Assessing the Effect of Slope Position on the Community Assemblage of Soil Diazotrophs and Root Arbuscular Mycorrhizal Fungi
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
2.2. Experimental Design and Sampling
2.3. Soil Physicochemical Analysis
2.4. DNA Extraction and Amplicon Sequencing
2.5. Sequence Analysis
2.6. Soil Physicochemical Analysis
2.6.1. Plant Diversity Analysis
2.6.2. Statistical Analysis
3. Results
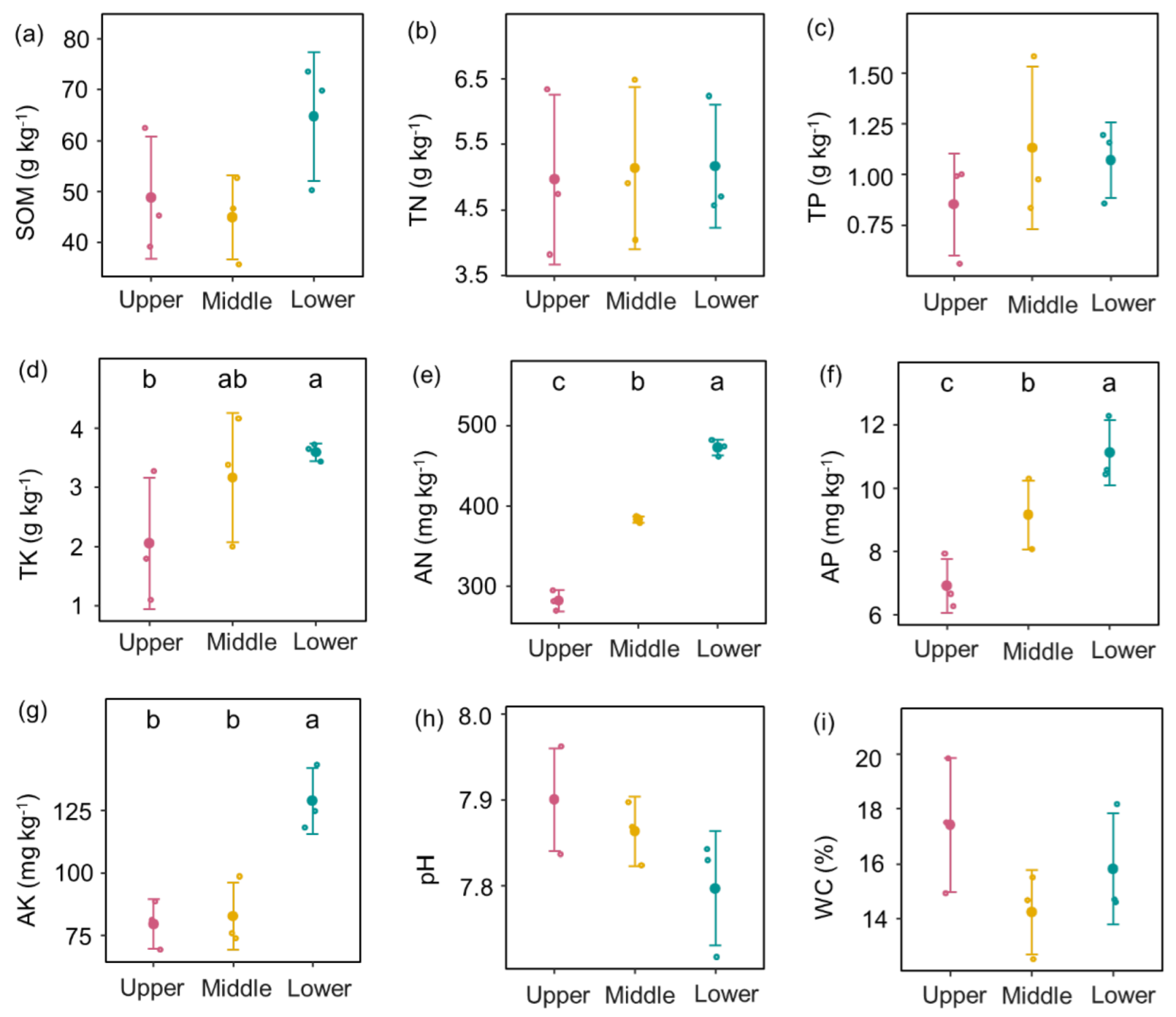
3.1. Change in Soil Properties, Plant Diversity, and Soil Diazotroph and Root AMF Diversity
3.2. Variations in Soil Diazotroph and Root AMF Community Compositions
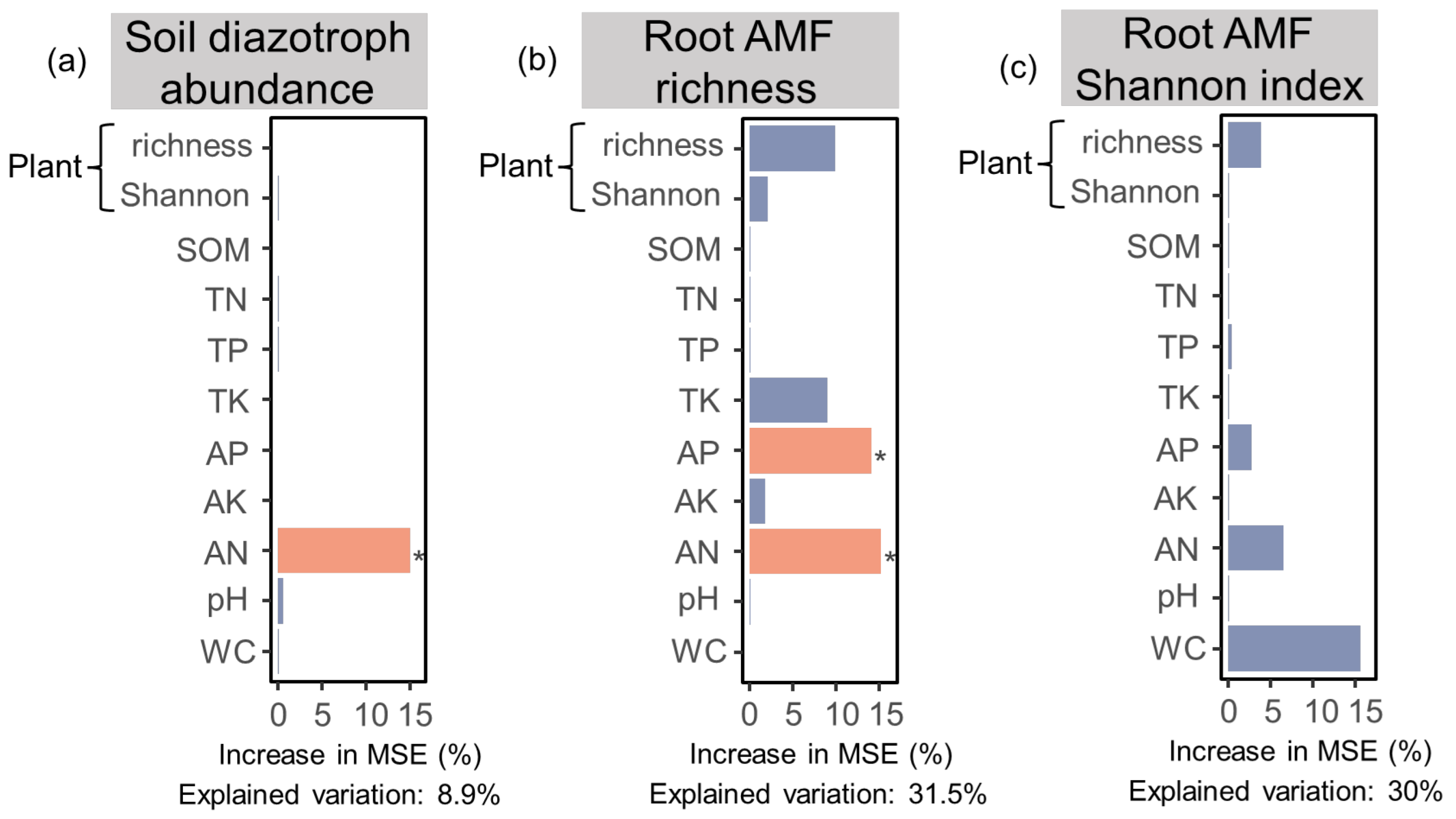
3.3. Relationships between Soil Properties, Plant Diversity, and Soil Diazotroph and Root AMF Communities
4. Discussion
4.1. Slope Position Effect on Soil Diazotroph and Root AMF Diversity
4.2. Responses of Soil Diazotroph and Root AMF Community Compositions to Slope Position
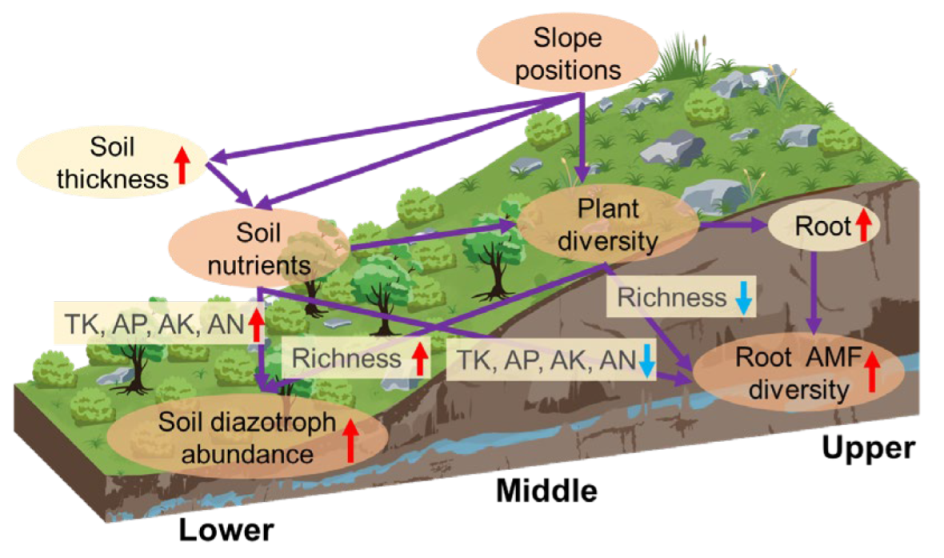
4.3. Slope Position with Different Soil Nutrients and Plant Diversity Driving Soil Diazotroph and Root AMF Properties
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.C.; Cleveland, C.C.; Townsend, A.R. Functional ecology of free-living nitrogen fixation: A contemporary perspective. Annu. Rev. Ecol. Evol. S. 2011, 42, 489–512. [Google Scholar] [CrossRef]
- Johnson, N.C.; Rowland, D.L.; Corkidi, L.; Egerton-Warburton, L.M.; Allen, E.B. Nitrogen enrichment alters mycorrhizal allocation at five mesic to semiarid grasslands. Ecology 2003, 84, 1895–1908. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Yu, H.; Liu, X.; Yang, C.; Peng, Y.; Yu, X.; Gu, H.; Zheng, X.; Wang, C.; Xiao, F.; Shu, L.; et al. Co-symbiosis of arbuscular mycorrhizal fungi (AMF) and diazotrophs promote biological nitrogen fixation in mangrove ecosystems. Soil Biol. Biochem. 2021, 161, 108382. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, G.; Luo, G.; Kong, Y.; Guo, J.; Wang, M.; Guo, S.; Ling, N.; Shen, Q. N-fertilizer-driven association between the arbuscular mycorrhizal fungal community and diazotrophic community impacts wheat yield. Agric. Ecosyst. Environ. 2018, 254, 191–201. [Google Scholar] [CrossRef]
