High-Throughput Microsatellite Markers Development for Genetic Characterization of Emerging Sporothrix Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Sporothrix Isolates and DNA Extraction
2.2. In Silico Genomic Characterization of Simple Sequence Repeats
2.3. Primer Design
2.4. PCR Optimization and Capillary Electrophoresis
2.5. Bioinformatic Analysis
2.6. Genetic Diversity Analysis and Linkage Disequilibrium
2.7. Structure Analysis
2.8. Analysis of Molecular Variance (AMOVA)
2.9. Characterization of the Mating-Type Idiomorphs and Mitochondrial DNA Typing
3. Results
3.1. In Silico Mining of SSR Markers
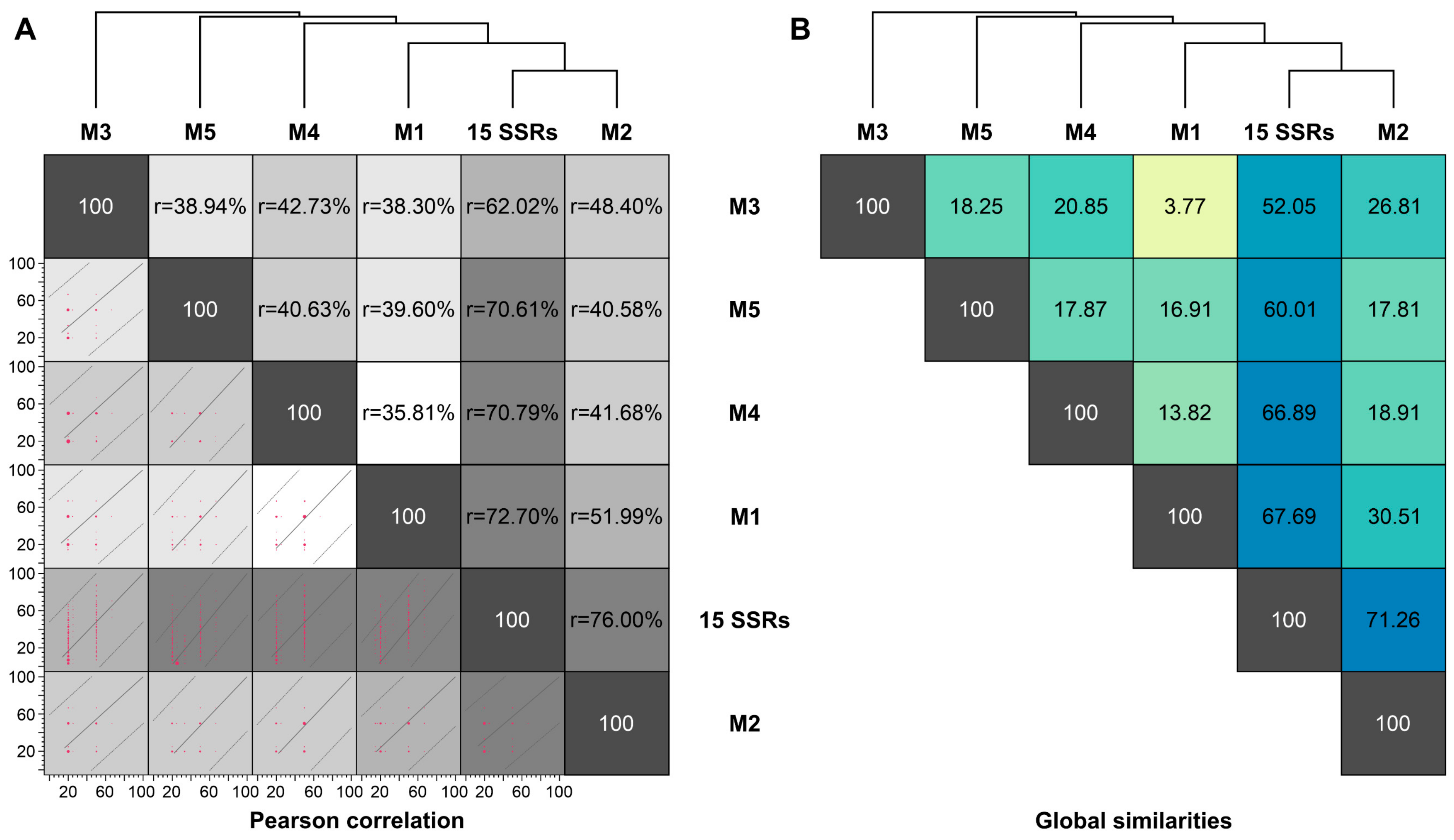
3.2. In Vitro Application of SSR Markers
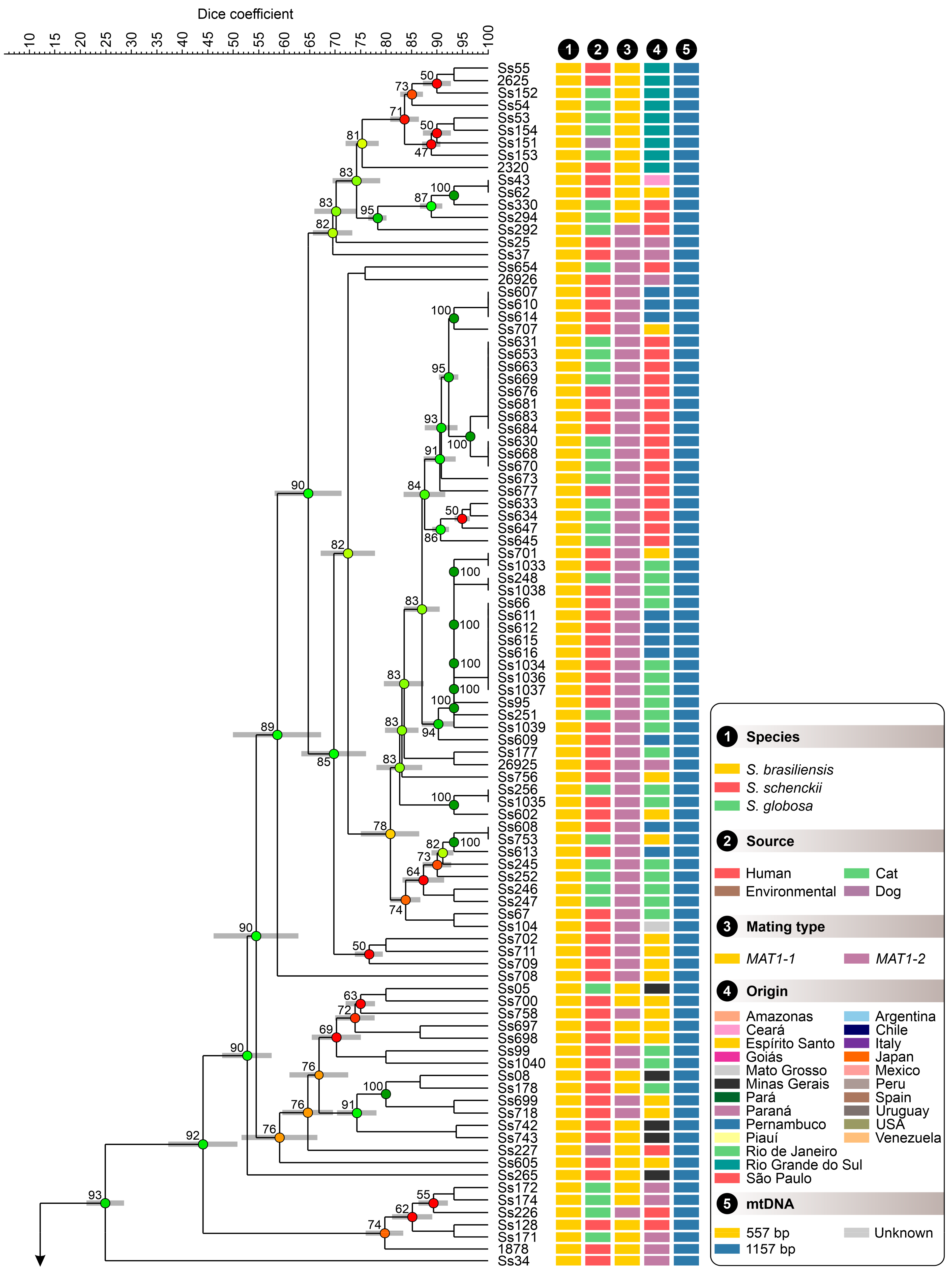
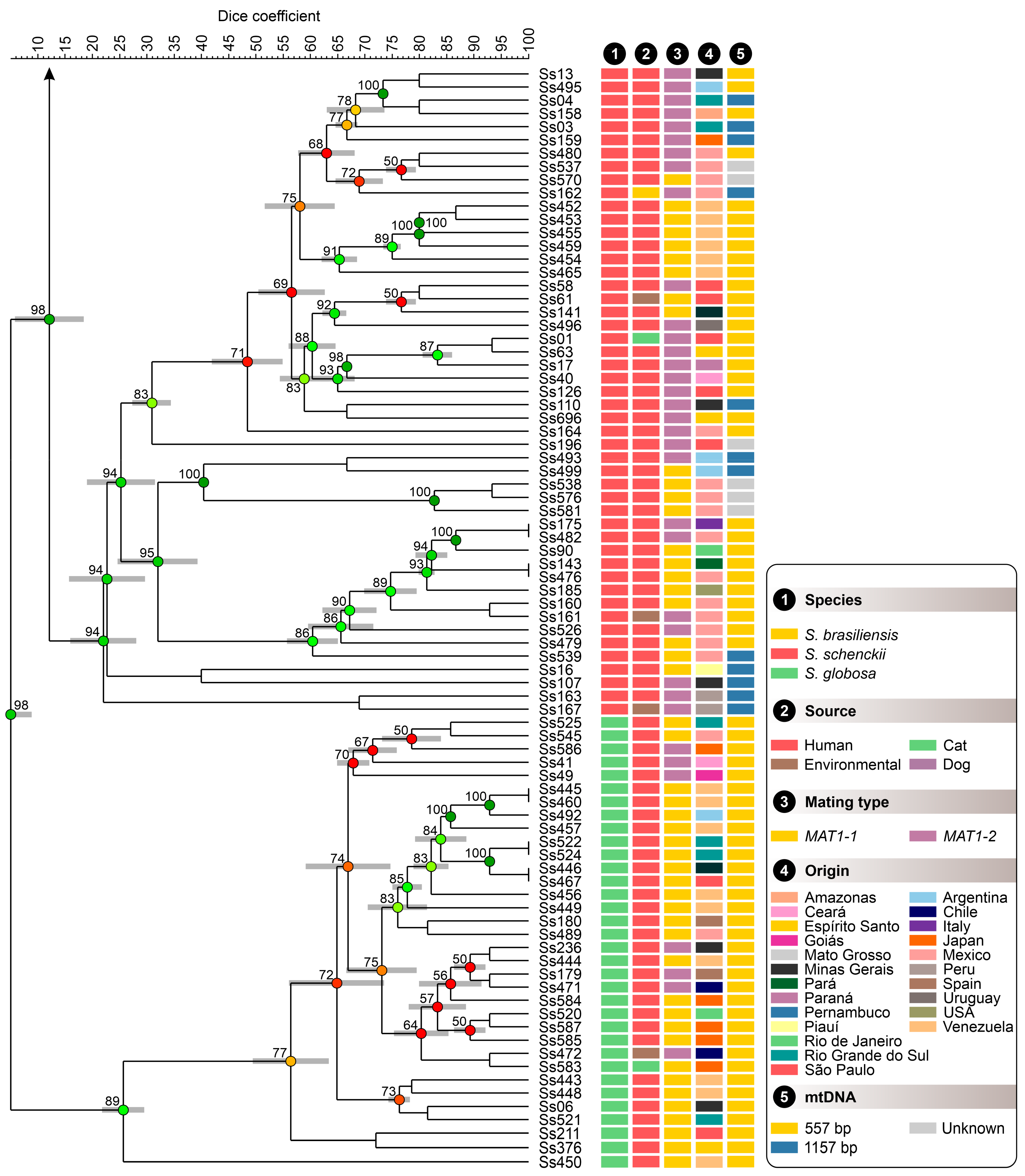
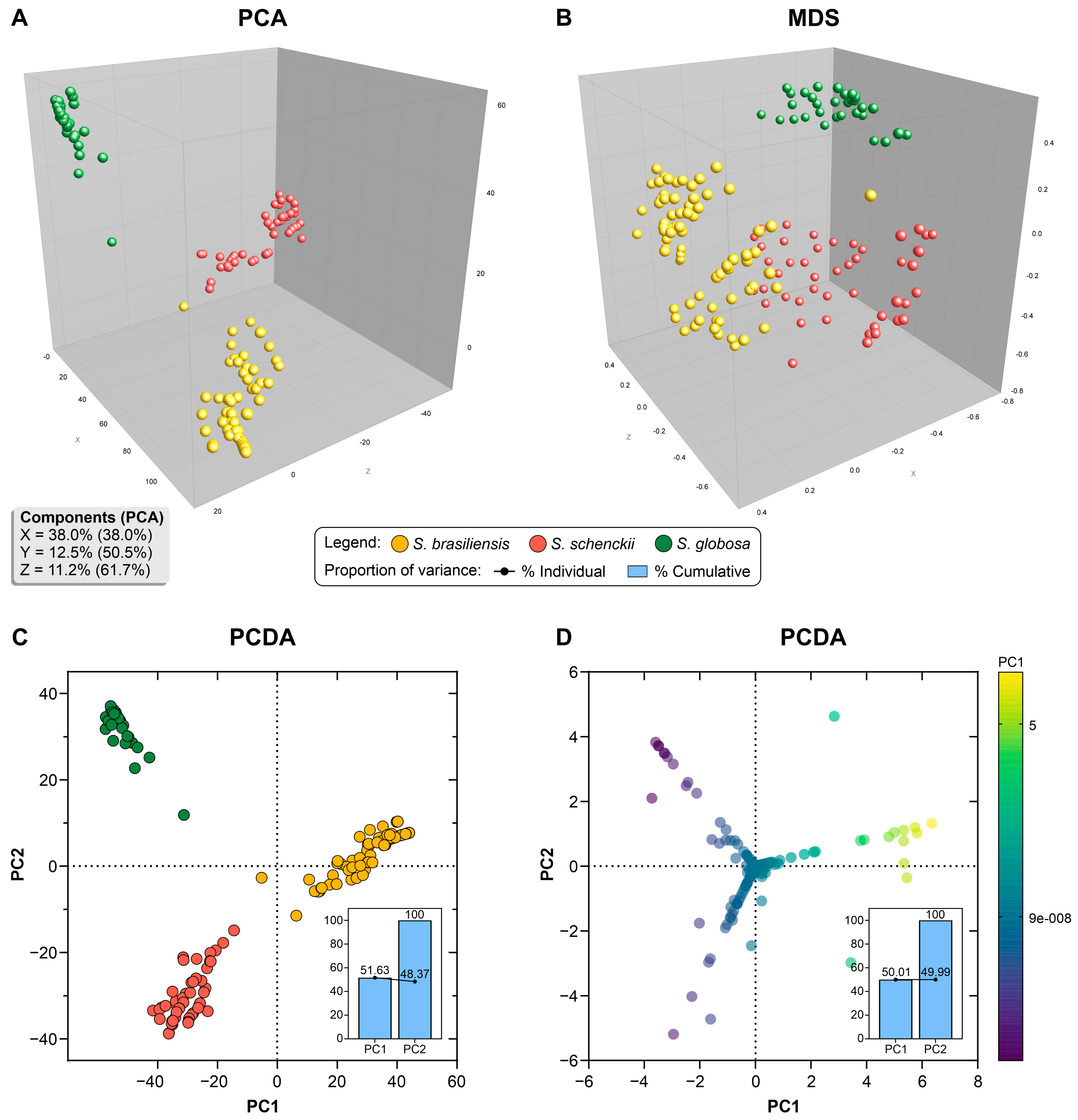
3.3. Population Structure Based on Multivariate Cluster Analysis
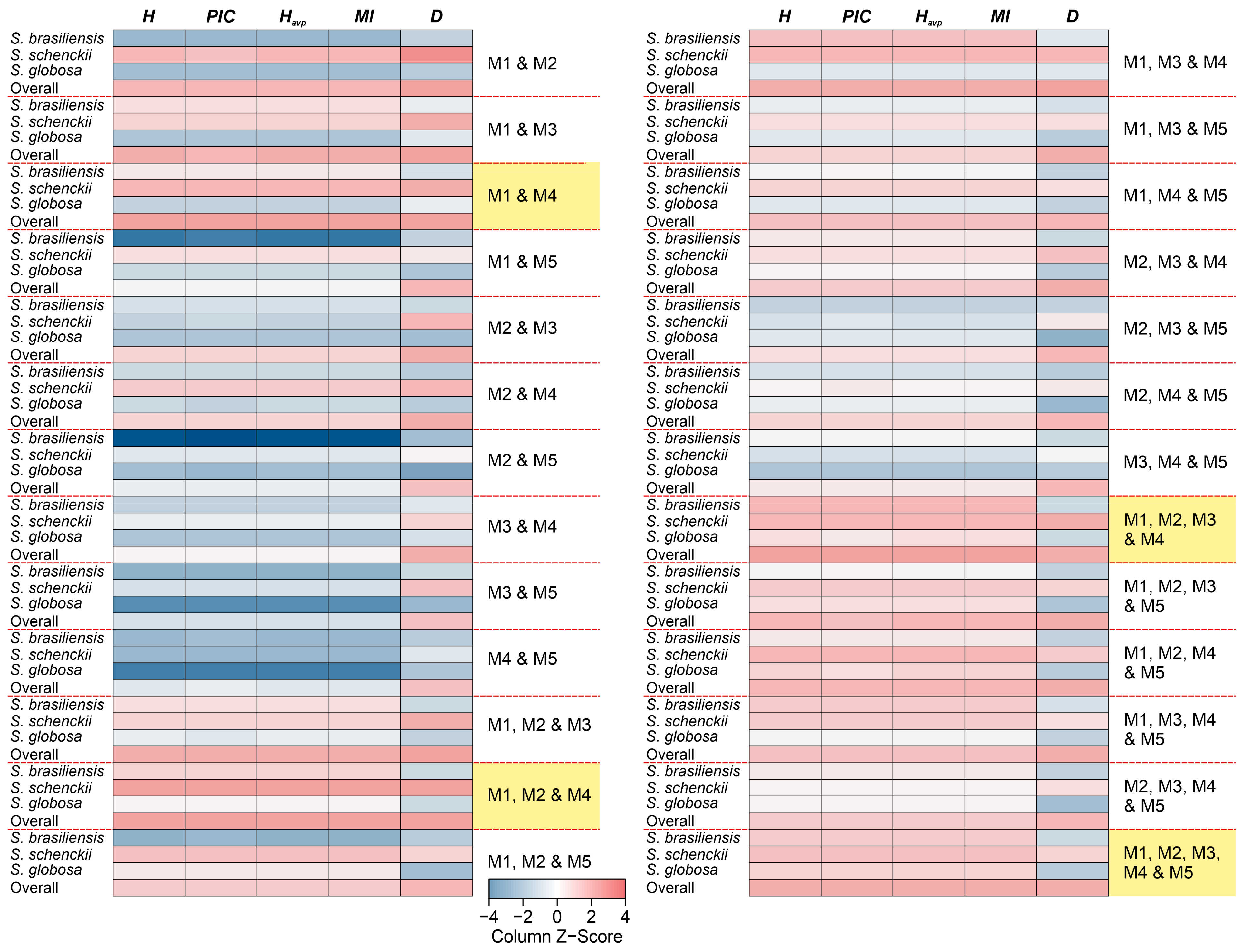
3.4. Population Genetics in Sporothrix Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Orofino-Costa, R.C.; Macedo, P.M.; Rodrigues, A.M.; Bernardes-Engemann, A.R. Sporotrichosis: An update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An. Bras. Dermatol. 2017, 92, 606–620. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Orofino-Costa, R.; de Camargo, Z.P. Sporothrix spp. In Pocket Guide to Mycological Diagnosis, 1st ed.; Cordeiro Rde, A., Ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 99–113. [Google Scholar]
- Orofino-Costa, R.; Freitas, D.F.S.; Bernardes-Engemann, A.R.; Rodrigues, A.M.; Talhari, C.; Ferraz, C.E.; Veasey, J.V.; Quintella, L.; Sousa, M.S.L.A.d.; Vettorato, R.; et al. Human sporotrichosis: Recommendations from the Brazilian Society of Dermatology for the clinical, diagnostic and therapeutic management. An. Bras. Dermatol. 2022, 97, 757–777. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Della Terra, P.P.; Gremiao, I.D.; Pereira, S.A.; Orofino-Costa, R.; de Camargo, Z.P. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia 2020, 185, 813–842. [Google Scholar] [CrossRef] [PubMed]
- Moussa, T.A.A.; Kadasa, N.M.S.; Al Zahrani, H.S.; Ahmed, S.A.; Feng, P.; Gerrits van den Ende, A.H.G.; Zhang, Y.; Kano, R.; Li, F.; Li, S.; et al. Origin and distribution of Sporothrix globosa causing sapronoses in Asia. J. Med. Microbiol. 2017, 66, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hagen, F.; Stielow, B.; Rodrigues, A.M.; Samerpitak, K.; Zhou, X.; Feng, P.; Yang, L.; Chen, M.; Deng, S.; et al. Phylogeography and evolutionary patterns in Sporothrix spanning more than 14,000 human and animal case reports. Persoonia 2015, 35, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Melo Teixeira, M.; de Hoog, G.S.; Schubach, T.M.P.; Pereira, S.A.; Fernandes, G.F.; Bezerra, L.M.L.; Felipe, M.S.; de Camargo, Z.P. Phylogenetic analysis reveals a high prevalence of Sporothrix brasiliensis in feline sporotrichosis outbreaks. PLoS Negl. Trop. Dis. 2013, 7, e2281. [Google Scholar] [CrossRef] [PubMed]
- Seyedmousavi, S.; Bosco, S.d.M.G.; de Hoog, S.; Ebel, F.; Elad, D.; Gomes, R.R.; Jacobsen, I.D.; Jensen, H.E.; Martel, A.; Mignon, B.; et al. Fungal infections in animals: A patchwork of different situations. Med. Mycol. 2018, 56, 165–187. [Google Scholar] [CrossRef]
