Effects of Carbon, Nitrogen, Ambient pH and Light on Mycelial Growth, Sporulation, Sorbicillinoid Biosynthesis and Related Gene Expression in Ustilaginoidea virens
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains, Plasmids and Culture Conditions
2.2. Effects of Carbon and Nitrogen Sources, Ambient pH, and Light Exposure
2.3. Mycelial Growth and Sporulation Assessments
2.4. Sorbicillinoid Production Determination
2.5. RNA Preparation, RT–qPCR and Sorbicillinoid BGC Genes Analyses
2.6. Gene Deletion and Phenotyping
2.7. Statistical Analysis
3. Results
3.1. Effects of Carbon Sources on Mycelial Growth, Sporulation and Sorbicillinoid Production
3.2. Effects of Nitrogen Sources on Mycelial Growth, Sporulation and Sorbicillinoid Production
3.3. Effects of Ambient pH on Mycelial Growth, Sporulation and Sorbicillinoid Production
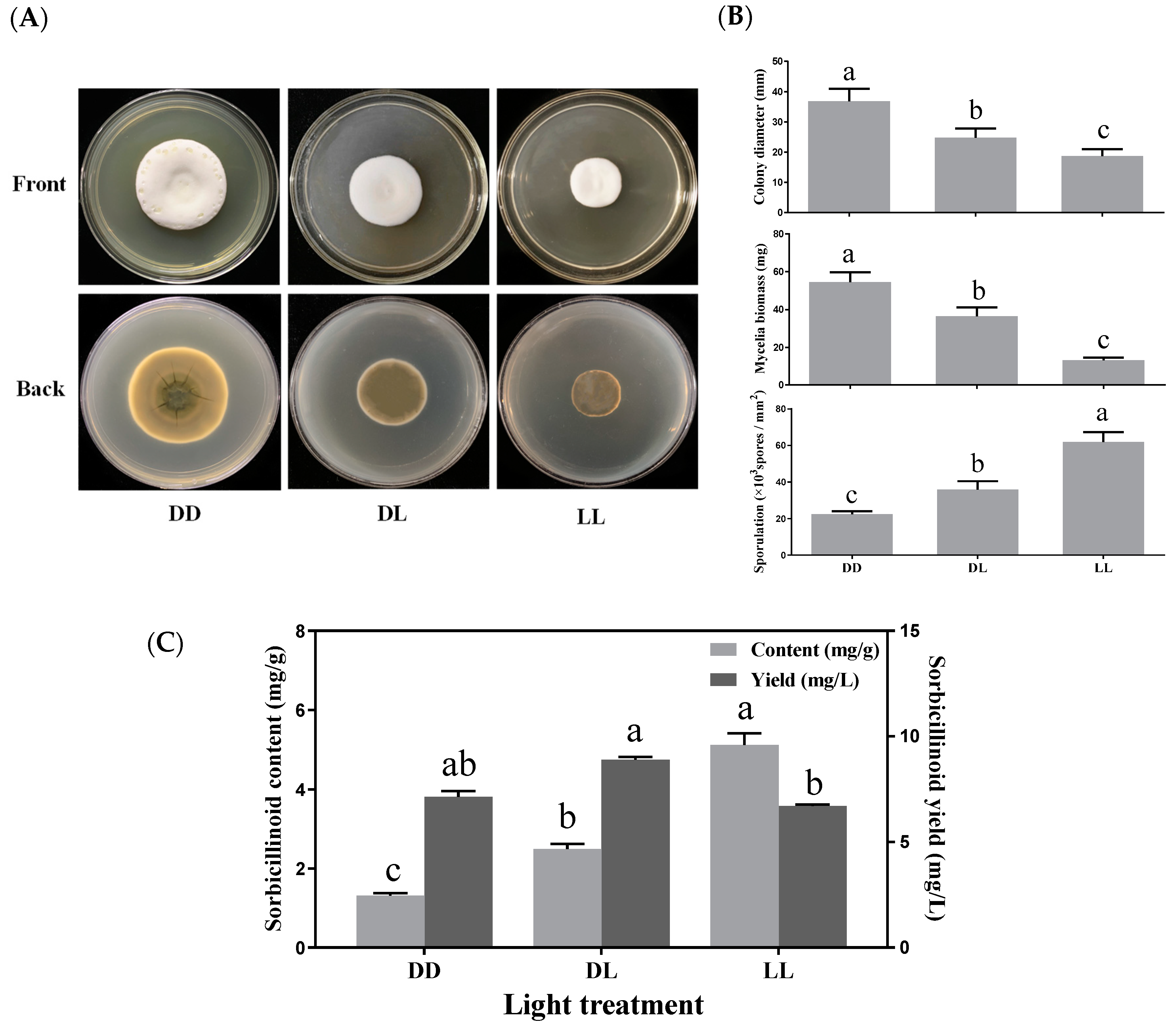
3.4. Effects of Light Exposure on Mycelial Growth, Sporulation and Sorbicillinoid Production
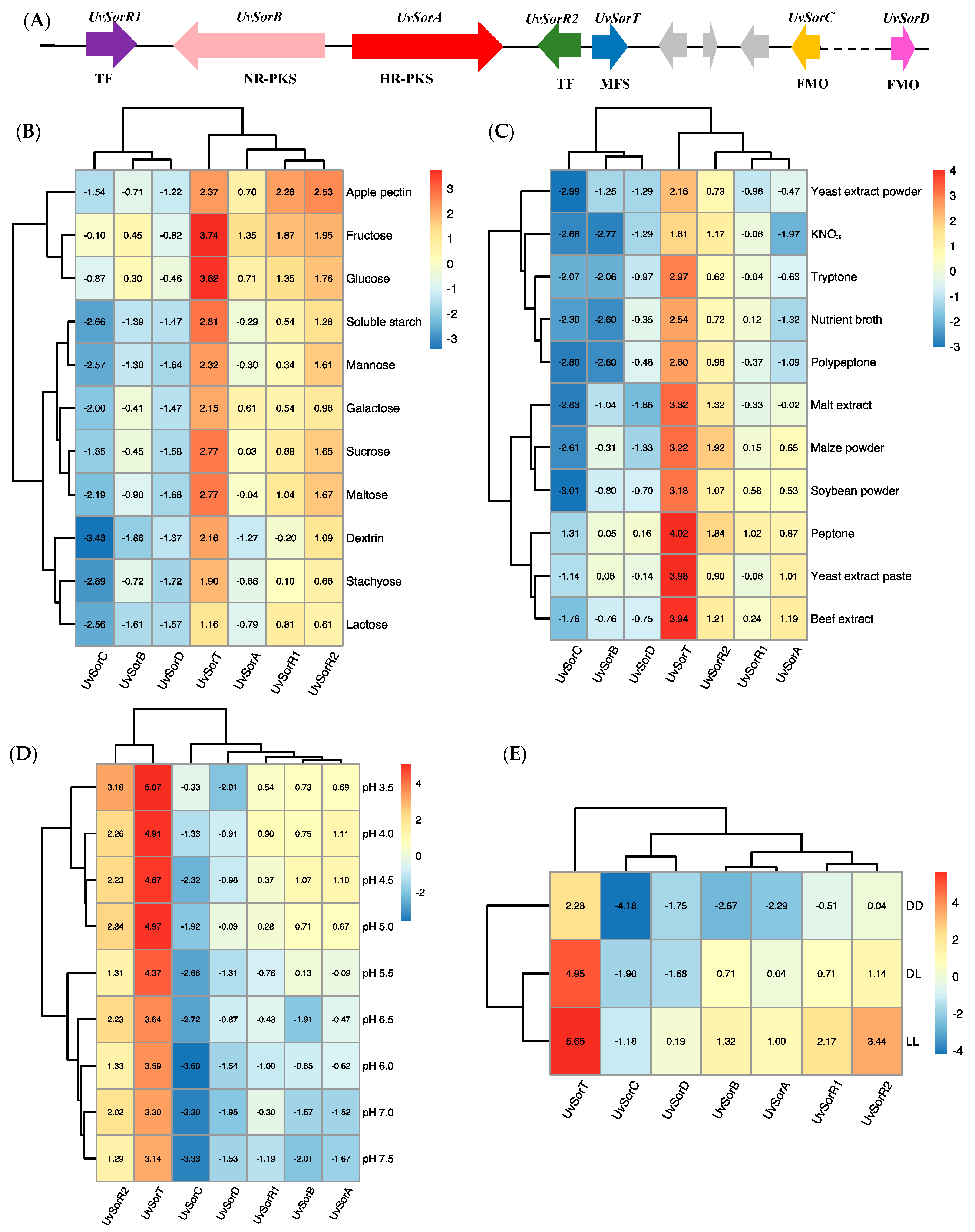
3.5. Relative Transcripts Levels of the Genes Involved in Sorbicillinoid Biosynthesis in Response to the Environmental Factors
3.6. Two Pathway-Specific Transcription Factor Genes UvSorR1 and UvSorR2 Involved in the Regulation of Sorbicillinoid Biosynthesis
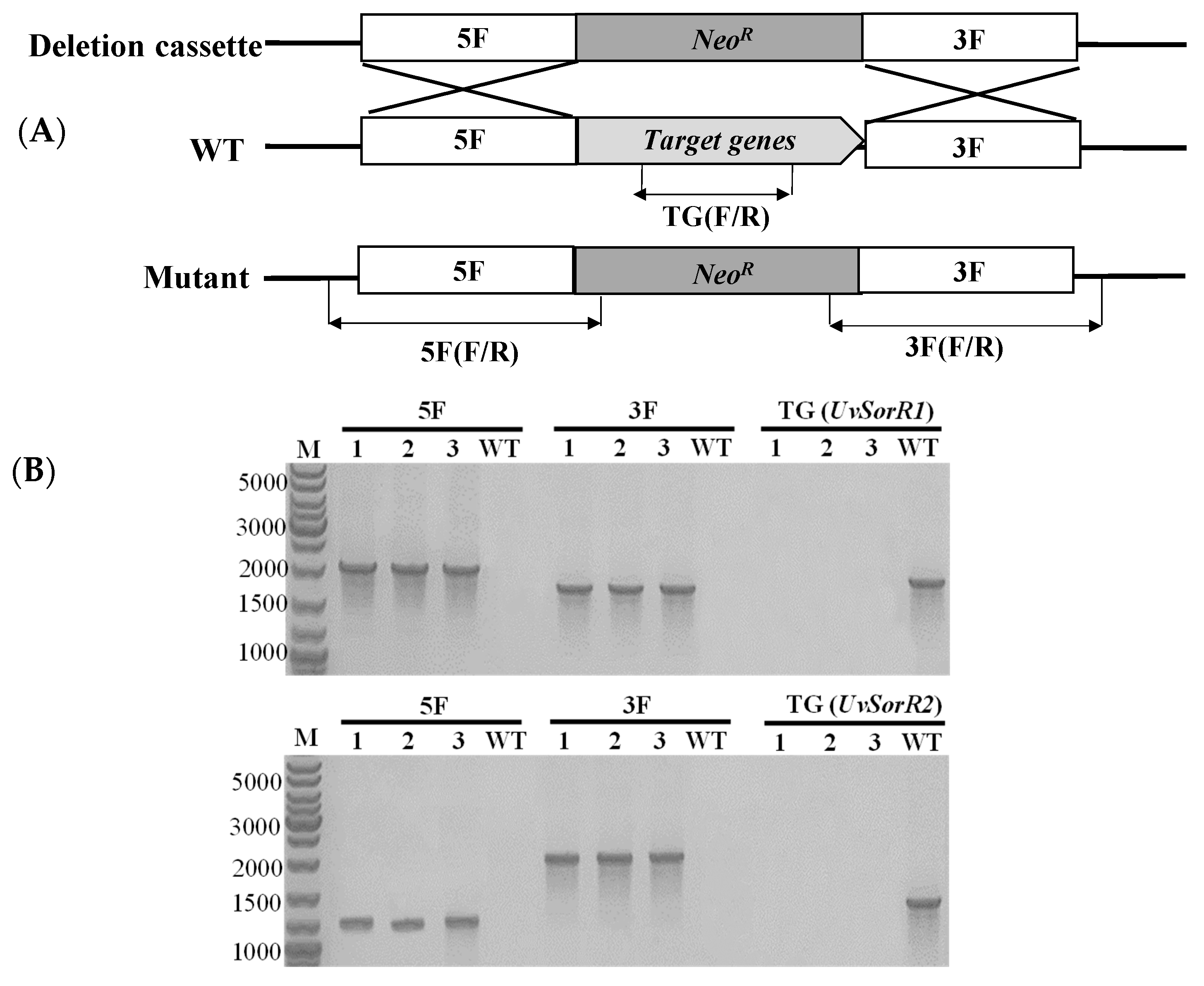
3.6.1. Deletion of Transcription Factor Genes UvSorR1 and UvSorR2
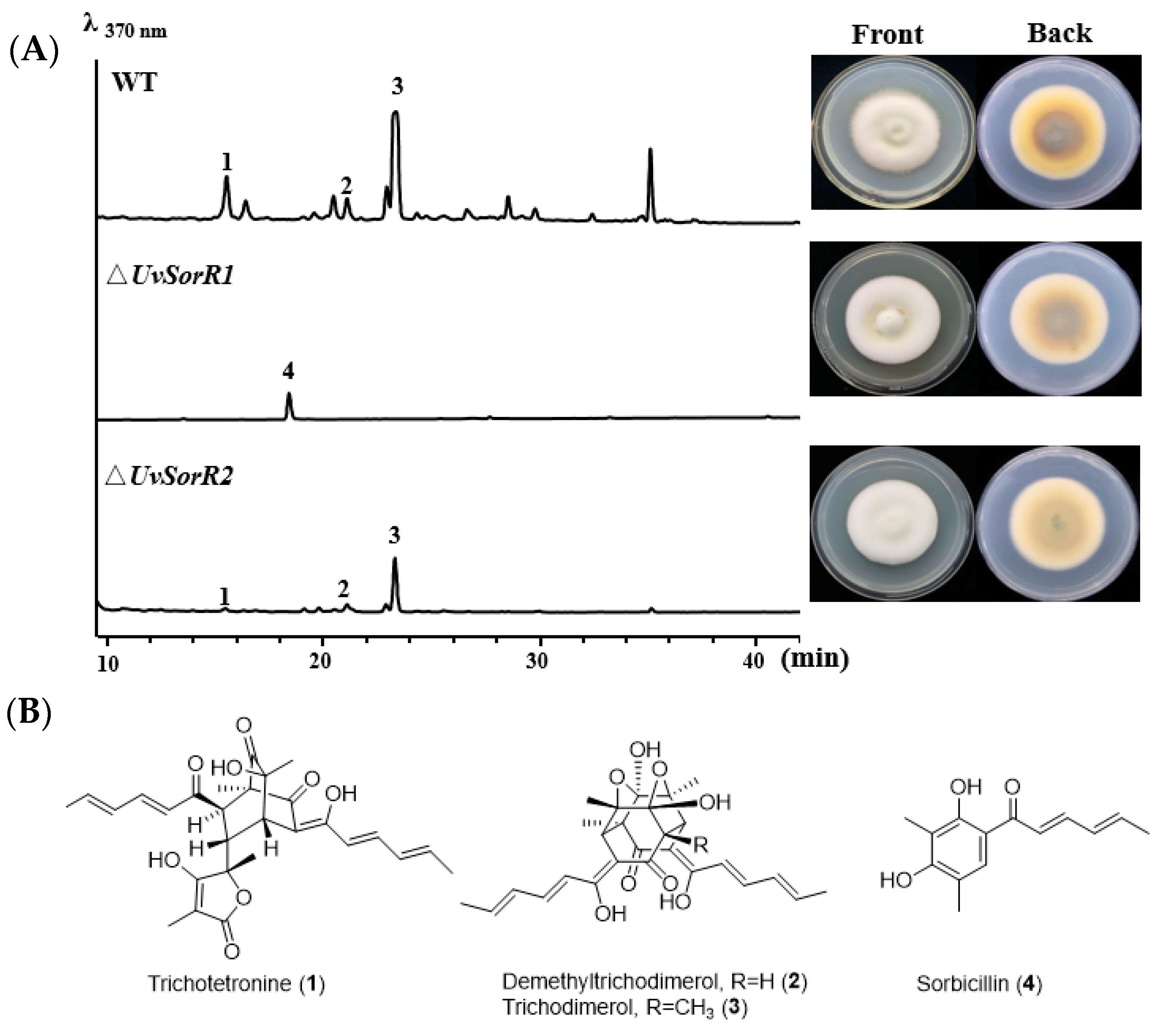
3.6.2. Regulation of UvSorR1 and UvSorR2 Involved in Sorbicillinoid Biosynthesis
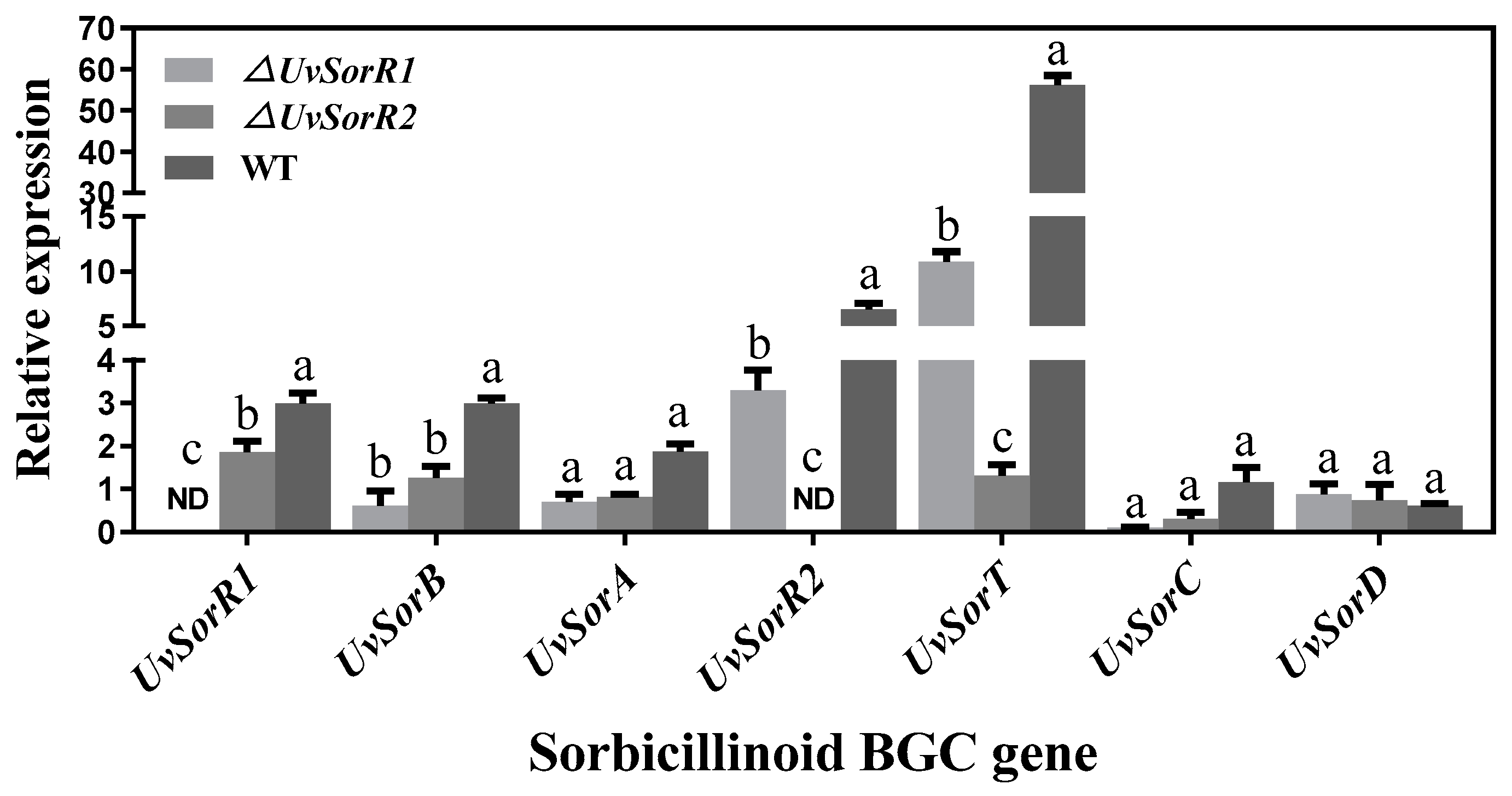
3.6.3. UvSorR1 and UvSorR2 Regulated Sorbicillinoid Biosynthesis at Transcriptional Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, J.; Yang, J.; Wang, Y.-Q.; Li, G.-B.; Li, Y.; Huang, F.; Wang, W.-M. Current understanding on Villosiclava virens, a unique flower-infecting fungus causing rice false smut disease. Mol. Plant Pathol. 2016, 17, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Meng, S.; Deng, Y.; Huang, S.; Kou, Y. Ustilaginoidea virens: A fungus infects rice flower and threats world rice production. Rice Sci. 2019, 26, 199–206. [Google Scholar]
- Sun, W.; Fan, J.; Fang, A.; Li, Y.; Tariqjaveed, M.; Li, D.; Hu, D.; Wang, W.-M. Ustilaginoidea virens: Insights into an emerging rice pathogen. Annu. Rev. Phytopathol. 2020, 58, 363–385. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, S.; Shan, T.; Wang, P.; Sun, W.; Chen, Z.; Wang, S. Chemistry and biology of mycotoxins from rice false smut pathogen. In Mycotoxins: Properties, Applications and Hazards; Melborn, B.J., Greene, J.C., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 109–130. [Google Scholar]