- Mahmoudi, N.; Caeiro, M.F.; Mahdhi, M.; Tenreiro, R.; Ulm, F.; Mars, M.; Cruz, C.; Dias, T. Arbuscular mycorrhizal traits are good indicators of soil multifunctionality in drylands. Geoderma 2021, 397, 115099. [Google Scholar] [CrossRef]
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef]
- Xiao, D.; Chen, Y.; He, X.; Xu, Z.; Bai, S.H.; Zhang, W.; Cheng, M.; Hu, P.; Wang, K. Management: Temperature and precipitation significantly influence the interactions between arbuscular mycorrhizal fungi and diazotrophs in karst ecosystems. Forest Ecol. Manag. 2021, 497, 119464. [Google Scholar] [CrossRef]
- Bomfim, B.; Silva, L.C.R.; Doane, T.A.; Horwath, W.R. Interactive effects of land-use change and topography on asymbiotic nitrogen fixation in the Brazilian Atlantic Forest. Biogeochemistry 2019, 142, 137–153. [Google Scholar] [CrossRef]
- Faghihinia, M.; Zou, Y.; Chen, Z.; Bai, Y.; Li, W.; Marrs, R.; Staddon, P.L. Environmental drivers of grazing effects on arbuscular mycorrhizal fungi in grasslands. Appl. Soil Ecol. 2020, 153, 103591. [Google Scholar] [CrossRef]
- Xu, X.; Wang, X.; Cleary, M.; Wang, P.; Lu, N.; Sun, Y.; Rönnberg, J. Slope position rather than thinning intensity affects arbuscular mycorrhizal fungi (AMF) community in Chinese fir plantations. Forests 2020, 11, 273. [Google Scholar] [CrossRef]
- Li, X.; McCarty, G.W.; Karlen, D.L.; Cambardella, C.A.; Effland, W. Soil organic carbon and isotope composition response to topography and erosion in Iowa. J. Geophys. Res. Biogeosci. 2018, 123, 3649–3667. [Google Scholar] [CrossRef]
- Yu, W.; Lawrence, N.C.; Sooksa-nguan, T.; Smith, S.D.; Tenesaca, C.; Howe, A.C.; Hall, S.J. Microbial linkages to soil biogeochemical processes in a poorly drained agricultural ecosystem. Soil Biol. Biochem. 2021, 156, 108228. [Google Scholar] [CrossRef]
- Tsui, C.C.; Chen, Z.S.; Hsieh, C.F. Relationships between soil properties and slope position in a lowland rain forest of southern Taiwan. Geoderma 2004, 123, 131–142. [Google Scholar] [CrossRef]
- Rui, J.; Hu, J.; Wang, F.; Zhao, Y.; Li, C. Altitudinal niches of symbiotic, associative and free-living diazotrophs driven by soil moisture and temperature in the alpine meadow on the Tibetan Plateau. Environ. Res. 2022, 211, 113033. [Google Scholar] [CrossRef]
