Echinocandin Nonsusceptible Candida Glabrata Bloodstream Infections: Data from a Large Multisite Population-Based Candidemia Surveillance Program, 2008–2014. Open Forum Infect. Dis. 2015, 2, ofv163. [Google Scholar] [CrossRef]
- Slavin, M.A.; Sorrell, T.C.; Marriott, D.; Thursky, K.A.; Nguyen, Q.; Ellis, D.H.; Morrissey, C.O.; Chen, S.C.A.; on behalf of the Australian Candidemia Study, Australasian Society for Infectious Diseases. Candidaemia in Adult Cancer Patients: Risks for Fluconazole-Resistant Isolates and Death. J. Antimicrob. Chemother. 2010, 65, 1042–1051. [Google Scholar] [CrossRef]
- Viscoli, C.; Girmenia, C.; Marinus, A.; Collette, L.; Martino, P.; Vandercam, B.; Doyen, C.; Lebeau, B.; Spence, D.; Krcmery, V.; et al. Candidemia in Cancer Patients: A Prospective, Multicenter Surveillance Study by the Invasive Fungal Infection Group (IFIG) of the European Organization for Research and Treatment of Cancer (EORTC). Clin. Infect. Dis. 1999, 28, 1071–1079. [Google Scholar] [CrossRef]

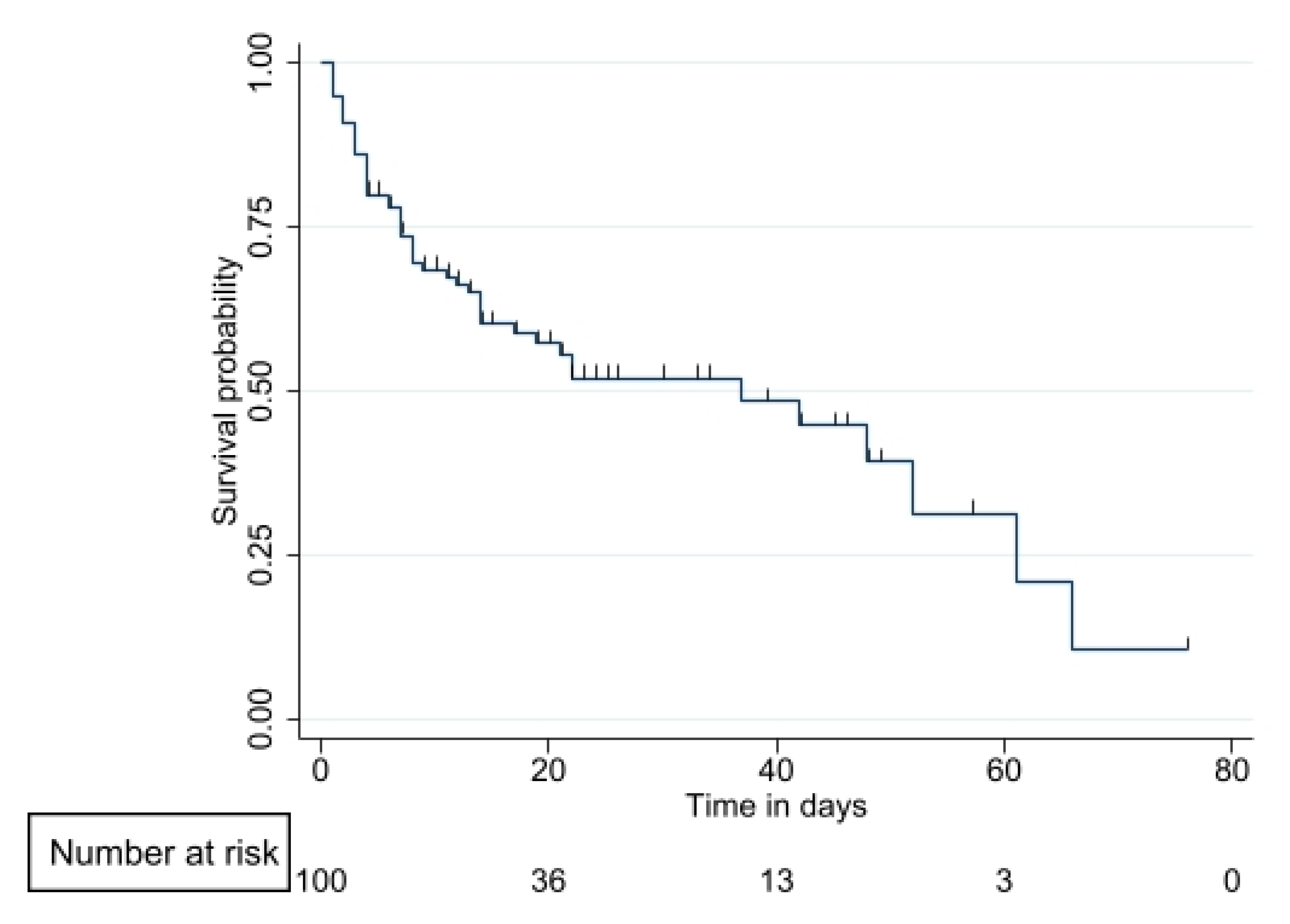
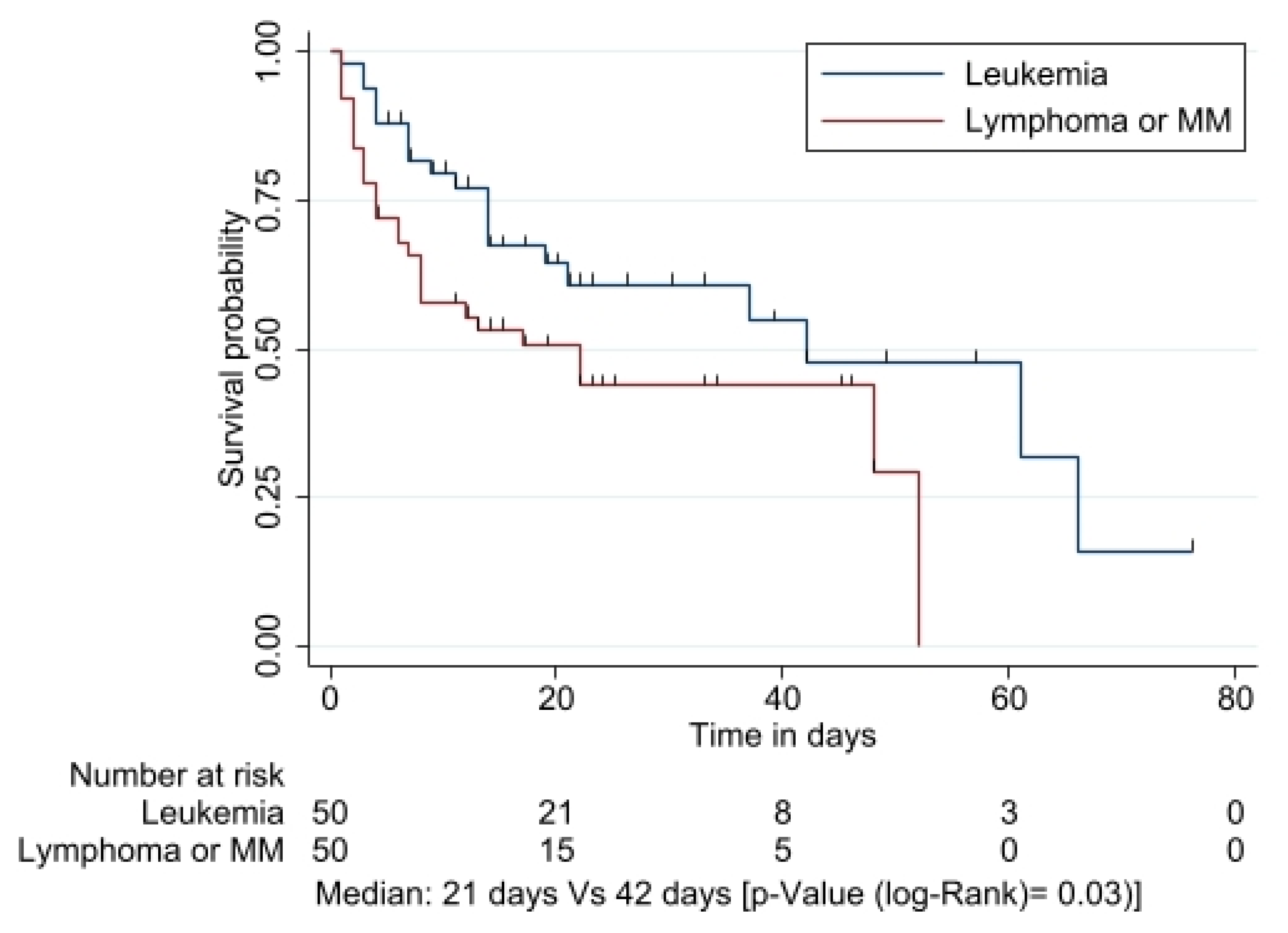
| Variable | Total n (%) |
|---|---|
| Patients included | 105 |
| Gender = female (%) | 55 (52.4) |
| Age = mean (SD) | 48.38 (19.03) |
| Diagnostic stage of hematologic malignancy (%) | |
| Active (new diagnosis/induction) | 54 (51.5) |
| Consolidation | 3 (2.9) |
| Relapse/refractory | 42 (40.0) |
| Remission | 6 (5.7) |
| Radiation therapy = yes (%) | 3 (2.9) |
| ECOG (%) | |
| 0–2 | 10 (9.5) |
| 3 | 43 (41.0) |
| 4 | 39 (37.1) |
| No data | 13 (12.4) |
| Antifungal prophylaxis = yes (%) | 32 (33.3) |
| ANC = mean (SD) | 260 (11.01) |
| FN = yes (%) | 64 (61.0) |
| Duration of neutropenia days = mean (SD) | 21.78 (16.13) |