- Lu, S.; Sun, W.; Meng, J.; Wang, A.; Wang, X.; Tian, J.; Fu, X.; Dai, J.; Liu, Y.; Lai, D.; et al. Bioactive bis-naphtho-γ-pyrones from rice false smut pathogen Ustilaginoidea virens. J. Agric. Food Chem. 2015, 63, 3501–3508. [Google Scholar] [CrossRef]
- Meng, J.; Sun, W.; Mao, Z.; Xu, D.; Wang, X.; Lu, S.; Lai, D.; Liu, Y.; Zhou, L.; Zhang, G. Main ustilaginoidins and their distribution in rice false smut balls. Toxins 2015, 7, 4023–4034. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, X.; Lin, F.; Sun, W.; Meng, J.; Wang, A.; Lai, D.; Zhou, L.; Liu, Y. The contents of ustiloxins A and B along with their distribution in rice false smut balls. Toxins 2016, 8, 262. [Google Scholar] [CrossRef]
- Sun, W.; Wang, A.; Xu, D.; Wang, W.; Meng, J.; Dai, J.; Liu, Y.; Lai, D.; Zhou, L. New ustilaginoidins from rice false smut balls caused by Villosiclava virens and their phytotoxic and cytotoxic activities. J. Agric. Food Chem. 2017, 65, 5151–5160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Lai, D.; Wang, W.; Dai, J.; Zhou, L.; Liu, Y. Ustiloxin G, a new cyclopeptide mycotoxin from rice false smut balls. Toxins 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Lai, D.; Meng, J.; Zhang, X.; Xu, D.; Dai, J.; Zhou, L. Ustilobisorbicillinol A, a cytotoxic sorbyl-containing aromatic polyketide from Ustilaginoidea virens. Org. Lett. 2019, 21, 1311–1314. [Google Scholar] [CrossRef]
- Meng, J.; Gu, G.; Dang, P.; Zhang, X.; Wang, W.; Dai, J.; Liu, Y.; Lai, D.; Zhou, L. Sorbicillinoids from the fungus Ustilaginoidea virens and their phytotoxic, cytotoxic, and antimicrobial activities. Front. Chem. 2019, 7, 435. [Google Scholar] [CrossRef]
- Meng, J.; Zhao, S.; Dang, P.; Zhou, Z.; Lai, D.; Zhou, L. Ustilaginoidin M1, a new bis-naphtho-γ-pyrone from the fungus Villosiclava virens. Nat. Prod. Res. 2021, 35, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Harned, A.M.; Volp, K.A. The sorbicillinoid family of natural products: Isolation, biosynthesis, and synthetic studies. Nat. Prod. Rep. 2011, 28, 1790–1810. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, X.; Xu, D.; Zhang, X.; Lai, D.; Zhou, L.; Zhang, G. Sorbicillinoids from fungi and their bioactivities. Molecules 2016, 21, 715. