Diversity of Fungi Isolated from Potato Nematode Cysts in Guizhou Province, China
Abstract
1. Introduction
2. Materials and Methods
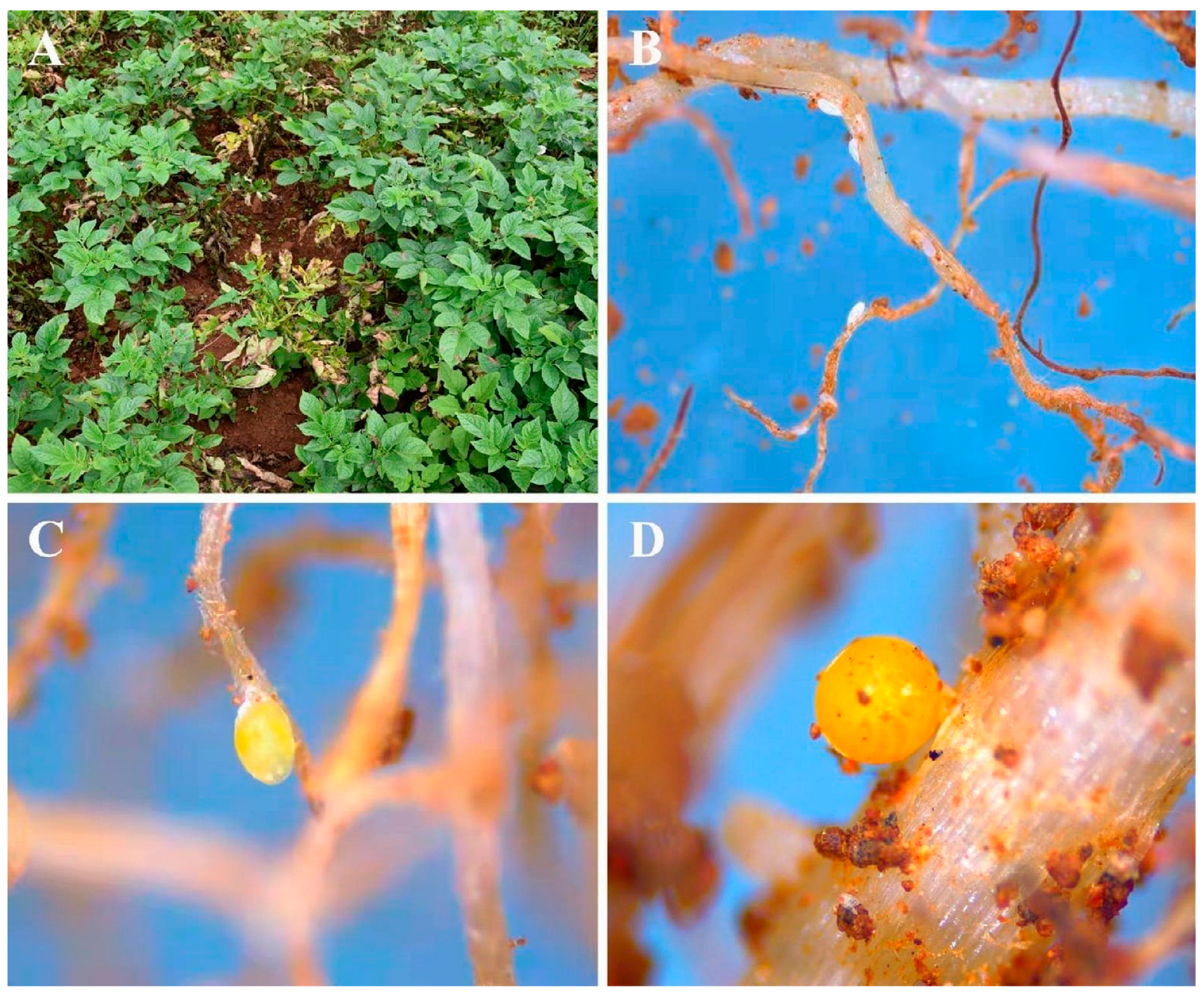
2.1. Nematode Collection
2.2. Isolation of Fungi from Cysts of G. rostochiensis
2.3. DNA Extraction, PCR, and Sequencing
2.4. Multigene Analyses
2.5. Diversity Indices and Functional Annotation Analysis
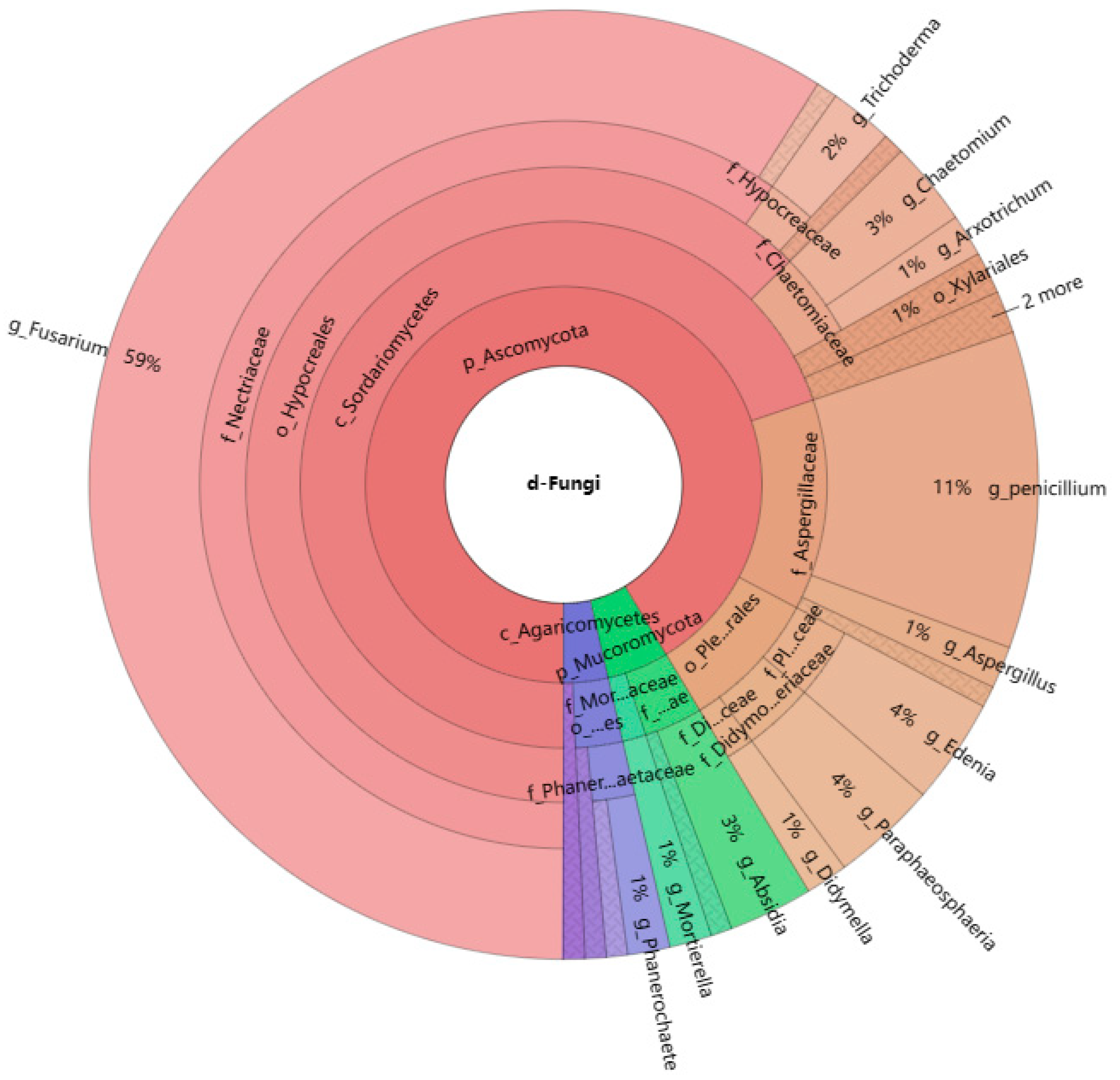
2.5.1. Dominant Taxa
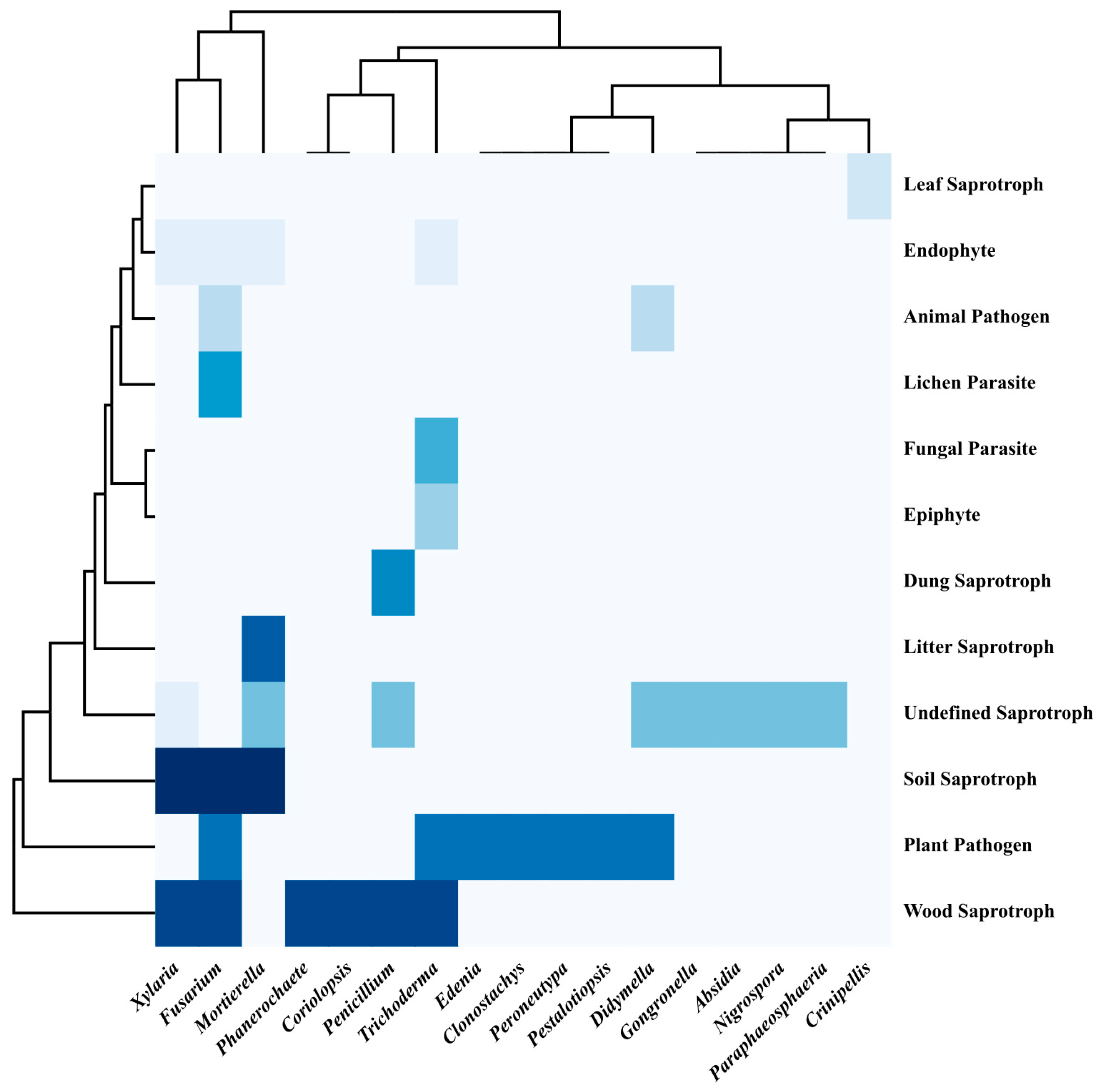
2.5.2. Lifestyle Diversity
2.6. In Vitro Parasitic Potential Tests of the Fungal Isolates towards Nematode Cysts
2.7. Data Analysis
3. Results
3.1. Fungi Associated with Cysts of G. rostochiensis
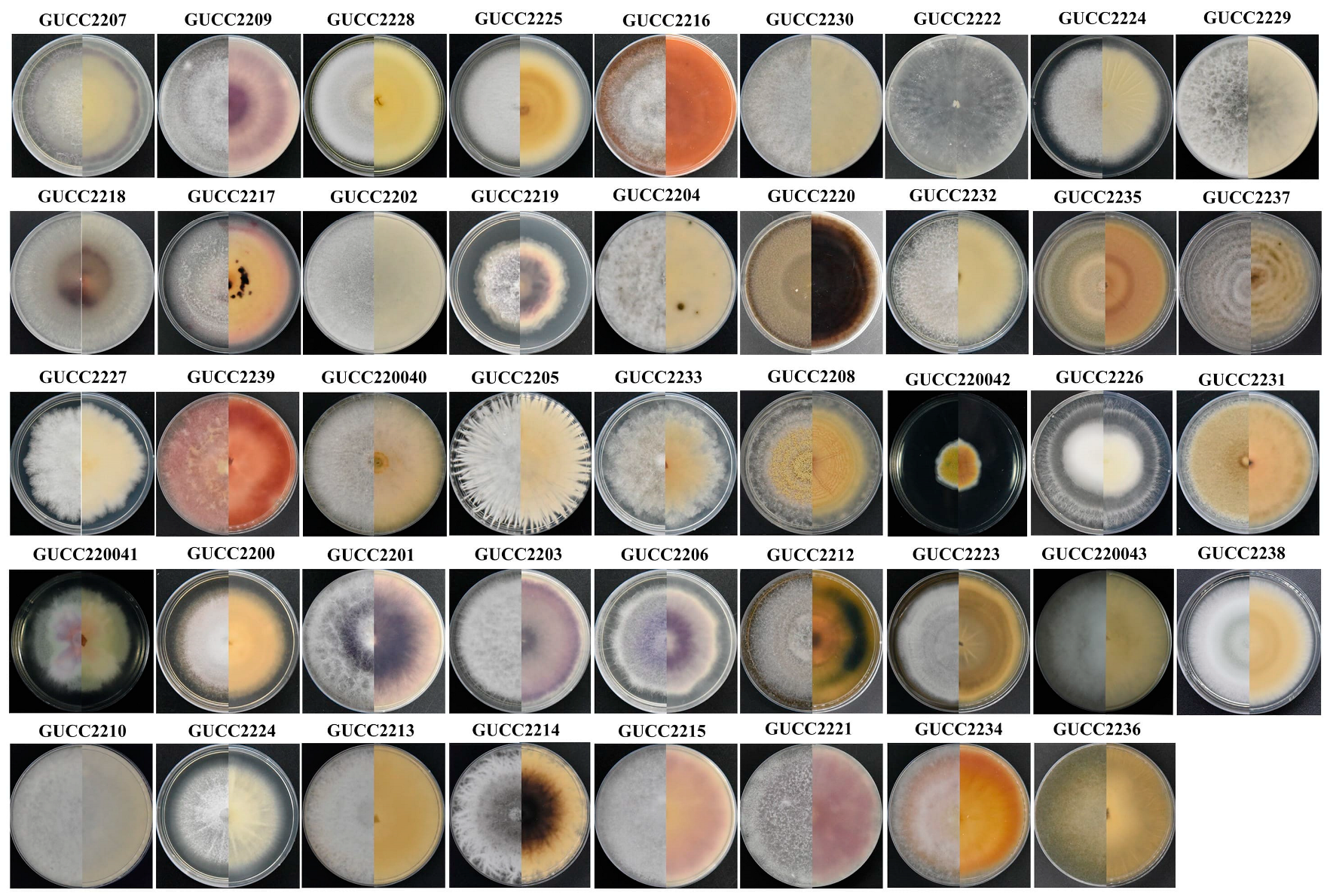
3.2. Diversity of Colonizing Fungi
3.2.1. Dominant Taxa
3.2.2. Lifestyle Diversity