- Gremião, I.D.; Miranda, L.H.; Reis, E.G.; Rodrigues, A.M.; Pereira, S.A. Zoonotic epidemic of sporotrichosis: Cat to human transmission. PLoS Pathog. 2017, 13, e1006077. [Google Scholar] [CrossRef]
- De Oliveira Bento, A.; de Sena Costa, A.S.; Lima, S.L.; do Monte Alves, M.; de Azevedo Melo, A.S.; Rodrigues, A.M.; da Silva-Rocha, W.P.; Milan, E.P.; Chaves, G.M. The spread of cat-transmitted sporotrichosis due to Sporothrix brasiliensis in Brazil towards the Northeast region. PLoS Negl. Trop. Dis. 2021, 15, e0009693. [Google Scholar] [CrossRef]
- Do Monte Alves, M.; Pipolo Milan, E.; da Silva-Rocha, W.P.; Soares de Sena da Costa, A.; Araújo Maciel, B.; Cavalcante Vale, P.H.; de Albuquerque, P.R.; Lopes Lima, S.; Salles de Azevedo Melo, A.; Messias Rodrigues, A.; et al. Fatal pulmonary sporotrichosis caused by Sporothrix brasiliensis in Northeast Brazil. PLoS Negl. Trop. Dis. 2020, 14, e0008141. [Google Scholar] [CrossRef]
- Aguiar, B.A.; Borges, I.L.; Silva, B.W.L.; Rodrigues, F.R.N.; Gonçalves, L.D.; do Rosário Casseb, A.; da Silva Brito, J.; de Pinheiro, A.Q.; Rocha, M.F.G.; de Araújo Viana, D. First case report of feline sporotrichosis caused by Sporothrix brasiliensis in the state of Ceará—Brazil. Med. Mycol. Case Rep. 2023, 40, 12–15. [Google Scholar] [CrossRef]
- García Duarte, J.M.; Wattiez Acosta, V.R.; Fornerón Viera, P.M.L.; Aldama Caballero, A.; Gorostiaga Matiauda, G.A.; Rivelli de Oddone, V.B.; Pereira Brunelli, J.G. Sporotrichosis transmitted by domestic cat. A family case report. Rev. Nac. 2017, 9, 67–76. [Google Scholar] [CrossRef]
- Aldama, A.; Aldama, J.G.; Pereira, J. Value of molecular techniques and risk factors in the diagnosis and evolution of sporotrichosis. About 2 cases of Sporothrix brasiliensis and S. globosa. An. Fac. Cienc. Médicas 2020, 53, 177–184. [Google Scholar] [CrossRef]
- Etchecopaz, A.N.; Lanza, N.; Toscanini, M.A.; Devoto, T.B.; Pola, S.J.; Daneri, G.L.; Iovannitti, C.A.; Cuestas, M.L. Sporotrichosis caused by Sporothrix brasiliensis in Argentina: Case report, molecular identification and in vitro susceptibility pattern to antifungal drugs. J. Mycol. Med. 2019, 30, 100908. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, S.; Isla, G.; Szusz, W.; Vivot, W.; Hevia, A.; Davel, G.; Canteros, C.E. Molecular identification and susceptibility profile of Sporothrix schenckii sensu lato isolated in Argentina. Mycoses 2018, 61, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.; González, C.; Blank, O.; Ramírez, V.; Río, C.D.; Santibáñez, S.; Pena, P. Sporotrichosis outbreak due to Sporothrix brasiliensis in domestic cats at Magallanes, Chile: A one-health-approach study. J. Fungi 2023, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- PAHO. Sporothrix brasiliensis, an Emerging Fungal Pathogen, Notable for Its Zoonotic Transmission and Epidemic Potential for Human and Animal Health in the Americas. Available online: https://www.someve.com.ar/images/noticias/2019/S-brasiliensis_lasAmericas_30082019_ES.pdf (accessed on 30 August 2019).
