Containment of Fusarium culmorum and Its Mycotoxins in Various Biological Systems by Antagonistic Trichoderma and Clonostachys Strains
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Dual Culture Bioassay
2.2.1. Potato Dextrose Agar (PDA) Dual Culture Bioassay
2.2.2. Solid Substrate Bioassay
2.2.3. Liquid Substrate Bioassay
2.3. Model Semi-Field and Field Experiments
2.3.1. Plant Material
2.3.2. Field and Semi-Field Experiment Designs
2.3.3. Inoculum Preparation and Inoculation
2.3.4. Sampling and Selected Yielding Parameters Calculation
2.4. Mycotoxin Identification
2.4.1. Chemicals and Reagents
2.4.2. Sample Preparation, Mycotoxins Extraction and HPLC Analysis
2.5. QPCR Quantification of F. culmorum Biomass
2.6. Microscopic Analysis
2.6.1. Pathogen-Antagonist Contact Zone Observation
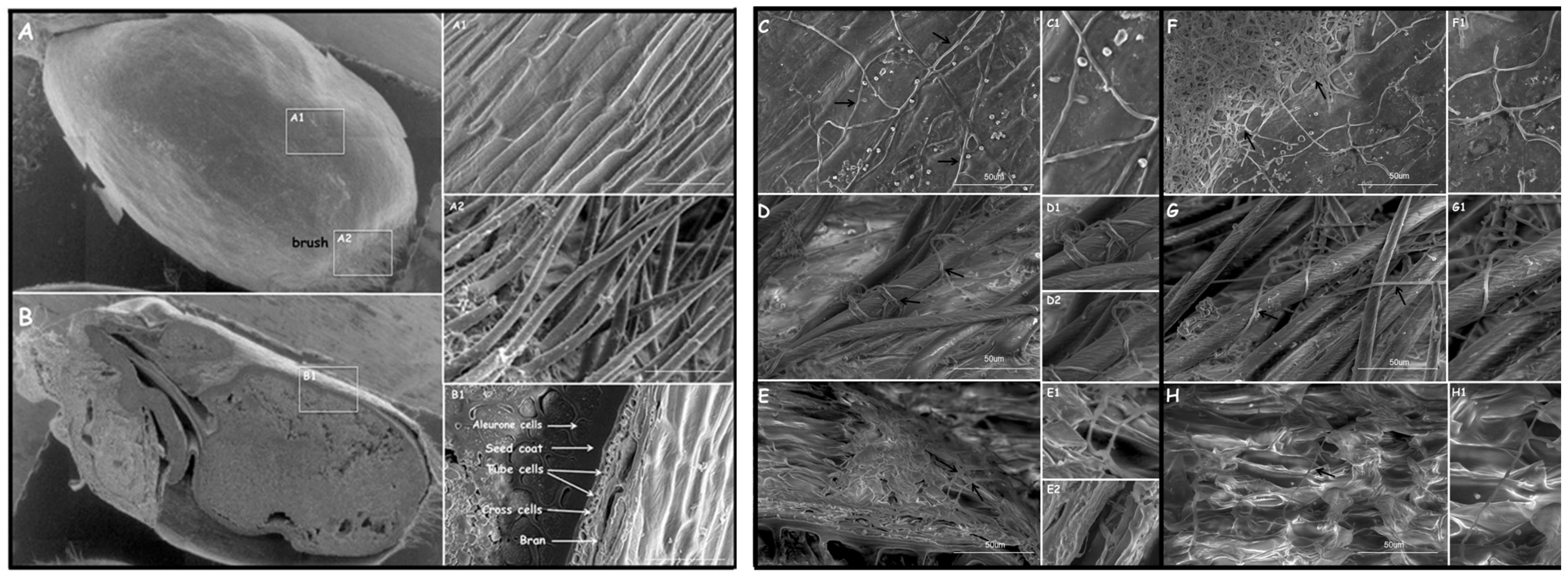
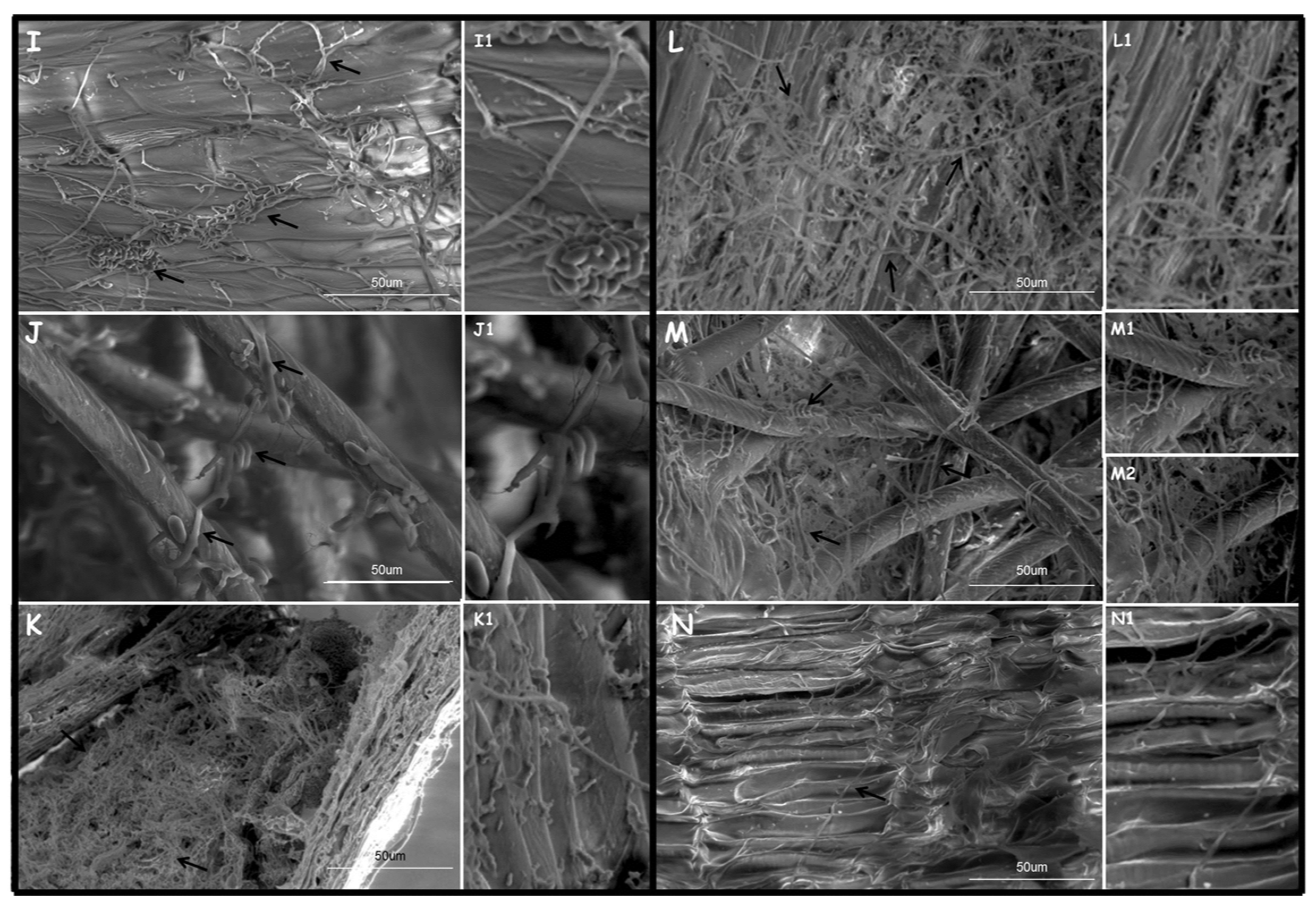
2.6.2. Fungi Wheat Kernel Colonization Observations
2.7. Statistical Analysis
3. Results
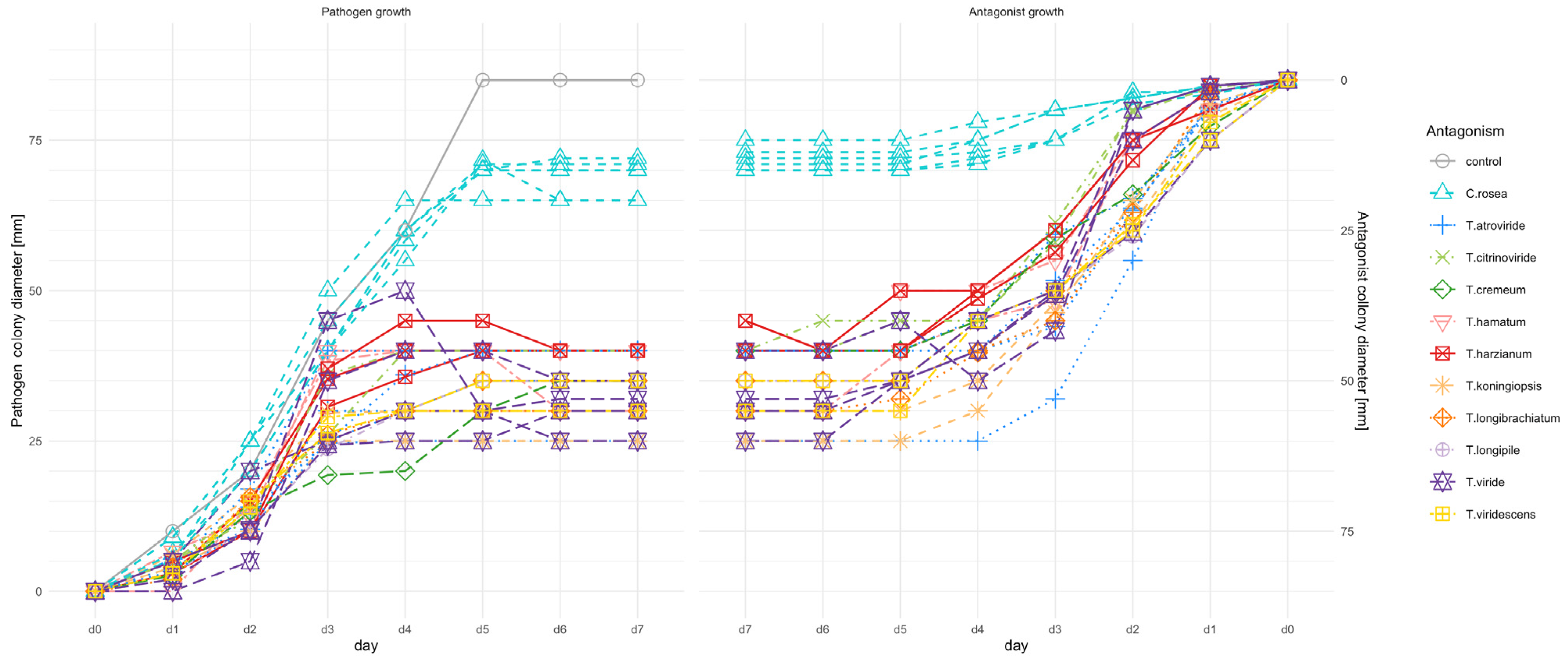
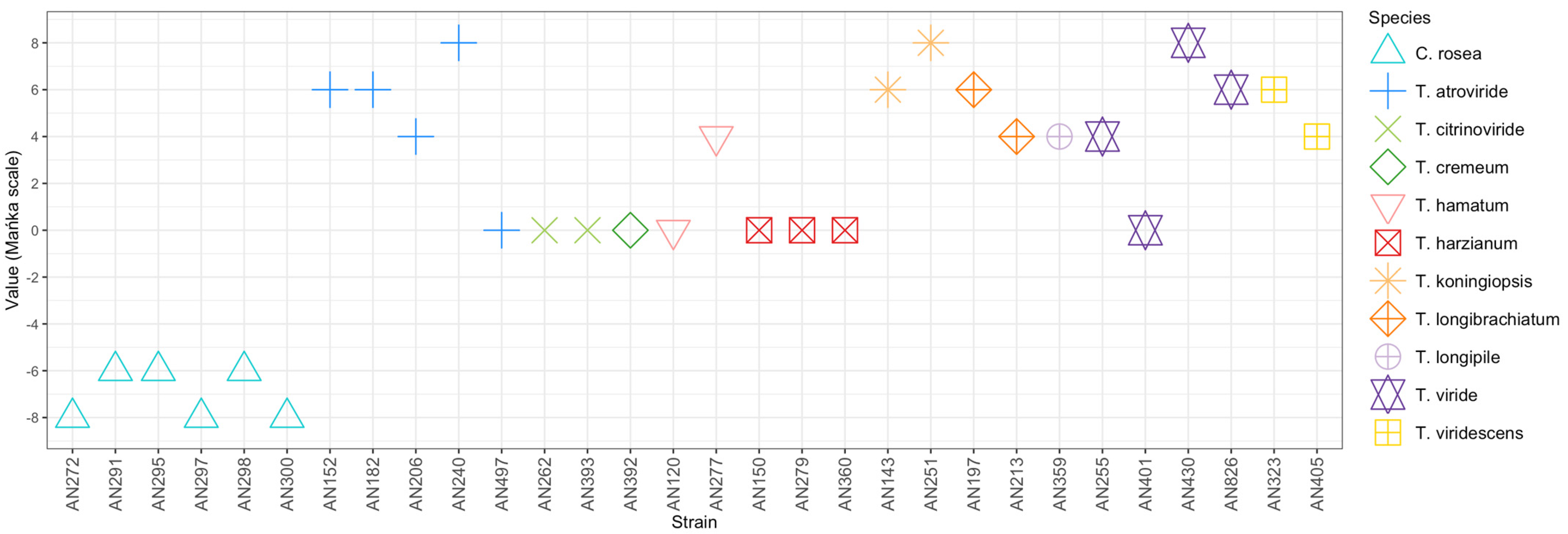
3.1. Assessment of the Antagonistic Potential of Trichoderma and Clonostachys Strains in a PDA Dual Culture Bioassay
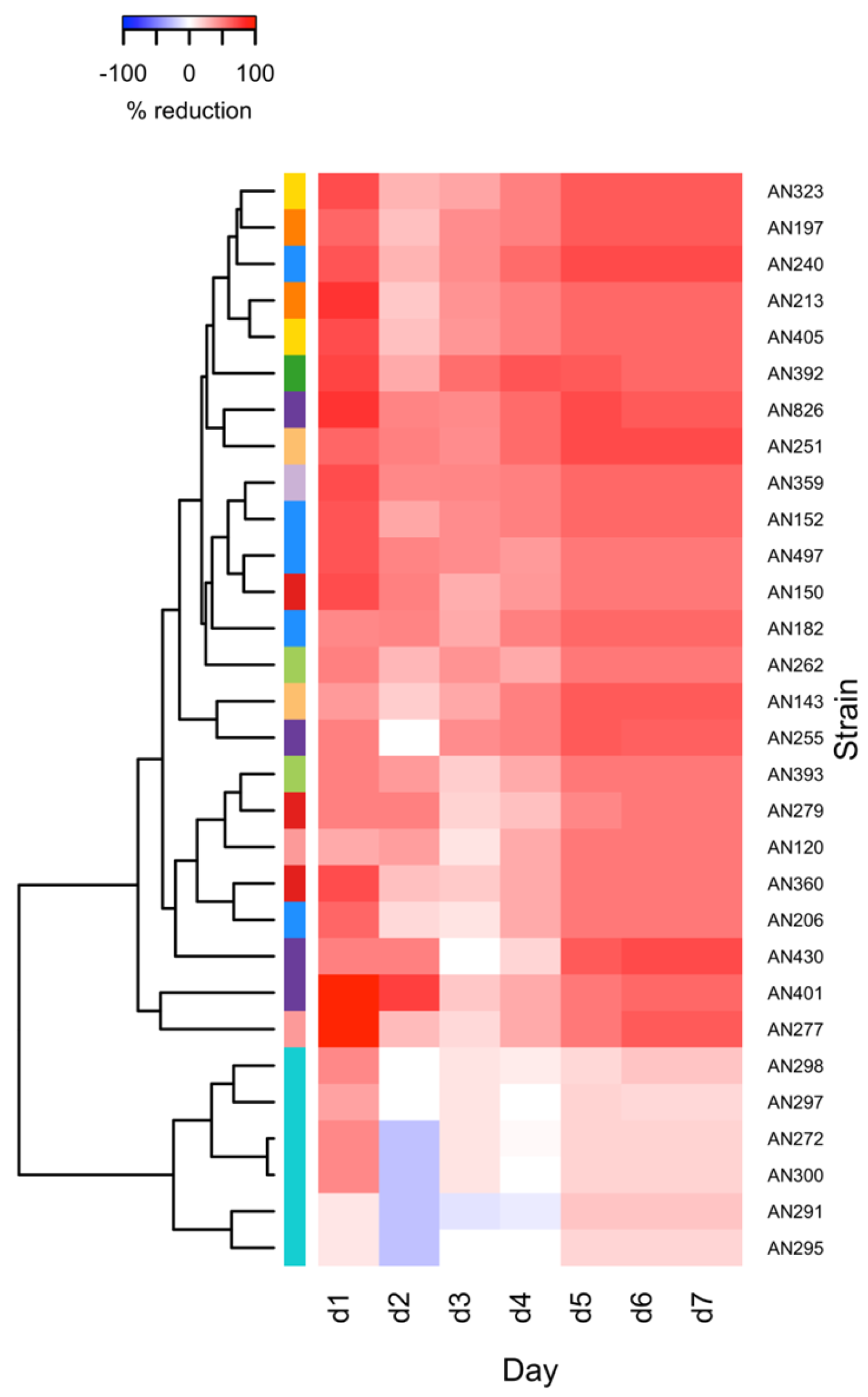
3.2. Assessment of the Potential of Selected Trichoderma and Clonostachys Strains to Reduce the Synthesis of Fusarium Mycotoxins in a Solid Substrate Bioassay
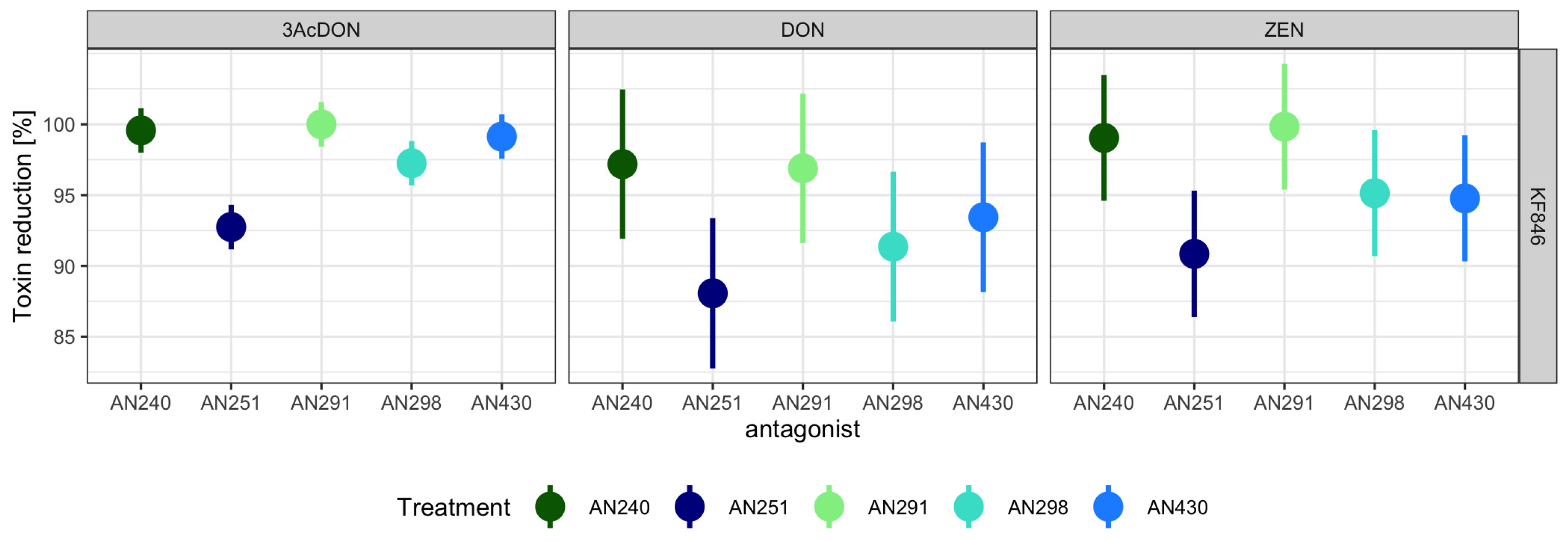
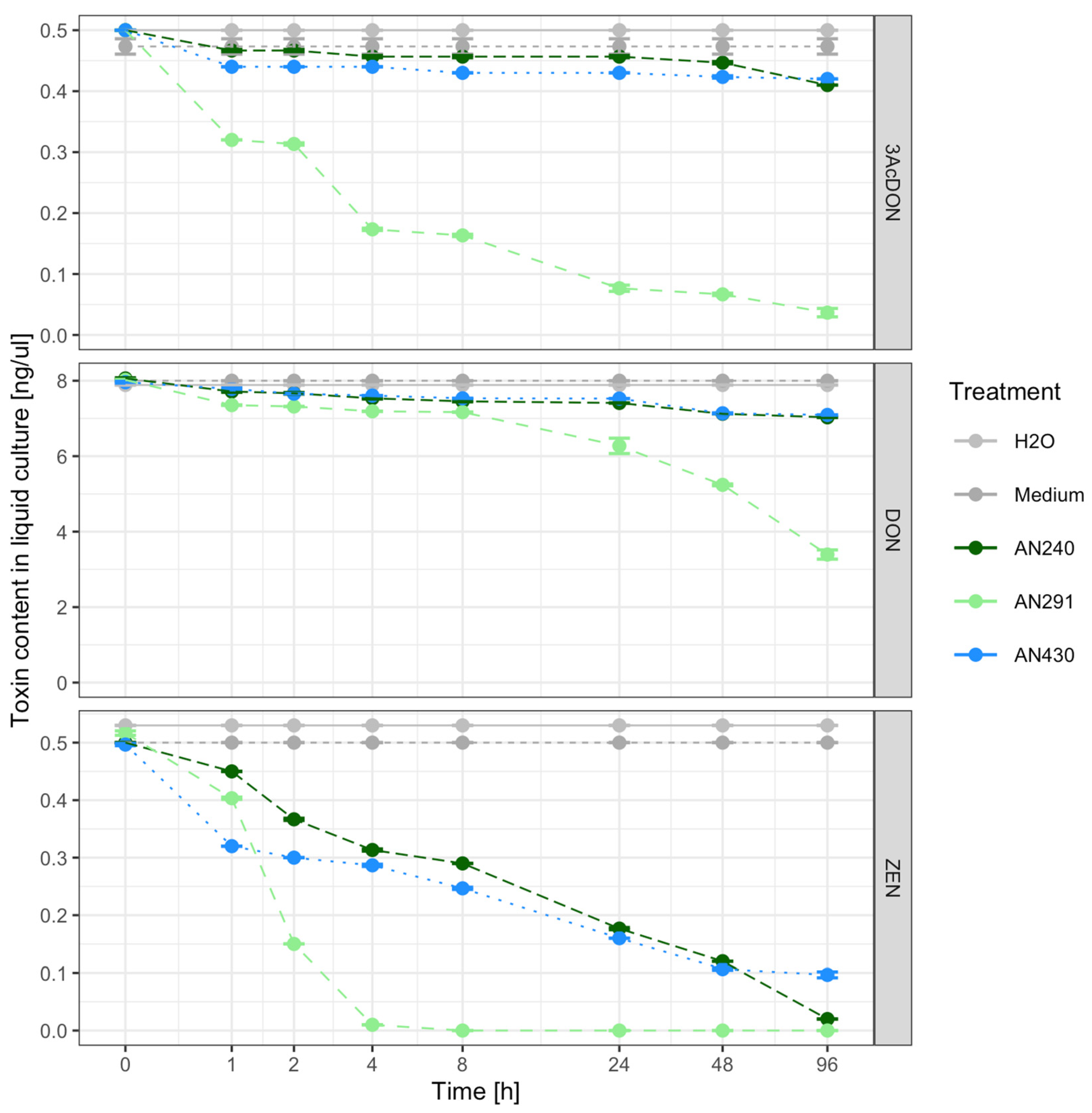
3.3. Assessment of the Ability of Trichoderma and Clonostachys Strains to Remove Mycotoxins in a Liquid Substrate Bioassay
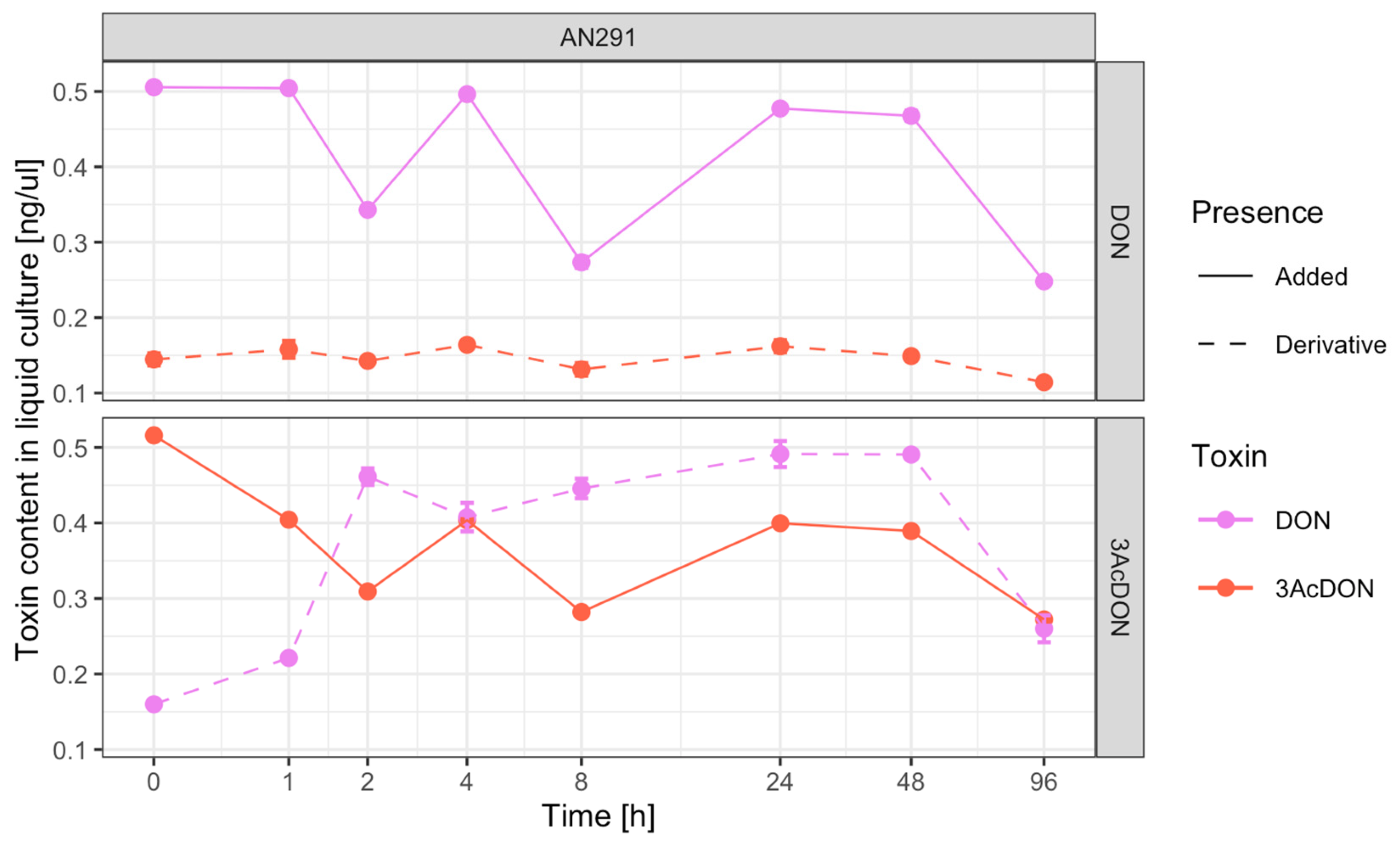
3.4. Toxin Transformations
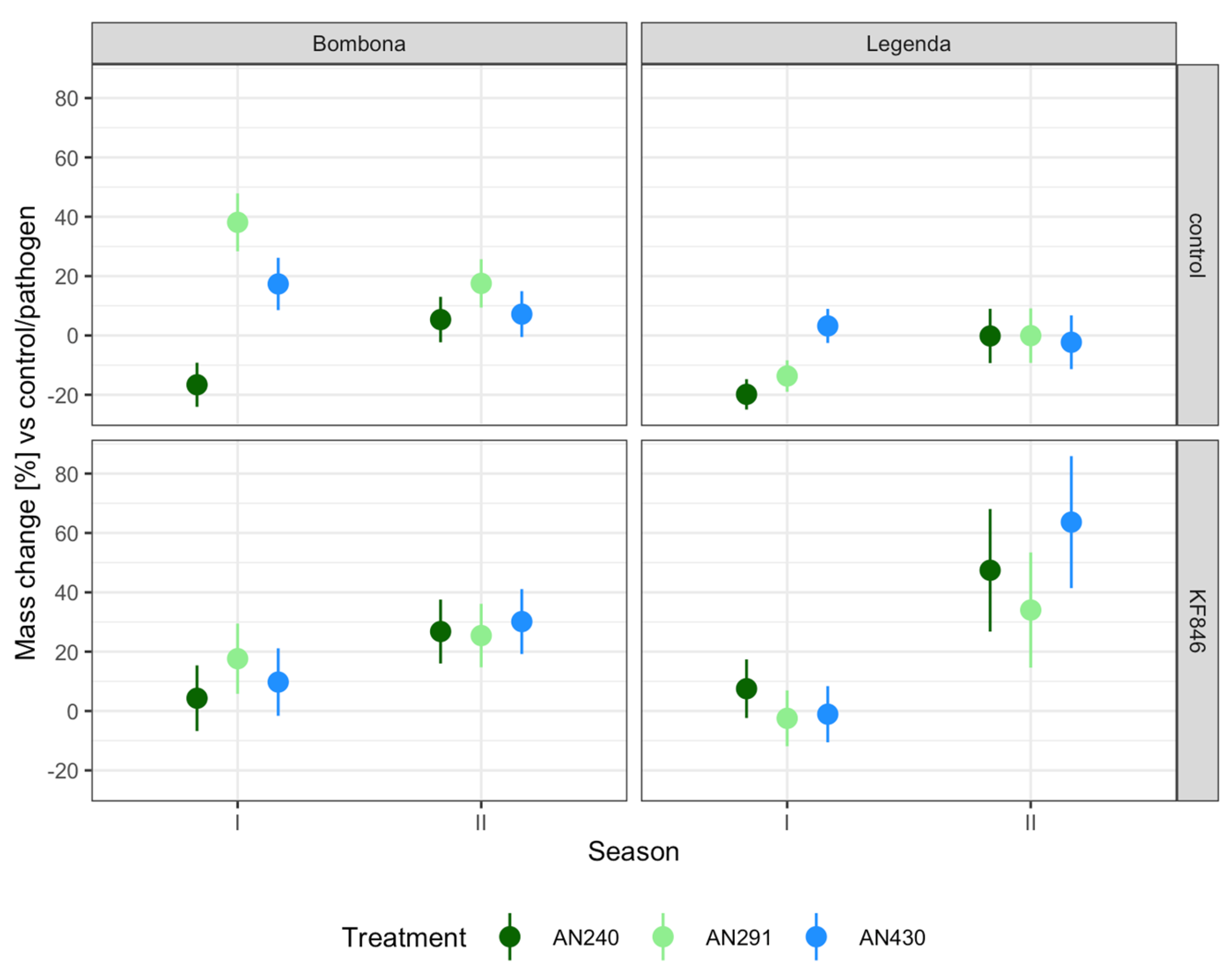
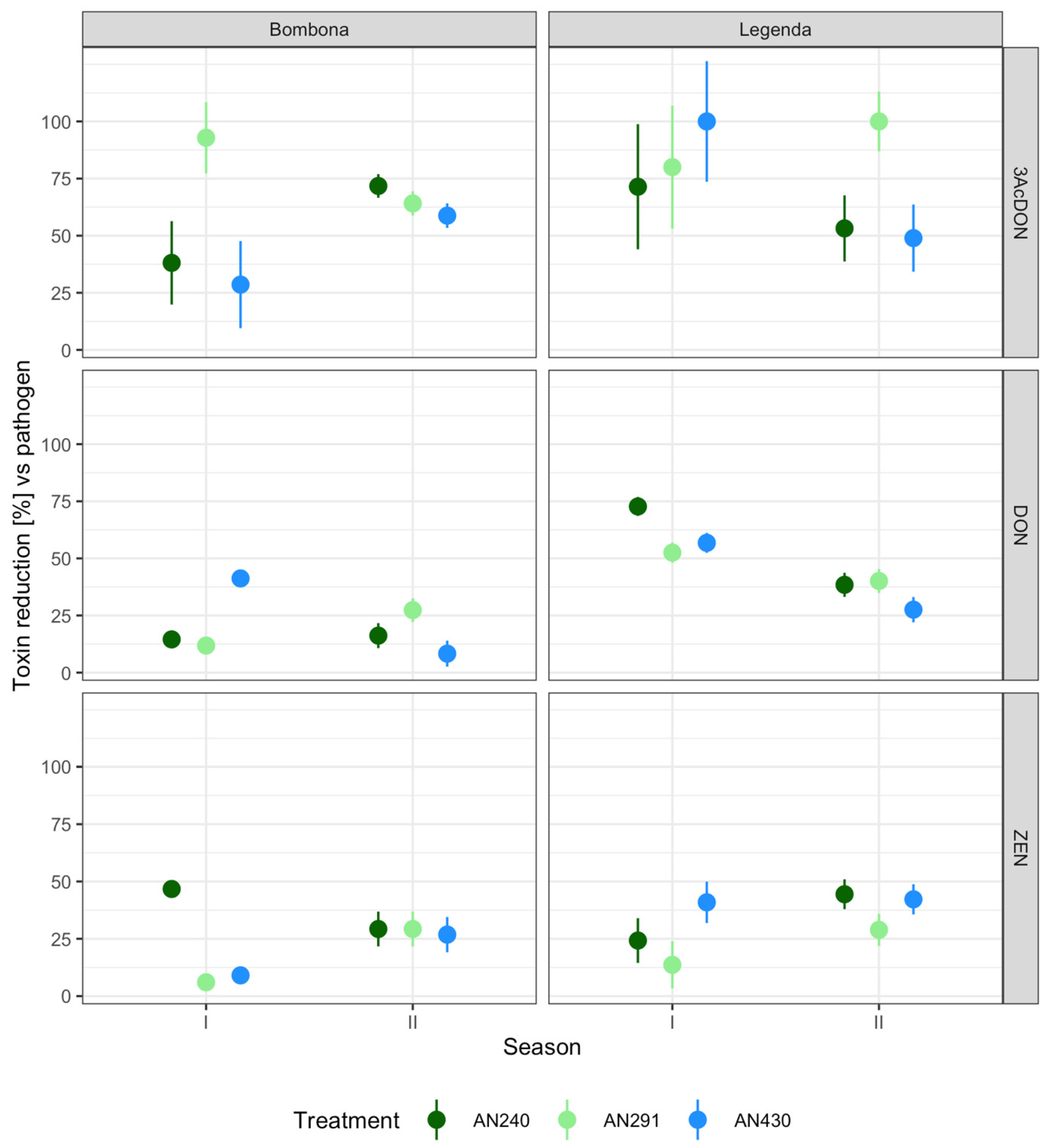
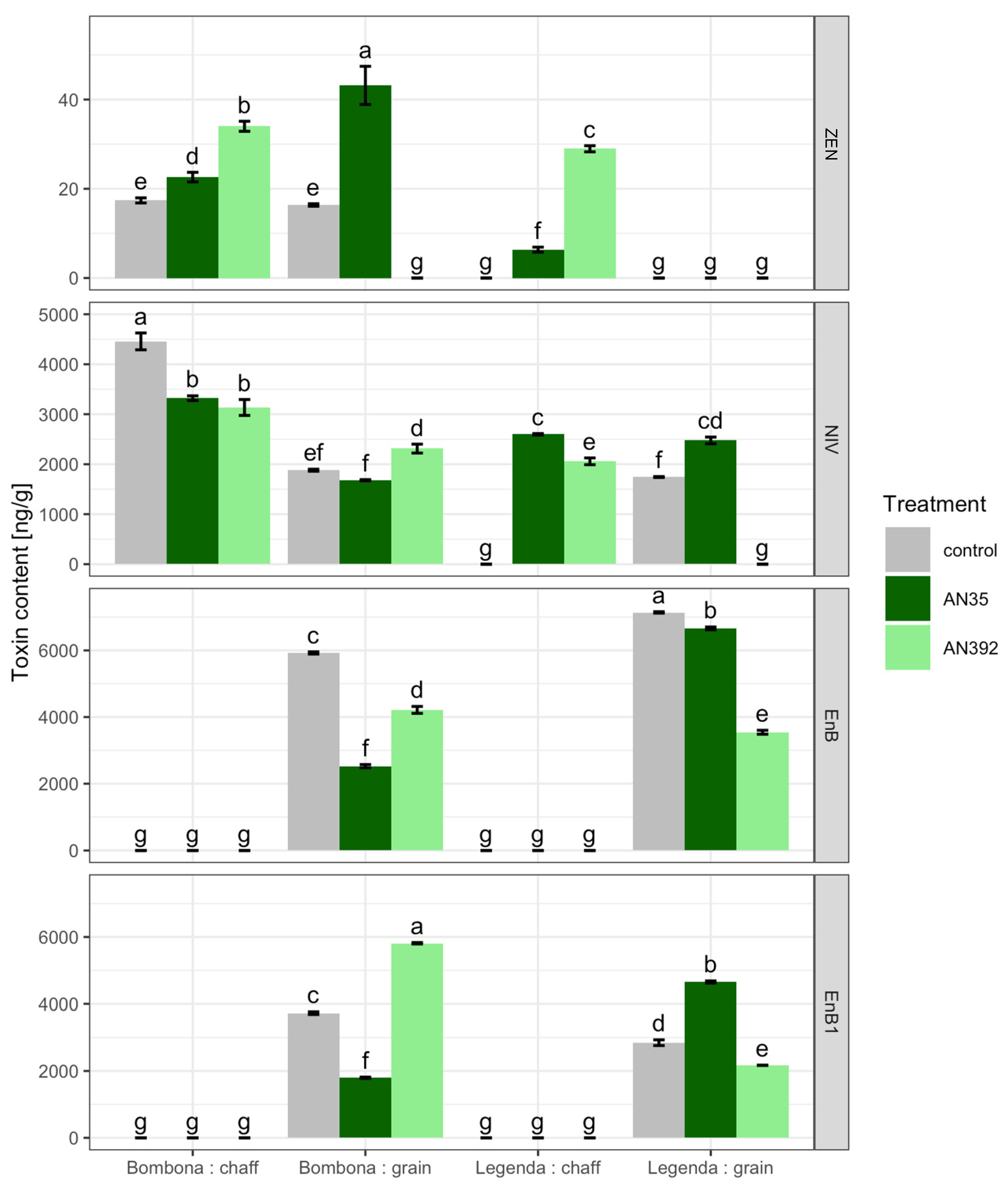
3.5. Semi-Field Experiment
3.6. F. culmorum KF 846 Biomass in Solid (Rice) Medium and in Wheat Kernels
3.7. Field Experiment
4. Discussion
4.1. PDA Dual Culture Bioassay: Preliminary Selection of Trichoderma and Clonostachys Strains Antagonistic to F. culmorum KF846
4.2. Solid Substrate Dual Culture Bioassay: Selection of Trichoderma and Clonostachys Strains with the Ability to Reduce Mycotoxin Synthesis by F. culmorum KF846
4.3. Liquid Substrate Bioassay: Determination of the Ability of Trichoderma and Clonostachys Strains to Eliminate Mycotoxins
4.4. Model Semi-Controlled Field and Field Experiment: The Effect of Trichoderma and Clonostachys on the Accumulation of Fusarium Mycotoxins in Wheat Tissues
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal Agent of Foot and Root Rot and Head Blight on Wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.-A.; Klink, H. Composition and Predominance of Fusarium Species Causing Fusarium Head Blight in Winter Wheat Grain Depending on Cultivar Susceptibility and Meteorological Factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, M.; Beyer, M.; Logrieco, A.; Audenaert, K.; Balmas, V.; Basler, R.; Boutigny, A.-L.; Chrpová, J.; Czembor, E.; Gagkaeva, T.; et al. A European Database of Fusarium graminearum and F. culmorum Trichothecene Genotypes. Front. Microbiol. 2016, 7, 00406. [Google Scholar] [CrossRef]