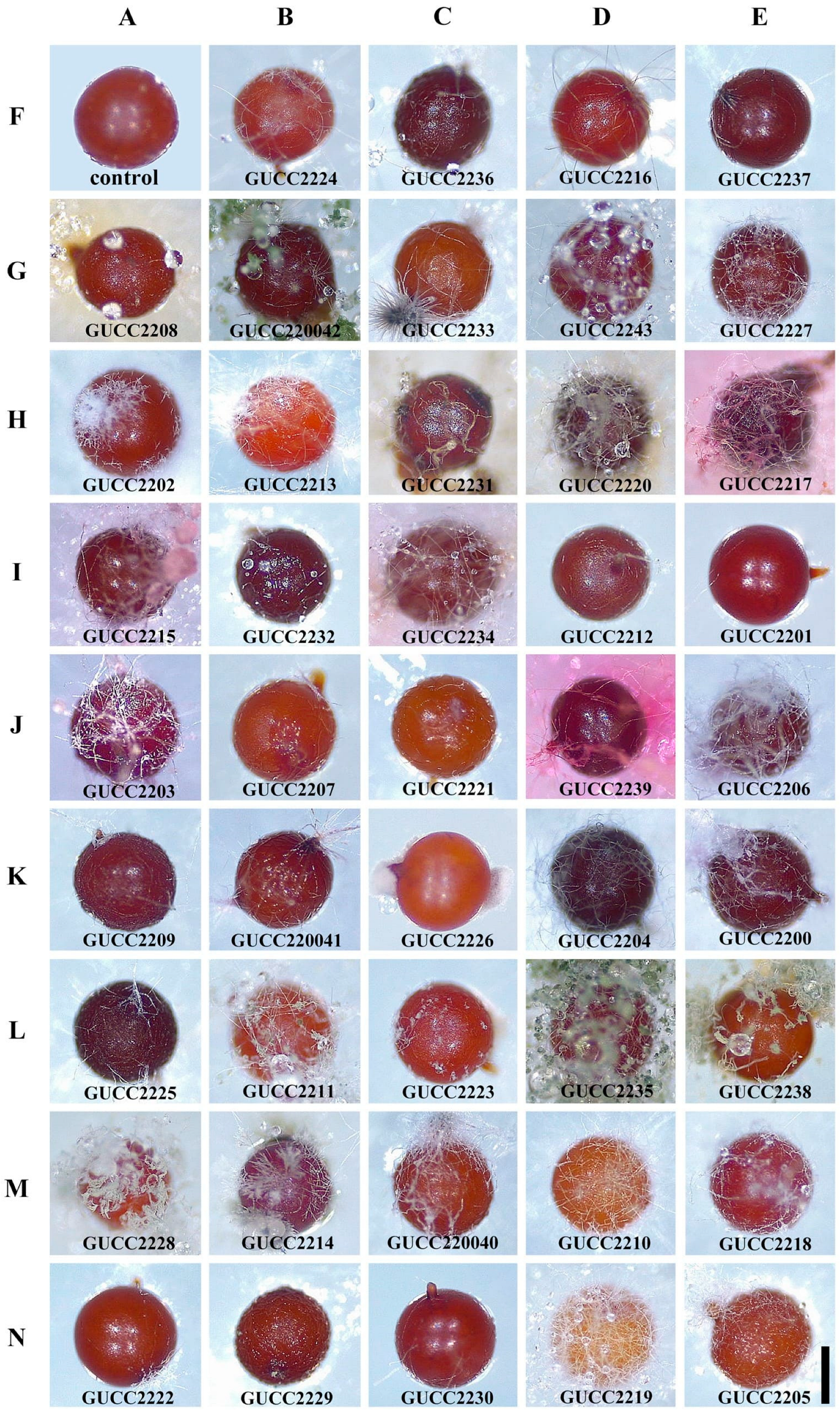
3.3. Parasitic Potential of the Fungal Isolates towards Nematode Cysts In Vitro
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zaheer, K.; Akhtar, M.H. Potato production, usage, and nutrition—A review. Crit. Rev. Food Sci. 2016, 56, 711–721. [Google Scholar] [CrossRef]
- Zhang, H.; Fen, X.U.; Yu, W.U.; Hu, H.H.; Dai, X.F. Progress of potato staple food research and industry development in China. J. Integr. Agr. 2017, 16, 2924–2932. [Google Scholar] [CrossRef]
- Barma, N.C.D.; Hossain, A.; Hakim, M.; Mottaleb, K.A.; Alam, M.; Reza, M.; Rohman, M. Progress and challenges of wheat production in the era of climate change: A Bangladesh perspective. In Wheat Production in Changing Environments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 615–679. [Google Scholar]
