Multi-Gene Phylogeny and Taxonomy of the Wood-Rotting Fungal Genus Phlebia sensu lato (Polyporales, Basidiomycota)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Herbarium Specimen Preparation
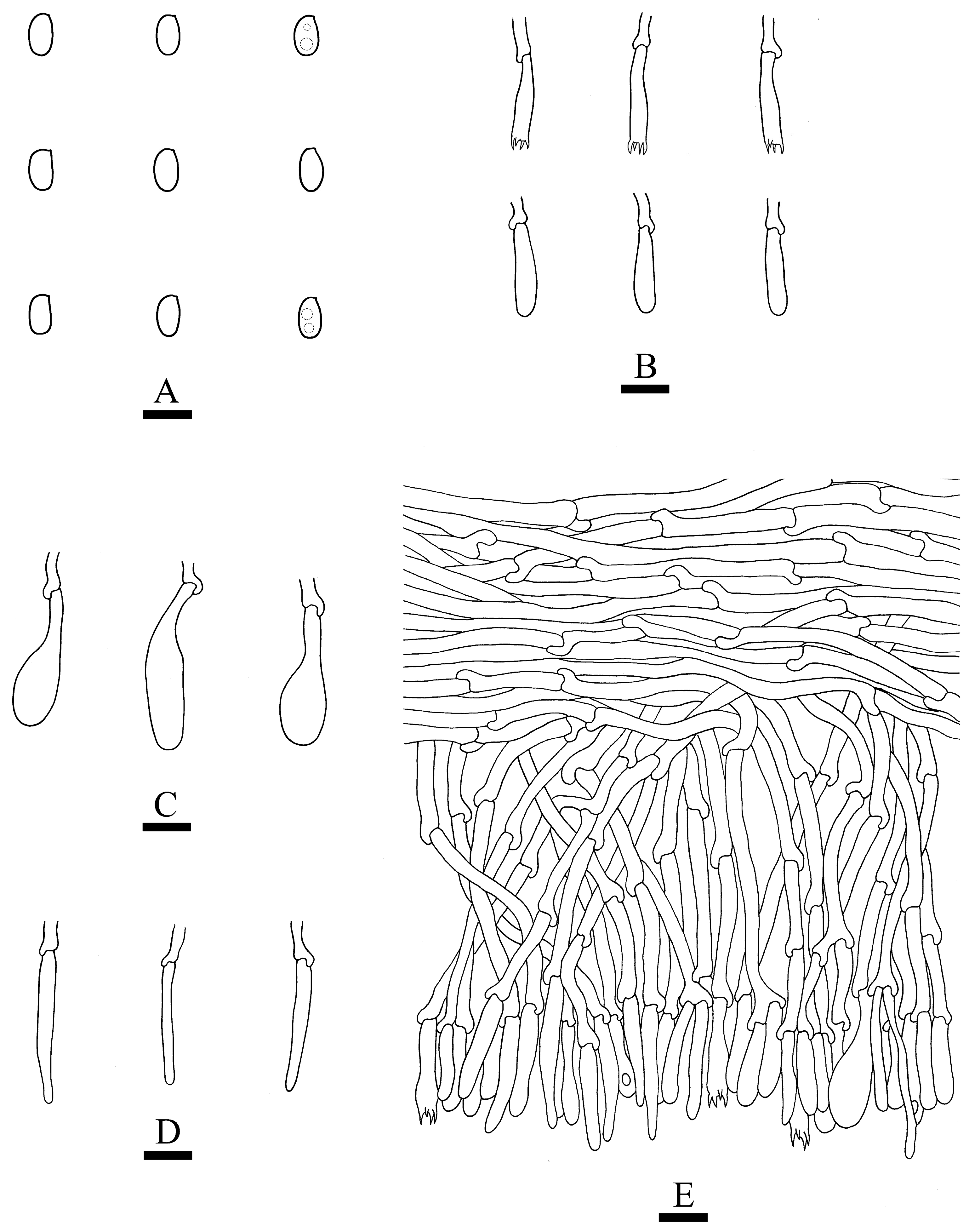
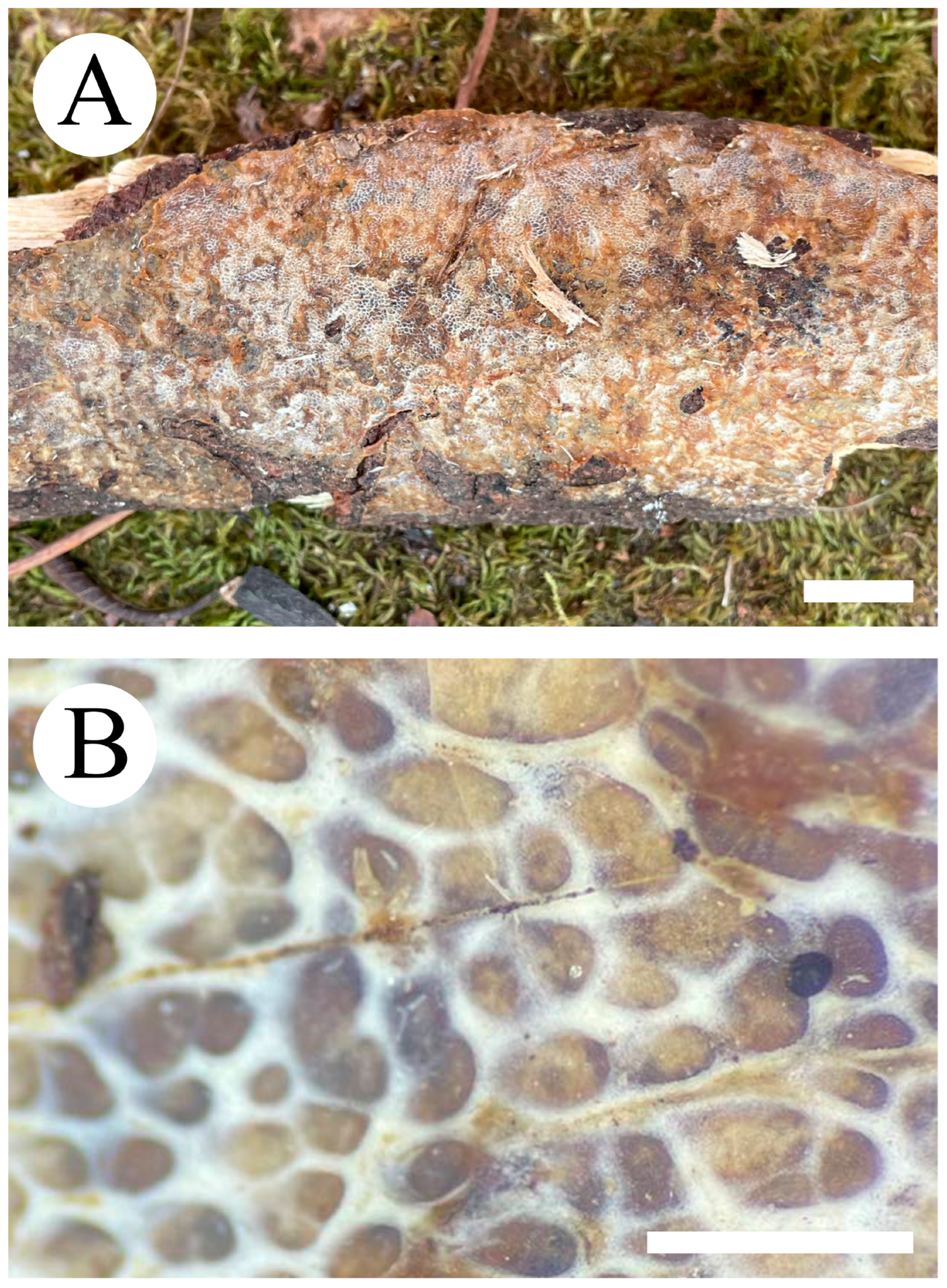
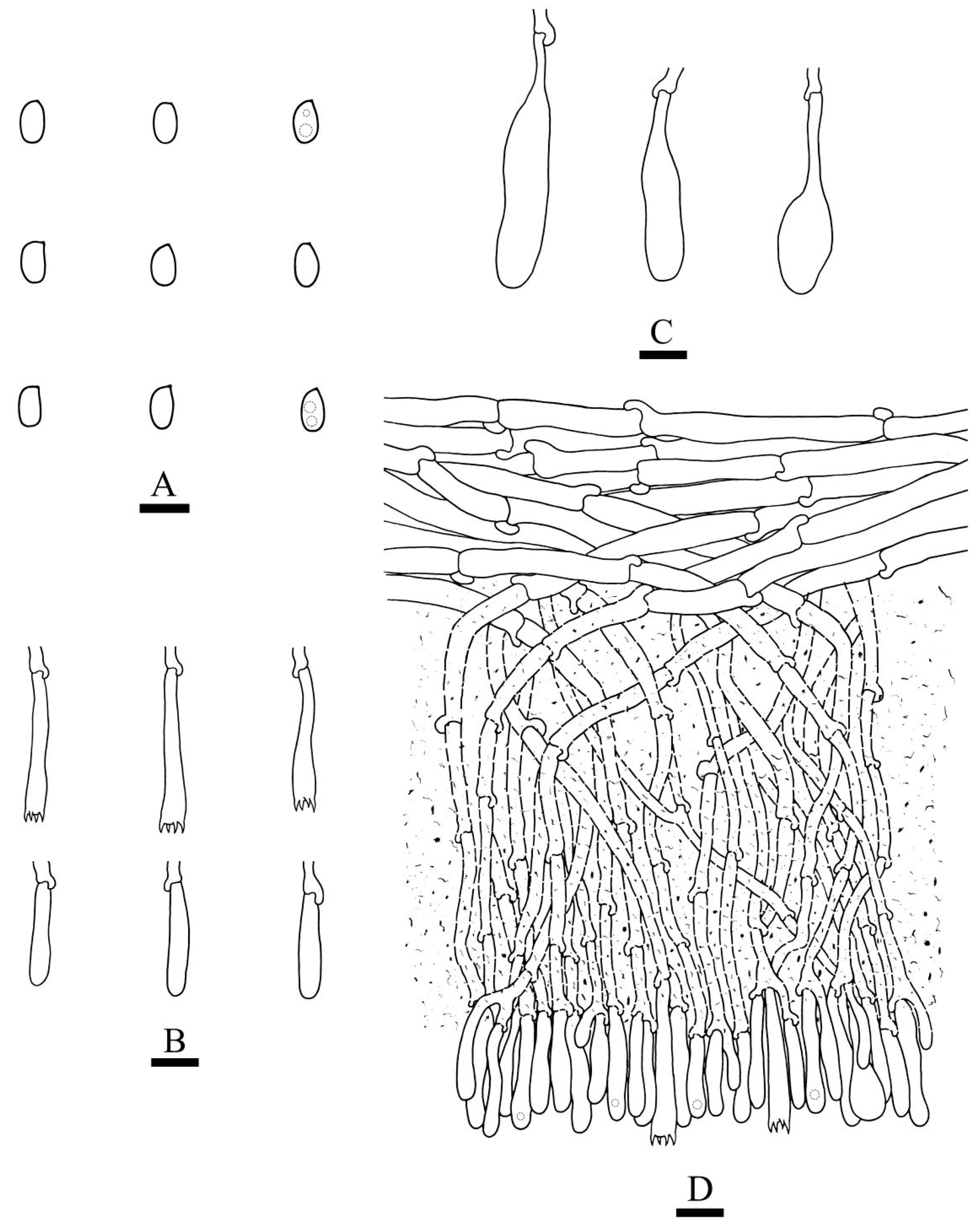
2.2. Morphology
2.3. DNA Extraction and Sequencing
2.4. Phylogenetic Analyses
3. Results
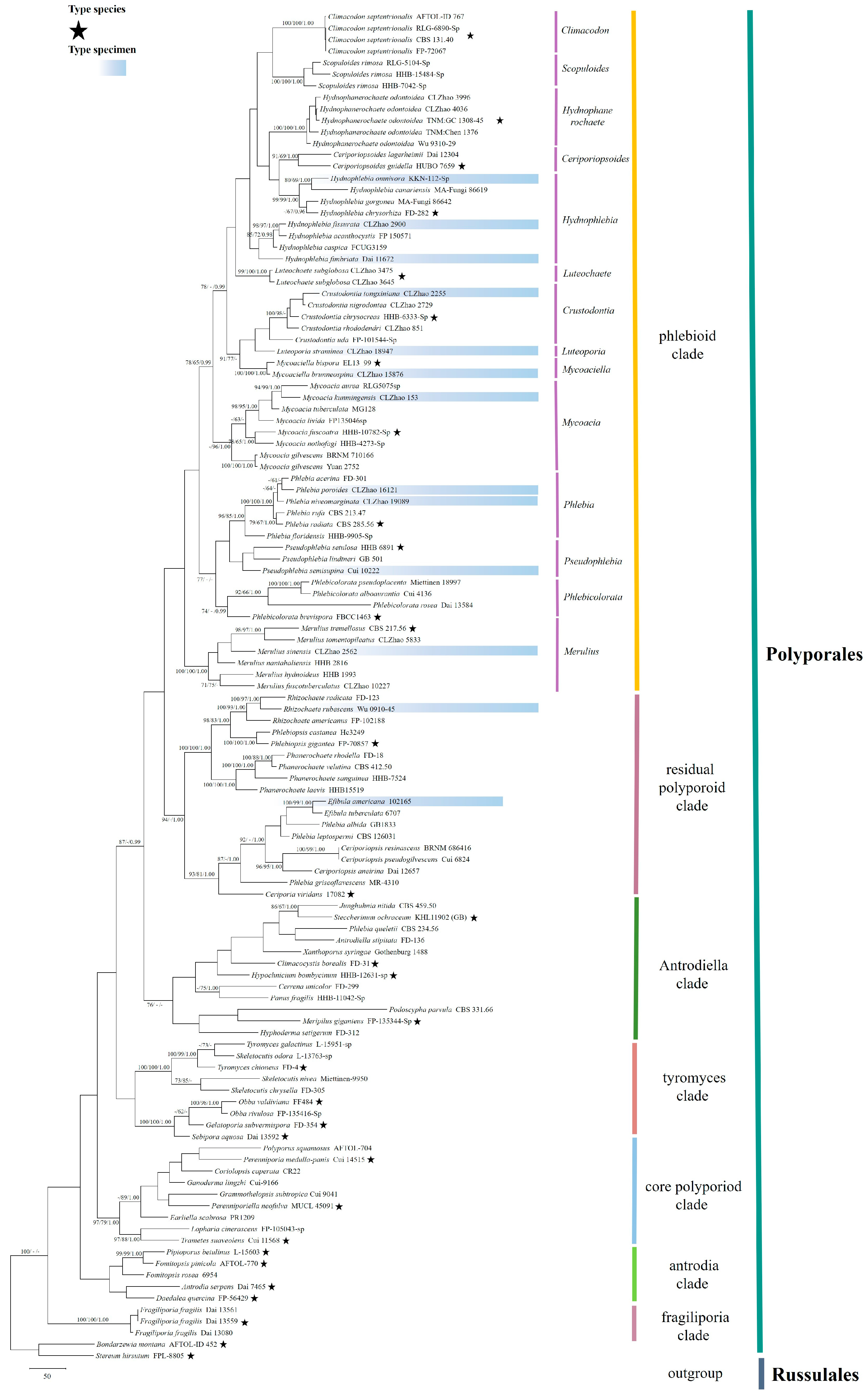
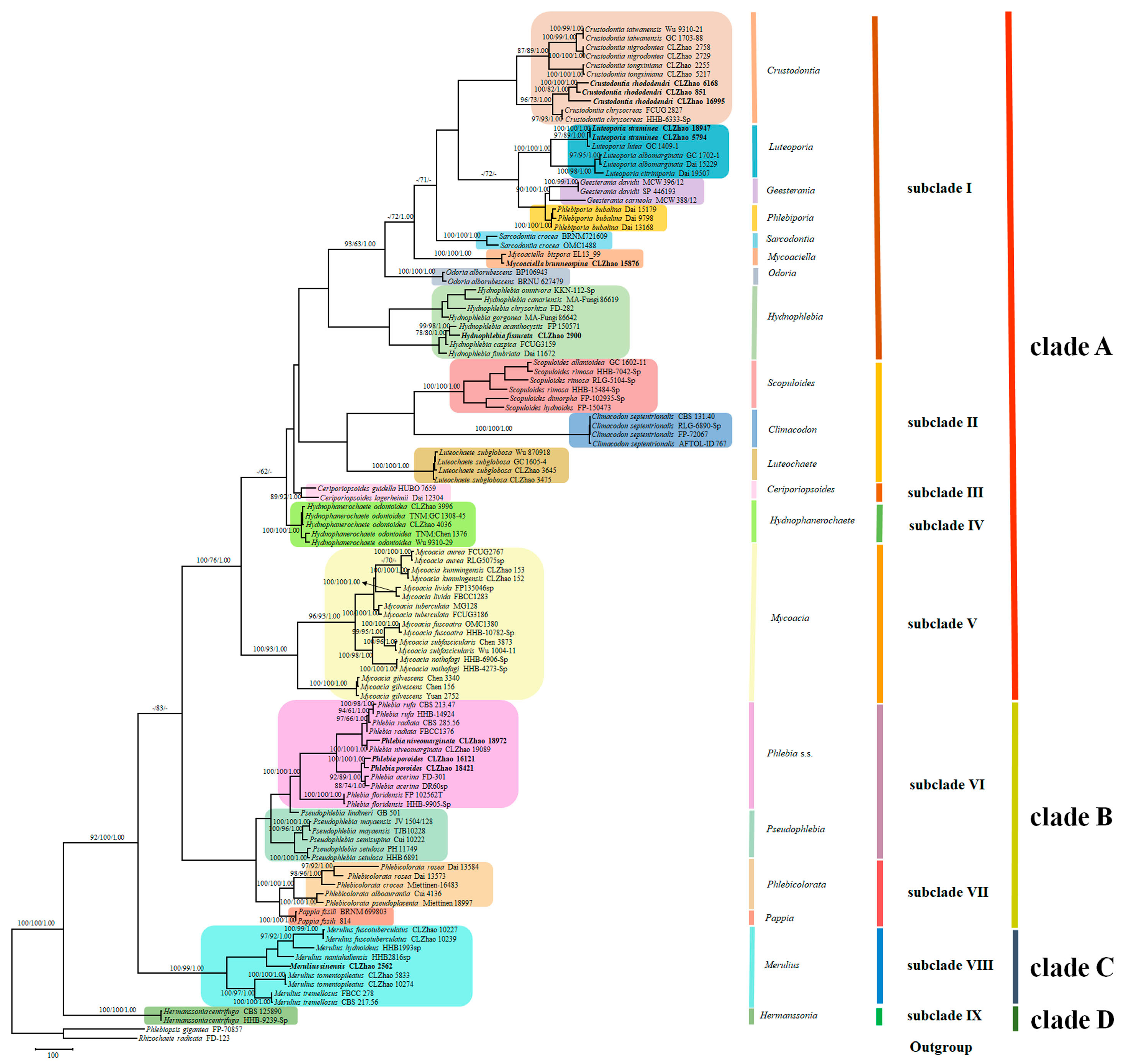
3.1. Phylogenetic Analyses
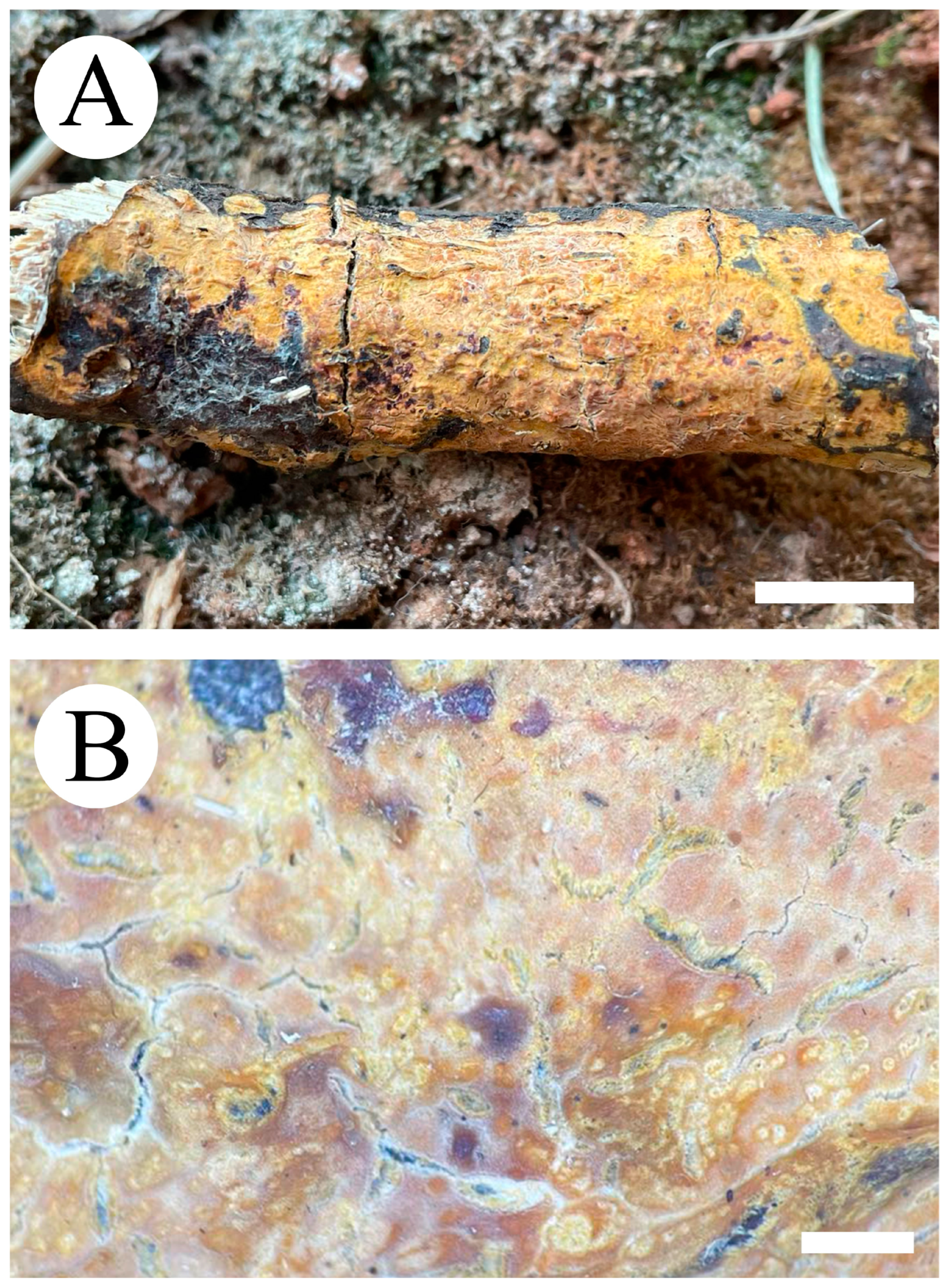
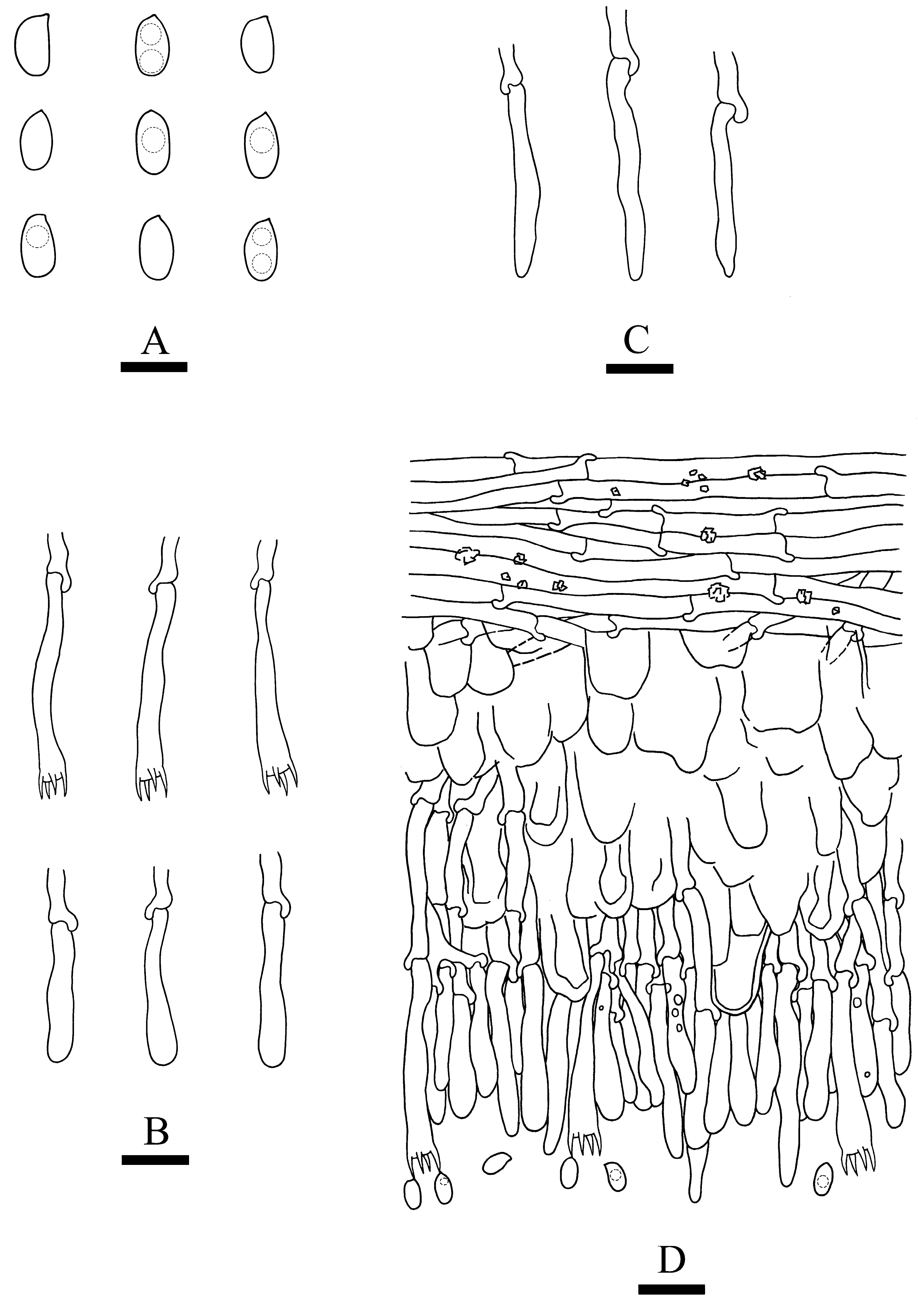
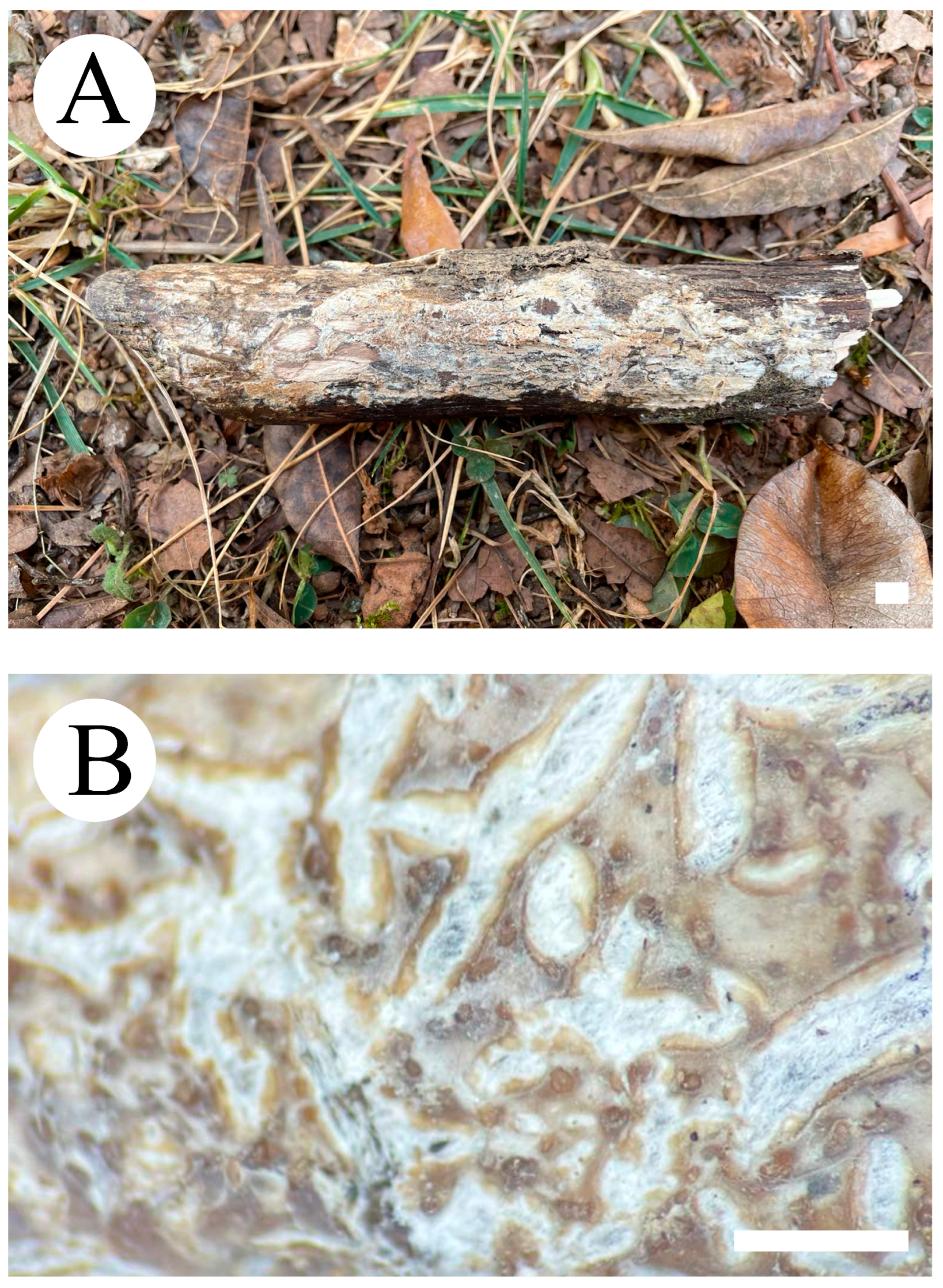
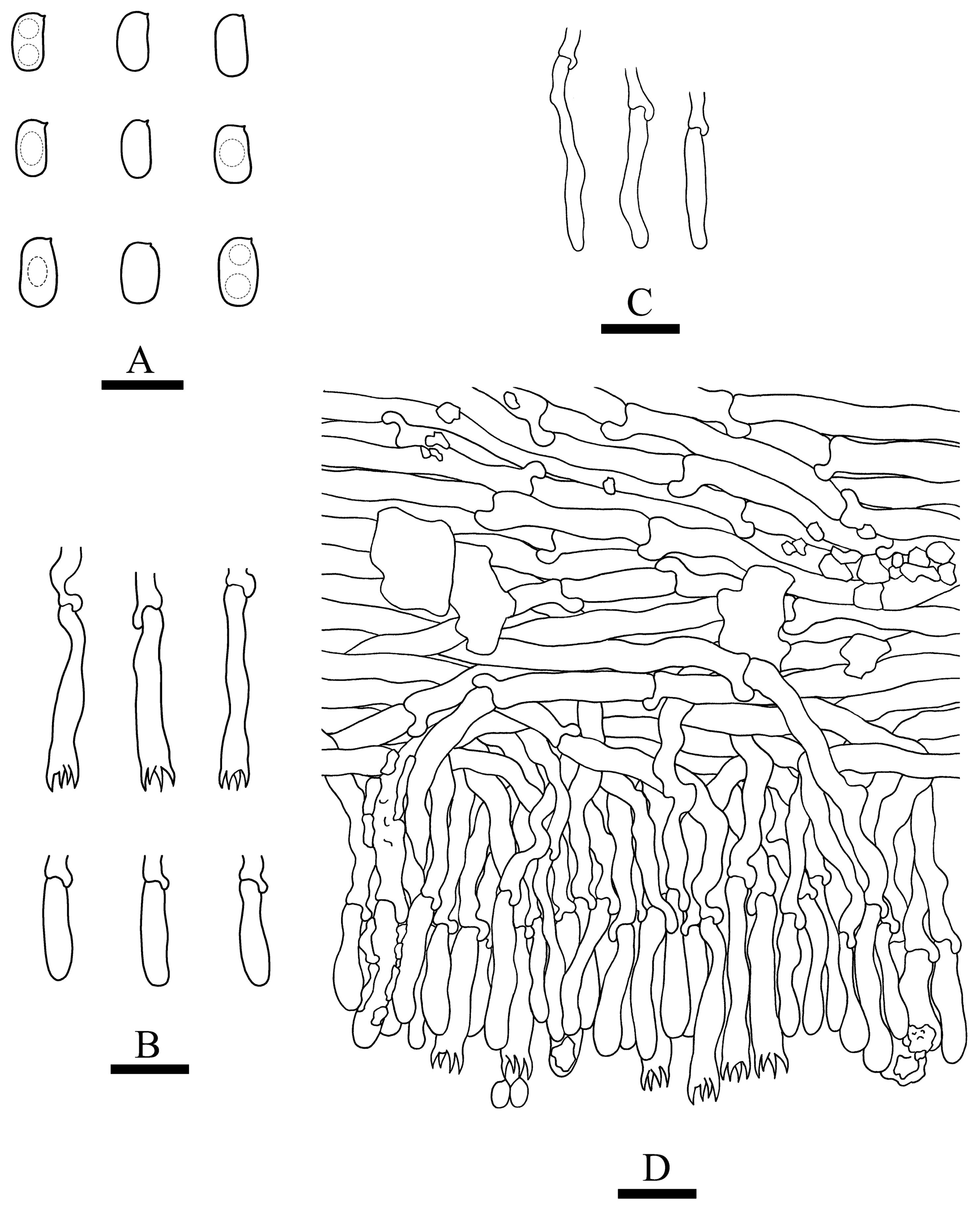
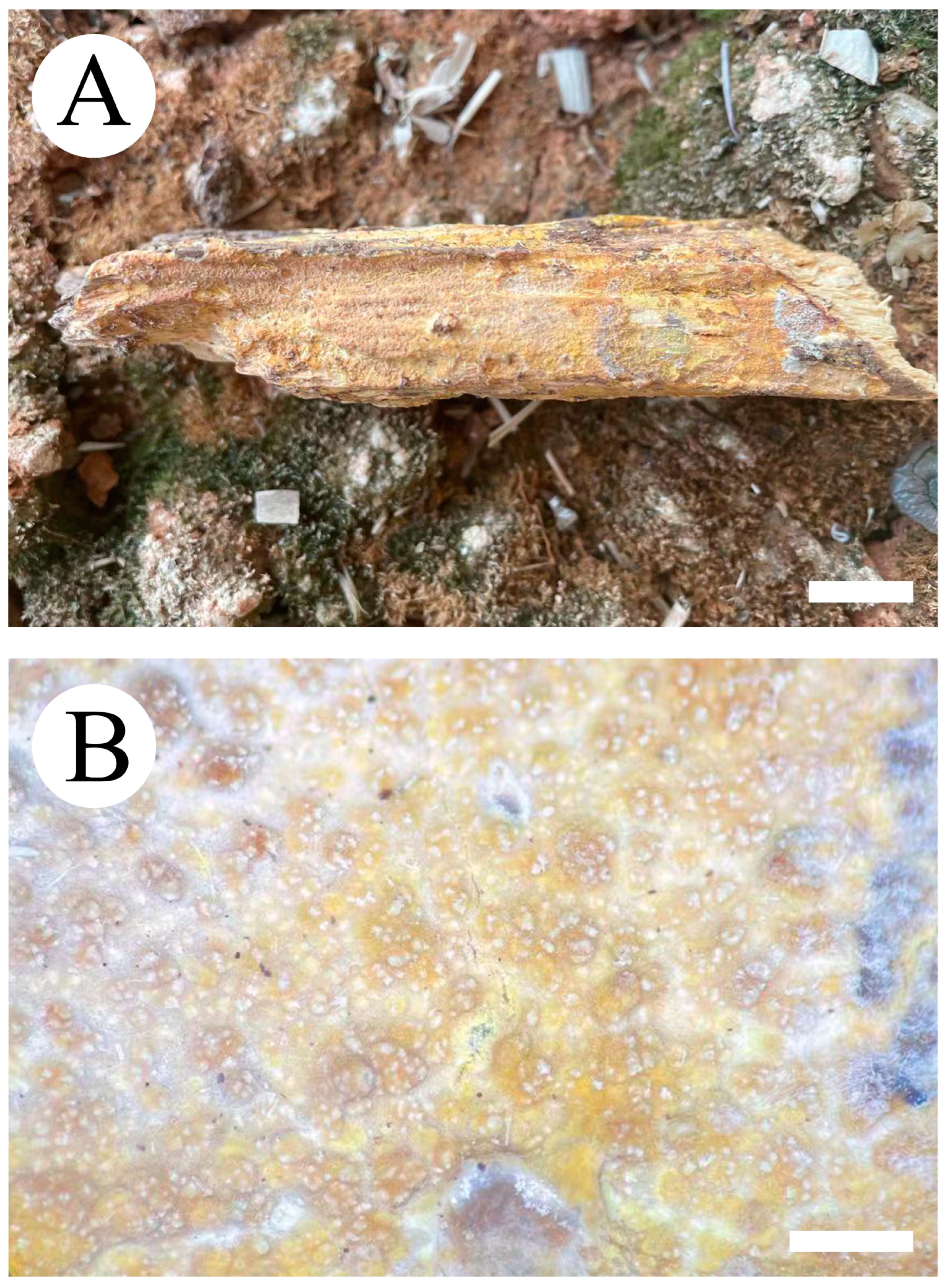
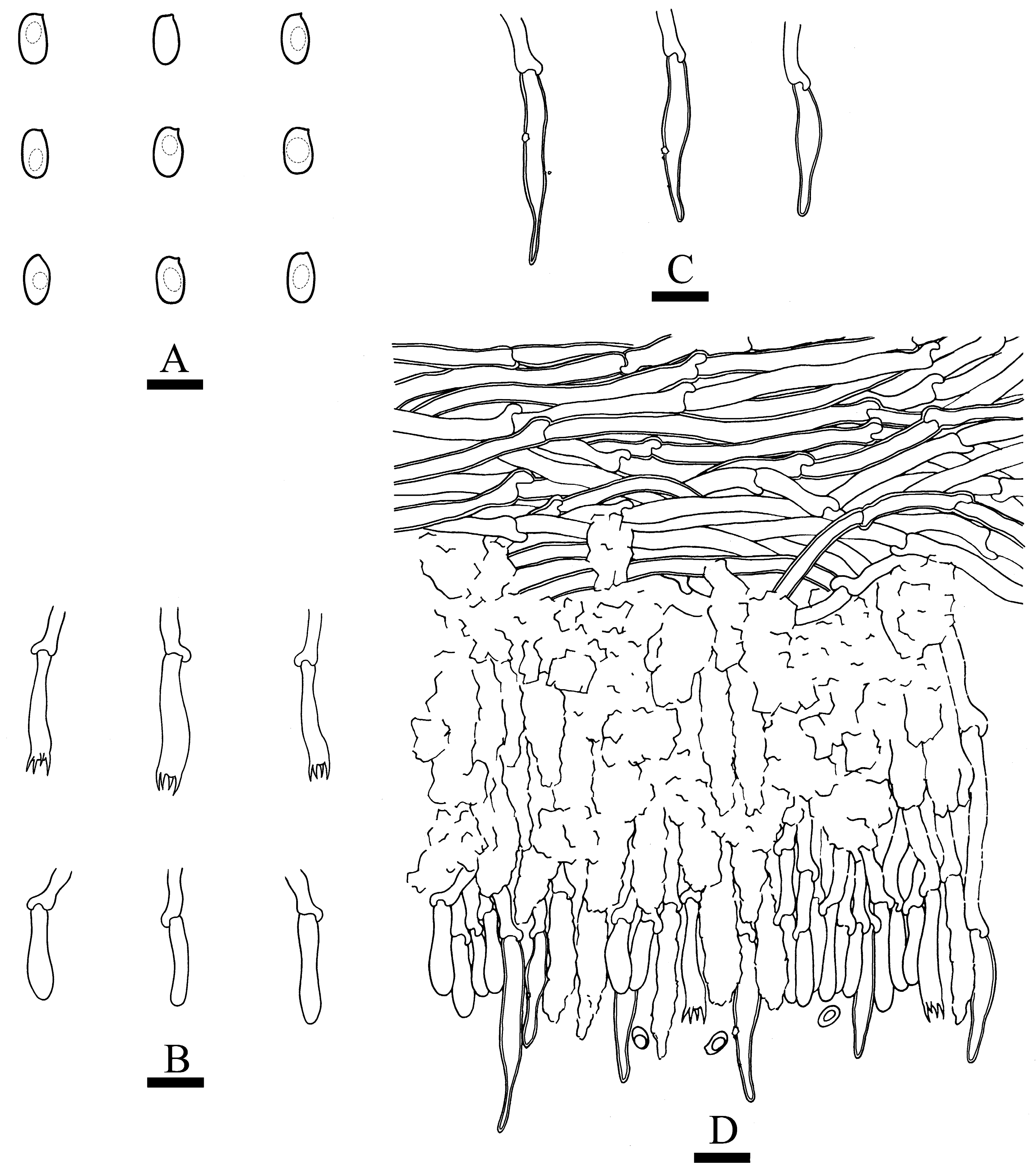
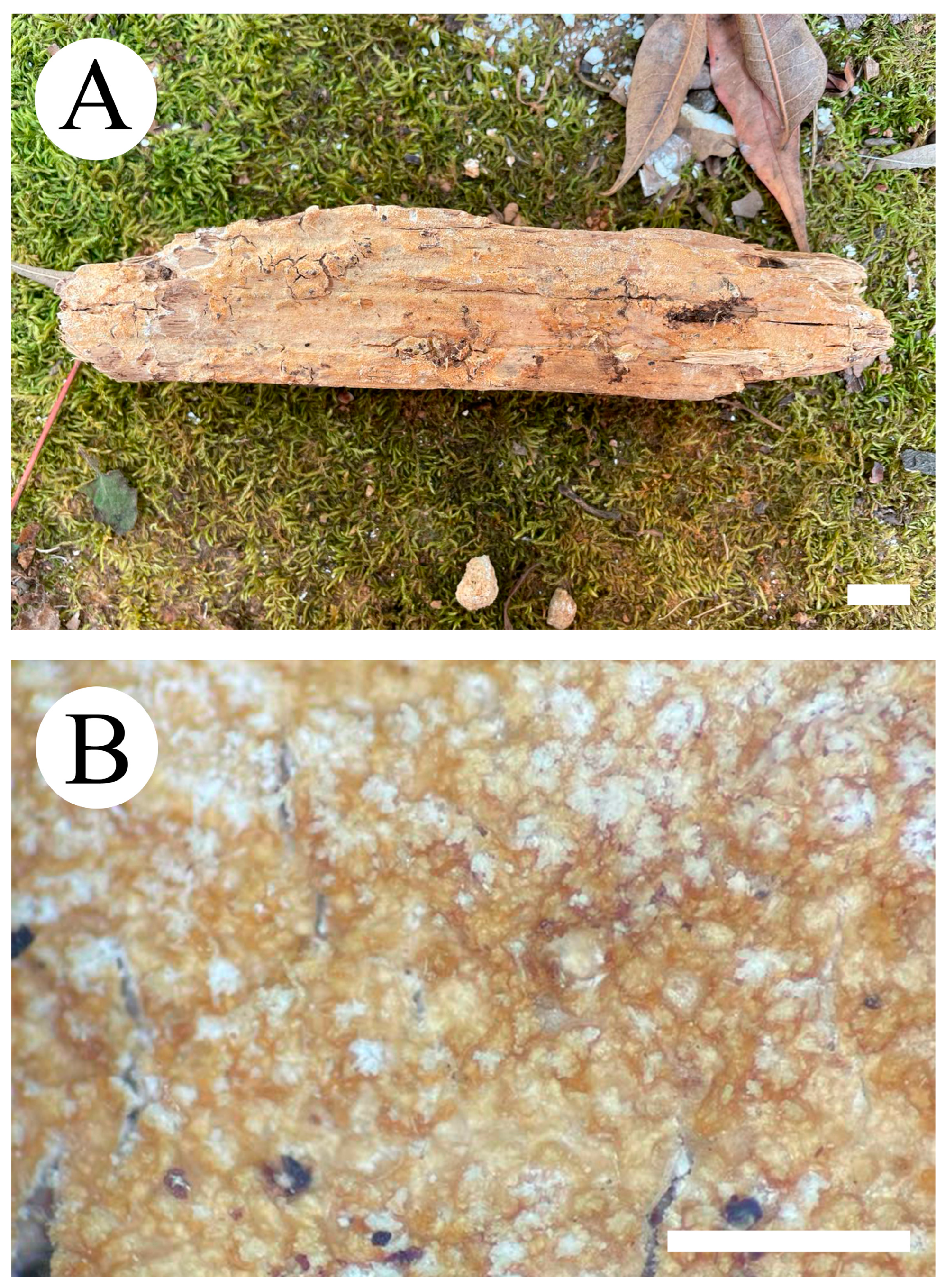
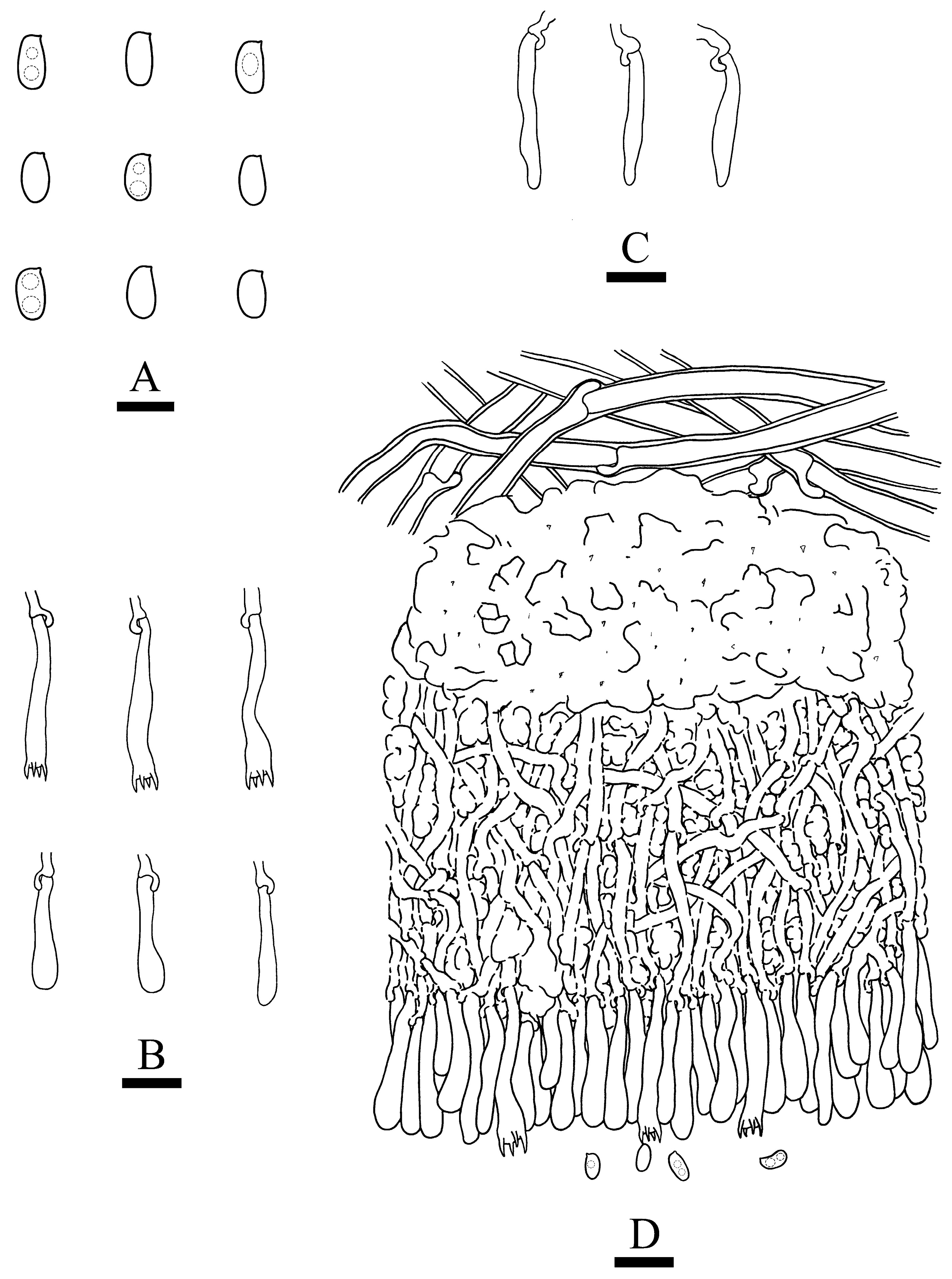
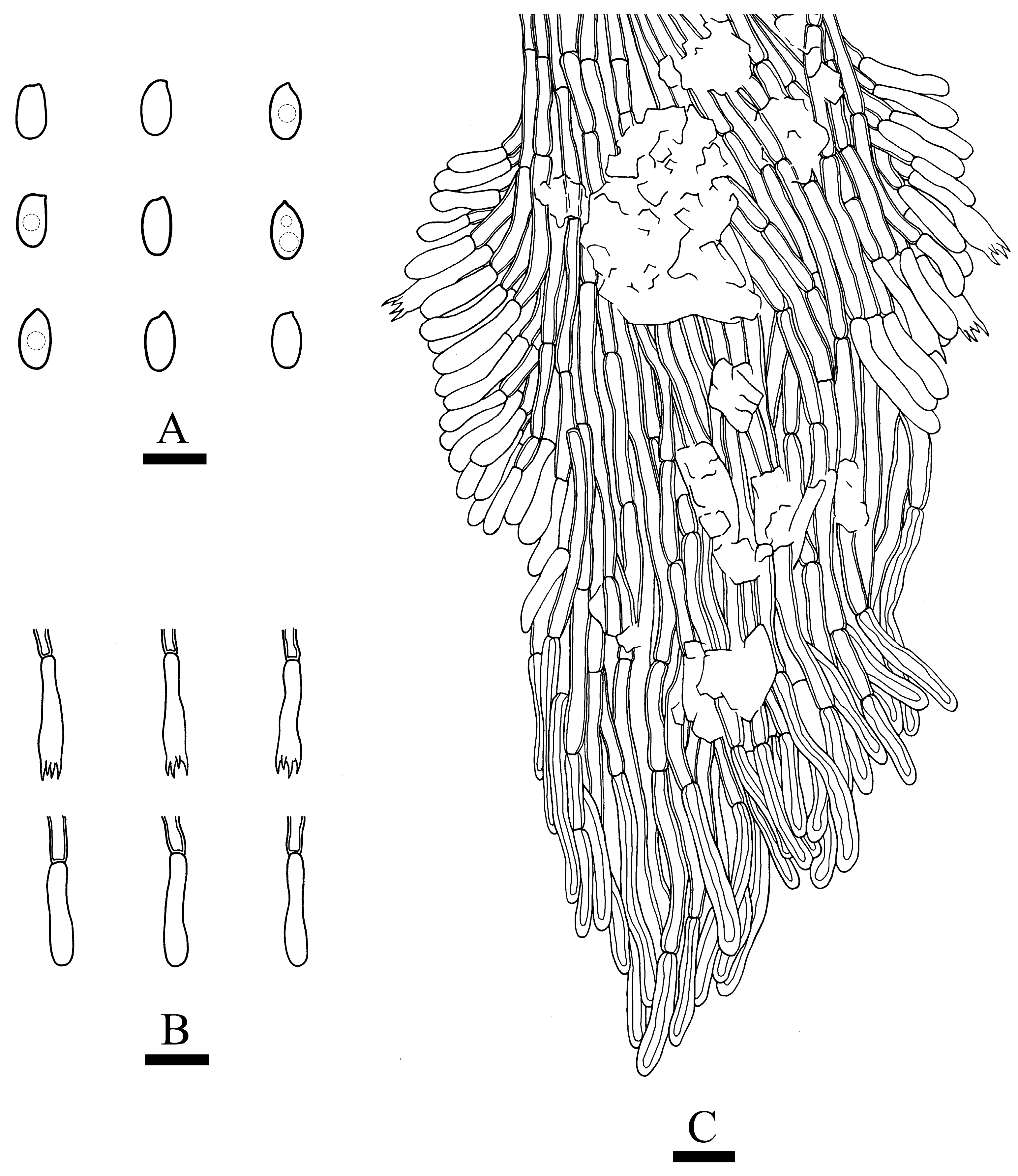
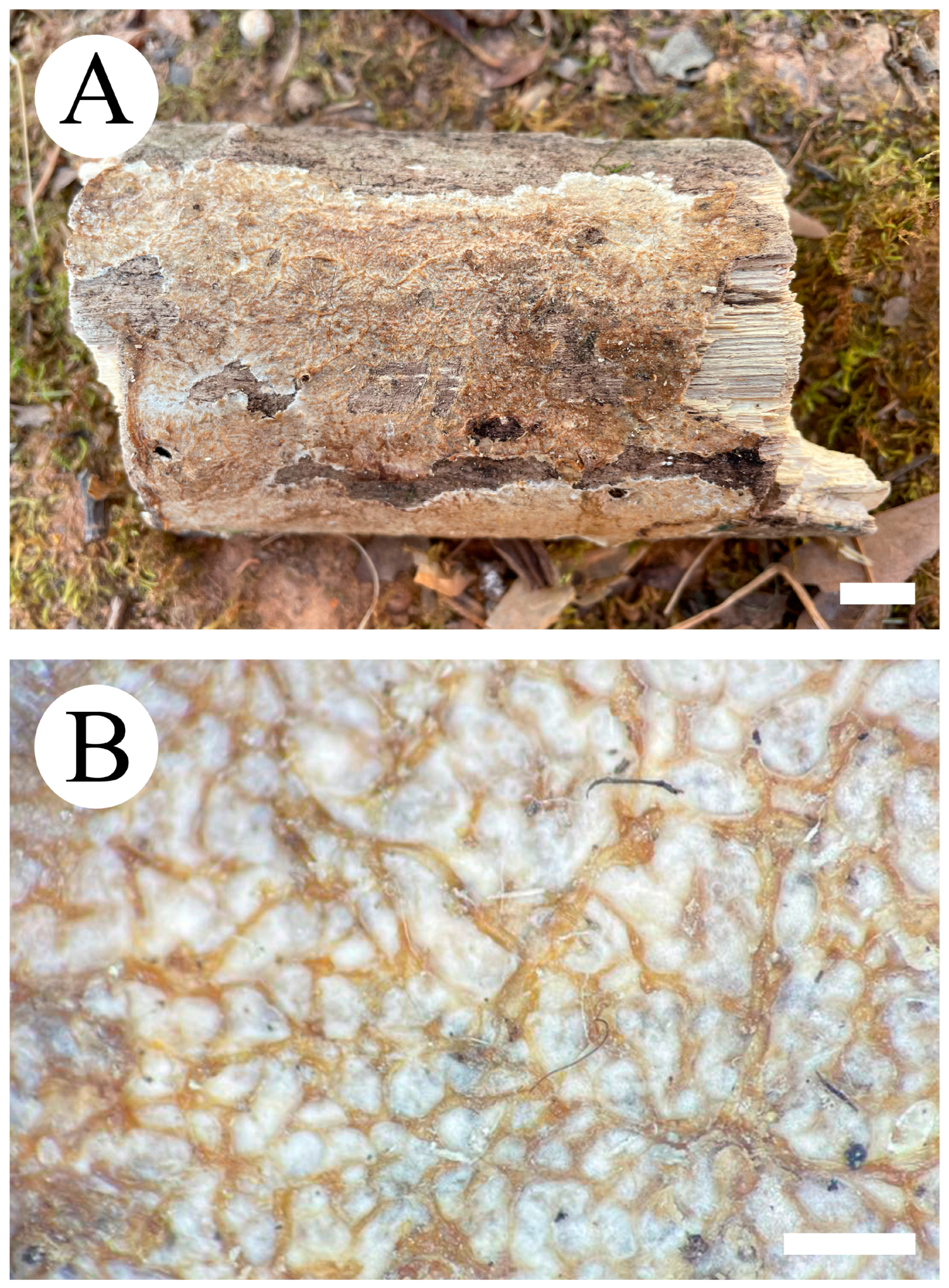
3.2. Taxonomy
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hibbett, D.; Abarenkov, K.; Kõljalg, U.; Öpik, M.; Chai, B.; Cole, J.R.; Wang, Q.; Crous, P.W.; Robert, V.A.R.G.; Helgason, T. Sequence-based classification and identification of Fungi. Mycologia 2016, 108, 1049–1068. [Google Scholar] [CrossRef] [PubMed]