| Previous episodes of FN = yes (%) | 29 (27.6) |
| Risk factor = yes (%) | 37 (35.2) |
| ANC < 500 = yes (%) | 57 (54.3) |
| HPCT = yes (%) | 9 (8.6) |
| Corticosteroids = yes (%) | 13 (12.4) |
| Cyclosporine = yes (%) | 10 (9.5) |
| Severe congenital immunodeficiency = yes (%) | 1 (1.0) |
| CVC = yes (%) | 70 (66.7) |
| Parenteral nutrition = yes (%) | 5 (4.8) |
| Mucositis = yes (%) | 17 916.2) |
| Disease | n (%) | Age Mean (SD) | Patients with Neutropenia < 500 n(%) | Neutrophil Count Median (IQR) | Days of Neutropenia Median (IQR) | Hospital Stay (Days) Median (IQR) | ICU Stay (Days) Median (IQR) | Non-Survivors n (%) | ECOG Median |
|---|---|---|---|---|---|---|---|---|---|
| ALL | 32 (30.4) | 34.34 (14.64) | 26 (81.2) | 95 (347.5) | 21.5 (19.5) | 44 (29.5) | 8 (16.75) | 14 (43.7) | 3 |
| DLBCL | 15 (14.2) | 57.46 (14.91) | 2 (20) | 6130 (10,735) | 9 (8) | 39 (25) | 10.5 (9.25) | 9 (60) | 4 |
| AML | 14 (13.3) | 48.64 (20.26) | 12 (100) | 25 (219.75) | 34 (19.25) | 43.5 (21.5) | 3.5 (3.75) | 6 (42.8) | 3 |
| Other lymphoma | 11 (10.5) | 61.27 (15.60) | 4 (36.3) | 2950 (6474) | 10.5 (7.5) | 41 (38.5) | 7.5 (8) | 3 (27.2) | 3 |
| Multiple myeloma | 8 (7.6) | 64.12 (9.38) | 0 | 4845 (4664) | – | 27.5 (41.5) | 18 (13) | 4 (50) | 4 |
| Hodgkin’s lymphoma | 6 (5.7) | 53 (19.35) | 2 (33.3) | 785 (5605) | 14 (6) | 56 (33.5) | 16 (29.5) | 4 (66.6) | 4 |
| PTCL | 5 (4.7) | 53.2 (19.57) | 2 (40) | 10,490 (8130) | 17 (0) | 19 (7) | 3 (3) | 5 (100) | 4 |
| Follicular lymphoma | 3 (2.8) | 49.11 (18.87) | 1 (33.3) | 740 (542) | 7.5 (7.5) | 28 (15.5) | 19.5 (2.5) | 2 (66.6) | 3 |
| CLL | 2 (1.9) | 75.5 (4.94) | 2 (100) | 15 (5) | 20.5 (1.5) | 45 (23) | 3 (0) | 2 (100) | 4 |