- Rachman, R.; Ligaj, M.; Chinthapalli, S.; Serafino Wani, R. Zoonotic acquisition of cutaneous Sporothrix brasiliensis infection in the UK. BMJ Case Rep. 2022, 15, e248418. [Google Scholar] [CrossRef]
- Barnacle, J.R.; Chow, Y.J.; Borman, A.M.; Wyllie, S.; Dominguez, V.; Russell, K.; Roberts, H.; Armstrong-James, D.; Whittington, A.M. The first three reported cases of Sporothrix brasiliensis cat-transmitted sporotrichosis outside South America. Med. Mycol. Case Rep. 2023, 39, 14–17. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global epidemiology of sporotrichosis. Med. Mycol. 2015, 53, 3–14. [Google Scholar] [CrossRef]
- De Beer, Z.W.; Duong, T.A.; Wingfield, M.J. The divorce of Sporothrix and Ophiostoma: Solution to a problematic relationship. Stud. Mycol. 2016, 83, 165–191. [Google Scholar] [CrossRef]
- Sasaki, A.A.; Fernandes, G.F.; Rodrigues, A.M.; Lima, F.M.; Marini, M.M.; Feitosa, L.d.S.; de Melo Teixeira, M.; Felipe, M.S.S.; da Silveira, J.F.; de Camargo, Z.P. Chromosomal polymorphism in the Sporothrix schenckii complex. PLoS ONE 2014, 9, e86819. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.F.; dos Santos, P.O.; Rodrigues, A.M.; Sasaki, A.A.; Burger, E.; de Camargo, Z.P. Characterization of virulence profile, protein secretion and immunogenicity of different Sporothrix schenckii sensu stricto isolates compared with S. globosa and S. brasiliensis species. Virulence 2013, 4, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, G.S.; Zhang, Y.; Camargo, Z.P. Emerging sporotrichosis is driven by clonal and recombinant Sporothrix species. Emerg. Microbes Infect. 2014, 3, e32. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, G.S.; de Cassia Pires, D.; Brilhante, R.S.N.; da Costa Sidrim, J.J.; Gadelha, M.F.; Colombo, A.L.; de Camargo, Z.P. Genetic diversity and antifungal susceptibility profiles in causative agents of sporotrichosis. BMC Infect. Dis. 2014, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Espinel-Ingroff, A.; Abreu, D.P.B.; Almeida-Paes, R.; Brilhante, R.S.N.; Chakrabarti, A.; Chowdhary, A.; Hagen, F.; Cordoba, S.; Gonzalez, G.M.; Govender, N.P.; et al. Multicenter and international study of MIC/MEC distributions for definition of epidemiological cutoff values (ECVs) for species of Sporothrix identified by molecular methods. Antimicrob. Agents Chemother. 2017, 61, e01057-17. [Google Scholar] [CrossRef]
- Marimon, R.; Gené, J.; Cano, J.; Trilles, L.; Dos Santos Lazéra, M.; Guarro, J. Molecular phylogeny of Sporothrix schenckii. J. Clin. Microbiol. 2006, 44, 3251–3256. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Rodrigues, A.M.; Feng, P.; Hoog, G.S. Global ITS diversity in the Sporothrix schenckii complex. Fungal Divers. 2014, 66, 153–165. [Google Scholar] [CrossRef]
- Ishizaki, H.; Kawasaki, M. Molecular epidemiology of Sporothrix schenckii. Nihon Ishinkin Gakkai Zasshi 2000, 41, 245–249. [Google Scholar] [CrossRef]
- Reis, R.S.; Almeida-Paes, R.; Muniz Mde, M.; Tavares, P.M.; Monteiro, P.C.; Schubach, T.M.; Gutierrez-Galhardo, M.C.; Zancopé-Oliveira, R.M. Molecular characterisation of Sporothrix schenckii isolates from humans and cats involved in the sporotrichosis epidemic in Rio de Janeiro, Brazil. Mem. Inst. Oswaldo Cruz 2009, 104, 769–774. [Google Scholar] [CrossRef]
- New, D.; Beukers, A.G.; Kidd, S.E.; Merritt, A.J.; Weeks, K.; van Hal, S.J.; Arthur, I. Identification of multiple species and subpopulations among Australian clinical Sporothrix isolates using whole genome sequencing. Med. Mycol. 2019, 57, 905–908. [Google Scholar] [CrossRef]
- Eudes Filho, J.; Santos, I.B.D.; Reis, C.M.S.; Patané, J.S.L.; Paredes, V.; Bernardes, J.; Poggiani, S.; Castro, T.C.B.; Gomez, O.M.; Pereira, S.A.; et al. A novel Sporothrix brasiliensis genomic variant in Midwestern Brazil: Evidence for an older and wider sporotrichosis epidemic. Emerg. Microbes Infect. 2020, 9, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.A.; Hagen, F.; Fisher, M.C.; de Camargo, Z.P.; Rodrigues, A.M. Genome-wide mapping using new AFLP markers to explore intraspecific variation among pathogenic Sporothrix species. PLoS Negl. Trop. Dis. 2020, 14, e0008330. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.A.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Kano, R.; Bonifaz, A.; Toriello, C.; Negroni, R.; Rego, R.S.M.; Gremiao, I.D.F.; et al. Trends in the molecular epidemiology and population genetics of emerging Sporothrix species. Stud. Mycol. 2021, 100, 100129. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, M.; Wang, Y.; Li, R.; He, L.; Wan, Z.; Li, F.; Zhang, J. Population structure and genetic diversity of Sporothrix globosa in China according to 10 novel microsatellite loci. J. Med. Microbiol. 2019, 68, 248–254. [Google Scholar] [CrossRef]
- Teixeira, M.M.; de Almeida, L.G.; Kubitschek-Barreira, P.; Alves, F.L.; Kioshima, E.S.; Abadio, A.K.; Fernandes, L.; Derengowski, L.S.; Ferreira, K.S.; Souza, R.C.; et al. Comparative genomics of the major fungal agents of human and animal Sporotrichosis: Sporothrix schenckii Sporothrix brasiliensis. BMC Genom. 2014, 15, 943. [Google Scholar] [CrossRef]
- Huang, L.; Gao, W.; Giosa, D.; Criseo, G.; Zhang, J.; He, T.; Huang, X.; Sun, J.; Sun, Y.; Huang, J.; et al. Whole-genome sequencing and in silico analysis of two strains of Sporothrix globosa. Genome Biol. Evol. 2016, 8, 3292–3296. [Google Scholar] [CrossRef]
- Gomez, O.M.; Alvarez, L.C.; Muñoz, J.F.; Misas, E.; Gallo, J.E.; Jimenez, M.D.P.; Arango, M.; McEwen, J.G.; Hernandez, O.; Clay, O.K. Draft genome sequences of two Sporothrix schenckii clinical isolates associated with human sporotrichosis in Colombia. Genome Announc. 2018, 6, e0049518. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, E.; Giosa, D.; Huang, L.; Zhang, J.; Gao, W.; Brankovics, B.; Oliveira, M.M.E.; Scordino, F.; Lo Passo, C.; Criseo, G.; et al. Draft genome sequence of the dimorphic fungus Sporothrix pallida, a nonpathogenic species belonging to Sporothrix, a genus containing agents of human and feline sporotrichosis. Genome Announc. 2016, 4, e00184-16. [Google Scholar] [CrossRef]