- Quarta, A.; Mita, G.; Haidukowski, M.; Santino, A.; Mulè, G.; Visconti, A. Assessment of Trichothecene Chemotypes of Fusarium culmorum Occurring in Europe. Food Addit. Contam. 2005, 22, 309–315. [Google Scholar] [CrossRef]
- Yörük, E.; Albayrak, G. Chemotyping of Fusarium graminearum and F. culmorum Isolates from Turkey by PCR Assay. Mycopathologia 2012, 173, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kammoun, L.G.; Gargouri, S.; Barreau, C.; Richard-Forget, F.; Hajlaoui, M.R. Trichothecene Chemotypes of Fusarium culmorum Infecting Wheat in Tunisia. Int. J. Food Microbiol. 2010, 140, 84–89. [Google Scholar] [CrossRef]
- Bentley, A.R.; Cromey, M.G.; Farrokhi-Nejad, R.; Leslie, J.F.; Summerell, B.A.; Burgess, L.W. Fusarium crown and root rot pathogens associated with wheat and grass stem bases on the South Island of New Zealand. Australas. Plant Pathol. 2006, 35, 495–502. [Google Scholar] [CrossRef]
- Scherm, B.; Orrù, M.; Balmas, V.; Spanu, F.; Azara, E.; Delogu, G.; Hammond, T.M.; Keller, N.P.; Migheli, Q. Altered Trichothecene Biosynthesis in TRI6-silenced Transformants of Fusarium culmorum Influences the Severity of Crown and Foot Rot on Durum Wheat Seedlings. Mol. Plant Pathol. 2011, 12, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Nešić, K.; Habschied, K.; Mastanjević, K. Possibilities for the Biological Control of Mycotoxins in Food and Feed. Toxins 2021, 13, 198. [Google Scholar] [CrossRef]
- Schiwek, S.; Alhussein, M.; Rodemann, C.; Budragchaa, T.; Beule, L.; von Tiedemann, A.; Karlovsky, P. Fusarium culmorum Produces NX-2 Toxin Simultaneously with Deoxynivalenol and 3-Acetyl-Deoxynivalenol or Nivalenol. Toxins 2022, 14, 456. [Google Scholar] [CrossRef]
- Wagacha, J.M.; Muthomi, J.W. Fusarium culmorum: Infection Process, Mechanisms of Mycotoxin Production and Their Role in Pathogenesis in Wheat. Crop Prot. 2007, 26, 877–885. [Google Scholar] [CrossRef]
- Senter, L.H.; Sanson, D.R.; Corley, D.G.; Tempesta, M.S.; Rottinghaus, A.A.; Rottinghaus, G.E. Cytotoxicity of Trichothecene Mycotoxins Isolated from Fusarium sporotrichioides (MC-72083) and Fusarium sambucinum in Baby Hamster Kidney (BHK-21) Cells. Mycopathologia 1991, 113, 127–131. [Google Scholar] [CrossRef] [PubMed]
- McCormick, S.P.; Kato, T.; Maragos, C.M.; Busman, M.; Lattanzio, V.M.T.; Galaverna, G.; Dall-Asta, C.; Crich, D.; Price, N.P.J.; Kurtzman, C.P. Anomericity of T-2 Toxin-Glucoside: Masked Mycotoxin in Cereal Crops. J. Agric. Food Chem. 2015, 63, 731–738. [Google Scholar] [CrossRef]