- James, T.Y.; Stajich, J.E.; Hittinger, C.T.; Rokas, A. Toward a fully resolved fungal tree of life. Annu. Rev. Microbiol. 2020, 74, 291–313. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, H.S.; Zhou, L.W. Polypore diversity in South China. Mycosystema 2020, 39, 653–682. [Google Scholar]
- Dai, Y.C.; Yang, Z.L.; Cui, B.K.; Wu, G.; Yuan, H.S.; Zhou, L.W.; He, S.H.; Ge, Z.W.; Wu, F.; Wei, Y.L.; et al. Diversity and systematics of the important macrofungi in Chinese forests. Mycosystema 2021, 40, 770–805. [Google Scholar] [CrossRef]
- He, M.Q.; Zhao, R.L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; Mckenzie, E.H.C.; Raspe, O.; Kakishima, M.; Sanchez-Ramrez, S. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Hyde, K.D.; Al-Ani, L.K.T.; Tedersoo, L.; Ags, S.F. Outline of Fungi and fungus-like taxa. Mycosphere 2020, 11, 1060–1456. [Google Scholar] [CrossRef]
- Bernicchia, A.; Gorjón, S.P. Fungi Europaei 12: Corticiaceaes s.l.; Edizioni Candusso: Salamanca, Spain, 2010; pp. 1–1007. [Google Scholar]
- Fries, E.M. Systema Mycologicum 1; Ex Officina Berlingiana: Lund, Sweden; Greifswald, Germany, 1821; Volume 1, pp. 1–520. [Google Scholar]
- Ginns, J.H. The genus Merulius 2. Species of Merulius and Phlebia proposed by Lloyd. Mycologia 1969, 61, 357–372. [Google Scholar] [CrossRef]
- Nakasone, K.K.; Burdsall, H.H. Merulius, a synonym of Phlebia. Mycotaxon 1984, 21, 241–246. [Google Scholar]