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, X.; Xue, M.; Zhao, Z.; Zhang, H.; Xu, D.; Lai, D.; Zhou, L. Recent advances in sorbicillinoids from fungi and their bioactivities (covering 2016–2021). J. Fungi 2022, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhu, T.; Li, Y.; Cai, S.; Zhao, B.; Gu, Q. Cytotoxic sorbicillinoids and bisorbicillinoids from a marine-derived fungus Trichoderma sp. Chem. Pharm. Bull. 2009, 57, 220–223. [Google Scholar] [CrossRef]
- Kawahara, T.; Takagi, M.; Shin-ya, K. JBIR-124: A novel antioxidative agent from a marine sponge-derived fungus Penicillium citrinum SpI080624G1f01. J. Antibiot. 2012, 65, 45–47. [Google Scholar] [CrossRef]
- Chen, G.; Chu, J. Characterization of two polyketide synthases involved in sorbicillinoid biosynthesis by Acremonium chrysogenum using the CRISPR/Cas9 System. Appl. Biochem. Biotechnol. 2019, 188, 1134–1144. [Google Scholar] [CrossRef]
- Kahlert, L.; Bassiony, E.F.; Cox, R.J.; Skellam, E.J. Diels–Alder reactions during the biosynthesis of sorbicillinoids. Angew. Chem. Int. Ed. 2020, 59, 5816–5822. [Google Scholar] [CrossRef]
- Kahlert, L.; Cox, R.J.; Skellam, E. The same but different: Multiple functions of the fungal flavin dependent monooxygenase SorD from Penicillium chrysogenum. Chem. Commun. 2020, 56, 10934. [Google Scholar] [CrossRef]
- Hinterdobler, W.; Bier, S.; Monroy, A.A.; Berger, H.; Dattenbock, C.; Schmoll, M. The G-protein coupled receptor GPR8 regulates secondary metabolism in Trichoderma reesei. Front. Bioeng. Biotechnol. 2020, 8, 558996. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, D.; Hou, X.; Wei, P.; Fu, J.; Zhao, Z.; Jing, M.; Lai, D.; Yin, W.; Zhou, L. UvSorA and UvSorB involved in sorbicillinoid biosynthesis contribute to fungal development, stress response and phytotoxicity in Ustilaginoidea virens. Int. J. Mol. Sci. 2022, 23, 11056. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2013, 11, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Assaf, C.E.H.; Zetina-Serrano, C.; Tahtah, N.; Khoury, A.E.; Atoui, A.; Oswald, I.P.; Puel, O.; Lorber, S. Regulation of secondary metabolism in the Penicillium genus. Int. J. Mol. Sci. 2020, 21, 9462. [Google Scholar] [CrossRef]
- Xue, M.; Hou, X.; Fu, J.; Zhang, J.; Wang, J.; Zhao, Z.; Xu, D.; Lai, D.; Zhou, L. Recent advances in search of bioactive secondary metabolites from fungi triggered by chemical epigenetic modifiers. J. Fungi 2023, 9, 172. [Google Scholar] [CrossRef] [PubMed]