- Suzuki, T.; Iwahashi, Y. Phytotoxicity Evaluation of Type B Trichothecenes Using a Chlamydomonas Reinhardtii Model System. Toxins 2014, 6, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.K.; Yoshizawa, T.; Shier, W.T. Cytotoxicity and Phytotoxicity of Trichothecene Mycotoxins Produced by Fusarium spp. Toxicon Off. J. Int. Soc. Toxinol. 2013, 74, 68–75. [Google Scholar] [CrossRef]
- Pinton, P.; Oswald, I.P. Effect of Deoxynivalenol and Other Type B Trichothecenes on the Intestine: A Review. Toxins 2014, 6, 1615–1643. [Google Scholar] [CrossRef]
- Ropejko, K.; Twarużek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the Toxicity, Occurrence, Metabolism, Detoxification, Regulations and Intake of Zearalenone: An Oestrogenic Mycotoxin. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Gromadzka, K.; Chełkowski, J.; Stepien, L.; Golinski, P. Occurrence of Zearalenone in Wheat and Maize Grain in Poland. Cereal Res. Commun. 2008, 36, 361–363. [Google Scholar]
- Gromadzka, K.; Waśkiewicz, A.; Goliński, P.; Świetlik, J. Occurrence of Estrogenic Mycotoxin—Zearalenone in Aqueous Environmental Samples with Various NOM Content. Water Res. 2009, 43, 1051–1059. [Google Scholar] [CrossRef]
- Wegulo, S.; Baenziger, P.; Nopsa, J.; Bockus, W.; Hallen-Adams, H. Management of Fusarium Head Blight of Wheat and Barley. Crop Prot. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- Czaja, K.; Góralczyk, K.; Struciński, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides--towards Increased Consumer Safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Sarmast, E.; Fallah, A.A.; Jafari, T.; Mousavi Khaneghah, A. Occurrence and Fate of Mycotoxins in Cereals and Cereal-Based Products: A Narrative Review of Systematic Reviews and Meta-Analyses Studies. Curr. Opin. Food Sci. 2021, 39, 68–75. [Google Scholar] [CrossRef]
- EUR-Lex—02009L0128-20091125—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/dir/2009/128/2009-11-25 (accessed on 29 January 2023).
- European Mission. Proposal for a Regulation of the European Parliament and of the Council on the Sustainable Use of Plant Protection Products and Amending Regulation (EU) 2021/2115; European Mission: Brussels, Belgium, 2022. [Google Scholar]
- Karlsson, M.; Durling, M.B.; Choi, J.; Kosawang, C.; Lackner, G.; Tzelepis, G.D.; Nygren, K.; Dubey, M.K.; Kamou, N.; Levasseur, A.; et al. Insights on the Evolution of Mycoparasitism from the Genome of Clonostachys rosea. Genome Biol. Evol. 2015, 7, 465–480. [Google Scholar] [CrossRef]
- Woo, S.L.; Hermosa, R.; Lorito, M.; Monte, E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, L.; Popiel, D.; Chełkowski, J.; Koczyk, G.; Samuels, G.J.; Sobieralski, K.; Siwulski, M. Species Diversity of Trichoderma in Poland. J. Appl. Genet. 2011, 52, 233–243. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Strakowska, J.; Chełkowski, J.; Gąbka-Buszek, A.; Kaczmarek, J. Trichoderma Species Occurring on Wood with Decay Symptoms in Mountain Forests in Central Europe: Genetic and Enzymatic Characterization. J. Appl. Genet. 2016, 57, 397–407. [Google Scholar] [CrossRef]