- Nakasone, K.K.; Burdsall, H.H. Phlebia species from eastern and southeastern United States. Mycotaxon 1995, 54, 335–359. [Google Scholar]
- Dhingra, G.S. Genus Phlebia Fr. in the eastern Himalaya. J. Indian Bot. Soc. 1989, 84, 111–117. [Google Scholar]
- Nakasone, K.K. Studies in Phlebia six species with teeth. Sydowia 1997, 49, 49–79. [Google Scholar]
- Nakasone, K.K. Mycoaciella, a synonym of Phlebia. Mycotaxon 2002, 81, 477–490. [Google Scholar]
- Nakasone, K.K. Type studies of resupinate hydnaceous Hymenomycetes described by Patouillard. Cryptogam. Mycol. 2003, 24, 131–145. [Google Scholar]
- Nakasone, K.K. Type studies of corticioid Hymenomycetes (Basidiomycota) with aculei. Sydowia 2009, 61, 273–285. [Google Scholar] [CrossRef]
- Hjortstam, K.; Ryvarden, L. Notes on the Corticiaceae of northern China. Acta Geol. Sin. 1988, 7, 77–88. [Google Scholar] [CrossRef]
- Roberts, P. Corticioid fungi from Korup National Park, Cameroon. Kew Bull. 2000, 55, 803–842. [Google Scholar] [CrossRef]
- Dai, Y.C.; Wei, Y.; Zhang, X.Q. An annotated checklist of non-poroid Aphyllophorales in China. Ann. Bot. Fenn. 2004, 41, 233–247. [Google Scholar]
- Gilbertson, R.L.; Hemmes, D.E. New species of lignicolous basidiomycetes from Hawaii. In Memoirs of the New York Botanical Garden; New York Botanical Garden: New York, NY, USA, 2004; Volume 89, pp. 81–92. [Google Scholar]