- Cuomo, C.A.; Rodriguez-Del Valle, N.; Perez-Sanchez, L.; Abouelleil, A.; Goldberg, J.; Young, S.; Zeng, Q.; Birren, B.W. Genome sequence of the pathogenic fungus Sporothrix schenckii (ATCC 58251). Genome Announc. 2014, 2, e00446-14. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, G.S.; Camargo, Z.P. Genotyping species of the Sporothrix schenckii complex by PCR-RFLP of calmodulin. Diagn. Microbiol. Infect. Dis. 2014, 78, 383–387. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; de Hoog, S.; de Camargo, Z.P. Emergence of pathogenicity in the Sporothrix schenckii complex. Med. Mycol. 2013, 51, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.; Silva, N.F.; Lima, R.A.; Caetano, E.P.; Alencar, L.P.; Castelo-Branco Dde, S.; Moreira, J.L.; Bandeira, S.P.; Camargo, Z.P.; Rodrigues, A.M.; et al. Easy storage strategies for Sporothrix spp. strains. Biopreserv. Biobank. 2015, 13, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl. Trop. Dis. 2015, 9, e0004190. [Google Scholar] [CrossRef] [PubMed]
- Della Terra, P.P.; Gonsales, F.F.; de Carvalho, J.A.; Hagen, F.; Kano, R.; Bonifaz, A.; Camargo, Z.P.; Rodrigues, A.M. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound Emerg. Dis. 2022, 69, e704–e716. [Google Scholar] [CrossRef]
- Du, L.; Zhang, C.; Liu, Q.; Zhang, X.; Yue, B.; Hancock, J. Krait: An ultrafast tool for genome-wide survey of microsatellites and primer design. Bioinformatics 2018, 34, 681–683. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L. GMATA: An Integrated software package for genome-scale SSR mining, marker development and viewing. Front. Plant Sci. 2016, 7, 1350. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef]
- Zuker, M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003, 31, 3406–3415. [Google Scholar] [CrossRef]
- Jaccard, P. The distribution of the flora in the Alpine. New Phytol. 1912, 11, 37–50. [Google Scholar] [CrossRef]
- Sokal, R.R.; Michener, C.D. A statistical method for evaluating systematic relationships. Univ. Kans. Sci. Bull. 1958, 38, 1409–1438. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. The comparison of dendrograms by objective methods. Taxon 1962, 11, 33–40. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Kohonen, T. Self-Organizing Maps, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2001; p. 502. [Google Scholar]
- Roberto, T.N.; De Carvalho, J.A.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Hahn, R.C.; de Camargo, Z.P.; Rodrigues, A.M. Exploring genetic diversity, population structure, and phylogeography in Paracoccidioides species using AFLP markers. Stud. Mycol. 2021, 100, 100131. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.L.; Skolnick, M.; Davis, R.W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar]
- Liu, B.H. Statistical Genomics: Linkage, Mapping, and QTL Analysis, 1st ed.; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Powell, W.; Morgante, M.; Andre, C.; Hanafey, M.; Vogel, J.; Tingey, S.; Rafalski, A. The comparison of RFLP, RAPD, AFLP and SSR (microsatellite) markers for germplasm analysis. Mol. Breed. 1996, 2, 225–238. [Google Scholar] [CrossRef]
- Varshney, R.K.; Chabane, K.; Hendre, P.S.; Aggarwal, R.K.; Graner, A. Comparative assessment of EST-SSR, EST-SNP and AFLP markers for evaluation of genetic diversity and conservation of genetic resources using wild, cultivated and elite barleys. Plant Sci. 2007, 173, 638–649. [Google Scholar] [CrossRef]
- Tessier, C.; David, J.; This, P.; Boursiquot, J.M.; Charrier, A. Optimization of the choice of molecular markers for varietal identification in Vitis vinifera L. Theor. Appl. Genet. 1999, 98, 171–177. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Bradbury, P.J.; Zhang, Z.; Kroon, D.E.; Casstevens, T.M.; Ramdoss, Y.; Buckler, E.S. TASSEL: Software for association mapping of complex traits in diverse samples. Bioinformatics 2007, 23, 2633–2635. [Google Scholar] [CrossRef] [PubMed]
- Slatkin, M. Linkage disequilibrium--understanding the evolutionary past and mapping the medical future. Nat. Rev. Genet. 2008, 9, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, J.K.; Stephens, M.; Donnelly, P. Inference of population structure using multilocus genotype data. Genetics 2000, 155, 945–959. [Google Scholar] [CrossRef]
- Evanno, G.; Regnaut, S.; Goudet, J. Detecting the number of clusters of individuals using the software STRUCTURE: A simulation study. Mol. Ecol. 2005, 14, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Earl, D.A.; vonHoldt, B.M. STRUCTURE HARVESTER: A website and program for visualizing STRUCTURE output and implementing the Evanno method. Conserv. Genet. Resour. 2012, 4, 359–361. [Google Scholar] [CrossRef]
- Jakobsson, M.; Rosenberg, N.A. CLUMPP: A cluster matching and permutation program for dealing with label switching and multimodality in analysis of population structure. Bioinformatics 2007, 23, 1801–1806. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.O.D.; Smouse, P.E. GenAlEx 6: Genetic analysis in Excel. Population genetic software for teaching and research. Mol Ecol Notes 2006, 6, 288–295. [Google Scholar] [CrossRef]
- Teixeira, H.; Rodríguez-Echeverría, S.; Nabais, C. Genetic diversity and differentiation of Juniperus thurifera in Spain and Morocco as determined by SSR. PLoS ONE 2014, 9, e88996. [Google Scholar] [CrossRef]
- De Carvalho, J.A.; Pinheiro, B.G.; Hagen, F.; Goncalves, S.S.; Negroni, R.; Kano, R.; Bonifaz, A.; de Camargo, Z.P.; Rodrigues, A.M. A new duplex PCR assay for the rapid screening of mating-type idiomorphs of pathogenic Sporothrix species. Fungal Biol. 2021, 125, 834–843. [Google Scholar] [CrossRef]
- Kawasaki, M.; Anzawa, K.; Mochizuki, T.; Ishizaki, H. New strain typing method with Sporothrix schenckii using mitochondrial DNA and polymerase chain reaction restriction fragment length polymorphism (PCR–RFLP) technique. J. Dermatol. 2012, 39, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory: New York, NY, USA, 2001. [Google Scholar]
