Chemistry in Fungal Bioluminescence: Theoretical Studies on Biosynthesis of Luciferin from Caffeic Acid and Regeneration of Caffeic Acid from Oxidized Luciferin
Abstract
1. Introduction
2. Computational Model and Method
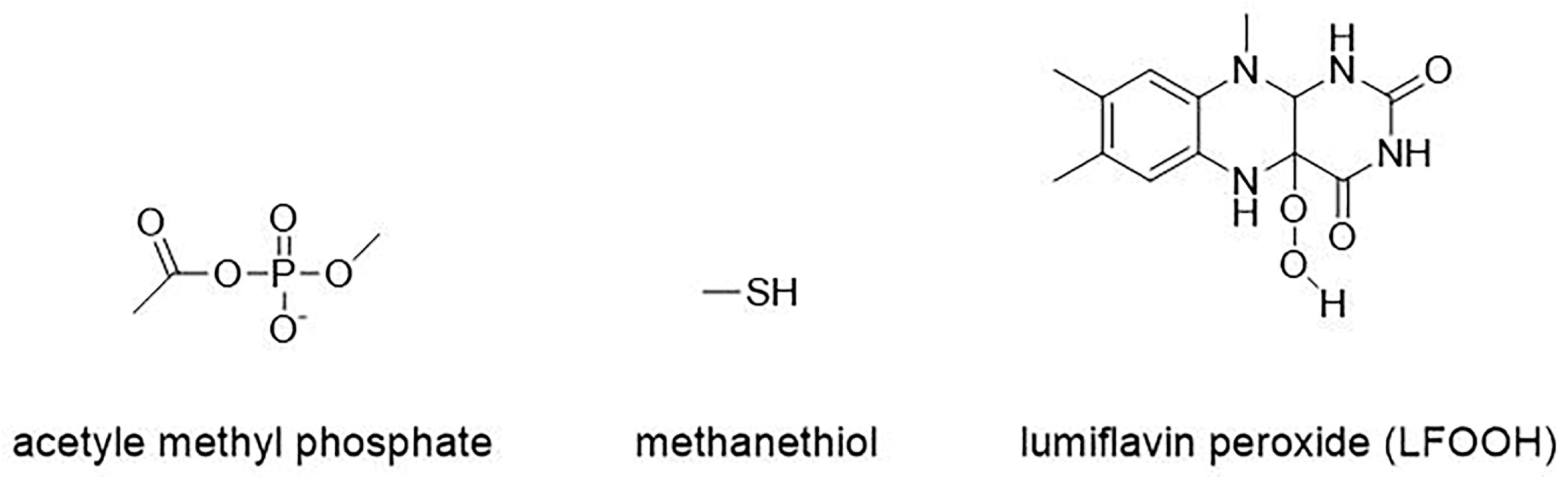
2.1. Computational Model of Stage 1
2.2. Computational Method
3. Results and Discussion
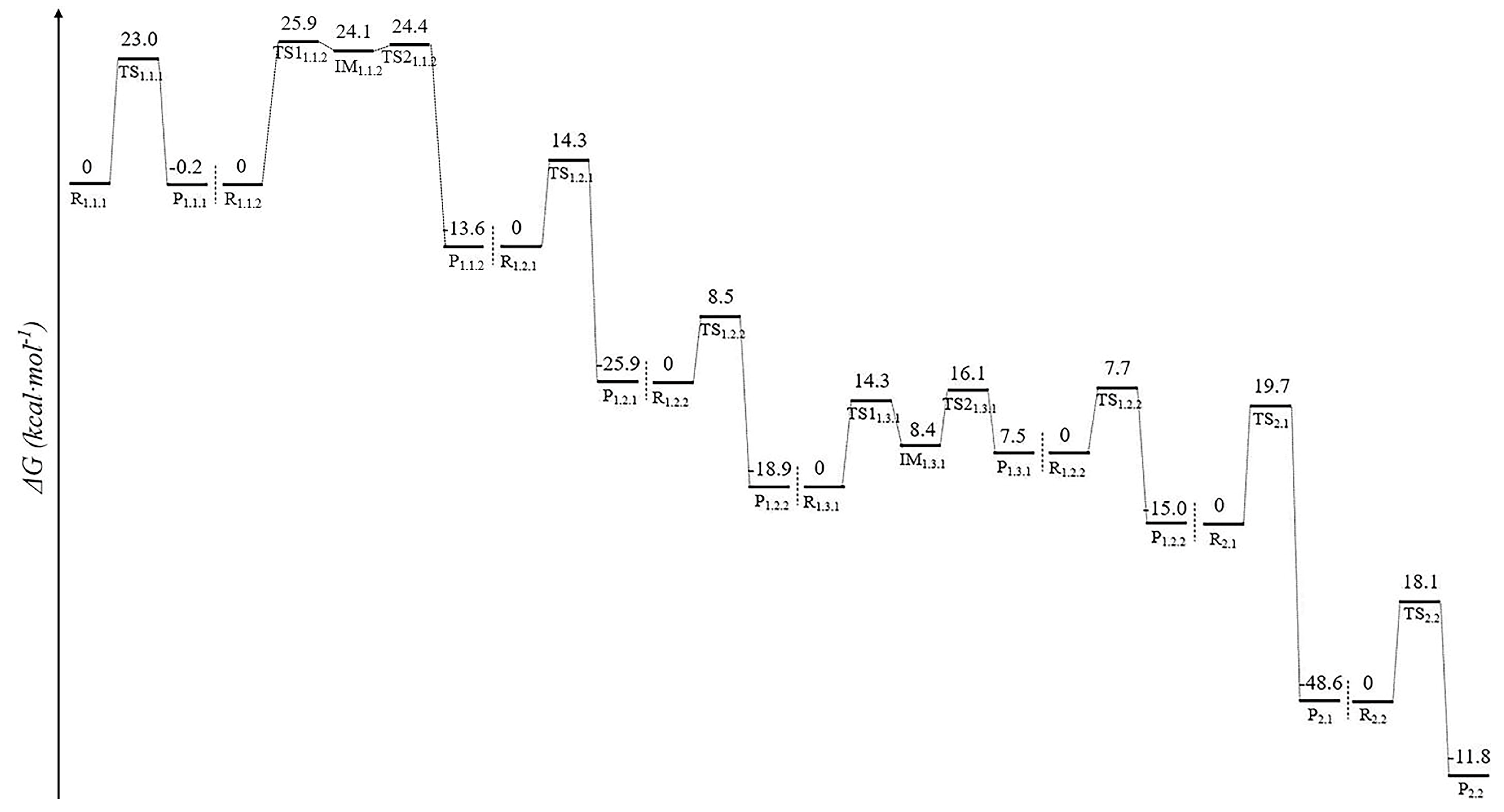
3.1. Stage 1: Biosynthesis of Fungal Luciferin—From Caffeic Acid to Luciferin
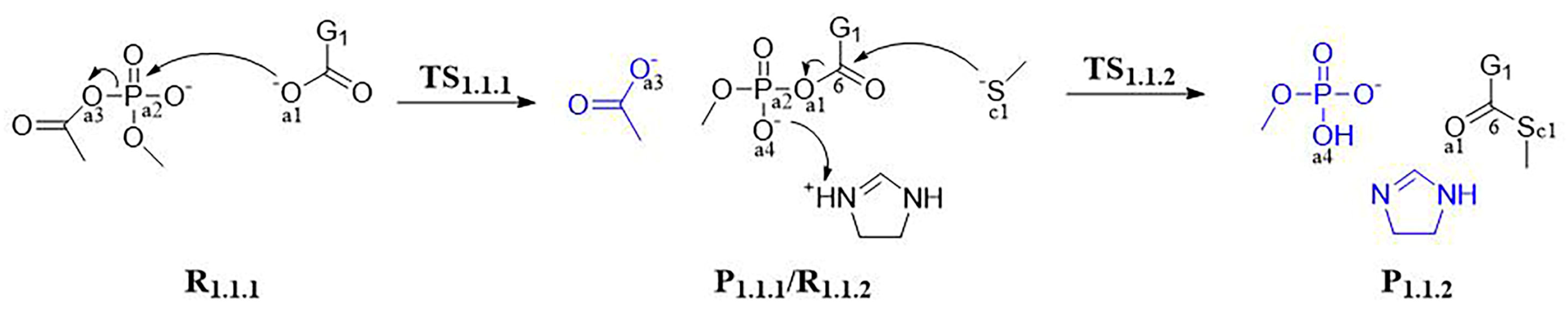
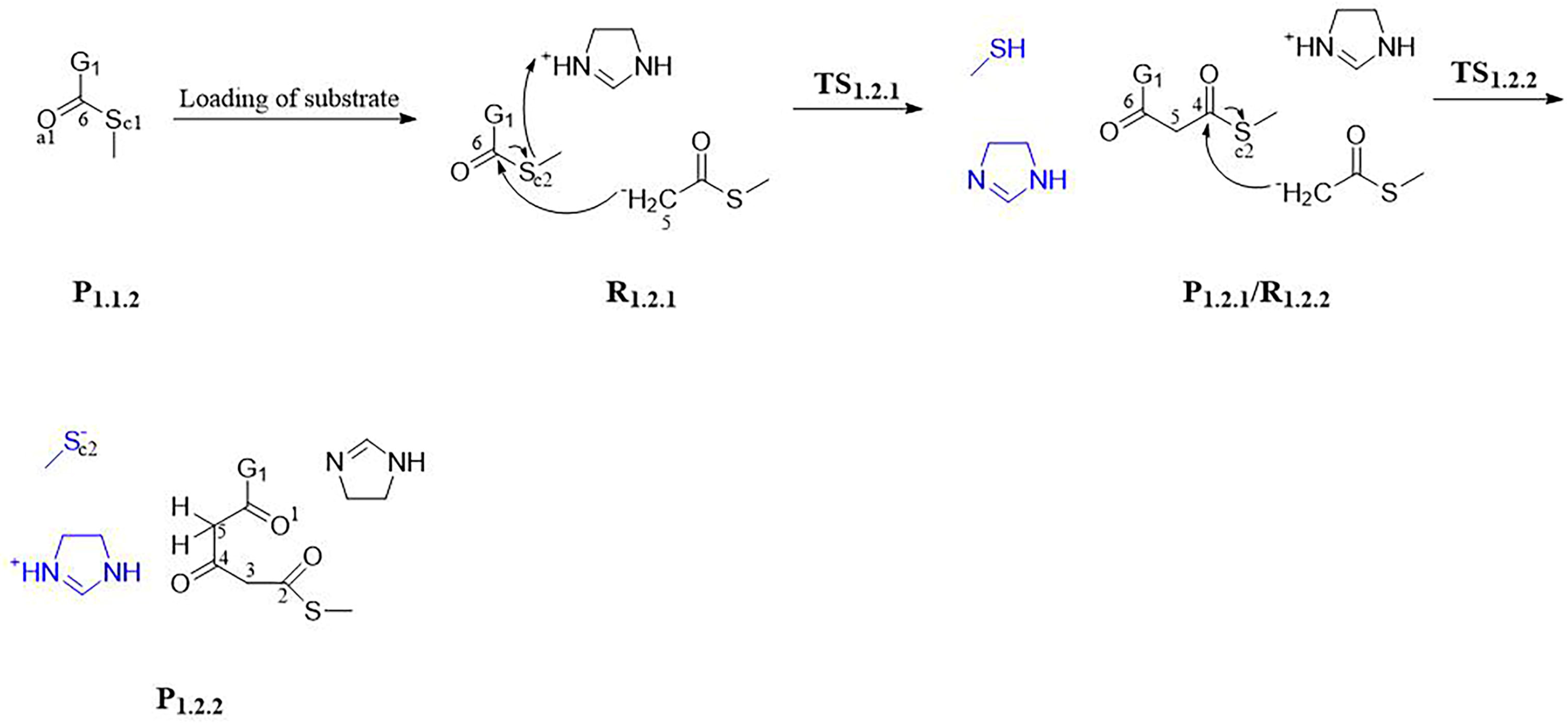
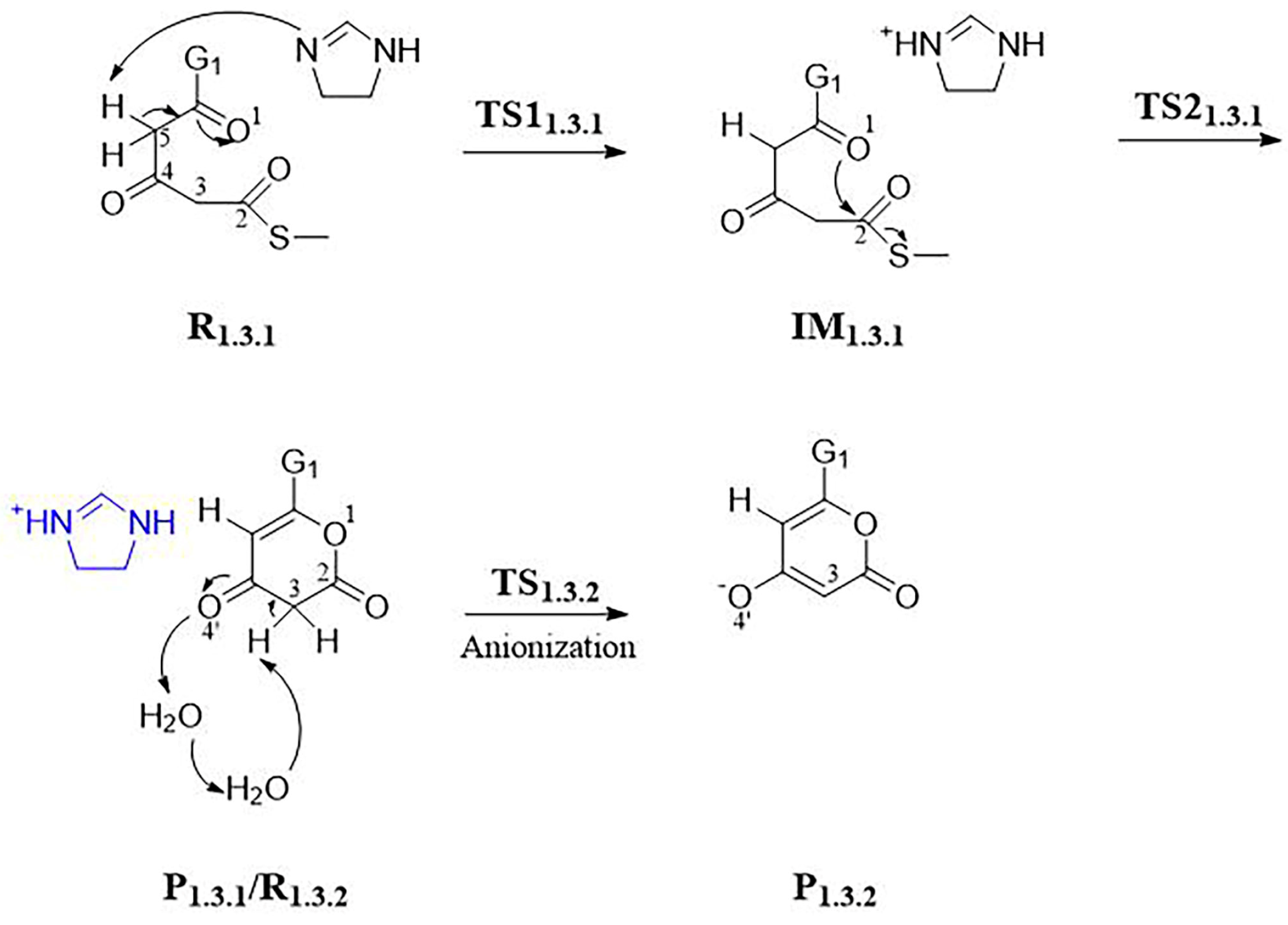
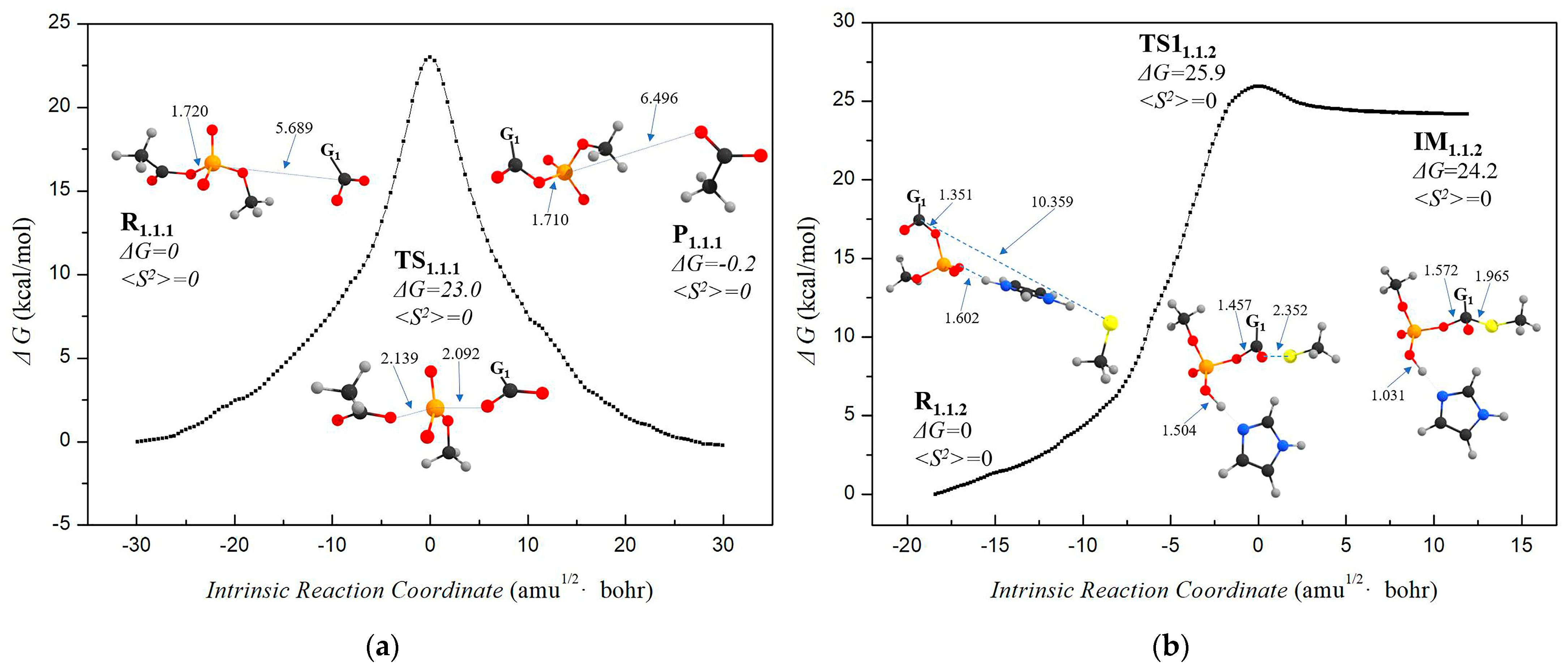
3.1.1. Step 1: Biosynthesis of Luciferin Precursors—From Caffeic Acid to Hispidin
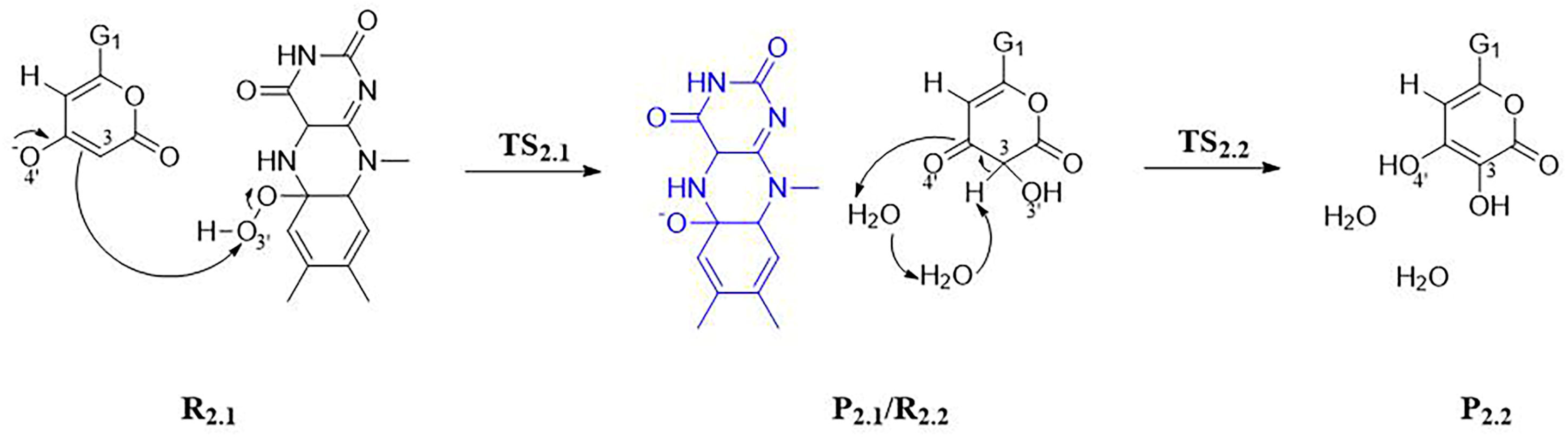
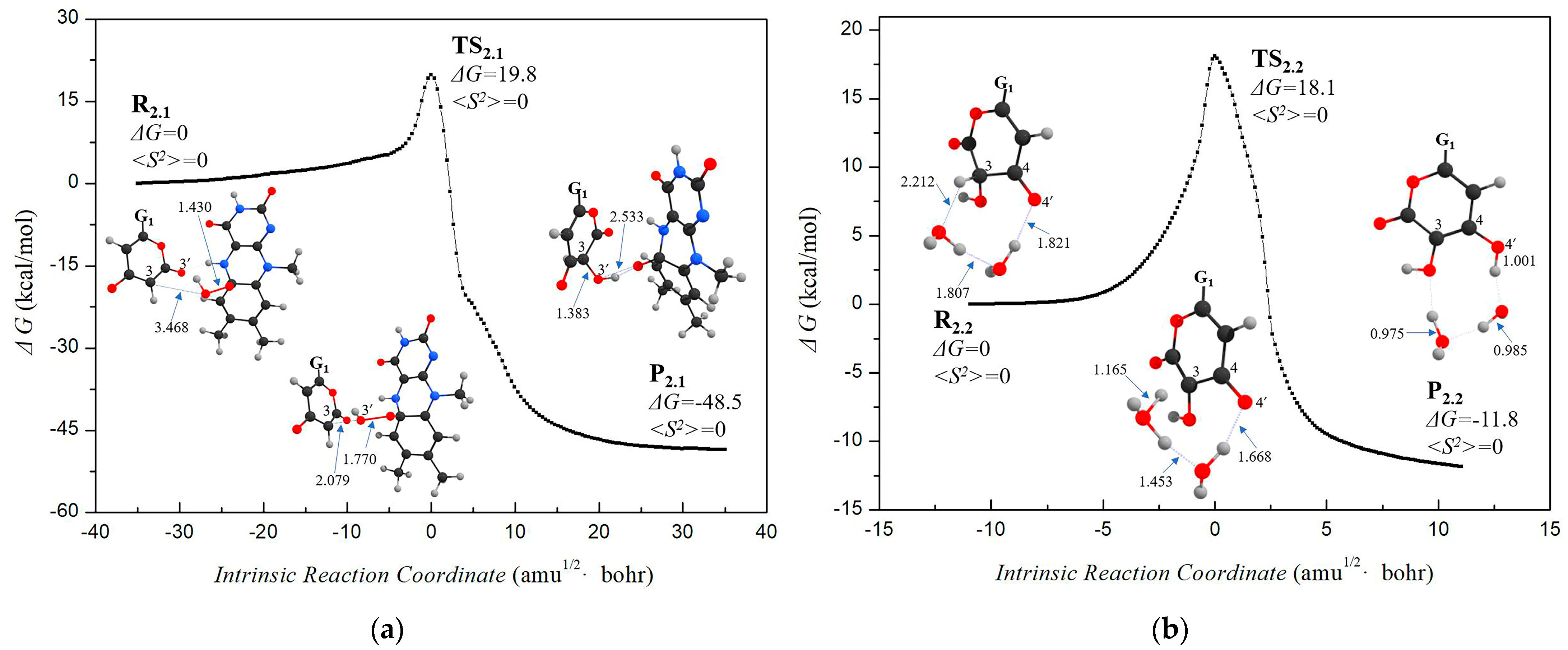
3.1.2. Step 2: Biosynthesis of Luciferin—From Hispidin to Luciferin
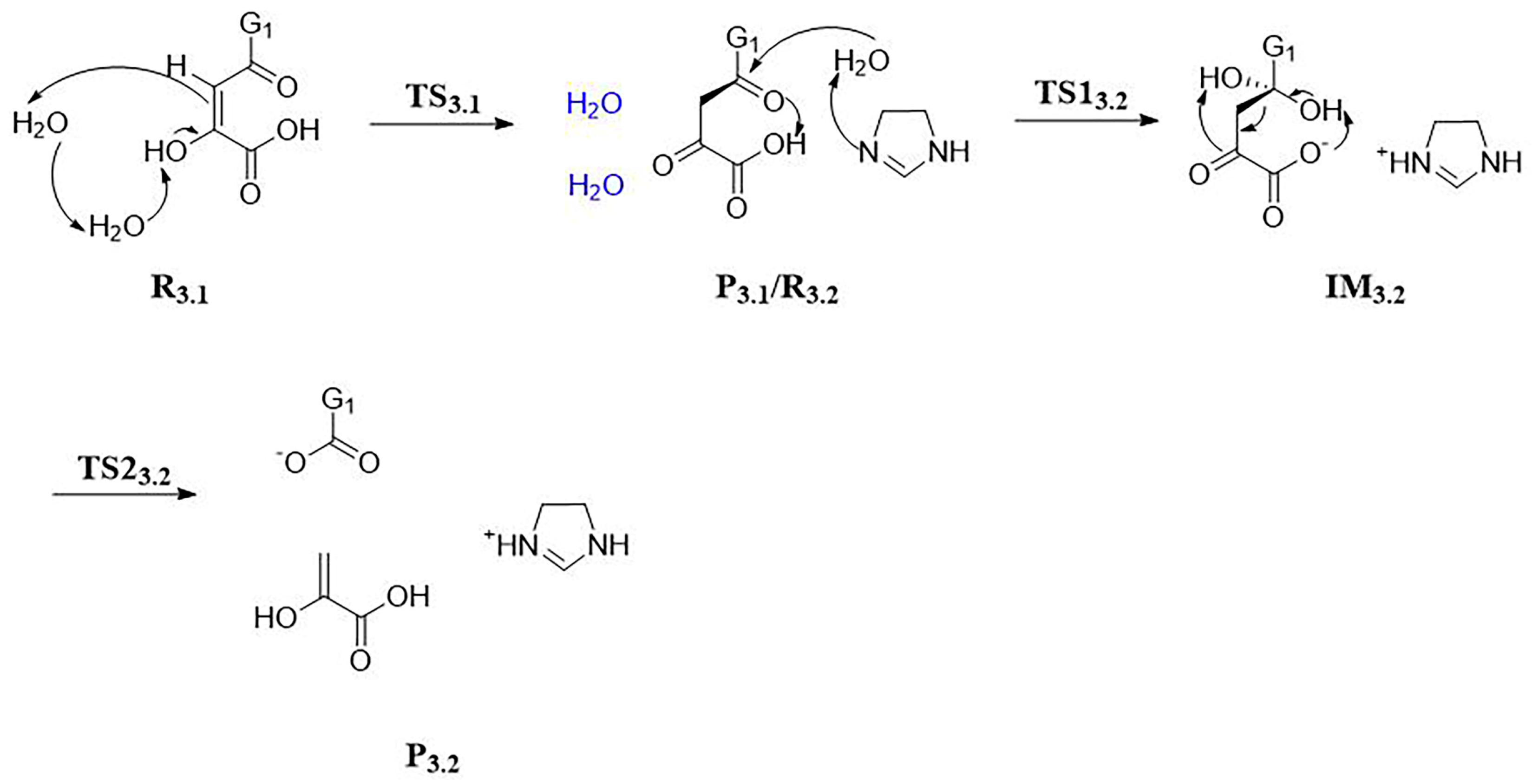
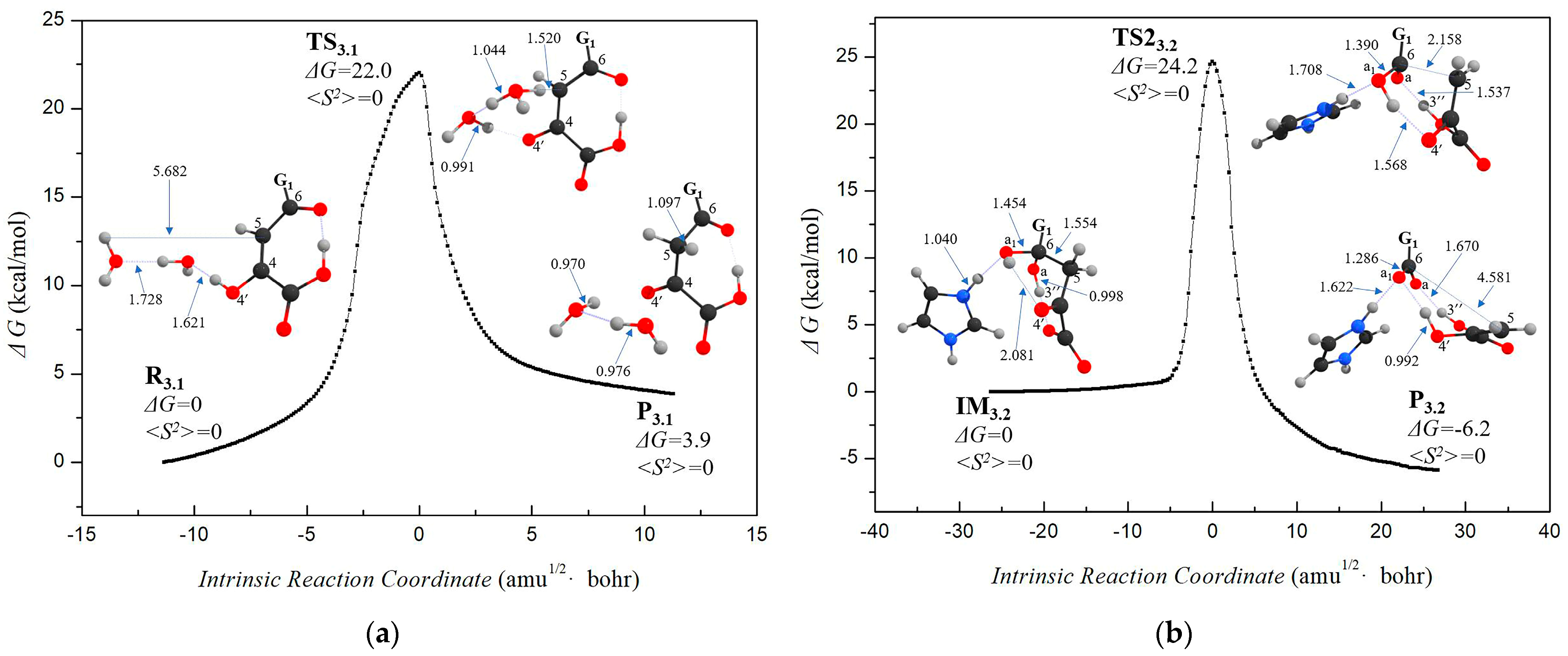