- Duhem, B.; Michel, H. Une espèce nouvelle de Phlebia possédant des fibres arboriformes. Cryptogam. Mycol. 2007, 28, 29–38. [Google Scholar]
- Duhem, B. Phlebia pyrenaica sp. nov., une nouvelle espèce méditerranéenne. Cryptogam. Mycol. 2009, 30, 319–328. [Google Scholar]
- Duhem, B. Phlebia rhodana sp. nov. et Phlebia jurassica sp. nov. (Agaricomycotina). Cryptogam. Mycol. 2013, 34, 291–301. [Google Scholar] [CrossRef]
- Singh, A.P.; Dhingra, G.S.; Singla, N. A new species of Phlebia (Basidiomycetes) from India. Mycotaxon 2010, 112, 21–24. [Google Scholar] [CrossRef]
- Westphalen, M.C.; Reck, M.A.; Silveira, R.M.B. First record of Phlebia incarnata from the southern hemisphere. Mycotaxon 2010, 114, 305–310. [Google Scholar] [CrossRef]
- Gorjón, S.P.; Greslebin, A.G. Type studies of the species of Odontia described by G.H. Cunningham. N. Z. J. Bot. 2012, 50, 289–301. [Google Scholar] [CrossRef]
- Binder, M.; Justo, A.; Riley, R.; Salamov, A.; LopezGiraldez, F.; Copeland, A.; Foster, B.; Sun, H.; Larsson, E.; Larsson, K. Phylogenetic and phylogenomic overview of the Polyporales. Mycologia 2013, 105, 1350–1373. [Google Scholar] [CrossRef]
- Zong, T.K.; Zhao, C.L. Morphological and molecular identification of two new species of Phlebiella (Polyporales, Basidiomycota) from southern China. Nov. Hedw. 2021, 112, 501–514. [Google Scholar] [CrossRef]