- Pries, C.E.H.; Sulman, B.N.; West, C.; O’Neill, C.; Poppleton, E.; Porras, R.C.; Castanha, C.; Zhu, B.; Wiedemeier, D.B.; Torn, M.S. Root litter decomposition slows with soil depth. Soil Biol. Biochem. 2018, 125, 103–114. [Google Scholar] [CrossRef]
- Bürgmann, H.; Meier, S.; Bunge, M.; Widmer, F.; Zeyer, J. Effects of model root exudates on structure and activity of a soil diazotroph community. Environ. Microbiol. 2005, 7, 1711–1724. [Google Scholar] [CrossRef]
- Liu, G.B.; Hou, X.L. Biomass and species diversity of herbosa at different position and aspects of slope in the hilly-gully region of Loess Plateau. Sci. Soil Water Conserv. 2009, 7, 67–73. [Google Scholar]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth-Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Li, S.L.; Liu, C.Q.; Chen, J.A.; Wang, S.J. Karst ecosystem and environment: Characteristics, evolution processes, and sustainable development. Agric. Ecosyst. Environ. 2021, 306, 107173. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Chen, H.; Yue, Y.; Zhang, W.; Zhang, M.; Qi, X.; Fu, Z. Karst landscapes of China: Patterns, ecosystem processes and services. Landscape Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef]
- Liang, Y.; He, X.; Chen, C.; Feng, S.; Liu, L.; Chen, X.; Zhao, Z.; Su, Y. Influence of plant communities and soil properties during natural vegetation restoration on arbuscular mycorrhizal fungal communities in a karst region. Ecol. Eng. 2015, 82, 57–65. [Google Scholar] [CrossRef]
- Xiao, D.; Che, R.; Liu, X.; Tan, Y.; Yang, R.; Zhang, W.; He, X.; Xu, Z.; Wang, K. Arbuscular mycorrhizal fungi abundance was sensitive to nitrogen addition but diversity was sensitive to phosphorus addition in karst ecosystems. Biol. Fert. Soils. 2019, 55, 457–469. [Google Scholar] [CrossRef]
- Xiao, D.; Xiao, L.; Che, R.; Tan, Y.; Liu, X.; Yang, R.; Zhang, W.; He, X.; Wang, K. Phosphorus but not nitrogen addition significantly changes diazotroph diversity and community composition in typical karst grassland soil. Agric. Ecosyst. Environ. 2020, 301, 106987. [Google Scholar] [CrossRef]
- Xiao, D.; He, X.; Zhang, W.; Cheng, M.; Hu, P.; Wang, K. Diazotroph and arbuscular mycorrhizal fungal diversity and community composition responses to karst and non-karst soils. Appl. Soil Ecol. 2022, 170, 104227. [Google Scholar] [CrossRef]