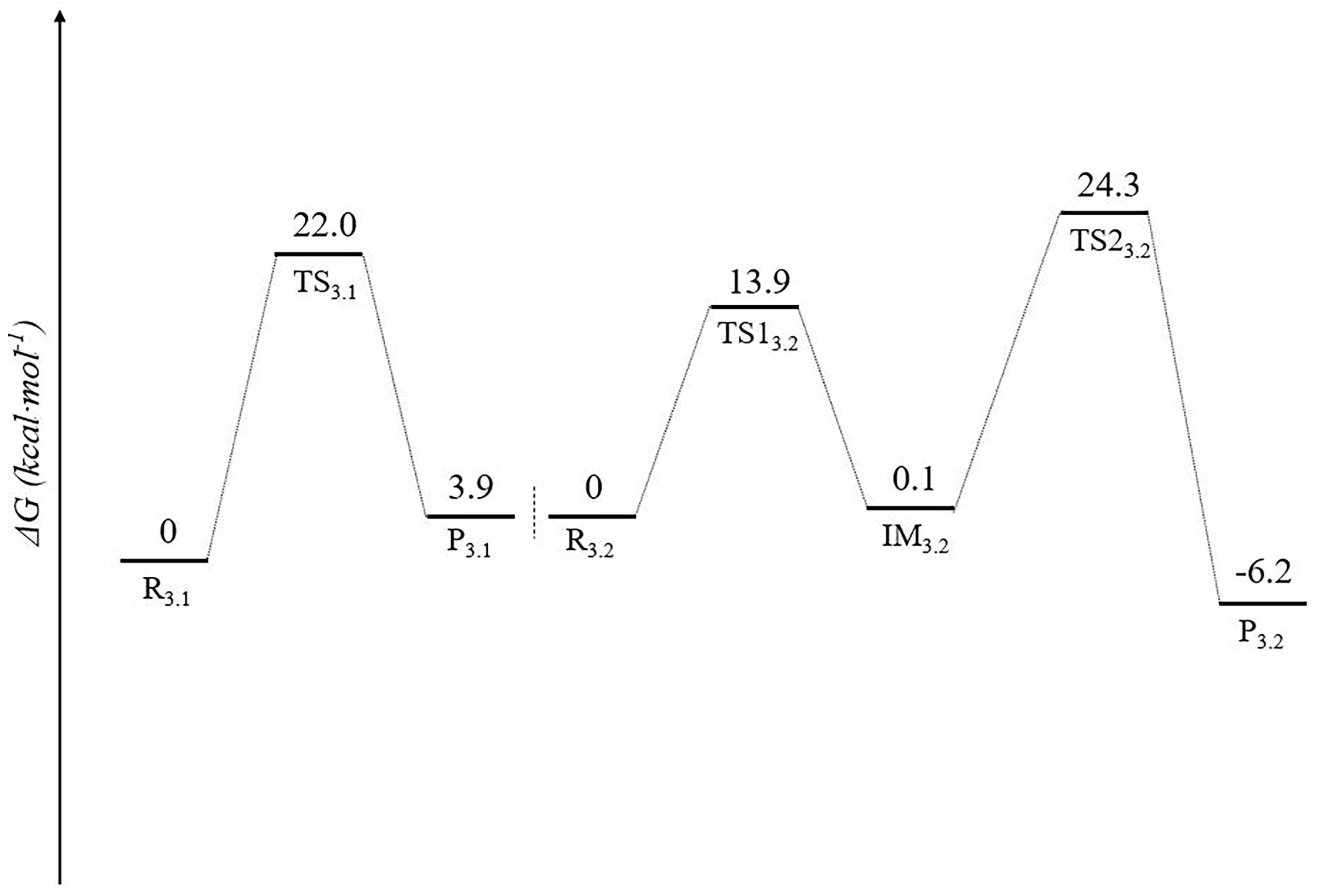
3.2. Stage 3: Recovery of Utilization of Oxidation Luciferin—From Oxidized Luciferin to Caffeic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harvey, E.N. A History of Luminescence from the Earliest Times until 1900; American Philosophical Society: Philadelphia, PA, USA, 1957. [Google Scholar]
- Mager, H.I.T.S. Biochemistry of Bacteria Bioluminescence. Photochem. Photobiol. 1995, 62, 607–614. [Google Scholar] [CrossRef]
- Wang, M.Y.; Liu, Y.J. Chemistry in Fungal Bioluminescence: A Theoretical Study from Luciferin to Light Emission. J. Org. Chem. 2021, 86, 1874–1881. [Google Scholar] [CrossRef]
- Kotlobay, A.A.; Sarkisyan, K.S.; Mokrushina, Y.A.; Yampolsky, I.V. Genetically encodable bioluminescent system from fungi. Proc. Natl. Acad. Sci. USA 2018, 115, 12728–12732. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O. Bioluminescence: Chemical Principles and Methods; World Scientific: Hackensack, NJ, USA, 2006. [Google Scholar]