- Kaur, G.; Singh, A.P.; Dhingra, G.S. Phlebia brevibasidia sp. nov. from India. Mycotaxon 2017, 132, 95–97. [Google Scholar] [CrossRef]
- Shen, S.; Ma, X.; Xu, T.M.; Zhao, C.L. Phiebia ailaoshanensis sp. nov. (Polyporales, Basidiomycota) evidenced by morphologicai characters and phylogenetic analyses. Phytotaxa 2018, 373, 184–196. [Google Scholar] [CrossRef]
- Huang, R.X.; Zhao, C.L. Three new species of Phlebia (Polyporales, Basidiomycota) based on the evidence from morphology and DNA sequence data. Mycol. Prog. 2020, 19, 753–767. [Google Scholar] [CrossRef]
- Huang, R.X.; Luo, K.Y.; Ma, R.X.; Zhao, C.L. Morphological and molecular identification of a new species of Phlebia (Polyporales, Basidiomycota) in China. Mycotaxon 2020, 135, 103–117. [Google Scholar] [CrossRef]
- Huang, R.X.; Luo, K.Y.; Zhao, C.L. Phlebia nigrodontea sp. nov. in Meruliaceae (Polyporales) with a black hymenial surface. Phytotaxa 2020, 458, 195–206. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, C.Y.; Wu, S.H. Species diversity, taxonomy and multi-gene phylogeny of phlebioid clade (Phanerochaetaceae, Irpicaceae, Meruliaceae) of Polyporales. Fungal Divers. 2021, 111, 337–442. [Google Scholar] [CrossRef]
- Mycobank. 2022. Available online: http://www.mycobank.org/Biolomics.aspx?Table=Mycobank (accessed on 20 August 2022).
- Index Fungorum. 2022. Available online: http://www.indexfungorum.org/names/Names.asp (accessed on 20 August 2022).