- Vieira, M.L.C.; Santini, L.; Diniz, A.L.; Munhoz, C.d.F. Microsatellite markers: What they mean and why they are so useful. Genet. Mol. Biol. 2016, 39, 312–328. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, J.A.; Monteiro, R.C.; Hagen, F.; Camargo, Z.P.; Rodrigues, A.M. Trends in molecular diagnostics and genotyping tools applied for emerging Sporothrix species. J. Fungi 2022, 8, 809. [Google Scholar] [CrossRef]
- Hancock, J.M. Genome size and the accumulation of simple sequence repeats: Implications of new data from genome sequencing projects. Genetica 2002, 115, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.T.; Zhang, Y.J.; Qiao, L.; Chen, B. Comparative analyses of simple sequence repeats (SSRs) in 23 mosquito species genomes: Identification, characterization and distribution (Diptera: Culicidae). Insect. Sci. 2019, 26, 607–619. [Google Scholar] [CrossRef]
- Mandal, K.; Dutta, S.; Upadhyay, A.; Panda, A.; Tripathy, S. Comparative genome analysis across 128 Phytophthora isolates reveal species-specific microsatellite distribution and localized evolution of compartmentalized genomes. Front. Microbiol. 2022, 13, 806398. [Google Scholar] [CrossRef]
- Karaoglu, H.; Lee, C.M.; Meyer, W. Survey of simple sequence repeats in completed fungal genomes. Mol. Biol. Evol. 2005, 22, 639–649. [Google Scholar] [CrossRef]
- Morgante, M.; Hanafey, M.; Powell, W. Microsatellites are preferentially associated with nonrepetitive DNA in plant genomes. Nat. Genet. 2002, 30, 194–200. [Google Scholar] [CrossRef]
- Dokholyan, N.V.; Buldyrev, S.V.; Havlin, S.; Stanley, H.E. Distributions of dimeric tandem repeats in non-coding and coding DNA sequences. J. Theor. Biol. 2000, 202, 273–282. [Google Scholar] [CrossRef]
- Tóth, G.; Gáspári, Z.; Jurka, J. Microsatellites in different eukaryotic genomes: Survey and analysis. Genome Res. 2000, 10, 967–981. [Google Scholar] [CrossRef]
- Wang, H.; Yang, B.; Wang, H.; Xiao, H. Impact of different numbers of microsatellite markers on population genetic results using SLAF-seq data for Rhododendron species. Sci. Rep. 2021, 11, 8597. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, G.S.; de Camargo, Z.P. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016, 12, e1005638. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Gamboa, L.; Martinez-Hernandez, F.; Maravilla, P.; Arenas-Guzman, R.; Flisser, A. Update of phylogenetic and genetic diversity of Sporothrix schenckii sensu lato. Med. Mycol. 2016, 54, 248–255. [Google Scholar] [CrossRef]
- Romeo, O.; Scordino, F.; Criseo, G. New insight into molecular phylogeny and epidemiology of Sporothrix schenckii species complex based on calmodulin-encoding gene analysis of Italian isolates. Mycopathologia 2011, 172, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Camacho, E.; León-Navarro, I.; Rodríguez-Brito, S.; Mendoza, M.; Niño-Vega, G.A. Molecular epidemiology of human sporotrichosis in Venezuela reveals high frequency of Sporothrix globosa. BMC Infect. Dis. 2015, 15, 94. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Beale, M.A.; Hagen, F.; Fisher, M.C.; Terra, P.P.D.; de Hoog, S.; Brilhante, R.S.N.; de Aguiar Cordeiro, R.; de Souza Collares Maia Castelo-Branco, D.; Rocha, M.F.G.; et al. The global epidemiology of emerging Histoplasma species in recent years. Stud. Mycol. 2020, 97, 100095. [Google Scholar] [CrossRef]
- Guevara, A.; Nery, A.F.; de Souza Carvalho Melhem, M.; Bonfietti, L.; Rodrigues, A.M.; Hagen, F.; de Carvalho, J.A.; de Camargo, Z.P.; de Souza Lima, B.J.; Vicente, V.A. Molecular epidemiology and clinical-laboratory aspects of chromoblastomycosis in Mato Grosso, Brazil. Mycoses 2022, 65, 1146–1158. [Google Scholar] [CrossRef]
- Al-Hatmi, A.M.; Hagen, F.; Menken, S.B.; Meis, J.F.; de Hoog, G.S. Global molecular epidemiology and genetic diversity of Fusarium, a significant emerging group of human opportunists from 1958 to 2015. Emerg. Microbes Infect. 2016, 5, e124. [Google Scholar] [CrossRef]
- Vatanshenassan, M.; Boekhout, T.; Mauder, N.; Robert, V.; Maier, T.; Meis, J.F.; Berman, J.; Then, E.; Kostrzewa, M.; Hagen, F. Evaluation of microsatellite typing, ITS Sequencing, AFLP fingerprinting, MALDI-TOF MS, and fourier-transform infrared spectroscopy analysis of Candida auris. J. Fungi 2020, 6, 146. [Google Scholar] [CrossRef]
- Marimon, R.; Cano, J.; Gené, J.; Sutton, D.A.; Kawasaki, M.; Guarro, J. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J. Clin. Microbiol. 2007, 45, 3198–3206. [Google Scholar] [CrossRef]
- Kano, R.; Okubo, M.; Siew, H.H.; Kamata, H.; Hasegawa, A. Molecular typing of Sporothrix schenckii isolates from cats in Malaysia. Mycoses 2015, 58, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Macêdo-Sales, P.A.; Souza, L.O.P.; Della-Terra, P.P.; Lozoya-Pérez, N.E.; Machado, R.L.D.; Rocha, E.M.d.S.d.; Lopes-Bezerra, L.M.; Guimarães, A.J.; Rodrigues, A.M.; Mora-Montes, H.M.; et al. Coinfection of domestic felines by distinct Sporothrix brasiliensis in the Brazilian sporotrichosis hyperendemic area. Fungal Genet. Biol. 2020, 140, 103397. [Google Scholar] [CrossRef]
- Macêdo-Sales, P.A.; Souto, S.R.L.S.; Destefani, C.A.; Lucena, R.P.; Machado, R.L.D.; Pinto, M.R.; Rodrigues, A.M.; Lopes-Bezerra, L.M.; Rocha, E.M.S.; Baptista, A.R.S. Domestic feline contribution in the transmission of Sporothrix in Rio de Janeiro State, Brazil: A comparison between infected and non-infected populations. BMC Vet. Res. 2018, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Gremião, I.D.F.; Oliveira, M.M.E.; Monteiro de Miranda, L.H.; Saraiva Freitas, D.F.; Pereira, S.A. Geographic expansion of sporotrichosis, Brazil. Emerg. Infect. Dis. 2020, 26, 621–624. [Google Scholar] [CrossRef] [PubMed]
- Gremião, I.D.F.; Miranda, L.H.M.; Pereira-Oliveira, G.R.; Menezes, R.C.; Machado, A.C.S.; Rodrigues, A.M.; Pereira, S.A. Advances and challenges in the management of feline sporotrichosis. Rev. Iberoam. Micol. 2022, 36, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Schubach, T.M.; Valle, A.C.; Gutierrez-Galhardo, M.C.; Monteiro, P.C.; Reis, R.S.; Zancope-Oliveira, R.M.; Marzochi, K.B.; Schubach, A. Isolation of Sporothrix schenckii from the nails of domestic cats (Felis catus). Med. Mycol. 2001, 39, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Schubach, T.M.; de Oliveira Schubach, A.; dos Reis, R.S.; Cuzzi-Maya, T.; Blanco, T.C.; Monteiro, D.F.; Barros, B.M.; Brustein, R.; Zancope-Oliveira, R.M.; Fialho Monteiro, P.C.; et al. Sporothrix schenckii isolated from domestic cats with and without sporotrichosis in Rio de Janeiro, Brazil. Mycopathologia 2002, 153, 83–86. [Google Scholar] [CrossRef]