- Oliveira, A.G.; Stevani, C.V.; Waldenmaier, H.E.; Viviani, V.; Emerson, J.M.; Loros, J.J.; Dunlap, J.C. Circadian control sheds light on fungal bioluminescence. Curr. Biol. 2015, 25, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Mitiouchkina, T.; Mishin, A.S.; Somermeyer, L.G.; Markina, N.M.; Chepurnyh, T.V.; Guglya, E.B.; Karataeva, T.A.; Palkina, K.A.; Shakhova, E.S.; Fakhranurova, L.I.; et al. Plants with genetically encoded autoluminescence. Nat. Biotechnol. 2020, 38, 944–946. [Google Scholar] [CrossRef]
- Krichevsky, A.; Meyers, B.; Vainstein, A.; Maliga, P.; Citovsky, V. Autoluminescent plants. PLoS ONE 2010, 5, e15461. [Google Scholar] [CrossRef] [PubMed]
- Khakhar, A.; Starker, C.G.; Chamness, J.C.; Lee, N.; Stokke, S.; Wang, C.; Swanson, R.; Rizvi, F.; Imaizumi, T.; Voytas, D. Building customizable auto-luminescent luciferase-based reporters in plants. Elife 2020, 9, e52786. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Desjardin, D.E.; Perry, B.A.; Stevani, C.V. Evidence that a single bioluminescent system is shared by all known bioluminescent fungal lineages. Photochem. Photobiol. Sci. 2012, 11, 848–852. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A At. Mol. Opt. Phys. 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter Mater. Phys. 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Pi, S.Q.; Li, Q.S.; Yue, L.; Liu, Y.J. Mechanistic investigation on chemiluminescent formaldehyde probes. Chem. Eur. J. 2021, 27, 5712–5720. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.Y.; Liu, Y.J. What exactly is the light emitter of a firefly? J. Chem. Theory Comput. 2015, 11, 5360–5370. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.W.; Liu, Y.J. Bioluminescence of Firefly Squid via Mechanism of Single Electron-Transfer Oxygenation and Charge-Transfer-Induced Luminescence. J. Am. Chem. Soc. 2017, 139, 1106–1119. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Gilson, M.K.; Honig, B.H. The Dielectric-Constant of a Folded Protein. Biopolymers 1986, 25, 2097–2119. [Google Scholar] [CrossRef]
- Pitera, J.W.; Falta, M.; van Gunsteren, W.F. Dielectric Properties of Proteins from Simulation. Biophys. J. 2001, 80, 2546–2556. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Ren, B.; Li, D.F.; Zheng, C.; Gai, Y.; Jiang, X.; Hu, Y. His-234 is an Essential Residue for Populus tomentosa 4 -coumarate: CoA Ligase Enzymatic Mechanisms. Acta Agron. Sin. 2013, 40, 1165–1172. [Google Scholar] [CrossRef]
- Gulick, A.M. Conformational Dynamics in the Acyl-CoA Synthetases, Adenylation Domains of Non-ribosomal Peptide Synthetases, and Firefly Luciferase. ACS Chem. Biol. 2009, 4, 811–827. [Google Scholar] [CrossRef] [PubMed]
- Austin, M.B.; Bowman, M.E.; Ferrer, J.L.; Schröder, J.; Noel, J.P. An Aldol Switch Discovered in Stilbene Synthases Mediates Cyclization Specificity of Type III Polyketide Synthases. Chem. Biol. 2004, 11, 1179–1194. [Google Scholar] [CrossRef] [PubMed]
- Tong, Y.; Trajkovic, M.; Savino, S.; van Berkel, W.J.; Fraaije, M.W. Substrate binding tunes the reactivity of hispidin 3-hydroxylase, a flavoprotein monooxygenase involved in fungal bioluminescence. J. Biol. Chem. 2020, 295, 16013–16022. [Google Scholar] [CrossRef]
- Brouns, S.J.; Barends, T.R.; Worm, P.; Akerboom, J.; Turnbull, A.P.; Salmon, L.; van der Oost, J. Structural insight into substrate binding and catalysis of a novel 2-keto-3-deoxy-D-arabinonate dehydratase illustrates common mechanistic features of the FAH superfamily. J. Mol. Biol. 2008, 379, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Seo, H.; Park, W.; Kim, K. Sequence, structure and function-based classification of the broadly conserved FAH superfamily reveals two distinct fumarylpyruvate hydrolase subfamilies. Environ. Microbiol. 