- Larsson, K.H. Re-thinking the classification of corticioid fungi. Mycol. Prog. 2007, 111, 1040–1063. [Google Scholar] [CrossRef]
- Justo, A.; Miettinen, O.; Floudas, D.; Ortiz-Santana, B.; Sjokvist, E.; Lindner, D.; Nakasone, K.; Niemel, T.; Larsson, K.H.; Ryvarden, L.; et al. A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol. 2017, 121, 798–824. [Google Scholar] [CrossRef] [PubMed]
- Hibbett, D.S.; Thorn, R.G. Basidiomycota: Homobasidiomycetes. In The Mycota VII, Part B.; McLaughlin, D.J., McLaughlin, E.G., Lemke, P.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 121–168. [Google Scholar]
- Larsson, K.H.; Larsson, E.; Koljalg, U. High phylogenetic diversity among corticioid homobasidiomycetes. Microbiol. Res. 2004, 108, 983–1002. [Google Scholar] [CrossRef] [PubMed]
- Tomšovský, M.; Menkis, A.; Vasaitis, R. Phylogenetic relationships in European Ceriporiopsis species inferred from nuclear and mitochondrial ribosomal DNA sequences. Fungal Biol. 2010, 114, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xu, T.M.; Song, C.G.; Zhao, C.L.; Wu, D.M.; Cui, B.K. Species diversity, molecular phylogeny and ecological habits of Cyanosporus (Polyporales, Basidiomycota) with an emphasis on Chinese collections. MycoKeys 2022, 86, 19–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.L.; Cui, B.K. Phylogeny and taxonomy of Ceriporiopsis (Polyporales) with descriptions of two new species from southem China. Phytotaxa 2014, 164, 17–28. [Google Scholar] [CrossRef]
- Zhao, C.L.; Cui, B.K.; Song, J.; Dai, Y.C. Fragiliporiaceae, a new family of Polyporales (Basidiomycota). Fungal Divers. 2015, 70, 115–126. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990. [Google Scholar]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Rehner, S.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium ana lysed from nuclear large subunit ribosomal DNA sequences. Microbiol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Kuuskeri, J.; Mäkelä, M.R.; Isotalo, J.; Oksanen, I.; Lundell, T. Lignocellulose-converting enzyme activity profiles correlate with molecular systematics and phylogeny grouping in the incoherent genus Phlebia (Polyporales, Basidiomycota). BMC Microbiol. 2015, 15, 217. [Google Scholar] [CrossRef]
- Matheny, P.B.; Liu, Y.J.; Ammirati, J.F.; Hallet, B.D. Using RPBI sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales). Am. J. Bot. 2002, 89, 688–698. [Google Scholar] [CrossRef]
- Matheny, P.B. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe, Agaricales). Mol. Phylogenet. Evol. 2005, 35, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Matheny, P.B.; Zheng, W.; Binder, M.; Curtis, J.M.; Hibbett, D.S. Contributions of rpb2 and tef1 to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol. Phylogenet. Evol. 2007, 43, 430–451. [Google Scholar] [CrossRef]
- Chen, C.C.; Chen, C.Y.; Lim, Y.W.; Wu, S.H. Phylogeny and taxonomy of Ceriporia and other related taxa and description of three new species. Mycologia 2020, 112, 64–84. [Google Scholar] [CrossRef]
- Wu, S.H. The Corticiaceae (Basidiomycetes) subamilies Phlebioideae, Phanerochaetoideae and Hyphodemmoideae in Taiwan. Acta Bot. Fenn. 1990, 142, 123. [Google Scholar]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Eberhardt, U.; Al-Hatmi, A. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2018, 92, 135–154. [Google Scholar] [CrossRef]
- Justo, A.; Hibbett, D.S. Phylogenetic classification of Trametes (Basidiomycota, Polyporales) based on a five marker dataset. Taxon 2011, 60, 1567–1583. [Google Scholar] [CrossRef]
- Ghobad-Nejhad, M.; Hallenberg, N. Multiple evidence for recognition of Phlebia tuberculata, a more widespread segregate of Phlebia livida (Polyporales, Basidiomycota). Mycol. Prog. 2012, 11, 27–35. [Google Scholar] [CrossRef]
- Floudas, D.; Hibbett, D.S. Revisiting the taxonomy of Phanerochaete (Polyporales, Basidiomycota) using a four gene dataset and extensive ITS sampling. Fungal Biol. 2015, 119, 679–719. [Google Scholar] [CrossRef] [PubMed]
- Westphalen, M.C.; Rajchenberg, M.; Tomšovský, M.; Gugliotta, A.M. A re-evaluation of neotropical Junghuhnia s. lat. (Polyporales, Basidiomycota) based on morphological and multigene analyses. Persoonia 2018, 41, 130–141. [Google Scholar] [CrossRef]
- Lygis, V.; Vasiliauskaite, I.; Matelis, A.; PliūRa, A.; Vasaitis, R. Fungi in living and dead stems and stumps of pinus mugo on coastal dunes of the baltic sea. Plant Prot. Sci. 2014, 50, 221–226. [Google Scholar] [CrossRef]
- Chen, C.C.; Wu, S.H.; Chen, C.Y. Hydnophanerochaete and Odontoefibula, two new genera of phanerochaetoid fungi (Polyporales, Basidiomycota) from East Asia. MycoKeys 2018, 39, 75–96. [Google Scholar] [CrossRef] [PubMed]
- Telleria, M.T.; Dueñas, M.; Martín, M.P. Three new species of Hydnophlebia (Polyporales, Basidiomycota) from the Macaronesian Islands. MycoKeys 2017, 27, 39–64. [Google Scholar] [CrossRef]
- Wu, F.; Yuan, Y.; Chen, J.J.; He, S.H. Luteoporia albomarginata gen. et sp. nov. (Meruliaceae, Basidiomycota) from tropical China. Phytotaxa 2016, 263, 31–41. [Google Scholar] [CrossRef]
- Liu, Z.B.; Yuan, Y. Luteoporia citriniporia sp. nov. (Polyporales, Basidiomycota), evidenced by morphological characters and phylogenetic analysis. Phytotaxa 2020, 461, 31–39. [Google Scholar] [CrossRef]
- Zhao, C.L.; Wu, Z.Q. Ceriporiopsis kunmingensis sp. nov. (Polyporales, Basidiomycota) evidenced by morphological characters and phylogenetic analysis. Mycol. Prog. 2017, 16, 93–100. [Google Scholar] [CrossRef]
- Miettinen, O.; Rajchenberg, M. Obba and Sebipora, new polypore genera related to Cinereomyces and Gelatoporia (Polyporales, Basidiomycota). Mycol. Prog. 2012, 11, 131–147. [Google Scholar] [CrossRef]
- Papp, V.; Dima, B. New systematic position of Aurantiporus alborubescens (Meruliaceae, Basidiomycota), a threatened old growth forest polypore. Mycol. Prog. 2017, 17, 319–332. [Google Scholar] [CrossRef]
- Dvořák, D.; Běťák, J.; Tomšovský, M. Aurantiporus alborubescens (Basidiomycota, Polyporales) first record in the Carpathians and notes on its systematic position. Czech Mycol. 2014, 66, 71–84. [Google Scholar] [CrossRef]
- Tomšovský, M. Delimitation of an almost forgotten species Spongipellis litschaueri (Polyporales, Basidiomycota) and its taxonomic position within the genus. Mycol. Prog. 2012, 11, 415–424. [Google Scholar] [CrossRef]
- Robledo, G.L.; Amalfi, M.; Castillo, G.; Rajchenberg, M.; Decock, C. Perenniporiella chaquenia sp. nov. and further notes on Perenniporiella and its relationships with Perenniporia (Poriales, Basidiomycota). Mycologia 2017, 101, 657–673. [Google Scholar] [CrossRef]
- Zhao, C.L.; Wu, F.; Liu, H.X.; Dai, Y.C. A phylogenetic and taxonomic study on Ceriporiopsis s. str. (Polyporales) in China. Nova Hedwig. 2015, 101, 403–417. [Google Scholar] [CrossRef]
- Wu, F.; Chen, J.J.; Ji, X.H.; Vlasák, J.; Dai, Y.C. Phylogeny and diversity of the morphologically similar polypore genera Rigidoporus, Physisporinus, Oxyporus and Leucophellinus. Mycologia 2017, 109, 749–765. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Cui, B.K. Phlebiporia bubalina gen. et. sp. nov. (Meruliaceae, Polyporales) from Southwest China with a preliminary phylogeny based on rDNA sequences. Mycol. Prog. 2014, 13, 563–573. [Google Scholar] [CrossRef]
- Ortiz-Santana, B.; Lindner, D.L.; Miettinen, O.; Justo, A.; Hibbett, D.S. A phylogenetic overview of the antrodia clade (Basidiomycota, Polyporales). Mycologia 2013, 105, 1391–1411. [Google Scholar] [CrossRef] [PubMed]
- Sjokvist, E.; Larsson, E.; Eberhardt, U.; Ryvarden, L.; Larsson, K.H. Stipitate stereoid basidiocarps have evolved multiple times. Mycologia 2012, 104, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Ginns, J.; Lindner, D.L.; Baronia, T.J.; Ryvarden, L. Aurantiopileus mayanensis a new genus and species of polypore (Polyporales, Basidiomycota) from Belize with connections to existing Asian species. N. Am. Fungi 2010, 5, 1–10. [Google Scholar] [CrossRef]
- Moreno, G.; Blanco, M.N.; Checa, J.; Platas, G.; Peláez, F. Taxonomic and phylogenetic revision of three rare irpicoid species within the Meruliaceae. Mycol. Prog. 2011, 10, 481–491. [Google Scholar] [CrossRef]
- Tomšovský, M. Arcodontia crocea (Basidiomycota, Polyporales) is unrelated to Spongipellis. Phytotaxa 2016, 288, 197–200. [Google Scholar] [CrossRef]
- Korhonen, A.; Seelan, J.; Miettinen, O. Cryptic species diversity in polypores: The skeletocutis nivea species complex. Mycokeys 2018, 36, 45–82. [Google Scholar] [CrossRef] [PubMed]
- Han, M.L.; Chen, Y.Y.; Shen, L.L.; Song, J.; Vlasák, J.; Dai, Y.C.; Cui, B.K. Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. 2016, 80, 343–373. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Swofford, D.L. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods); Version 4.0b10; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Felsenstein, J. Confidence intervals on phylogenetics: An approach using bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.A.; Pfeiffer, W.; Schwartz, T. The CIPRES Science Gateway: Enabling high-impact science for phylogenetics researchers with limited resources. Assoc. Comput. Mach. 2012, 39, 1–8. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program Distributed by the Author; Evolutionary Biology Centre, Uppsala University: Uppsala, Sweden, 2004. [Google Scholar]
- Ronquist, F.; Huelsenbeck, J.P. MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef]
- Hjortstam, K.; Ryvarden, L. New taxa and new combinations in tropical corticioid fungi (Basidiomycotina, Aphyllophorales). Synop. Fung 2005, 20, 33–41. [Google Scholar]
- Berkeley, M.J. Notices of North American fungi. Grevillea 1873, 1, 177–180. [Google Scholar]
- Ryvarden, L.; Hjortstam, K.; Iturriaga, T. Studies in corticioid fungi from Venezuela II (Basidiomycotina, Aphyllophorales). Synop. Fung 2005, 20, 42–78. [Google Scholar]
- Nakasone, K.K.; Gilbertson, R.L. Three resupinate hydnaceous basidiomycetes from Hawaii. Folia Cryptog. Estonica 1998, 33, 85–92. [Google Scholar]
- Hallenberg, N. New taxa of corticiaceae from N, Iran (Basidiomycetes). Mycotaxon 1980, 11, 447–475. [Google Scholar]
- Donk, M.A. Revisie van de Nederlandse Heterobasidiomycetae en Homobasidiomycetae-Aphyllophoraceae. Meded Ned. Mycol. Ver. 1931, 1820, 68–200. [Google Scholar]
- Eriksson, J.; Hjortstam, K.; Ryvarden, L. The Corticiaceae of North Europe, Vol 5; Fungiflora: Oslo, Norway, 1978; p. 1047. [Google Scholar]
- Stalpers, J.A. Notes on Mycoacia—I. Persoonia 1976, 9, 145–148. [Google Scholar]
- Sytsma, N.K.J. Biosystematic Studies on Phlebia acerina, P. rufa, and P. radiata in North America. Mycologia 1993, 85, 996–1016. [Google Scholar] [CrossRef]
- Nakasone, K.K.; Eslyn, W.E. A new species, Phlebia brevispora, a cause of internal decay in utility poles. Mycologia 1981, 73, 803–811. [Google Scholar] [CrossRef]