- Gelsen, R. Molecular monitoring of environmental conditions influencing the induction of ochratoxin A biosynthesis genes in Penicillium nordicum. Mol. Nutr. Food Res. 2004, 48, 532–540. [Google Scholar]
- Jiao, F.; Kawakami, A.; Nakajima, T. Effects of different carbon sources on trichothecene productionand Tri gene expression by Fusarium graminearum in liquid culture. FEMS Microbiol. Lett. 2008, 285, 212–219. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Magan, N.; Geisen, R. Stress induction of mycotoxin biosynthesis genes by abiotic factors. FEMS Microbiol. Lett. 2008, 284, 142–149. [Google Scholar] [CrossRef]
- Penalva, M.A.; Arst, H.N. Recent Advances in the characterization of ambient pH regulation of gene expression in filamentous fungi yeasts. Annu. Rev. Microbiol. 2004, 58, 425–451. [Google Scholar] [CrossRef]
- Penalva, M.A.; Tilburn, J.; Bignell, E.; Arst, H.N. Ambient pH gene regulation in fungi: Making connections. Trends Microbiol. 2008, 16, 291–300. [Google Scholar] [CrossRef]
- Bayram, O.; Braus, G.H. Coordination of secondary metabolism and development in fungi: The velvet family of regulatory proteins. FEMS Microbiol. Rev. 2012, 36, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Cepeda-Garcia, C.; Domminguez-Santos, R.; Garcia-Rico, R.O.; Garcia-Estrada, C.; Cajiao, A.; Fierro, F.; Mrtin, J.F. Direct involvement of the CreA transcription factor in penicillin biosynthesis and expression of the pcbAB gene in Penicillium chrysogenum. Appl. Microbiol. Biotechnol. 2014, 98, 7113–7124. [Google Scholar] [CrossRef] [PubMed]
- Tudzynski, B. Nitrogen regulation of fungal secondary metabolism in fungi. Front. Microbiol. 2014, 5, 656. [Google Scholar] [CrossRef] [PubMed]
- Todd, R.B.; Andrianopoulos, A. Evolution of a fungal regulatory gene family: The Zn(II)2Cys6 binuclear cluster DNA binding motif. Fungal Genet. Biol. 1997, 21, 388–405. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, S.; Larochelle, M.; Turcotte, B. A fungal family of transcriptional regulators: The zinc cluster proteins. Microbiol. Mol. Biol. Rev. 2006, 70, 583–604. [Google Scholar] [CrossRef]
- Yang, K.; Tian, J.; Keller, N.P. Post-translational modifications drive secondary metabolite biosynthesis in Aspergillus: A review. Environ. Microbiol. 2022, 24, 2857–2881. [Google Scholar] [CrossRef]