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Frużyńska-Jóźwiak, D. Diversity of Trichoderma spp. Causing Pleurotus Green Mould Diseases in Central Europe. Folia Microbiol. 2013, 58, 325–333. [Google Scholar] [CrossRef]
- Gromadzka, K.; Błaszczyk, L.; Chełkowski, J.; Waskiewicz, A. Occurrence of Mycotoxigenic Fusarium Species and Competitive Fungi on Preharvest Maize Ear Rot in Poland. Toxins 2019, 11, 224. [Google Scholar] [CrossRef]
- Wiśniewska, H.; Kowalczyk, K. Resistance of Cultivars and Breeding Lines of Spring Wheat to Fusarium culmorum and Powdery mildew. J. Appl. Genet. 2005, 46, 35–40. [Google Scholar] [PubMed]
- Ochodzki, P.; Twardawska, A.; Wiśniewska, H.; Góral, T. Resistance to Fusarium Head Blight, Kernel Damage, and Concentrations of Fusarium Mycotoxins in the Grain of Winter Wheat Lines. Agronomy 2021, 11, 1690. [Google Scholar] [CrossRef]
- Stępień, Ł.; Popiel, D.; Koczyk, G.; Chełkowski, J. Wheat-Infecting Fusarium Species in Poland--Their Chemotypes and Frequencies Revealed by PCR Assay. J. Appl. Genet. 2008, 49, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Nirenberg, H. Untersuchungen Uber Die Morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola; Berlin-Dahlem. Mitt Biol Bundesanst f Land-Forstwirt 1976, 169, 1–117. [Google Scholar]
- Błaszczyk, L.; Basińska-Barczak, A.; Ćwiek-Kupczyńska, H.; Gromadzka, K.; Popiel, D.; Stępień, Ł. Suppressive Effect of Trichoderma spp. on Toxigenic Fusarium Species. Pol. J. Microbiol. 2017, 66, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Edgington, L.V.; Khew, K.I.; Barron, G. Fungitoxic Spectrum of Benzimidazole Compounds. Phytopathology 1971, 61, 42. [Google Scholar] [CrossRef]
- Mańka, K. Zbiorowiska grzybów jako kryterium oceny wpływu środowiska na choroby roślin. [Fungal communities as a criterion for estimating the effect of the environment on plant diseases.—Engl. summary]. Zesz. Probl. Postępów Nauk Rol. 1974, 160, 9–23. [Google Scholar]
- Basińska-Barczak, A.; Błaszczyk, L.; Szentner, K. Plant Cell Wall Changes in Common Wheat Roots as a Result of Their Interaction with Beneficial Fungi of Trichoderma. Cells 2020, 9, 2319. [Google Scholar] [CrossRef]
- Kostecki, M.; Wisniewska, H.; Perrone, G.; Ritieni, A.; Golinski, P.; Chelkowski, J.; Logrieco, A. The Effects of Cereal Substrate and Temperature on Production of Beauvericin, Moniliformin and Fusaproliferin by Fusarium Subglutinans ITEM-1434. Food Addit. Contam. 1999, 16, 361–365. [Google Scholar] [CrossRef]
- Tomczak, M.; Wiśniewska, H.; Stepien, L.; Kostecki, M.; Chełkowski, J.; Goliński, P. Deoxynivalenol, Nivalenol and Moniliformin in Wheat Samples with Head Blight (Scab) Symptoms in Poland (1998–2000). Eur. J. Plant Pathol. 2002, 108, 625–630. [Google Scholar] [CrossRef]
- Yang, D.; Geng, Z.M.; Yao, J.B.; Zhang, X.; Zhang, P.P.; Ma, H.X. Simultaneous Determination of Deoxynivalenol, and 15- and 3-Acetyldeoxynivalenol in Cereals by HPLC-UV Detection. World Mycotoxin J. 2013, 6, 117–125. [Google Scholar] [CrossRef]