- Vlasák, J.; Vlasák, J., Jr.; Ryvarden, L. Four new polypore species from the westerm United States. Mycotaxon 2012, 119, 217–231. [Google Scholar] [CrossRef]
- Miettinen, O.; Spirin, V.; Vlasák, J.; Rivoire, B.; Hibbett, D. Polypores and genus concepts in Phanerochaetaceae (Polyporales, Basidiomycota). MycoKeys 2016, 17, 1–46. [Google Scholar] [CrossRef]
- Nilsson, R.H.; Ryberg, M.; Kristiansson, E.; Abarenkov, K.; Larsson, K.H.; Kõljalg, U. Taxonomic reliability of DNA sequences in public sequence databases: A fungal perspective. PLoS ONE 2006, 1, e59. [Google Scholar] [CrossRef]
- Hofstetter, V.; Buyck, B.; Eyssartier, G.; Schnee, S.; Gindro, K. The unbearable lightness of sequenced-based identification. Fungal Divers. 2019, 96, 243–284. [Google Scholar] [CrossRef]
- Liu, D.M.; Xu, Y.L.; Li, Y.; Liu, W.H.; He, S.H. Two new species of Hydnophlebia (Meruliaceae, Polyporales) from China based on morphological and molecular evidence. Phytotaxa 2020, 477, 35–46. [Google Scholar] [CrossRef]
- Hjortstam, K.; Ryvarden, L. Tropical distribution of species of Mycoaciella (Basidiomycotina). Synop. Fung 2009, 26, 7–9. [Google Scholar]
- Schulzer, S.; Kanitz, A.; Knapp, J.A. Die bisher bekannten Pflanzen Slavoniens, ein Versuch. Verh. Zool. Bot. Ges. Wien 1866, 16, 3–172. [Google Scholar]
- Wu, S.H. Six new species of Phanerochaete from Taiwan. Botan. Bull. Acad. Sin. 2000, 41, 165–174. [Google Scholar]
- Eriksson, J.; Ryvarden, L. The Corticiaceae of North Europe 4. In Hyphodermella-Mycoacia; Fungifora: Oslo, Norway, 1973. [Google Scholar]
- Zmitrovich, I.V. Conspectus Systematis Polyporacearum v. 1.0.; Folia Cryptogamica Petropolitana: Petersburg, Russia, 2018; Volume 6, pp. 1–145. [Google Scholar]
- Dai, Y.C. A revised checklist of corticioid and hydnoid fungi in China for 2010. Mycoscience 2011, 52, 69–79. [Google Scholar] [CrossRef]
- Wu, F.; Man, X.W.; Tohtirjap, A.; Dai, Y.C. A comparison of polypore funga and species composition in forest ecosystems of China, North America, and Europe. For. Ecosyst. 2022, 9, 100051. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.D.; Wang, Y.R.; Zhou, M.; Qiu, J.Z.; Li, D.W.; Vlasák, J.; Liu, H.G.; Dai, Y.C. Two new forest pathogens in Phaeolus (Polyporales, Basidiomycota) on Chinese coniferous trees were confirmed by molecular phylogeny. Front. Microbiol. 2022, 13, 942603. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Wu, Y.D.; Vlasák, J.; Yuan, Y.; Dai, Y.C. Phylogenetic analysis demonstrating four new species in Megasporoporia sensu lato (Polyporales, Basidiomycota). Mycosphere 2021, 12, 1012–1037. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, J.J.; Korhonen, K.; Martin, F.; Dai, Y.C. An updated global species diversity and phylogeny in the forest pathogenic genus Heterobasidion (Basidiomycota, Russulales). Front. Microbiol. 2021, 11, 596393. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Liu, Z.B.; Liu, H.G.; Si, J. Two new corticioid species of Phanerochaetaceae (Polyporales, Basidiomycota) from Southwest China. Front. Cell. Infect. Microbiol. 2023, 13, 1105918. [Google Scholar] [CrossRef]
- Sun, Y.F.; Xing, J.H.; He, X.L.; Wu, D.M.; Song, C.G.; Liu, S.; Vlasák, J.; Gates, G.; Gibertoni, T.B.; Cui, B.K. Species diversity, systematic revision and molecular phylogeny of Ganodermataceae (Polyporales, Basidiomycota) with an emphasis on Chinese collections. Stud. Mycol. 2022, 101, 287–415. [Google Scholar] [CrossRef]

| Locus | Primer | Primer Sequences 5′ to 3′ | Annealing Temperature | Orientation | References |
|---|---|---|---|---|---|
| internal transcribed spacer (ITS) | ITS5 | GGA AGTAAA AGT CGTAACAAGG | 58 | Forward | [45] |
| ITS4 | TCCTCCGCTTATTGATATGC | 58 | Reverse | [45] | |
| large subunit ribosomal DNA (LSU) | LR0R | ACCCGCTGA ACTTAAGC | 48 | Forward | [45] |
| LR7 | TACTACCACCAAGATCT | 48 | Reverse | [45] | |
| translation elongation factor 1-alpha (tef 1-α) | EF1-983F | GCYCCYGGHCAYCGTGAYTTYAT | 60 | Forward | [47] |
| EF1-2218R | ATGACACCRACRGCRACRGTYTG | 60 | Reverse | [47] | |
| small subunit of mitochondrial rRNA gene (mtSSU) | MS1 | CAGCAGTCAAGAATATTAGTCAATG | 52 | Forward | [45] |
| MS2 | GCGGATTATCGAATTAAATAAC | 52 | Reverse | [45] | |
| Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) | GAPDH-F | ATGGTCTACATGTTCAAGTACGAC | 50 | Forward | [48] |
| GAPDH-R | TCGACGAGGGGATGATGT T | 50 | Reverse | [48] | |
| RNA polymerase II largest subunit (rpb1) | RPB1-Af | GARTGYCCDGGDCAYTTYGG | 60 | Forward | [49] |
| RPB1-Cr | CCNGCDATNTCRTTRTCCATRTA | 60 | Reverse | [49] | |
| RNA polymerase II second largest subunit (rpb2) | bRPB2-6F | TGGGGYATGGTNTGYCCYGC | 52 | Forward | [50] |
| bRPB2-7.1R | CCCATRGCYTGYTTMCCCATDGC | 52 | Reverse | [50] |
| Species Name | Sample No. | GenBank Accession No. | References | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ITS | nLSU | RPB1 | RPB2 | TEF1 | GAPDH | mt-SSU | |||
| Antrodiella stipitate | FD-136 | KP135314 | KP135197 | KP134886 | — | — | — | — | [28] |
| Bondarzewia montana | AFTOL-ID 452 | DQ200923 | DQ234539 | DQ256049 | AY218474 | DQ059044 | — | — | [51] |
| Ceriporia viridans | GC 1708-211 | LC427027 | LC427049 | LC427062 | — | — | — | — | [52] |
| Ceriporiopsis aneirina | Dai 12657 | KF845952 | KF845945 | — | — | — | — | — | [43] |
| C. pseudogilvescens | Cui 6824 | KU509523 | — | — | — | — | — | — | [53] |
| C. resinascens | BRNM 686416 | FJ496679 | FJ496703 | — | — | — | — | FJ496737 | [41] |
| Ceriporiopsoides guidella | HUBO 7659 | FJ496687 | FJ496722 | — | — | — | — | FJ496740 | [41] |
| C. lagerheimii | Dai 12304 | KX161647 | KX161651 | — | — | — | — | — | Unpublished |
| Cerrena unicolor | FD-299 | KP135304 | KP135209 | KP134874 | KP134968 | — | — | — | [28] |
| Climacocystis borealis | FD-31 | KP135308 | KP135210 | KP134882 | KP134895 | — | — | — | [28] |
| Climacodon septentrionalis | AFTOL-767 | AY854082 | AY684165 | AY864873 | AY780941 | AY885151 | — | — | Unpublished |
| C. septentrionalis | CBS 131.40 | MH856064 | MH867555 | — | — | — | — | — | [54] |
| C. septentrionalis | FP-72067 | KP135345 | — | — | — | — | — | — | [28] |
| C. septentrionalis | RLG-6890-Sp | KP135344 | — | — | — | — | — | — | [28] |
| Coriolopsis caperata | CR 22 | JN164999 | JN164789 | — | — | — | — | — | [55] |
| Crustodontia chrysocreas | HHB-6333-Sp | KP135358 | KP135263 | KP134861 | KP134908 | — | — | — | [28] |
| C. chrysocreas | FCUG2827 | HQ153411 | — | — | — | — | — | — | [56] |
| C. nigrodontea | CLZhao 2729 | MT896823 | MT896819 | ON960280 * | — | ON892520 * | — | — | [33]; Present study |
| C. nigrodontea | CLZhao 2758 | MT896824 | — | — | — | — | — | — | [33] |
| C. rhododendri | CLZhao 851 | MW732399 | MW724791 | ON942236 * | ON918559 * | — | — | MW732759 | Present study |
| C. rhododendri | CLZhao 6168 | MW732400 | MW724792 | ON950240 * | — | ON892523 * | ON892530 * | MW732760 | Present study |
| C. rhododendri | CLZhao 16995 | MW732396 | MW724788 | — | — | — | — | MW732768 | Present study |
| C. taiwanensis | GC 1703-88 | MZ636944 | MZ637106 | MZ748466 | OK136049 | — | — | — | [28] |
| C. taiwanensis | Wu 9310-21 | MZ636945 | MZ637107 | — | — | — | — | — | [34] |
| C. tongxiniana | CLZhao 2255 | MT020773 | MT020751 | — | — | — | — | — | [31] |
| C. tongxiniana | CLZhao 5217 | MT020778 | MT020756 | ON892526 * | ON918558 * | ON892521 * | — | MW732754 | [31]; Present study |
| C. uda | FP-101544-Sp | KP135361 | KP135232 | KP134859 | KP134909 | MZ913649 | — | — | [57] |
| Daedalea quercina | FP56429 | KY948809 | KY948883 | KY948989 | — | — | — | — | [38] |
| Earliella scabrosa | PR1209 | JN165009 | JN164793 | JN164819 | JN164866 | JN164894 | — | — | [55] |
| Efibula americana | FP-102165 | KP135016 | KP135256 | KP134808 | KP134916 | — | — | — | [28] |
| E. tuberculate | OM-6707 | KP135017 | — | KP134807 | — | — | — | — | [28] |
| Fomitopsis pinicola | AFTOL-770 | AY854083 | AY684164 | AY864875 | AY786056 | AY885152 | — | FJ436112 | Unpublished |
| Fragiliporia fragilis | Dai 13080 | KJ734260 | KJ734264 | — | KJ790248 | KJ790245 | — | KJ734268 | [44] |
| F. fragilis | Dai 13559 | KJ734261 | KJ734265 | — | KJ790249 | KJ790246 | — | KJ734269 | [44] |
| F. fragilis | Dai 13561 | KJ734262 | KJ734266 | — | KJ790250 | KJ790247 | — | KJ734270 | [44] |
| Ganoderma lingzhi | Cui-9166 | MG732955 | — | — | — | MH127978 | — | — | Unpublished |
| Geesterania carneola | MCW 388/12 | KY174999 | KY174999 | — | KY175011 | KY175013 | — | — | [58] |
| G. carneola | SP 446193 | NR_158508 | — | — | — | — | — | — | [58] |
| G. davidii | MCW 396/12 | KY174998 | KY174998 | — | KY175012 | KY175016 | — | — | [58] |
| Gelatopori subvermispora | FD-354 | KP135312 | KP135212 | KP134879 | KP134961 | — | — | — | [28] |
| Grammothelopsis subtropica | Cui 9041 | JQ845096 | JQ845099 | — | — | — | — | — | Unpublished |
| Hermanssonia centrifuga | CBS 125890 | MH864088 | MH875547 | — | — | — | — | — | [54] |
| H. centrifuga | HHB-9239-Sp | KP135380 | KP135262 | KP134844 | KP134974 | MZ913721 | — | — | [28] |
| Heterobasidion annosum | VL-296 | JF440572 | — | — | — | — | — | — | [59] |
| Hydnophanerochaete odontoidea | CLZhao 3996 | MH784926 | MH784936 | — | — | — | — | — | [30] |
| H. odontoidea | CLZhao 4036 | MH784927 | MH784937 | — | — | — | — | — | [30] |
| H. odontoidea | Wu 9310-29 | LC379002 | — | — | — | — | — | — | [60] |
| H. odontoidea | TNM: GC 1308-45 | LC363486 | LC363492 | LC363497 | — | — | — | — | [60] |
| H. odontoidea | TNM: Chen 1376 | LC363485 | LC363491 | LC363496 | — | — | — | — | [60] |
| Hydnophlebia acanthocystis | FP 150571 | KY948767 | KY948844 | KY948914 | — | — | — | — | [38] |
| H. canariensis | MA-Fungi 86619 | KF483009 | KF528100 | — | — | — | — | — | [61] |
| H. caspica | FCUG3159 | HQ153410 | — | — | — | — | — | — | [56] |
| H. chrysorhiza | FD-282 | KP135338 | KP135217 | KP134848 | KP134897 | — | — | — | [28] |
| H. fimbriata | Dai 11672 | KJ698633 | KJ698637 | — | — | — | — | — | [44] |
| H. fissurata | CLZhao 2900 | MW732402 | MW724794 | ON892527 * | ON892536 * | ON968926 * | — | MW732762 | Present study |
| H. gorgonea | MA-Fungi 86642 | KF483031 | KF528122 | — | — | — | — | — | [61] |
| H. omnivora | KKN-112-Sp | KP135334 | KP135216 | KP134846 | — | — | — | — | [28] |
| Hyphoderma setigerum | FD-312 | KP135297 | KP135222 | KP134871 | — | — | — | — | [28] |
| Hypochnicium bombycinum | HHB-12631-sp | KY948801 | — | KY948930 | — | — | — | — | [38] |
| Junghuhnia nitida | CBS 459.50 | MH856708 | MH868226 | — | — | — | — | — | [54] |
| Lopharia cinerascens | FP-105043-sp | JN165019 | JN164813 | — | — | — | — | — | [55] |
| Luteochaete subglobosa | CLZhao 3475 | MK881897 | MK881787 | — | — | — | — | — | Unpublished |
| L. subglobosa | CLZhao 3645 | MK881899 | MK881789 | — | — | ON892522 * | — | MW732758 | Present study |