| CML | 2 (1.9) | 55 (8.48) | 1 (50) | 18,965 (18,885) | 10 (10) | 40 (2) | 16 (0) | 1 (50) | 2 |
| APL | 2 (1.9) | 49.2 (12.02) | 0 | 12,230 (8120) | – | 10.2 (44) | 33.5 (8.5) | 1 (50) | 3 |
| Burkitt’s lymphoma | 1 (0.9) | 32 | 0 | 10 | – | 39 | 5 | 0 | 3 |
| Malignant hystyocytosis | 1(0.9) | 27 | 1(100) | 10 | 31 | 93 | 0 | 1 (100) | 3 |
| Lymphoblastic lymphoma | 1(0.9) | 21 | 1 (100) | 2 | 13 | 43 | – | 0 | 3 |
| Mycosis fungoides | 1(0.9) | 53 | 0 | 8290 | – | 84 | – | 0 | 4 |
| Other leukemia | 1(0.9) | 26 | 0 | 200 | – | 34 | 11 | 1 (100) | 4 |
| Microorganism | n (%) | |
|---|---|---|
| 1 | Candida tropicalis | 29 (28) |
| 2 | Candida albicans | 22 (21) |
| 3 | Candida parapsilosis | 18 (17) |
| 4 | Candida krusei | 13 (12) |
| 5 | Cryptococcus neoformans | 9 (8.5) |
| 6 | Candida glabrata | 3 (2.8) |
| 7 | Trichosporum asahii | 3 (2.8) |
| 8 | Fusarium spp. | 2 (1.9) |
| 9 | Candida guillermondii, Cryptococcus laurentii, Geotrichum spp., Malassezia pachydermatis, Rhodotorula mucilaginosa, Trichosporum beigelii | 1 (1) |
| Variable | HR | IC 95% | p-Value |
|---|---|---|---|
| Age | 1.01 | 1–1.03 | 0.04 * |
| Gender | 0.67 | 0.39–1.17 | 0.16 |
| Diagnosis by groups: lymphoma/MM | 1.91 | 1.08–3.36 | 0.02 * |
| ECOG | 1 | 0.99–1 | 0.83 |
| ICU admission | 3.14 | 1.74–5.67 | <0.001 ** |
| ANC < 500 | 0.55 | 0.31–0.95 | 0.03 |
| Septic shock | 2.6 | 1.5–4.52 | <0.001 ** |
| CVC | 1.38 | 0.76–2.51 | 0.27 |
| Relapsed/refractory clinical status | 0.71 | 0.4–2.27 | 0.25 |
| Variable | Coefficient | HR | z | p-Value |
|---|---|---|---|---|
| Diagnosis by groups: lymphoma/MM | 0.583 | 1.792 | 2.039 | 0.04 * |