- Ferrara, M.; Perrone, G.; Gallo, A. Recent advances in biosynthesis and regulatory mechanisms of principal mycotoxins. Curr. Opin. Food Sci. 2022, 48, 100923. [Google Scholar] [CrossRef]
- Guzman-Chavez, F.; Salo, O.; Nygard, Y.; Lankhorst, P.P.; Bovenberg, R.A.L.; Driessen, A.J.M. Mechanism and regulation of sorbicillin biosynthesis by Penicillium chrysogenum. Microb. Biotechnol. 2017, 10, 958–968. [Google Scholar] [CrossRef]
- Guzman-Chavez, F.; Salo, O.; Samol, M.; Ries, M.; Kuipers, J.; Bovenberg, R.A.L.; Vreeken, R.J.; Driessen, A.J.M. Deregulation of secondary metabolism in a histone deacetylase mutant of Penicillium chrysogenum. MicrobiologyOpen. 2018, 7, e00598. [Google Scholar] [CrossRef]
- Yu, J.; Han, H.; Zhang, X.; Ma, C.; Sun, C.; Che, Q.; Gu, Q.; Zhu, T.; Zhang, G.; Li, D. Discovery of two new sorbicillinoids by overexpression of the global regulator LaeA in a marine-derived fungus Penicillium dipodomyis YJ-11. Mar. Drugs 2019, 17, 446. [Google Scholar] [CrossRef]
- Seiboth, B.; Karimi, R.A.; Phatale, P.A.; Linke, R.; Hartl, L.; Sauer, D.G.; Smith, K.M.; Baker, S.E.; Freitag, M.; Kubicek, C.P. The putative protein methyltransferase LAE1 controls cellulase gene expression in Trichoderma reesei. Mol. Microbiol. 2012, 84, 1150–1164. [Google Scholar] [CrossRef] [PubMed]
- Derntl, C.; Rassinger, A.; Srebotnik, E.; Mach, R.L.; Mach-Algner, A.R. Identification of the main regulator responsible for synthesis of the typical yellow pigment produced by Trichoderma reesei. Appl. Environ. Microbiol. 2016, 82, 6247–6257. [Google Scholar] [CrossRef] [PubMed]
- Monroy, A.A.; Stappler, E.; Schuster, A.; Sulyok, M.; Schmoll, M. A CRE1- regulated cluster is responsible for light dependent production of dihydrotrichotetronin in Trichoderma reesei. PLoS ONE 2017, 12, e0182530. [Google Scholar] [CrossRef] [PubMed]
- Hitzenharmmer, E.; Busch, C.; Sulyok, M.; Schunhmacher, R.; Kluger, B.; Wischnitzki, E.; Scholl, M. YPR2 is a regulator of light modulated carbon and secondary metabolism in Trichoderma reesei. BMC Genom. 2019, 20, 211. [Google Scholar]
- Hinterdobler, W.; Schuster, A.; Tisch, D.; Ozkan, E.; Bazafkan, H.; Schinnerl, J.; Brecker, L.; Bohmdorfer, S.; Schmoll, M. The role of PKAc1 in gene regulation and trichodimerol production in Trichoderma reesei. Fungal Biol. Biotechnol. 2019, 6, 12. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, R.; Zheng, F.; Meng, X.; Zhang, W.; Liu, W. Dual regulatory role of chromatin remodeler ISW1 in coordinating cellulase and secondary metabolite biosynthesis in Trichoderma reesei. mBio 2022, 13, e03456-21. [Google Scholar] [CrossRef]