2020, 22, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Pircher, H.; Straganz, G.D.; Ehehalt, D.; Morrow, G.; Tanguay, R.M.; Jansen-Dürr, P. Identification of human fumarylacetoacetate hydrolase domain-containing protein 1 (FAHD1) as a novel mitochondrial acylpyruvase. J. Biol. Chem. 2011, 286, 36500–36508. [Google Scholar] [CrossRef] [PubMed]
- Pircher, H.; von Grafenstein, S.; Diener, T.; Metzger, C.; Albertini, E.; Taferner, A.; Unterluggauer, H.; Kramer, C.; Liedl, K.R.; Jansen-Dürr, P. Identification of FAH domain-containing protein 1 (FAHD1) as oxaloacetate decarboxylase. J. Biol. Chem. 2015, 290, 6755–6762. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, M.; Liu, Y. Chemistry in Fungal Bioluminescence: Theoretical Studies on Biosynthesis of Luciferin from Caffeic Acid and Regeneration of Caffeic Acid from Oxidized Luciferin. J. Fungi 2023, 9, 369. https://doi.org/10.3390/jof9030369
Liu X, Wang M, Liu Y. Chemistry in Fungal Bioluminescence: Theoretical Studies on Biosynthesis of Luciferin from Caffeic Acid and Regeneration of Caffeic Acid from Oxidized Luciferin. Journal of Fungi. 2023; 9(3):369. https://doi.org/10.3390/jof9030369
Chicago/Turabian StyleLiu, Xiayu, Mingyu Wang, and Yajun Liu. 2023. "Chemistry in Fungal Bioluminescence: Theoretical Studies on Biosynthesis of Luciferin from Caffeic Acid and Regeneration of Caffeic Acid from Oxidized Luciferin" Journal of Fungi 9, no. 3: 369. https://doi.org/10.3390/jof9030369
APA StyleLiu, X., Wang, M., & Liu, Y. (2023). Chemistry in Fungal Bioluminescence: Theoretical Studies on Biosynthesis of Luciferin from Caffeic Acid and Regeneration of Caffeic Acid from Oxidized Luciferin. Journal of Fungi, 9(3), 369. https://doi.org/10.3390/jof9030369