- Liu, L.; He, X.; Wang, K.; Xie, Y.; Xie, Q.; O’Donnell, A.G.; Chen, C. The Bradyrhizobium-legume symbiosis is dominant in the shrubby ecosystem of the Karst region, Southwest China. Eur. J. Soil Biol. 2015, 68, 1–8. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1996; pp. 961–1010. [Google Scholar]
- Lu, R. Methods for Soil Agro-Chemistry Analysis; China Agricultural Science and Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Kanehiro, Y.; Sherman, G.D. Fusion with sodium carbonate for total elemental analysis. In Methods of Soil Analysis, Part 2, Agronomy 9; Black, C.A., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA, 1965; pp. 952–958. [Google Scholar]
- Liaw, A.; Wiener, M. Classification and regression by random Forest. R News 2002, 2, 22. [Google Scholar]
- Van Langenhove, L.; Depaepe, T.; Vicca, S.; Berge, J.V.D.; Stahl, C.; Courtois, E.; Weedon, J.; Urbina, I.; Grau, O.; Asensio, D.; et al. Regulation of nitrogen fixation from free-living organisms in soil and leaf litter of two tropical forests of the Guiana shield. Plant Soil. 2020, 450, 93–110. [Google Scholar] [CrossRef] [PubMed]
- Doetterl, S.; Six, J.; Van Wesemael, B.; Van Oost, K. Carbon cycling in eroding landscapes: Geomorphic controls on soil organic C pool composition and C stabilization. Global Chang. Biol. 2012, 18, 2218–2232. [Google Scholar] [CrossRef]
- Weintraub, S.R.; Taylor, P.G.; Porder, S.; Cleveland, C.C.; Asner, G.P.; Townsend, A.R. Topographic controls on soil nitrogen availability in a lowland tropical forest. Ecology 2015, 96, 1561–1574. [Google Scholar] [CrossRef]
- Zhao, Y.D.; Hu, X.; Pan, P.Y. Positive feedback relationship between shrub encroachment and arbuscular mycorrhizal fungi in the Inner Mongolia grassland of northern China. Appl. Soil Ecol. 2022, 177, 104525. [Google Scholar] [CrossRef]
- Zhu, J.; Jansen-Willems, A.; Müller, C.; Dörsch, P. Topographic differences in nitrogen cycling mediate nitrogen retention in a subtropical, N-saturated forest catchment. Soil Biol. Biochem. 2021, 159, 108303. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, D.; Li, D.; Huang, L.; Wang, M.; Liao, C.; Liu, F. Variation of soil carbon accumulation across a topographic gradient in a humid subtropical mountain forest. Biogeochemistry 2020, 149, 337–354. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Liancourt, P.; Spence, L.A.; Song, D.S.; Lkhagva, A.; Sharkhuu, A.; Boldgiv, B.; Helliker, B.R.; Petraitis, P.S.; Casper, B.B. Plant response to climate change varies with topography, interactions with neighbors, and ecotype. Ecology 2013, 94, 444–453. [Google Scholar] [CrossRef]