- Li, N.; Chen, Y.; Shen, Y.; Wang, W. Roles of PKAc1 and CRE1 in cellulose degradation, conidiation, and yellow pigment synthesis in Trichoderma reesei QM6a. Biotechnol. Lett. 2022, 44, 1465–1475. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Li, X.; Lyu, X.; Liu, Z.; Zhao, H.; Jiao, X.; Zhang, W.; Xie, J.; Liu, W. A histone H3K9 methyltransferase Dim5 mediates repression of sorbicillinoid biosynthesis in Trichoderma reesei. Microb. Biotechnol. 2022, 15, 2533–2546. [Google Scholar] [CrossRef]
- Zong, Y.; Li, B.; Tian, S. Effects of carbon, nitrogen and ambient pH on patulin production and related gene expression in Penicillium expansum. Int. J. Food Microbiol. 2015, 206, 102–108. [Google Scholar] [CrossRef]
- Barda, O.; Maor, U.; Sadhasivam, S.; Bi, Y.; Zakin, V.; Prusky, D.; Sionov, E. The pH-responsive transcription factor PacC governs pathogenicity and ochratoxin A biosynthesis in Aspergillus carbonarius. Front. Microbiol. 2020, 11, 210. [Google Scholar] [CrossRef]
- Zou, M.; Xin, B.; Sun, X.; Lin, R.; Lu, J.; Qi, J.; Xie, B.; Cheng, X. URA3 as a selectable marker for disruption and functional assessment of PacC gene in the entomopathogenic fungus Isaria javanica. J. Fungi 2023, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, Y.; Zhang, Q.; Zhang, X.; Shen, D.; Yu, J.; Yu, M.; Pan, X.; Cao, H.; Yong, M.; et al. The N-terminus of an Ustilaginoidea virens Ser-Thr-rich glycosylphosphatidylinositol-anchored protein elicits plant immunity as a MAMP. Nat. Commun. 2021, 12, 2451. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Fang, A.; Zheng, X.; Wang, S.; Wang, J.; Fan, J.; Song, Z.; Gao, H.; Yang, J.; Zeng, Q.; et al. Ustilaginoidea virens nuclear effector SCRE4 suppresses rice immunity via inhibiting expression of a positive immune regulator OsARF17. Int. J. Mol. Sci. 2022, 23, 10527. [Google Scholar] [CrossRef]
- Gu, Z.; Ding, Z.; Chen, X.; Guo, L.; Zeng, D.; Qian, Q.; Ma, B. Reference gene selection of Ustilaginoidea virens by real-time PCR. Chin. J. Rice Sci. 2012, 26, 615–618. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, Y.; Han, Y.; Xu, J.-R.; Wang, C. HvHOG1 is important for hyphal growth and stress responses in the rice false smut fungus Ustilaginoidea virens. Sci. Rep. 2016, 6, 24824. [Google Scholar] [CrossRef]