| ICU admission | 1.128 | 3.089 | 3.749 | <0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Espíndola, L.A.; Cuervo-Maldonado, S.I.; Enciso-Olivera, J.L.; Gómez-Rincón, J.C.; Jiménez-Cetina, L.; Sánchez-Pedraza, R.; García-Guzmán, K.; López-Mora, M.J.; Álvarez-Moreno, C.A.; Cortés, J.A.; et al. Fungemia in Hospitalized Adult Patients with Hematological Malignancies: Epidemiology and Risk Factors. J. Fungi 2023, 9, 400. https://doi.org/10.3390/jof9040400
Vargas-Espíndola LA, Cuervo-Maldonado SI, Enciso-Olivera JL, Gómez-Rincón JC, Jiménez-Cetina L, Sánchez-Pedraza R, García-Guzmán K, López-Mora MJ, Álvarez-Moreno CA, Cortés JA, et al. Fungemia in Hospitalized Adult Patients with Hematological Malignancies: Epidemiology and Risk Factors. Journal of Fungi. 2023; 9(4):400. https://doi.org/10.3390/jof9040400
Chicago/Turabian StyleVargas-Espíndola, Luz Alejandra, Sonia I. Cuervo-Maldonado, José L. Enciso-Olivera, Julio C. Gómez-Rincón, Leydy Jiménez-Cetina, Ricardo Sánchez-Pedraza, Katherine García-Guzmán, María José López-Mora, Carlos A. Álvarez-Moreno, Jorge Alberto Cortés, and et al. 2023. "Fungemia in Hospitalized Adult Patients with Hematological Malignancies: Epidemiology and Risk Factors" Journal of Fungi 9, no. 4: 400. https://doi.org/10.3390/jof9040400
APA StyleVargas-Espíndola, L. A., Cuervo-Maldonado, S. I., Enciso-Olivera, J. L., Gómez-Rincón, J. C., Jiménez-Cetina, L., Sánchez-Pedraza, R., García-Guzmán, K., López-Mora, M. J., Álvarez-Moreno, C. A., Cortés, J. A., Garzón-Herazo, J. R., Martínez-Vernaza, S., Sierra-Parada, C. R., & Murillo-Sarmiento, B. A. (2023). Fungemia in Hospitalized Adult Patients with Hematological Malignancies: Epidemiology and Risk Factors. Journal of Fungi, 9(4), 400. https://doi.org/10.3390/jof9040400