- Teixeira, M.d.M.; Rodrigues, A.M.; Tsui, C.K.M.; de Almeida, L.G.P.; Van Diepeningen, A.D.; Gerrits van den Ende, B.; Fernandes, G.F.; Kano, R.; Hamelin, R.C.; Lopes-Bezerra, L.M.; et al. Asexual propagation of a virulent clone complex in human and feline outbreak of sporotrichosis. Eukaryot. Cell 2015, 14, 158–169. [Google Scholar] [CrossRef]
- Montenegro, H.; Rodrigues, A.M.; Galvão Dias, M.A.; da Silva, E.A.; Bernardi, F.; Camargo, Z.P. Feline sporotrichosis due to Sporothrix brasiliensis: An emerging animal infection in São Paulo, Brazil. BMC Vet. Res. 2014, 10, 269. [Google Scholar] [CrossRef]
- Maschio-Lima, T.; Marques, M.D.R.; Lemes, T.H.; Brizzotti-Mazuchi, N.S.; Caetano, M.H.; de Almeida, B.G.; Bianco, L.M.; Monteiro, R.C.; Rodrigues, A.M.; de Camargo, Z.P.; et al. Clinical and epidemiological aspects of feline sporotrichosis caused by Sporothrix brasiliensis and in vitro antifungal susceptibility. Vet. Res. Commun. 2021, 45, 171–179. [Google Scholar] [CrossRef]
- Ribeiro-Marques, M.D.; Maschio-Lima, T.; Lemes, T.H.; Siqueira, J.P.Z.; Brizzotti-Mazuchi, N.S.; Caetano, M.H.; Almeida, B.G.; Mozaner, L.Q.; Monteiro, R.C.; Camargo, Z.P.; et al. Sporothrix pathogenic clade: Molecular analysis of animal and human clinical isolates. Med. Mycol. 2023, 61, myac096. [Google Scholar] [CrossRef]
- Rediguieri, B.C.; da Cruz Bahiense, I.; de Carvalho, J.A.; Leite, G.R.; Falqueto, A.; Rodrigues, A.M.; Goncalves, S.S. Clinical, Epidemiological, and Epizootic Features of Sporothrix brasiliensis in Espirito Santo, Brazil. Ecohealth 2022, 19, 124–134. [Google Scholar] [CrossRef]
- Da Cruz Bahiense Rocha, I.; Terra, P.P.D.; Cardoso de Oliveira, R.; Lubianca Zanotti, R.; Falqueto, A.; de Camargo, Z.P.; Rodrigues, A.M.; Goncalves, S.S. Molecular-based assessment of diversity and population structure of Sporothrix spp. clinical isolates from Espirito Santo-Brazil. Mycoses 2021, 64, 420–427. [Google Scholar] [CrossRef]
- Sanchotene, K.O.; Madrid, I.M.; Klafke, G.B.; Bergamashi, M.; Terra, P.P.D.; Rodrigues, A.M.; de Camargo, Z.P.; Xavier, M.O. Sporothrix brasiliensis outbreaks and the rapid emergence of feline sporotrichosis. Mycoses 2015, 58, 652–658. [Google Scholar] [CrossRef]
- Baes Pereira, S.; dos Reis Gomes, A.; Bressan Waller, S.; Batista Xavier, J.R.; Messias Rodrigues, A.; Kutscher Ripoll, M.; Ferreira, M.R.A.; Rochedo Conceição, F.; Osório de Faria, R.; Pascoti Bruhn, F.R. Sporotrichosis in dogs: Epidemiological and clinical-therapeutic profile and the emergence of itraconazole-resistant isolates. Med. Mycol. 2022, 60, myac089. [Google Scholar] [CrossRef]
- Rodrigues, A.M.; Gonçalves, S.S.; de Carvalho, J.A.; Borba-Santos, L.P.; Rozental, S.; de Camargo, Z.P. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J. Fungi 2022, 8, 776. [Google Scholar] [CrossRef]
- Porras-Hurtado, L.; Ruiz, Y.; Santos, C.; Phillips, C.; Carracedo, A.; Lareu, M.V. An overview of STRUCTURE: Applications, parameter settings, and supporting software. Front. Genet. 2013, 4, 98. [Google Scholar] [CrossRef]
- Ishizaki, H.; Kawasaki, M.; Anzawa, K.; Mochizuki, T.; Chakrabarti, A.; Ungpakorn, R.; Torres Guererro, H.; Toriello, C.; Arenas, R. Mitochondrial DNA analysis of Sporothrix schenckii in India, Thailand, Brazil, Colombia, Guatemala and Mexico. Nihon Ishinkin Gakkai Zasshi 2009, 50, 19–26. [Google Scholar] [CrossRef]
- Watanabe, S.; Kawasaki, M.; Mochizuki, T.; Ishizaki, H. RFLP Analysis of the Internal Transcribed Spacer regions of Sporothrix schenckii. Nihon Ishinkin Gakkai Zasshi 2004, 45, 165–175. [Google Scholar] [CrossRef]
- Ishizaki, H.; Kawasaki, M.; Aoki, M.; Wu, S.; Lin, J.; Kim, J.A.; Won, Y.H.; Calvo, C.R. Mitochondrial DNA analysis of Sporothrix schenckii from China, Korea and Spain. Nihon Ishinkin Gakkai Zasshi 2004, 45, 23–25. [Google Scholar] [CrossRef]
- Ishizaki, H.; Kawasaki, M.; Mochizuki, T.; Jin, X.Z.; Kagawa, S. Environmental isolates of Sporothrix schenckii in China. Nihon Ishinkin Gakkai Zasshi 2002, 43, 257–260. [Google Scholar] [CrossRef]
- Ishizaki, H.; Kawasaki, M.; Aoki, M.; Vismer, H.; Muir, D. Mitochondrial DNA analysis of Sporothrix schenckii in South Africa and Australia. Med. Mycol. 2000, 38, 433–436. [Google Scholar] [CrossRef]
- Ishizaki, H.; Kawasaki, M.; Aoki, M.; Matsumoto, T.; Padhye, A.A.; Mendoza, M.; Negroni, R. Mitochondrial DNA analysis of Sporothrix schenckii in North and South America. Mycopathologia 1998, 142, 115–118. [Google Scholar] [CrossRef]
- Takeda, Y.; Kawasaki, M.; Ishizaki, H. Phylogeny and molecular epidemiology of Sporothrix schenckii in Japan. Mycopathologia 1991, 116, 9–14. [Google Scholar] [CrossRef]
- Suzuki, K.; Kawasaki, M.; Ishizaki, H. Analysis of restriction profiles of mitochondrial DNA from Sporothrix schenckii and related fungi. Mycopathologia 1988, 103, 147–151. [Google Scholar] [CrossRef]
- Mochizuki, H.; Anzawa, K.; Mochizuki, T. Genotyping of intraspecies polymorphisms of Sporothrix globosa using partial sequence of mitochondrial DNA. J. Dermatol. 2022, 49, 263–271. [Google Scholar] [CrossRef]
- Zhao, L.; Cui, Y.; Zhen, Y.; Yao, L.; Shi, Y.; Song, Y.; Chen, R.; Li, S. Genetic variation of Sporothrix globosa isolates from diverse geographic and clinical origins in China. Emerg. Microbes Infect. 2017, 6, e88. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Shankarnarayan, S.A.; Hemashetter, B.M.; Verma, S.; Chauhan, S.; Nath, R.; Savio, J.; Capoor, M.; Kaur, H.; Ghosh, A.K.; et al. Phenotypic and molecular characterisation of Sporothrix globosa of diverse origin from India. Braz. J. Microbiol. 2021, 52, 91–100. [Google Scholar] [CrossRef]