- Liang, Y.; Han, Y.; Wang, C.; Jiang, C.; Xu, J.-R. Targeted deletion of the USTA and UvSLT2 genes efficiently in Ustilaginoidea virens with the CRISPR-Cas9 system. Front. Plant Sci. 2018, 9, 699. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Li, G.-B.; Gong, Z.-Y.; Li, Y.; Huang, F.; Fan, J.; Wang, W.-M. Stachyose is a preferential carbon source utilized by the rice false smut pathogen, Villosiclava virens. Physiol. Mol. Plant Pathol. 2016, 96, 69–76. [Google Scholar] [CrossRef]
- Fu, R.; Yin, C.; Liu, Y.; Ding, L.; Zhu, J.; Zheng, A.; Li, P. The influence of nutrient and environmental factors on mycelium growth and conidium of false smut Villosiclava virens. Afr. J. Microbiol. Res. 2013, 7, 825–833. [Google Scholar]
- Kumar, S.N.; Devi, G.U.; Jagadeeshwar, R.; Ladhalakshmi, D. Physiological and viability studies of pseudomorphs of false smut of rice caused by Ustilaginoidea virens. Res. J. Agric. Sci. 2015, 6, 697–699. [Google Scholar]
- Keller, N.P.; Nesbitt, C.; Sarr, B.; Phillips, T.D.; Burow, G.B. pH regulation of sterigmatocystin and aflatoxin biosynthesis in Aspergillus spp. Phytopathology 1997, 87, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Merhej, J.; Rihard-Forget, F.; Barreau, C. The pH regulatory factor Pac1 regulates Tri gene expression and trichothecene production in Fusarium graminearum. Fungal Genet. Biol. 2011, 48, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lin, F.; Sun, W.; Yuan, S.; Zhou, Z.; Wu, F.-G.; Chen, Z. Constitutive hyperproduction of sorbicillinoids in Trichoderma reesei ZC121. Biotechnol. Biofuels 2018, 11, 291. [Google Scholar] [CrossRef]
- Fasoyin, O.E.; Wang, B.; Qiu, M.; Han, X.; Chung, K.-R.; Wang, S. Carbon catabolite repression gene creA regulates morphology, aflatoxin biosynthesis and virulence in Aspergillus flavus. Fungal Genet. Biol. 2018, 115, 41–51. [Google Scholar] [CrossRef]
- Ruiz-Villafan, B.; Cruz-Bautista, R.; Manzo-Ruiz, M.; Passari, A.K.; Villarreal-Gomez, K.; Rodriguez-Sanoja, R.; Sanchez, S. Carbon catabolite regulation of secondary metabolite formation, an old but not well-established regulatory system. Microb. Biotechnol. 2022, 15, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Ries, L.N.A.; Beattie, S.R.; Espeso, E.A.; Cramer, R.A.; Goldman, G.H. Diverse regulation of the CreA carbon catabolite repressor in Aspergillus nidulans. Genetics 2016, 203, 335–352. [Google Scholar] [CrossRef]