- Tajik, S.; Ayoubi, S.; Lorenz, N. Soil microbial communities affected by vegetation, topography and soil properties in a forest ecosystem. Appl. Soil Ecol. 2020, 149, 103514. [Google Scholar] [CrossRef]
- Jackson, R.B.; Canadell, J.; Ehleringer, J.R.; Mooney, H.A.; Sala, O.; Schulze, E.D. A global analysis of root distributions for terrestrial biomes. Oecologia 1996, 108, 389–411. [Google Scholar] [CrossRef]
- Xiao, D.; Tan, Y.; Liu, X.; Yang, R.; Zhang, W.; He, X.; Xu, Z.; Wang, K. Responses of soil diazotrophs to legume species and density in a karst grassland, southwest China. Agric. Ecosyst. Environ. 2020, 288, 106707. [Google Scholar] [CrossRef]
- Chen, W.X.; Wang, E.T. Rhizobia in China; Science Press: Beijing, China, 2011. [Google Scholar]
- Oldroyd, G.E.D.; Downie, J.A. Coordinating nodule morphogenesis with rhizobial infection in legumes. Annu. Rev. Plant Biol. 2008, 59, 519–546. [Google Scholar] [CrossRef]
- Dodds, W.K.; Gudder, D.A.; Mollenhauer, D. The ecology of Nostoc. J. Phycol. 1995, 31, 2–18. [Google Scholar] [CrossRef]
- Che, R.; Deng, Y.; Wang, F.; Wang, W.; Xu, Z.; Hao, Y.; Xue, K.; Zhang, B.; Tang, L.; Zhou, H.; et al. Autotrophic and symbiotic diazotrophs dominate nitrogen-fixing communities in Tibetan grassland soils. Sci. Total Environ. 2018, 639, 997–1006. [Google Scholar] [CrossRef]
- Zhang, L.; Unteregelsbacher, S.; Hafner, S.; Xu, X.; Schleuss, P.M.; Miehe, G.; Kuzyakov, Y. Fate of organic and inorganic nitrogen in crusted and non-crusted Kobresia grasslands. Land Degrad. Dev. 2017, 28, 166–174. [Google Scholar] [CrossRef]
- Strom, N.; Hu, W.; Haarith, D.; Chen, S.; Bushley, K. Interactions between soil properties, fungal communities, the soybean cyst nematode, and crop yield under continuous corn and soybean monoculture. Appl. Soil Ecol. 2020, 147, 103388. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Li, D.; Xu, C.; Xiang, X. Differential responses of arbuscular mycorrhizal fungal communities to mineral and organic fertilization. MicrobiologyOpen 2020, 9, e00920. [Google Scholar] [CrossRef]