- Eppinger, M.; Pearson, T.; Koenig, S.S.K.; Pearson, O.; Hicks, N.; Agrawal, S.; Sanjar, F.; Galens, K.; Daugherty, S.; Crabtree, J.; et al. Genomic epidemiology of the Haitian cholera outbreak: A single introduction followed by rapid, extensive, and continued spread characterized the onset of the epidemic. mBio 2014, 5, e01721-14. [Google Scholar] [CrossRef]
- Rydholm, C.; Szakacs, G.; Lutzoni, F. Low genetic variation and no detectable population structure in Aspergillus fumigatus compared to closely related Neosartorya species. Eukaryot. Cell 2006, 5, 650–657. [Google Scholar] [CrossRef]
- Sewell, T.R.; Zhu, J.; Rhodes, J.; Hagen, F.; Meis, J.F.; Fisher, M.C.; Jombart, T. Nonrandom Distribution of Azole Resistance across the Global Population of Aspergillus fumigatus. mBio 2019, 10, e01721-14. [Google Scholar] [CrossRef]
- Klaassen, C.H.; Gibbons, J.G.; Fedorova, N.D.; Meis, J.F.; Rokas, A. Evidence for genetic differentiation and variable recombination rates among Dutch populations of the opportunistic human pathogen Aspergillus fumigatus. Mol. Ecol. 2012, 21, 57–70. [Google Scholar] [CrossRef]
- Lofgren, L.A.; Ross, B.S.; Cramer, R.A.; Stajich, J.E. The pan-genome of Aspergillus fumigatus provides a high-resolution view of its population structure revealing high levels of lineage-specific diversity driven by recombination. PLoS Biol. 2022, 20, e3001890. [Google Scholar] [CrossRef]
- Weir, B.S. Inferences about linkage disequilibrium. Biometrics 1979, 35, 235–254. [Google Scholar] [CrossRef]
- Stukenbrock, E.H.; Dutheil, J.Y. Fine-Scale Recombination Maps of Fungal Plant Pathogens Reveal Dynamic Recombination Landscapes and Intragenic Hotspots. Genetics 2018, 208, 1209–1229. [Google Scholar] [CrossRef]
- Schurko, A.M.; Neiman, M.; Logsdon, J.M., Jr. Signs of sex: What we know and how we know it. Trends Ecol. Evol. 2009, 24, 208–217. [Google Scholar] [CrossRef]
- Ruggiero, M.V.; D’Alelio, D.; Ferrante, M.I.; Santoro, M.; Vitale, L.; Procaccini, G.; Montresor, M. Clonal expansion behind a marine diatom bloom. ISME J. 2018, 12, 463–472. [Google Scholar] [CrossRef]
- Tibayrenc, M.; Ayala, F.J. Reproductive clonality of pathogens: A perspective on pathogenic viruses, bacteria, fungi, and parasitic protozoa. Proc. Natl. Acad. Sci. USA 2012, 109, E3305–E3313. [Google Scholar] [CrossRef]
- Úbeda, F.; Haig, D.; Patten, M.M. Stable linkage disequilibrium owing to sexual antagonism. Proc. Biol. Sci. 2011, 278, 855–862. [Google Scholar] [CrossRef]
- Little, T.J.; Ebert, D. Sex, linkage disequilibrium and patterns of parasitism in three species of cyclically parthenogenetic Daphnia (Cladocera: Crustacea). Heredity 2000, 85, 257–265. [Google Scholar] [CrossRef]
- Hosid, E.; Grishkan, I.; Yusim, E.; Frenkel, Z.; Wasser, S.P.; Nevo, E.; Korol, A. The mode of reproduction in natural populations of ascomycetous fungus, Emericella nidulans, from Israel. Genet. Res. 2010, 92, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kano, R.; Anzawa, K.; Mochizuki, T.; Nishimoto, K.; Hiruma, M.; Kamata, H.; Hasegawa, A. Sporothrix schenckii (sensu strict S. globosa) mating type 1-2 (MAT1-2) gene. J. Dermatol. 2013, 40, 726–730. [Google Scholar] [CrossRef]
- Kano, R.; Tsui, C.K.; Hamelin, R.C.; Anzawa, K.; Mochizuki, T.; Nishimoto, K.; Hiruma, M.; Kamata, H.; Hasegawa, A. The MAT1-1:MAT1-2 ratio of Sporothrix globosa isolates in Japan. Mycopathologia 2015, 179, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Tsui, C.K.M.; Beauseigle, S.; Ojeda Alayon, D.I.; Rice, A.V.; Cooke, J.E.K.; Sperling, F.A.H.; Roe, A.D.; Hamelin, R.C. Fine-scale genetic diversity and relatedness in fungi associated with the mountain pine beetle. Can. J. For. Res. 2019, 49, 933–941. [Google Scholar] [CrossRef]
- Talas, F.; McDonald, B.A. Genome-wide analysis of Fusarium graminearum field populations reveals hotspots of recombination. BMC Genom. 2015, 16, 996. [Google Scholar] [CrossRef]
- Singh, N.K.; Karisto, P.; Croll, D. Population-level deep sequencing reveals the interplay of clonal and sexual reproduction in the fungal wheat pathogen Zymoseptoria tritici. Microb. Genom. 2021, 7, 678. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Leyval, C.; Bonnin, I. High genetic diversity in arbuscular mycorrhizal fungi: Evidence for recombination events. Heredity 2001, 87, 243–253. [Google Scholar] [CrossRef]
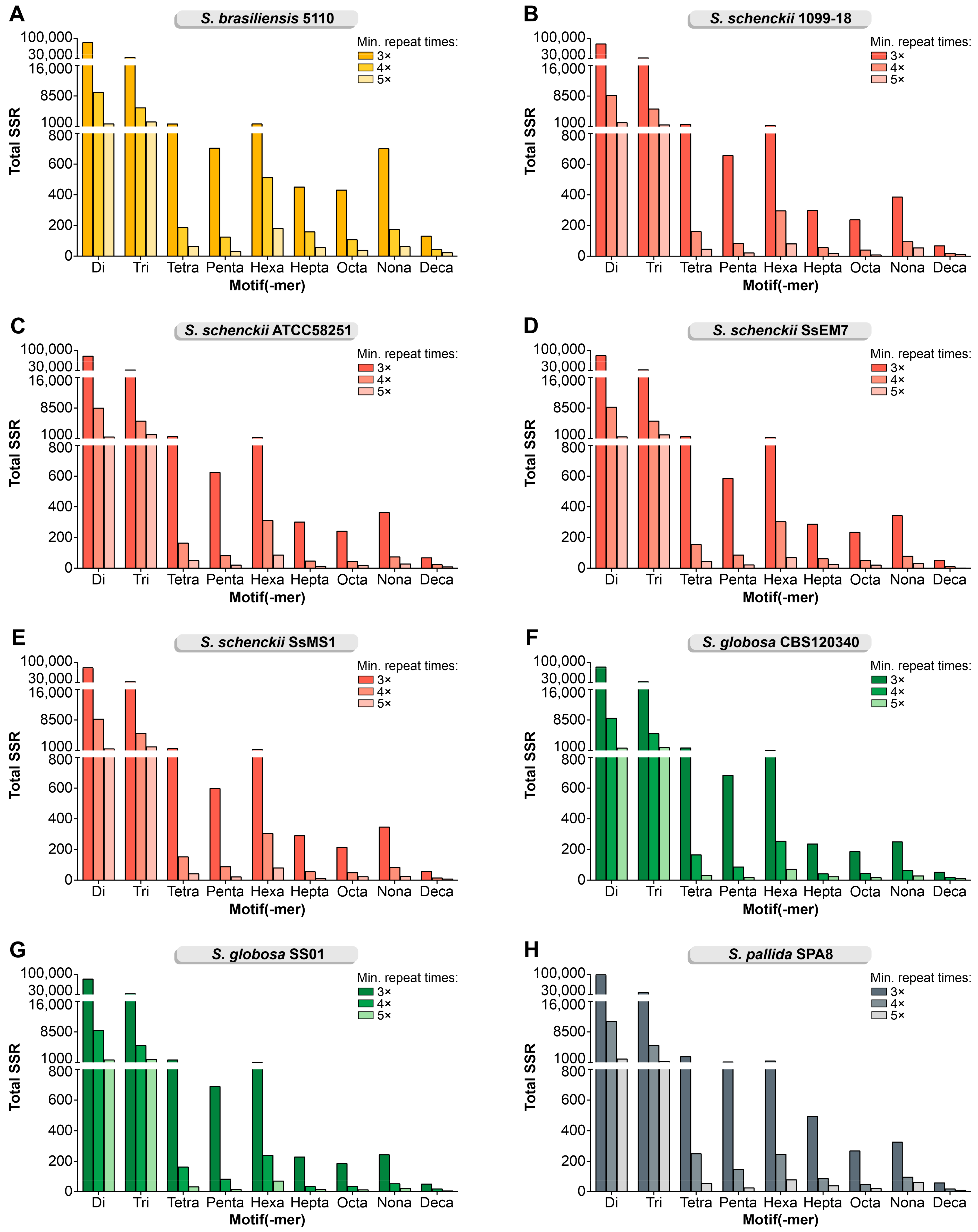
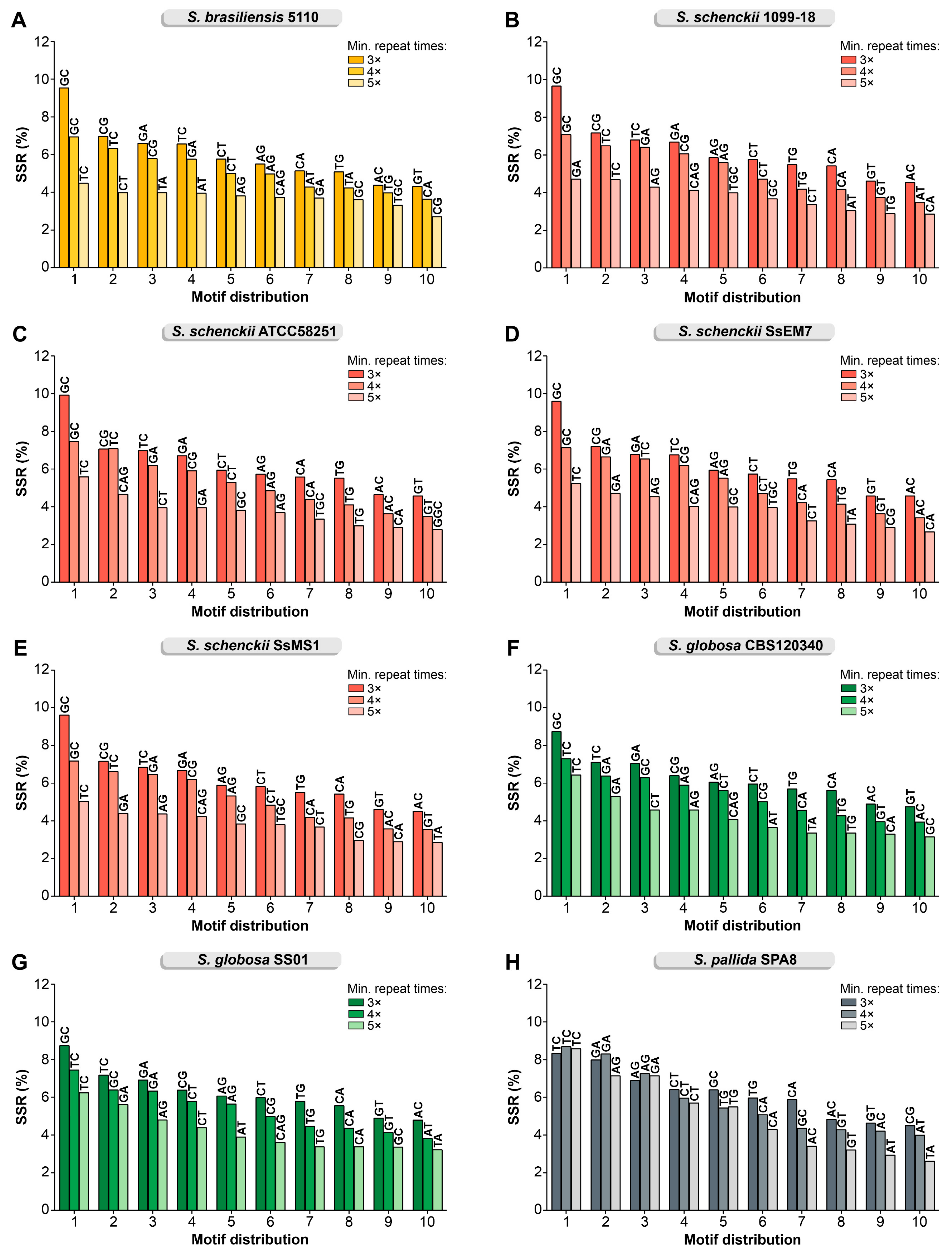

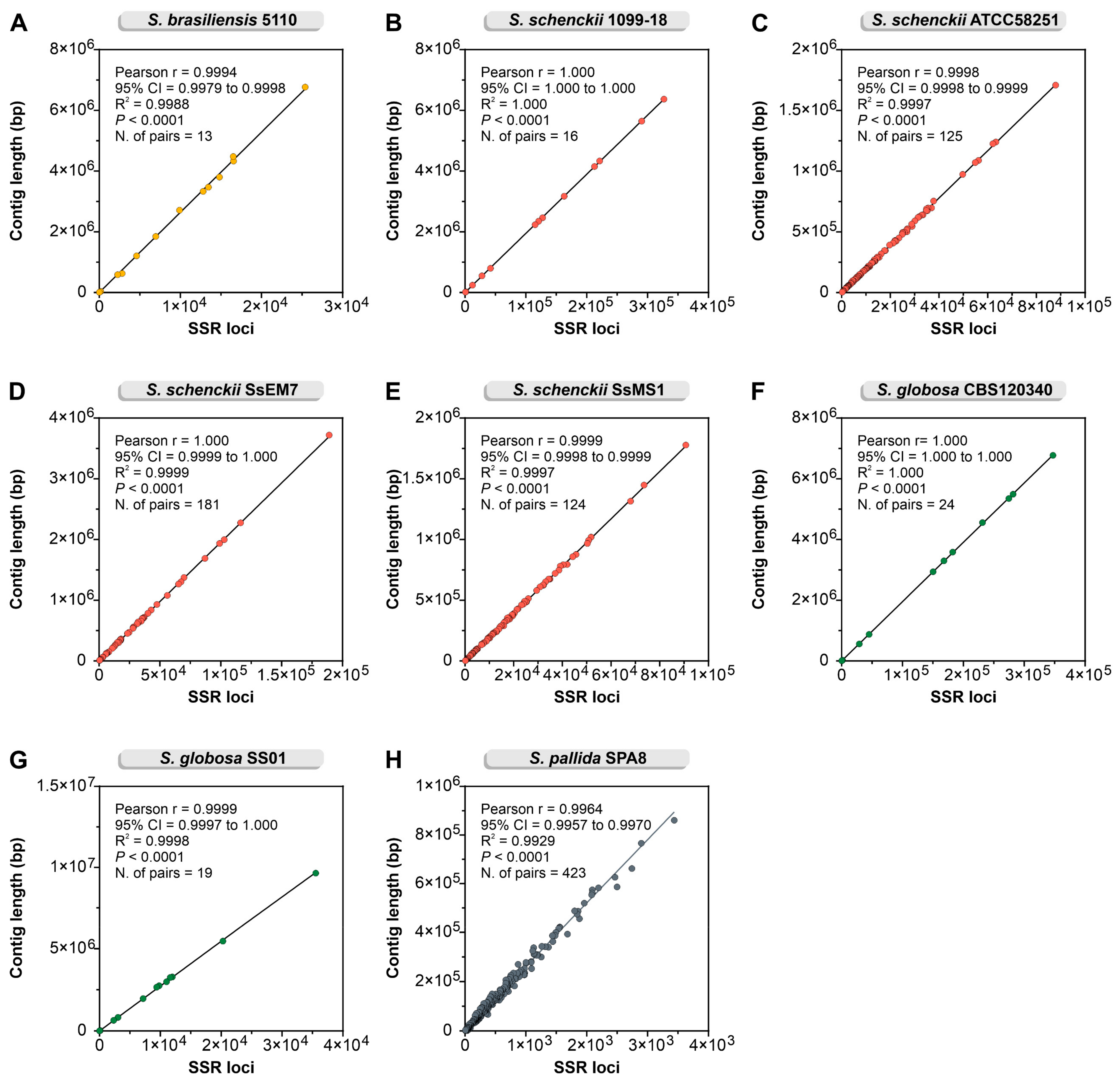
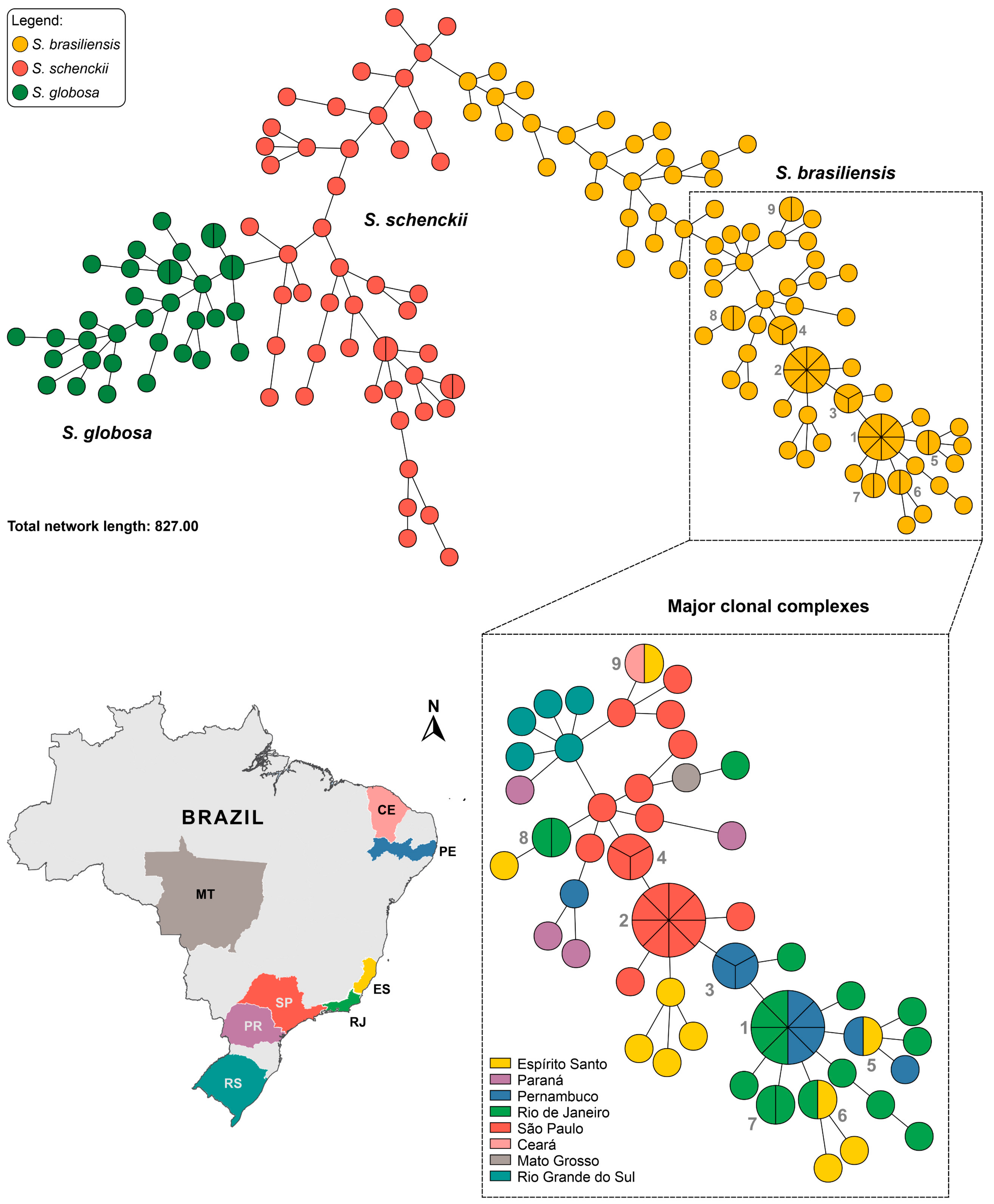
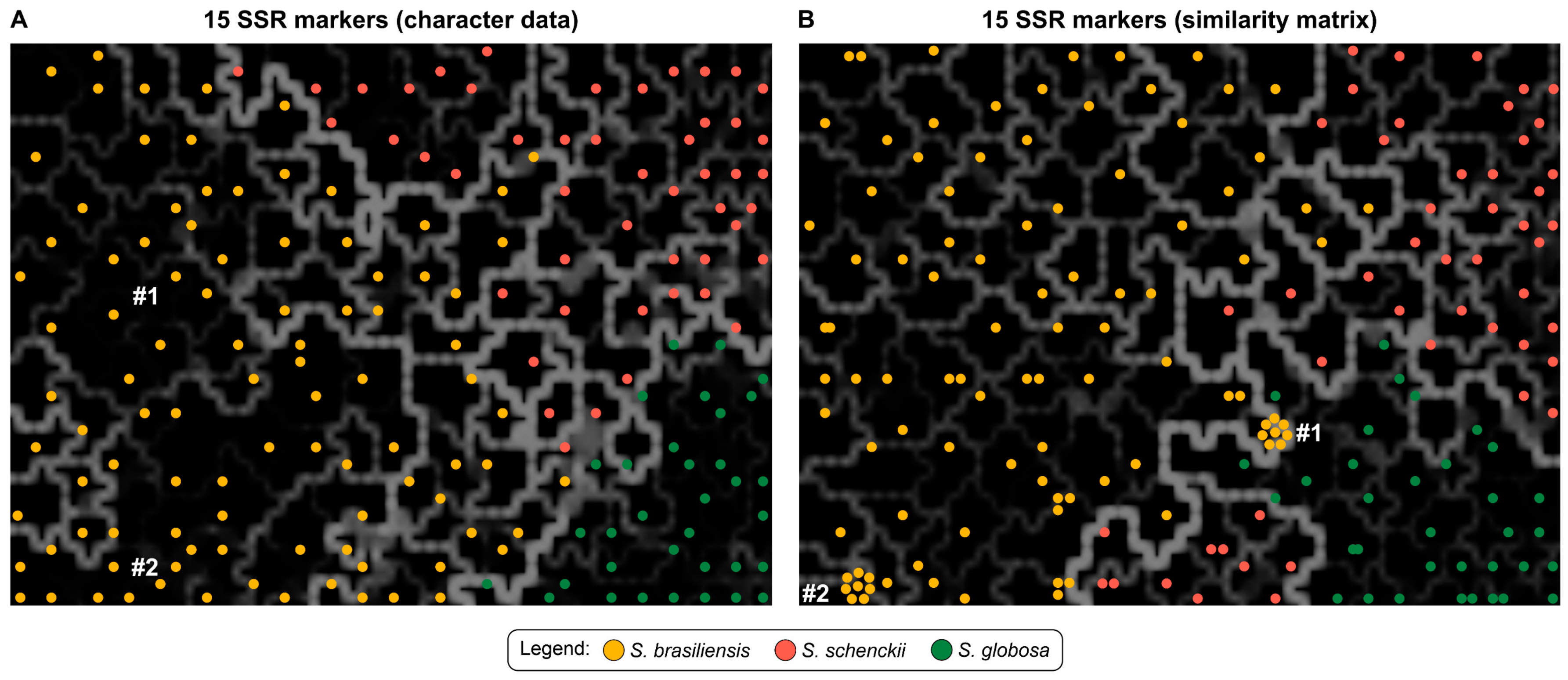
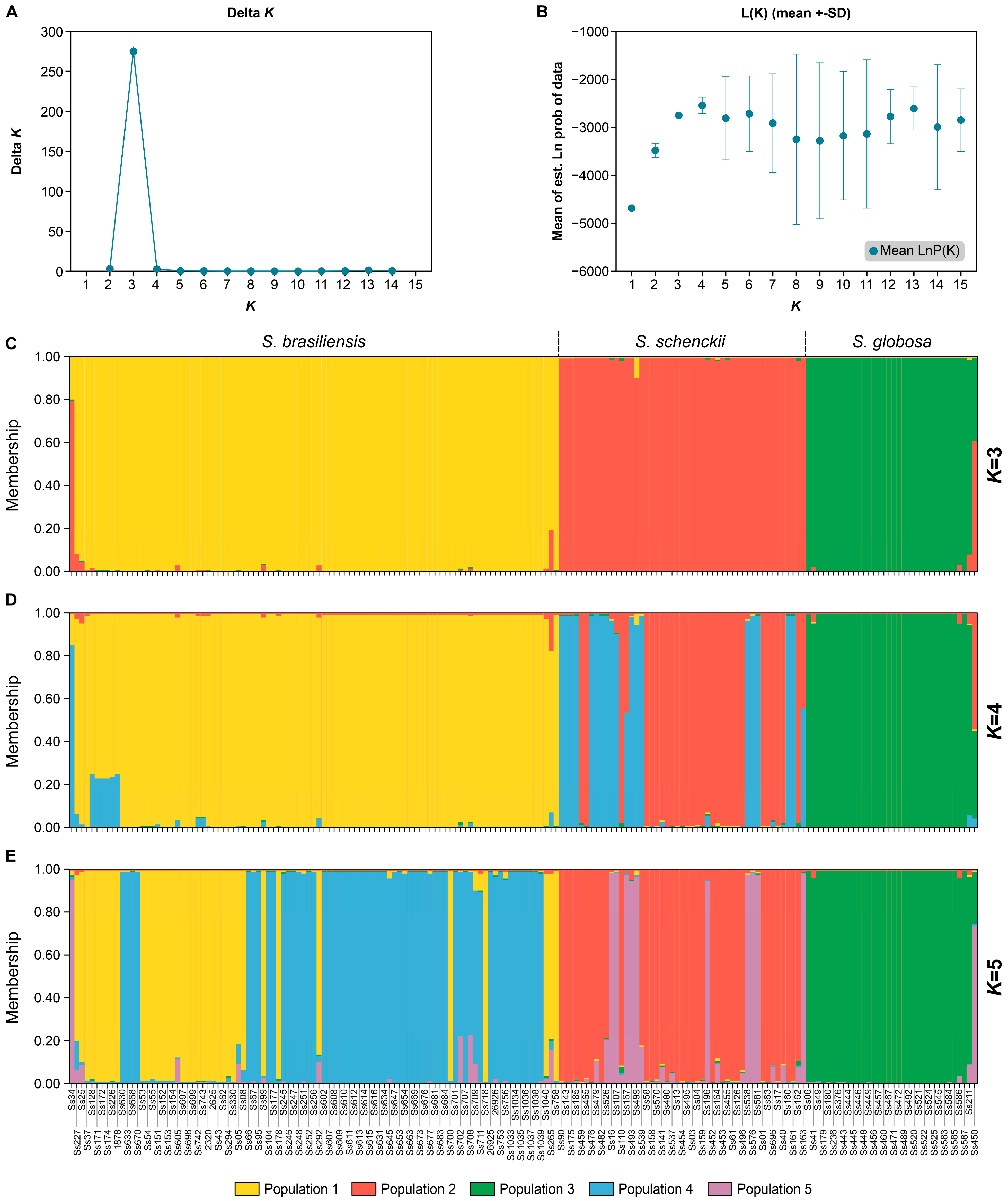

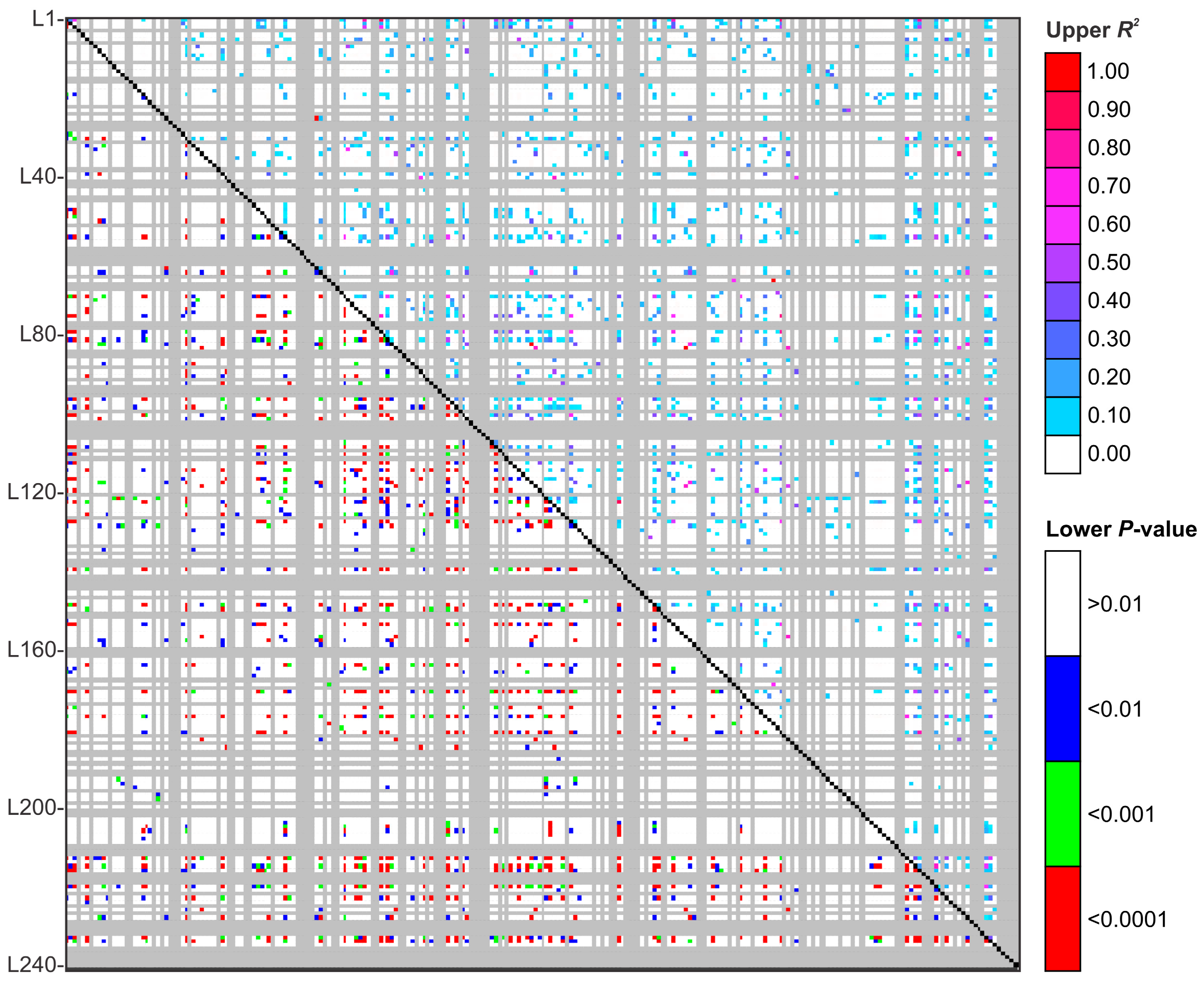
| Strains | Total SSR Loci | Frequency (SSRs/Mb) * | Total SSR Loci with Primer Pair Designed | Total SSR Loci Without Primer Pair Designed | Total Number of Unique Markers |
|---|---|---|---|---|---|
| 5110 | 126,286 | 3803.12 | 124,525 (98.60%) | 1761 (1.39%) | 86,689 |
| 1099-18 | 119,583 | 3693.67 | 118,265 (98.89%) | 1318 (1.10%) | 83,104 |
| ATCC58251 | 118,140 | 3670.30 | 117,648 (99.58%) | 492 (0.41%) | 82,841 |
| SsEM7 | 120,585 | 3668.41 | 119,435 (99.04%) | 1150 (0.95%) | 83,825 |
| SsMS1 | 120,118 | 3676.89 | 119,136 (99.18%) | 982 (0.81%) | 83,891 |
| CBS120340 | 122,335 | 3654.67 | 121,189 (99.06%) | 1146 (0.93%) | 85,279 |
| SS01 | 122,302 | 3651.55 | 121,251 (99.14%) | 1051 (0.85%) | 85,487 |
| SPA8 | 143,912 | 3804.65 | 141,266 (98.16%) | 2646 (1.83%) | 98,369 |
| SSR Loci | 5′ Dye | Primer Sequence (5′-3′) F: Forward; R: Reverse | Motif | Tm (°C) | Expected Amplicon Size (bp) * | ||
|---|---|---|---|---|---|---|---|
| 5110 | 1099-18 | CBS 120340 | |||||
| SSR11 | NED | F: TGGAAGCAAAACCATGGTGCCTTTC | CAGTCGCCCC | 60.5 | 87 | 125 | 96 |
| - | R: GAGATCTGCCAAAACGACCGTC | 58.2 | |||||
| SSR50 | VIC | F: TTCGACGCCACTAAAGAAGCCG | TGTAAGTGGA | 59.7 | 144 | 143 | 195 |
| - | R: ATTCATTTCTTTTCCAGCCTTGGT | 55.4 | |||||
| SSR61 | FAM | F: TTTTCTCGTGAGAAGGTCCCTCCC | TTCTTTTGCC | 60.3 | 136 | 208 | 139 |
| - | R: CCAAAGACGGCGGGAACAAGCG | 63.4 | |||||
| SSR150 | FAM | F: TGGAGCACGACACTCTACCTCACC | CAACGG | 62.0 | 389 | 383 | 389 |
| - | R: GTCGAGGCGGCAATACGAGATG | 60.1 | |||||
| SSR181 | NED | F: AGGACTTGGACGCATCGGTG | GAGCCA | 59.8 | 167 | 167 | 209 |
| - | R: CAATGTGACCCAGACCACCTTTG | 58.3 | |||||