- Price, J.A.; Coyne, D.; Blok, V.C.; Jones, J.T. Potato cyst nematodes Globodera rostochiensis and G. pallida. Mol. Plant Pathol. 2021, 22, 495–507. [Google Scholar] [CrossRef]
- Turner, S. The origins, global distribution and biology of potato cyst nematodes (Globodera rostochiensis (Woll.) and Globodera pallida Stone). In Potato Cyst Nematodes, Biology, Distribution and Control; Centre for Agriculture and Bioscience International: Wallingford, UK, 1998. [Google Scholar]
- Evans, K.; Stone, A.R. A review of the distribution and biology of the potato cyst-nematodes Globodera rostochiensis and G. pallida. Pans 1977, 23, 178–189. [Google Scholar] [CrossRef]
- Nicol, J.M.; Turner, S.J.; Coyne, D.L.; Den Nijs, L.; Hockland, S.; Maafi, Z.T. Current nematode threats to world agriculture. In Genomics and Molecular Genetics of Plant-Nematode Interactions; Jones, J.T., Gheysen, G., Fenoll, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 21–44. [Google Scholar]
- Pineda, O.; Bonierbale, M.W.; Plaisted, R.L.; Brodie, B.B.; Tanksley, S.D. Identification of RFLP markers linked to the H1 gene conferring resistance to the potato cyst nematode Globodera rostochiensis. Genome 1993, 36, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Devrajan, K.; Prabhu, S.; Seenivasan, N.; Sudha, A.; Ramakrishnan, S.; Anita, B. Occurrence of native microbial antagonists against potato cyst nematodes in the Nilgiri Hills of Tamil Nadu. Potato J. 2011, 38, 67–72. [Google Scholar]
- Krishna Prasad, K.S.; Singh, D.B. Note on the parasitic nematodes associated with potato in Karnataka State, India. Inter. Nematol. Network Newsletter 1986, 3, 11–13. [Google Scholar]