- Visconti, A.; Pascale, M. Determination of Zearalenone in Corn by Means of Immunoaffinity Clean-up and High-Performance Liquid Chromatography with Fluorescence Detection. J. Chromatogr. A 1998, 815, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Waalwijk, C.; Kastelein, P.; de Vries, I.; Kerényi, Z.; van der Lee, T.; Hesselink, T.; Köhl, J.; Kema, G. Major Changes in Fusarium spp. in Wheat in the Netherlands. Eur. J. Plant Pathol. 2003, 109, 743–754. [Google Scholar] [CrossRef]
- Rahman, M.A.; Begum, M.F.; Alam, M.F. Screening of Trichoderma Isolates as a Biological Control Agent against Ceratocystis Paradoxa Causing Pineapple Disease of Sugarcane. Mycobiology 2009, 37, 277–285. [Google Scholar] [CrossRef]
- Abdellatif, L.; Fernandez, M.R.; Lokuruge, P. Mode of Action of Potential Biocontrol Agents against Fusarium Species and Cochliobolus sativus. Mycologia 2022, 114, 476–486. [Google Scholar] [CrossRef]
- Pellan, L.; Durand, N.; Martinez, V.; Fontana, A.; Schorr-Galindo, S.; Strub, C. Commercial Biocontrol Agents Reveal Contrasting Comportments Against Two Mycotoxigenic Fungi in Cereals: Fusarium graminearum and Fusarium verticillioides. Toxins 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Geiger, A.; Karácsony, Z.; Geml, J.; Váczy, K.Z. Mycoparasitism Capability and Growth Inhibition Activity of Clonostachys rosea Isolates against Fungal Pathogens of Grapevine Trunk Diseases Suggest Potential for Biocontrol. PLoS ONE 2022, 17, e0273985. [Google Scholar] [CrossRef]
- Kostrzewska-Szlakowska, I.; Kiersztyn, B. Microbial Biomass and Enzymatic Activity of the Surface Microlayer and Subsurface Water in Two Dystrophic Lakes. Pol. J. Microbiol. 2017, 66, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Modrzewska, M.; Błaszczyk, L.; Stępień, Ł.; Urbaniak, M.; Waśkiewicz, A.; Yoshinari, T.; Bryła, M. Trichoderma versus Fusarium—Inhibition of Pathogen Growth and Mycotoxin Biosynthesis. Molecules 2022, 27, 8146. [Google Scholar] [CrossRef]
- Gimeno, A.; Stanley, C.E.; Ngamenie, Z.; Hsung, M.-H.; Walder, F.; Schmieder, S.S.; Bindschedler, S.; Junier, P.; Keller, B.; Vogelgsang, S. A Versatile Microfluidic Platform Measures Hyphal Interactions between Fusarium graminearum and Clonostachys rosea in Real-Time. Commun. Biol. 2021, 4, 1–10. [Google Scholar] [CrossRef]
- Sun, Z.-B.; Li, S.-D.; Ren, Q.; Xu, J.-L.; Lu, X.; Sun, M.-H. Biology and Applications of Clonostachys rosea. J. Appl. Microbiol. 2020, 129, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Lv, B.; Uddin, M.J.; Chen, Y.; Fan, L.; Sun, Z.; Sun, M.; Li, S. Monitoring Mycoparasitism of Clonostachys rosea against Botrytis cinerea Using GFP. J. Fungi 2022, 8, 567. [Google Scholar] [CrossRef]