- Jose de Assis, L.; Silva, L.P.; Bayram, O.; Dowling, P.; Kniemeyer, O.; Kruger, T.; Brakhage, A.A.; Chen, Y.Y.; Dong, L.; Tan, K.; et al. Carbon catabolite repression in filamentous fungi is regulated by phosphorylation of the transcription factor CreA. mBio 2021, 12, e03146-20. [Google Scholar] [CrossRef]
- Yu, M.; Yu, J.; Cao, H.; Pan, X.; Song, T.; Qi, Z.; Du, Y.; Huang, S.; Liu, Y. The velvet protein UvVEA regulates conidiation and chlamydospore formation in Ustilaginoidea virens. J. Fungi 2022, 8, 479. [Google Scholar] [CrossRef]
- Derntl, C.; Kluger, B.; Bueschl, C.; Schuhmacher, R.; Mach, R.L.; Mach-Aigner, A.R. Transcription factor Xpp1 is a switch between primary and secondary fungal metabolism. Proc. Natl. Acad. Sci. USA 2017, 114, E560–E569. [Google Scholar] [CrossRef]
- Zhou, X.; Li, G.; Xu, J.-R. Efficient approaches for generating GFP fusion and epitope-tagging constructs in filamentous fungi. Methods Mol. Biol. 2011, 722, 199–212. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Hou, X.; Xu, D.; Xue, M.; Zhang, J.; Wang, J.; Yang, Y.; Lai, D.; Zhou, L. Effects of Carbon, Nitrogen, Ambient pH and Light on Mycelial Growth, Sporulation, Sorbicillinoid Biosynthesis and Related Gene Expression in Ustilaginoidea virens. J. Fungi 2023, 9, 390. https://doi.org/10.3390/jof9040390
Zhang X, Hou X, Xu D, Xue M, Zhang J, Wang J, Yang Y, Lai D, Zhou L. Effects of Carbon, Nitrogen, Ambient pH and Light on Mycelial Growth, Sporulation, Sorbicillinoid Biosynthesis and Related Gene Expression in Ustilaginoidea virens. Journal of Fungi. 2023; 9(4):390. https://doi.org/10.3390/jof9040390
Chicago/Turabian StyleZhang, Xuping, Xuwen Hou, Dan Xu, Mengyao Xue, Jiayin Zhang, Jiacheng Wang, Yonglin Yang, Daowan Lai, and Ligang Zhou. 2023. "Effects of Carbon, Nitrogen, Ambient pH and Light on Mycelial Growth, Sporulation, Sorbicillinoid Biosynthesis and Related Gene Expression in Ustilaginoidea virens" Journal of Fungi 9, no. 4: 390. https://doi.org/10.3390/jof9040390
APA StyleZhang, X., Hou, X., Xu, D., Xue, M., Zhang, J., Wang, J., Yang, Y., Lai, D., & Zhou, L. (2023). Effects of Carbon, Nitrogen, Ambient pH and Light on Mycelial Growth, Sporulation, Sorbicillinoid Biosynthesis and Related Gene Expression in Ustilaginoidea virens. Journal of Fungi, 9(4), 390. https://doi.org/10.3390/jof9040390