| L. subglobosa | GC 1605-4 | MZ636995 | MZ637156 | MZ748455 | OK136053 | MZ913645 | — | — | [34] |
| L. subglobosa | Wu 870918 | MZ636996 | GQ470662 | MZ748456 | OK136054 | MZ913646 | — | — | [34] |
| Luteoporia albomarginata | Dai 15229 | KU598873 | KU598878 | — | — | — | — | — | [62] |
| L. albomarginata | GC 1702-1 | LC379003 | LC379155 | LC379160 | LC387358 | LC387377 | — | — | [60] |
| L. citriniporia | Dai 19507 | MT872218 | MT872216 | — | — | — | — | — | [63] |
| L. lutea | GC 1409-1 | MZ636998 | MZ637158 | MZ748467 | OK136050 | MZ913656 | — | — | [34] |
| L. straminea | CLZhao 5794 | OM897115 * | OM897114 * | — | — | — | — | — | Present study |
| L. straminea | CLZhao 18947 | MW732407 | MW724799 | — | — | — | — | MW732765 | Unpublished |
| Merulius fuscotuberculata | CLZhao 10227 | MT020759 | MT020737 | ON892524 * | ON892537 * | ON855009 * | ON634683 * | — | Present study |
| M. fuscotuberculata | CLZhao 10239 | MT020760 | MT020738 | ON892525 * | ON892538 * | ON936910 * | ON980563 * | — | Present study |
| M. giganteus | FP-135344-Sp | KP135307 | KP135228 | — | — | — | — | — | [28] |
| M. hydnoidea | HHB 1993sp | KY948778 | KY948853 | KY948921 | — | — | — | — | [38] |
| M. nantahaliensis | HHB 2816sp | KY948777 | KY948852 | KY948920 | — | — | — | — | [38] |
| M. sinensis | CLZhao 2562 | MW732401 | MW724793 | — | — | — | ON892532 * | MW732761 | Present study |
| M. tomentopileata | CLZhao 5833 | MT020761 | MT020739 | — | — | — | — | — | [31] |
| M. tomentopileata | CLZhao 10274 | MT020771 | MW732469 | — | — | — | ON892531 * | MW732752 | [31]; Present study |
| M. tremellosus | CBS 217.56 | MH857589 | MH869138 | — | — | — | — | — | [54] |
| M. tremellosus | FBCC278 | LN611126 | LN611126 | — | LN611035 | — | LN611072 | — | [48] |
| Mycoacia aurea | FCUG 2767 | HQ153409 | — | — | — | — | — | — | [56] |
| M. aurea | RLG 5075sp | KY948759 | — | KY948918 | — | — | — | — | [38] |
| M. fuscoatra | HHB-10782-Sp | KP135365 | KP135365 | KP134857 | KP134910 | — | — | — | [28] |
| M. fuscoatra | OMC 1380 | KY948754 | — | — | — | — | — | — | [38] |
| M. gilvescens | BRNM 710166 | FJ496684 | FJ496720 | — | — | — | — | — | [41] |
| M. gilvescens | Chen 156 | MZ636935 | MZ637098 | — | — | — | — | — | [34] |
| M. gilvescens | Chen 3340 | MZ636936 | MZ637099 | MZ748446 | OK136039 | MZ913651 | — | — | [34] |
| M. gilvescens | Yuan 2752 | KF845953 | KF845946 | — | — | — | — | — | [43] |
| M. kunmingensis | CLZhao 152 | KX081072 | KX081074 | — | — | — | — | — | [64] |
| M. kunmingensis | CLZhao 153 | KX081073 | KX081075 | — | — | — | — | — | [64] |
| M. livida | FP 135046 sp | KY948758 | KY948850 | KY948917 | — | — | — | — | [38] |
| M. livida | FBCC 1283 | LN611123 | LN611123 | — | LN611033 | — | — | — | [48] |
| M. nothofagi | HHB-4273-Sp | KP135369 | KP135266 | KP134858 | KP134911 | — | — | — | [28] |
| M. nothofagi | HHB-6906-Sp | KP135368 | — | — | — | — | — | — | [28] |
| M. subfascicularis | Chen 3873 | MZ637007 | MZ637168 | — | — | — | — | — | [34] |
| M. subfascicularis | Wu 1004-11 | MZ637008 | — | MZ748448 | OK136044 | MZ913653 | — | — | [34] |
| M. tuberculata | MG 128 | HQ153425 | — | — | — | — | — | — | [56] |
| M. tuberculata | FCUG 3186 | HQ153418 | — | — | — | — | — | — | [56] |
| Mycoaciella bispora | EL 13_99 | AY463446 | AY586692 | — | — | — | — | — | [40] |
| M. brunneospina | CLZhao 15876 | MW732404 | MW724796 | ON892515 * | — | — | — | MW732764 | Present study |
| Obba rivulosa | FP-135416-Sp | KP135309 | KP135208 | KP134878 | KP134962 | — | — | — | [28] |
| O. valdiviana | FF484 | HQ659236 | — | — | — | — | — | — | [65] |
| Odoria alborubescens | BP 106943 | MG097864 | MG097867 | MG213724 | MG213723 | — | — | — | [66] |
| O. alborubescens | BRNU 627479 | JQ821319 | JQ821318 | — | — | — | — | — | [67] |
| Panus fragilis | HHB-11042-Sp | KP135328 | KP135233 | KP134877 | KP134970 | — | — | — | [28] |
| Pappia fssilis | 814 | HQ728291 | HQ729001 | — | — | — | — | — | [68] |
| P. fssilis | BRNM 699803 | HQ728292 | HQ729002 | — | — | — | — | — | [68] |
| Perenniporia medulla-panis | Cui 14515 | MG847214 | MG847223 | — | — | — | — | — | Present study |
| Perenniporiella neofulva | MUCL 45091 | FJ411080 | FJ393852 | — | — | — | — | — | [69] |
| Phanerochaete laevis | HHB 15519 | KP135149 | KP135249 | KP134836 | KP134952 | — | — | — | [28] |
| P. rhodella | FD-18 | KP135187 | KP135258 | KP134832 | KP134948 | — | — | — | [28] |
| P. sanguinea | HHB-7524 | KP135101 | KP135244 | KP134825 | KP134943 | — | — | — | [28] |
| P. velutina | CBS 412.50 | MH856692 | MH868209 | — | — | — | — | — | [54] |
| Phlebia acerina | FD-301 | KP135378 | KP135260 | KP134862 | — | — | — | — | [28] |
| P. acerina | DR 60sp | KY948773 | — | KY948924 | — | — | — | — | [38] |
| P. albida | GB 1833 | KY948748 | KY948889 | KY948960 | — | — | — | — | [38] |
| P. griseoflavescens | MR-4310 | KY948797 | KY948888 | KY948963 | — | — | — | — | [38] |
| P. floridensis | HHB-9905 | KP135383 | KP135264 | KP134863 | KP134899 | — | — | — | [28] |
| P. floridensis | FP 102562T | KP135386 | — | — | — | — | — | — | [28] |
| P. leptospermi | CBS 126031 | MH863894 | MH875355 | — | — | — | — | — | [54] |
| P. niveomarginata | CLZhao 18972 | MW732409 | MW724801 | ON892518 * | ON925000 * | ON892529 * | ON892519 * | — | Present study |
| P. niveomarginata | CLZhao 19089 | MW732410 | MW724802 | — | — | — | ON892535 * | — | Present study |
| P. ochraceofulva | FBCC 360 | LN611117 | LN611117 | — | LN611028 | — | LN651203 | — | [48] |
| P. poroides | CLZhao 16121 | MW732405 | MW724797 | ON892516 * | ON918560 * | — | ON892533 * | — | Present study |
| P. poroides | CLZhao 18421 | MW732406 | MW724798 | ON892517 * | ON924999 * | ON892528 * | ON892534 * | — | Present study |
| P. queletii | CBS 234.56 | MH857600 | MH869148 | — | — | — | — | — | [54] |
| P. radiata | CBS 285.56 | MH857642 | MH869187 | — | — | — | — | — | [54] |
| P. radiata | FBCC 1376 | LN611102 | LN611102 | — | LN611014 | — | LN611061 | — | [48] |
| P. rufa | CBS 213.47 | MH856224 | MH867751 | — | — | — | — | — | [54] |
| P. rufa | HHB-14924 | KP135374 | — | — | — | — | — | — | [48] |
| Phlebicolorata alboaurantia | Cui 4136 | KF845955 | KF845948 | — | — | — | — | — | [43] |
| P. brevispora | FBCC 1463 | LN611135 | LN611135 | — | LN611041 | — | LN611081 | — | [48] |
| P. crocea | Miettinen-16483 | KY948745 | KY948901 | KY948927 | — | — | — | — | [38] |
| P. pseudoplacenta | Miettinen 18997 | KY948744 | KY948902 | KY948926 | — | — | — | — | [38] |
| P. rosea | Dai 13584 | KJ698636 | KJ698640 | — | — | — | — | — | [70] |
| P. rosea | Dai 13573 | KJ698635 | KJ698639 | — | — | — | — | — | [70] |
| Phlebiopsis castanea | He 3249 | MT386375 | — | — | — | — | — | — | Unpublished |
| P. gigantea | FP-70857 | KP135390 | KP135272 | KP134821 | KP134930 | — | — | — | [28] |
| Phlebiporia bubalina | Dai 9798 | KY131842 | KY131901 | — | — | — | — | — | [71] |
| P. bubalina | Dai 13168 | KC782526 | KC782528 | — | — | — | — | — | [72] |
| P. bubalina | Dai 15179 | KY131843 | KY131902 | — | — | — | — | — | [71] |
| Piptoporus betulinus | L-15603-Sp | KC585373 | KC585202 | KY949005 | — | — | — | — | [73] |
| Podoscypha parvula | CBS 331.66 | JN649361 | JN649361 | — | — | — | — | — | [74] |
| Polyporus squamosus | AFTOL-704 | DQ267123 | AY629320 | DQ831023 | DQ408120 | DQ028601 | — | JN710743 | Unpublished |
| Pseudophlebia lindtneri | GB 501 | KY948772 | KY948847 | KY948923 | — | — | — | — | [38] |
| P. mayaensis | JV 1504/128 | KT156706 | — | — | — | — | — | — | Unpublished |
| P. mayaensis | TJB 10228 | HM772140 | HM772139 | — | — | — | — | — | [75] |
| P. semisupina | Cui 10222 | KF845956 | KF845949 | — | — | — | — | — | [43] |
| P. setulosa | HHB-6891-Sp | KP135382 | KP135267 | KP134864 | KP134901 | MZ913650 | — | — | [28] |
| P. setulosa | PH 11749 | GU461312 | GU461312 | — | — | — | — | — | [76] |
| Rhizochaete americanus | FP-102188 | KP135409 | KP135277 | KP134815 | KP134934 | — | — | — | [28] |
| R. radicata | FD-123 | KP135407 | KP135279 | KP134816 | KP134937 | — | — | — | [28] |
| R. rubescens | Wu 0910-45 | LC387335 | MF110294 | LC387348 | LC387370 | LC270925 | — | — | [60] |
| Sarcodontia crocea | BRNM 721609 | KX831470 | KX831472 | — | — | — | — | — | [77] |
| S. crocea | OMC 1488 | KY948798 | KY948903 | KY948928 | — | — | — | — | [38] |
| Scopuloides allantoidea | GC 1602-11 | MZ637080 | MZ637278 | — | — | — | — | — | [34] |
| S. dimorpha | FP-102935-Sp | KP135353 | KP135285 | KP134855 | KP134905 | — | — | — | [28] |
| S. hydnoides | FP-150473 | KP135355 | KP135284 | KP134854 | — | — | — | — | [28] |
| S. rimosa | HHB-7042-Sp | KP135350 | KP135282 | KP134853 | KP134903 | — | — | — | [28] |
| S. rimosa | HHB-15484-Sp | KP135352 | KP135281 | KP134851 | KP134902 | MZ913665 | — | — | [28] |
| S. rimosa | RLG-5104-Sp | KP135351 | KP135283 | KP134852 | KP134904 | — | — | — | [28] |
| Sebipora aquosa | Dai 13592 | KU376422 | KX161660 | — | — | — | — | — | Unpublished |
| Skeletocutis chrysella | FD-305 | KP135310 | KP135286 | KP134890 | KP134976 | — | — | — | [28] |
| S. nivea | Miettinen-9950 | KY953045 | KY953045 | KY948969 | — | — | — | — | [78] |
| S. odora | L-13763-sp | KY948830 | KY948893 | KY949046 | — | — | — | — | [38] |
| Steccherinum ochraceum | KHL11902 | JQ031130 | JQ031130 | — | — | — | — | — | [27] |
| Stereum hirsutum | FPL-8805 | AY854063 | AF393078 | AY864886 | — | AY885159 | — | — | Unpublished |
| Trametes suaveolens | Cui 11568 | KR605823 | KR605766 | — | — | — | — | — | [79] |
| Tyromyces chioneus | FD-4 | KP135311 | KP135291 | KP134804 | KP134977 | — | — | — | [28] |
| T. galactinus | L-15951-sp | KY948829 | KY948892 | KY948966 | — | — | — | — | [38] |
| Xanthoporus syringae | Gothenburg 1488 | JN710607 | — | — | — | — | — | — | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, C.; Qu, M.; Huang, R.; Karunarathna, S.C. Multi-Gene Phylogeny and Taxonomy of the Wood-Rotting Fungal Genus Phlebia sensu lato (Polyporales, Basidiomycota). J. Fungi 2023, 9, 320. https://doi.org/10.3390/jof9030320
Zhao C, Qu M, Huang R, Karunarathna SC. Multi-Gene Phylogeny and Taxonomy of the Wood-Rotting Fungal Genus Phlebia sensu lato (Polyporales, Basidiomycota). Journal of Fungi. 2023; 9(3):320. https://doi.org/10.3390/jof9030320
Chicago/Turabian StyleZhao, Changlin, Menghan Qu, Ruoxia Huang, and Samantha C. Karunarathna. 2023. "Multi-Gene Phylogeny and Taxonomy of the Wood-Rotting Fungal Genus Phlebia sensu lato (Polyporales, Basidiomycota)" Journal of Fungi 9, no. 3: 320. https://doi.org/10.3390/jof9030320
APA StyleZhao, C., Qu, M., Huang, R., & Karunarathna, S. C. (2023). Multi-Gene Phylogeny and Taxonomy of the Wood-Rotting Fungal Genus Phlebia sensu lato (Polyporales, Basidiomycota). Journal of Fungi, 9(3), 320. https://doi.org/10.3390/jof9030320