- Atanasova, L.; Crom, S.L.; Gruber, S.; Coulpier, F.; Seidl-Seiboth, V.; Kubicek, C.P.; Druzhinina, I.S. Comparative Transcriptomics Reveals Different Strategies of Trichoderma mycoparasitism. BMC Genom. 2013, 14, 121. [Google Scholar] [CrossRef] [PubMed]
- Chaverri, P.; Samuels, G.J. Evolution of Habitat Preference and Nutrition Mode in a Cosmopolitan Fungal Genus with Evidence of Interkingdom Host Jumps and Major Shifts in Ecology. Evolution 2013, 67, 2823–2837. [Google Scholar] [CrossRef]
- del Carmen, H.; Rodríguez, M.; Evans, H.C.; de Abreu, L.M.; de Macedo, D.M.; Ndacnou, M.K.; Bekele, K.B.; Barreto, R.W. New Species and Records of Trichoderma Isolated as Mycoparasites and Endophytes from Cultivated and Wild Coffee in Africa. Sci. Rep. 2021, 11, 5671. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, M.; Atanasova, L.; Jensen, D.F.; Zeilinger, S. Necrotrophic Mycoparasites and Their Genomes. In The Fungal Kingdom; Heitman, J., Crous, P.W., Stukenbrock, E.H., James, T.Y., Gow, N.A.R., Eds.; American Society for Microbiology: Washington, DC, USA, 2017; pp. 1005–1026. [Google Scholar]
- Hue, A.G.; Voldeng, H.D.; Savard, M.E.; Fedak, G.; Tian, X.; Hsiang, T. Biological Control of Fusarium Head Blight of Wheat with Clonostachys rosea Strain ACM941. Can. J. Plant Pathol. 2009, 31, 169–179. [Google Scholar] [CrossRef]
- Demissie, Z.A.; Witte, T.; Robinson, K.A.; Sproule, A.; Foote, S.J.; Johnston, A.; Harris, L.J.; Overy, D.P.; Loewen, M.C. Transcriptomic and Exometabolomic Profiling Reveals Antagonistic and Defensive Modes of Clonostachys rosea Action Against Fusarium graminearum. Mol. Plant-Microbe Interact. 2020, 33, 842–858. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, F.M.; De Boevre, M.; Landschoot, S.; De Saeger, S.; Haesaert, G.; Audenaert, K. Fungal Endophytes Control Fusarium graminearum and Reduce Trichothecenes and Zearalenone in Maize. Toxins 2018, 10, 493. [Google Scholar] [CrossRef]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium Head Blight: Interactions between Trichoderma and Mycotoxigenic Fusarium. Microbiology 2012, 158, 98–106. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, Y.; Liu, N.; Yan, Z.; Liao, Y.; Chen, J.; De Saeger, S.; Yang, H.; Zhang, Q.; Wu, A. Detoxification of Deoxynivalenol via Glycosylation Represents Novel Insights on Antagonistic Activities of Trichoderma When Confronted with Fusarium graminearum. Toxins 2016, 8, 335. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, Y.; Yan, Z.; Liao, Y.; Chen, J.; De Boevre, M.; De Saeger, S.; Wu, A. Antagonistic and Detoxification Potentials of Trichoderma Isolates for Control of Zearalenone (ZEN) Producing Fusarium graminearum. Front. Microbiol. 2018, 8, 02710. [Google Scholar] [CrossRef]
- Shantha, T. Fungal Degradation of Aflatoxin B1. Nat. Toxins 1999, 7, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Mann, R.; Rehm, H.J. Degradation Products from Aflatoxin B1 by Corynebacterium rubrum, Aspergillus niger, Trichoderma viride and Mucor ambiguus. Eur. J. Appl. Microbiol. Biotechnol. 1976, 2, 297–306. [Google Scholar] [CrossRef]