| SSR199 | NED | F: CATGCTGTTGTAGATGGGCAGATG | CGCTGC | 58.1 | 570 | 543 | 579 |
| - | R: CAGGTGCAGCAAGAGTATGCGG | 60.6 | |||||
| SSR235 | FAM | F: TTGGTCCCGAGAAAGCCCAG | GATCCA | 59.7 | 477 | 453 | 429 |
| - | R: CTGGTGGCGGTCGATGGTGTC | 62.8 | |||||
| SSR307 | VIC | F: CTGTAAGGGTGTGTTGTACGTCGTTG | AGCAGA | 59.4 | 355 | 309 | 309 |
| - | R: GATCGCGCAGGTTGCCTATT | 58.2 | |||||
| SSR343 | FAM | F: GTGGCCACTGTAGTACGCGAC | TGC | 60.1 | 268 | 235 | 230 |
| - | R: CATACTCGCGGAGTTTCGGATCTC | 59.0 | |||||
| SSR391 | NED | F: ACTGTTCCATTCTCCCGACCGAG | CTACAC | 60.6 | 272 | 302 | - |
| - | R: GTGCTCAAGTCGTGCAAGGGC | 61.5 | |||||
| SSR408 | NED | F: TTGGAAGAAGCGGCAACAAGTGG | CTGGGA | 60.4 | 273 | 292 | 265 |
| - | R: TAGGGCGGCAGGACGGGAAG | 64.2 | |||||
| SSR538 | VIC | F: CTTGGAGGTGTTGATGAGTGTCTGC | ACCAGG | 59.6 | 250 | 256 | 243 |
| - | R: CACATCACAGACGACGCTGGAC | 60.1 | |||||
| SSR637 | VIC | F: TCGGTTTCCTCGACCAATTCC | ACCG | 57.2 | 191 | 183 | 178 |
| - | R: CATGGTTGCGGTGGCTTATGTCG | 60.8 | |||||
| SSR646 | VIC | F: TCTTGTGGCGGAGTGGCTGTC | CAC | 62.1 | 362 | 362 | 374 |
| - | R: GCACATCCTGTGTCCAGCGAAC | 60.8 | |||||
| SSR661 | FAM | F: TACCGTTGCGCTTTGCCCCATTTG | TGTTT | 62.8 | 371 | 253 | 253 |
| - | R: CATCGGCAGCCATTCCATTTGTG | 59.3 | |||||
| Multiplex ID | Species (n) | Alleles (n) | H | PIC | E | Havp | MI | D |
|---|---|---|---|---|---|---|---|---|
| M1 | S. brasiliensis (97) | 26 | 0.7908 | 0.7644 | 1.0000 | 0.7908 | 0.7908 | 0.4045 |
| S. schenckii (49) | 43 | 0.8980 | 0.8899 | 1.0000 | 0.8980 | 0.8980 | 0.8370 | |
| S. globosa (34) | 13 | 0.8089 | 0.7845 | 1.0000 | 0.8089 | 0.8089 | 0.4908 | |
| Overall (180) | 64 | 0.9010 | 0.8941 | 1.0000 | 0.9010 | 0.9010 | 0.7813 | |
| M2 | S. brasiliensis (97) | 13 | 0.7291 | 0.6796 | 1.0000 | 0.7291 | 0.7291 | 0.2531 |
| S. schenckii (49) | 29 | 0.8740 | 0.8628 | 1.0000 | 0.8740 | 0.8740 | 0.7633 | |
| S. globosa (34) | 8 | 0.7141 | 0.6599 | 1.0000 | 0.7141 | 0.7141 | 0.1468 | |
| Overall (180) | 40 | 0.8759 | 0.8637 | 1.0000 | 0.8759 | 0.8759 | 0.7183 | |
| M3 | S. brasiliensis (97) | 18 | 0.8245 | 0.8029 | 1.0000 | 0.8245 | 0.8245 | 0.4825 |
| S. schenckii (49) | 28 | 0.8032 | 0.7821 | 1.0000 | 0.8032 | 0.8032 | 0.5986 | |
| S. globosa (34) | 9 | 0.7803 | 0.7473 | 1.0000 | 0.7803 | 0.7803 | 0.3571 | |
| Overall (180) | 40 | 0.8650 | 0.8524 | 1.0000 | 0.8650 | 0.8650 | 0.7202 | |
| M4 | S. brasiliensis (97) | 39 | 0.7785 | 0.7522 | 1.0000 | 0.7785 | 0.7785 | 0.3736 |
| S. schenckii (49) | 27 | 0.8523 | 0.8371 | 1.0000 | 0.8523 | 0.8523 | 0.6366 | |
| S. globosa (34) | 17 | 0.7797 | 0.7525 | 1.0000 | 0.7797 | 0.7797 | 0.4504 | |
| Overall (180) | 67 | 0.8636 | 0.8519 | 1.0000 | 0.8636 | 0.8636 | 0.7172 | |
| M5 | S. brasiliensis (97) | 20 | 0.6023 | 0.5381 | 1.0000 | 0.6023 | 0.6023 | 0.2673 |
| S. schenckii (49) | 16 | 0.7420 | 0.7004 | 1.0000 | 0.7420 | 0.7420 | 0.2449 | |
| S. globosa (34) | 3 | 0.6699 | 0.5987 | 1.0000 | 0.6699 | 0.6699 | 0.0927 | |
| Overall (180) | 29 | 0.7683 | 0.7392 | 1.0000 | 0.7683 | 0.7683 | 0.6265 | |
| 15 SSRs | S. brasiliensis (97) | 116 | 0.9057 | 0.8987 | 1.0000 | 0.9057 | 0.9057 | 0.3562 |
| S. schenckii (49) | 143 | 0.9077 | 0.9009 | 1.0000 | 0.9077 | 0.9077 | 0.6161 | |
| S. globosa (34) | 50 | 0.8993 | 0.8904 | 1.0000 | 0.8993 | 0.8993 | 0.3075 | |
| Overall (180) | 240 | 0.9159 | 0.9101 | 1.0000 | 0.9159 | 0.9159 | 0.7127 |
| Group | Source of Variation | df | SS | MS | Est. Var. | % | p-Value |
|---|---|---|---|---|---|---|---|
| Sporothrix | Among population | 2 | 1609.687 | 804.843 | 14.677 | 54% | 0.0001 |
| (n = 180) | Within population | 177 | 2217.469 | 12.528 | 12.528 | 46% | 0.0001 |
| Total | 179 | 3827.156 | 27.205 | 100% | |||
| S. brasiliensis | Among population | 2 | 83.248 | 41.624 | 1.416 | 12% | 0.0001 |
| (n = 96) * | Within population | 93 | 934.752 | 10.051 | 10.051 | 88% | 0.0001 |
| Total | 95 | 1018.000 | 11.467 | 100% |
| LD Characteristic | Clinical Clade (n = 180) | S. brasiliensis (n = 97) | S. schenckii (n = 49) | S. globosa (n = 34) |
|---|---|---|---|---|
| Total number of markers | 240 | 240 | 240 | 240 |
| Number of markers pairs | 28,560 | 28,560 | 28,560 | 28,560 |
| Number of markers pairs at 0 ≤ p ≤ 0.001 | 871 (3.40%) | 102 (0.35%) | 87 (0.30%) | 7 (0.024%) |
| Number of markers pairs at 0.001 ≤ p ≤ 0.01 | 330 (1.15%) | 59 (0.20%) | 74 (0.25%) | 10 (0.035%) |
| Number of markers pairs at r2 = 1 | 4 (0.014%) | 2 (0.007%) | 8 (0.028%) | 0 |
| Mean r2 | 0.02714 | 0.04104 | 0.04957 | 0.05840 |
| Mean D′ | 0.8910 | 0.7750 | 0.8405 | 0.6170 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losada, L.C.d.M.L.; Monteiro, R.C.; de Carvalho, J.A.; Hagen, F.; Fisher, M.C.; Spruijtenburg, B.; Meis, J.F.; de Groot, T.; Gonçalves, S.S.; Negroni, R.; et al. High-Throughput Microsatellite Markers Development for Genetic Characterization of Emerging Sporothrix Species. J. Fungi 2023, 9, 354. https://doi.org/10.3390/jof9030354
Losada LCdML, Monteiro RC, de Carvalho JA, Hagen F, Fisher MC, Spruijtenburg B, Meis JF, de Groot T, Gonçalves SS, Negroni R, et al. High-Throughput Microsatellite Markers Development for Genetic Characterization of Emerging Sporothrix Species. Journal of Fungi. 2023; 9(3):354. https://doi.org/10.3390/jof9030354
Chicago/Turabian StyleLosada, Luiza Chaves de Miranda Leonhardt, Ruan Campos Monteiro, Jamile Ambrósio de Carvalho, Ferry Hagen, Matthew C. Fisher, Bram Spruijtenburg, Jacques F. Meis, Theun de Groot, Sarah Santos Gonçalves, Ricardo Negroni, and et al. 2023. "High-Throughput Microsatellite Markers Development for Genetic Characterization of Emerging Sporothrix Species" Journal of Fungi 9, no. 3: 354. https://doi.org/10.3390/jof9030354
APA StyleLosada, L. C. d. M. L., Monteiro, R. C., de Carvalho, J. A., Hagen, F., Fisher, M. C., Spruijtenburg, B., Meis, J. F., de Groot, T., Gonçalves, S. S., Negroni, R., Kano, R., Bonifaz, A., de Camargo, Z. P., & Rodrigues, A. M. (2023). High-Throughput Microsatellite Markers Development for Genetic Characterization of Emerging Sporothrix Species. Journal of Fungi, 9(3), 354. https://doi.org/10.3390/jof9030354