- Sánchez-Castro, I.; Gianinazzi-Pearson, V.; Cleyet-Marel, J.; Baudoin, E.; van Tuinen, D. Glomeromycota communities survive extreme levels of metal toxicity in an orphan mining site. Sci. Total Environ. 2017, 598, 121–128. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Pan, F.; Li, D.; Chen, H.; Wang, K. Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in southwest China. Plant Soil. 2015, 391, 77–91. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bakker, R.; Verwaal, J.; Scheublin, T.R.; Rutten, M.; Van Logtestijn, R.; Staehelin, C. Symbiotic bacteria as a determinant of plant community structure and plant productivity in dune grassland. FEMS Microbiol. Ecol. 2006, 56, 178–187. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Shen, Z.; Rui, J.; Jin, D.; Li, J.; Li, X. Community assemblage of free-living diazotrophs along the elevational gradient of Mount Gongga. Soil Ecol. Lett. 2019, 1, 136–146. [Google Scholar] [CrossRef]
- Fabianska, I.; Sosa-Lopez, E.; Bucher, M. The role of nutrient balance in shaping plant root-fungal interactions: Facts and speculation. Curr. Opin. Microbiol. 2019, 49, 90–96. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, D.; Hong, T.; Chen, M.; He, X.; Wang, K. Assessing the Effect of Slope Position on the Community Assemblage of Soil Diazotrophs and Root Arbuscular Mycorrhizal Fungi. J. Fungi 2023, 9, 394. https://doi.org/10.3390/jof9040394
Xiao D, Hong T, Chen M, He X, Wang K. Assessing the Effect of Slope Position on the Community Assemblage of Soil Diazotrophs and Root Arbuscular Mycorrhizal Fungi. Journal of Fungi. 2023; 9(4):394. https://doi.org/10.3390/jof9040394
Chicago/Turabian StyleXiao, Dan, Tao Hong, Meifeng Chen, Xunyang He, and Kelin Wang. 2023. "Assessing the Effect of Slope Position on the Community Assemblage of Soil Diazotrophs and Root Arbuscular Mycorrhizal Fungi" Journal of Fungi 9, no. 4: 394. https://doi.org/10.3390/jof9040394
APA StyleXiao, D., Hong, T., Chen, M., He, X., & Wang, K. (2023). Assessing the Effect of Slope Position on the Community Assemblage of Soil Diazotrophs and Root Arbuscular Mycorrhizal Fungi. Journal of Fungi, 9(4), 394. https://doi.org/10.3390/jof9040394