- el-Sharkawy, S.; Abul-Hajj, Y.J. Microbial Cleavage of Zearalenone. Xenobiotica Fate Foreign Compd. Biol. Syst. 1988, 18, 365–371. [Google Scholar] [CrossRef]
- Takahashi-Ando, N.; Kimura, M.; Kakeya, H.; Osada, H.; Yamaguchi, I. A Novel Lactonohydrolase Responsible for the Detoxification of Zearalenone: Enzyme Purification and Gene Cloning. Biochem. J. 2002, 365, 1–6. [Google Scholar] [CrossRef]
- Kosawang, C.; Karlsson, M.; Vélëz, H.; Rasmussen, P.H.; Collinge, D.B.; Jensen, B.; Jensen, D.F. Zearalenone Detoxification by Zearalenone Hydrolase Is Important for the Antagonistic Ability of Clonostachys rosea against Mycotoxigenic Fusarium graminearum. Fungal Biol. 2014, 118, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, A.; Musa, T.; Voegele, R.T.; Vogelgsang, S. The Potential of Antagonistic Fungi for Control of Fusarium graminearum and Fusarium crookwellense Varies Depending on the Experimental Approach. J. Appl. Microbiol. 2015, 118, 1165–1179. [Google Scholar] [CrossRef]
- Almeida, F.B.d.R.; Cerqueira, F.M.; Silva, R.d.N.; Ulhoa, C.J.; Lima, A.L. Mycoparasitism Studies of Trichoderma Harzianum Strains against Rhizoctonia Solani: Evaluation of Coiling and Hydrolytic Enzyme Production. Biotechnol. Lett. 2007, 29, 1189–1193. [Google Scholar] [CrossRef]
- Anees, M.; Tronsmo, A.; Edel-Hermann, V.; Hjeljord, L.G.; Héraud, C.; Steinberg, C. Characterization of Field Isolates of Trichoderma Antagonistic against Rhizoctonia solani. Fungal Biol. 2010, 114, 691–701. [Google Scholar] [CrossRef]
- Jackowiak, H.; Packa, D.; Wiwart, M.; Perkowski, J. Scanning Electron Microscopy of Fusarium Damaged Kernels of Spring Wheat. Int. J. Food Microbiol. 2005, 98, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Sarrocco, S.; Matarese, F.; Moncini, L.; Pachetti, G.; Ritieni, A.; Moretti, A.; Vannacci, G. Biocontrol of Fusarium Head Blight by Spike Application of Trichoderma gamsii. J. Plant Pathol. 2013, 95, 19–27. [Google Scholar]
- Alukumbura, A.S.; Bigi, A.; Sarrocco, S.; Fernando, W.G.D.; Vannacci, G.; Mazzoncini, M.; Bakker, M.G. Minimal Impacts on the Wheat Microbiome When Trichoderma Gamsii T6085 Is Applied as a Biocontrol Agent to Manage Fusarium Head Blight Disease. Front. Microbiol. 2022, 13, 972016. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.G.; Chen, Y.; Voldeng, H.D.; Fedak, G.; Savard, M.E.; Längle, T.; Zhang, J.; Harman, G.E. Concentration and Cultivar Effects on Efficacy of CLO-1 Biofungicide in Controlling Fusarium Head Blight of Wheat. Biol. Control 2014, 73, 2–7. [Google Scholar] [CrossRef]
- Jeleń, H.; Błaszczyk, L.; Chełkowski, J.; Rogowicz, K.; Strakowska, J. Formation of 6-n-Pentyl-2H-Pyran-2-One (6-PAP) and Other Volatiles by Different Trichoderma Species. Mycol. Prog. 2014, 13, 589–600. [Google Scholar] [CrossRef]
- Buśko, M.; Chełkowski, J.; Popiel, D.; Perkowski, J. Solid Substrate Bioassay to Evaluate Impact of Trichoderma on Trichothecene Mycotoxin Production by Fusarium Species. J. Sci. Food Agric. 2008, 88, 536–541. [Google Scholar] [CrossRef]
- Popiel, D.; Kwasna, A.; Chelkowski, J.; Stepien, L.; Laskowska, M. Impact of Selected Antagonistic Fungi on Fusarium Species—Toxigenic Cereal Pathogens. Acta Mycol. 2008, 43, 29–40. [Google Scholar] [CrossRef]
- Wiśniewska, H.; Stępień, Ł.; Waśkiewicz, A.; Beszterda, M.; Góral, T.; Belter, J. Toxigenic Fusarium Species Infecting Wheat Heads in Poland. Open Life Sci. 2014, 9, 163–172. [Google Scholar] [CrossRef]
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waśkiewicz, A.; Stępień, Ł. Fusarium Head Blight Incidence and Mycotoxin Accumulation in Three Durum Wheat Cultivars in Relation to Sowing Date and Density. Sci. Nat. 2017, 105, 2. [Google Scholar] [CrossRef]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-Beneficial Effects of Trichoderma and of Its Genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef]
- Sui, L.; Li, J.; Philp, J.; Yang, K.; Wei, Y.; Li, H.; Li, J.; Li, L.; Ryder, M.; Toh, R.; et al. Trichoderma atroviride Seed Dressing Influenced the Fungal Community and Pathogenic Fungi in the Wheat Rhizosphere. Sci. Rep. 2022, 12, 9677. [Google Scholar] [CrossRef]
- Xue, A.G.; Wei, G.; YuanHong, C.; Siddiqui, I.; Marchand, G.; JingHui, L.; ChangZhong, R. Effect of Seed Treatment with Novel Strains of Trichoderma spp. on Establishment and Yield of Spring Wheat. Crop Prot. 2017, 96, 97–102. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Błaszczyk, L.; Ćwiek-Kupczyńska, H.; Hoppe Gromadzka, K.; Basińska-Barczak, A.; Stępień, Ł.; Kaczmarek, J.; Lenc, L. Containment of Fusarium culmorum and Its Mycotoxins in Various Biological Systems by Antagonistic Trichoderma and Clonostachys Strains. J. Fungi 2023, 9, 289. https://doi.org/10.3390/jof9030289
Błaszczyk L, Ćwiek-Kupczyńska H, Hoppe Gromadzka K, Basińska-Barczak A, Stępień Ł, Kaczmarek J, Lenc L. Containment of Fusarium culmorum and Its Mycotoxins in Various Biological Systems by Antagonistic Trichoderma and Clonostachys Strains. Journal of Fungi. 2023; 9(3):289. https://doi.org/10.3390/jof9030289
Chicago/Turabian StyleBłaszczyk, Lidia, Hanna Ćwiek-Kupczyńska, Karolina Hoppe Gromadzka, Aneta Basińska-Barczak, Łukasz Stępień, Joanna Kaczmarek, and Leszek Lenc. 2023. "Containment of Fusarium culmorum and Its Mycotoxins in Various Biological Systems by Antagonistic Trichoderma and Clonostachys Strains" Journal of Fungi 9, no. 3: 289. https://doi.org/10.3390/jof9030289
APA StyleBłaszczyk, L., Ćwiek-Kupczyńska, H., Hoppe Gromadzka, K., Basińska-Barczak, A., Stępień, Ł., Kaczmarek, J., & Lenc, L. (2023). Containment of Fusarium culmorum and Its Mycotoxins in Various Biological Systems by Antagonistic Trichoderma and Clonostachys Strains. Journal of Fungi, 9(3), 289. https://doi.org/10.3390/jof9030289