- Ramana, K.V.; Mohandas, C. Occurrence of potato cyst nematode Globodera pallida (Stone, 1973) in Kerala. Indian J. Nematol. 1988, 18, 141. [Google Scholar]
- Nagachandrabose, S. Combined application of Pseudomonas fluorescens and Purpureocillium lilacinum liquid formulations to manage Globodera spp. on potato. Crop Prot. 2017, 6, 529–537. [Google Scholar]
- Jiang, R.; Huan, P.; Li, Y. First record of the golden potato nematode Globodera rostochiensis in Yunnan and Sichuan provinces of China. J. Integr. Agr. 2022, 21, 898–899. [Google Scholar] [CrossRef]
- Peng, D.L.; Liu, H.; Peng, H.; Jiang, R.; Li, Y.Q.; Wang, X.; Ge, J.J.; Zhao, S.Q.; Feng, X.D.; Feng, M.Y. First detection of the potato cyst nematode (Globodera rostochiensis) in a major potato production region of China. Plant Dis. 2022, 107, 233. [Google Scholar] [CrossRef] [PubMed]
- Gray, N.F. Ecology of nematophagous fungi: Distribution and habitat. Ann. Appl. Biol. 1983, 102, 501–509. [Google Scholar] [CrossRef]
- Yu, Q.; Coosemans, J. Fungi associated with cysts of Globodera rostochiensis, G. pallida, and Heterodera schachtii; and egg masses and females of Meloidogyne hapla in Belgium. Phytoprotection 1998, 79, 63–69. [Google Scholar] [CrossRef]
- Kerry, B.R. An assessment of progress toward microbial control of plant-parasitic nematodes. J. Nematol. 1990, 22, 621–631. [Google Scholar]
- Fresenius, G. Beitrage zur Mykologie. J. Cell Sci. 1854, S1–2, 118–122. [Google Scholar] [CrossRef]
- Kerry, B.R. Fungal parasites of cyst nematodes. Agric. Ecosyst. Environ. 1988, 24, 293–305. [Google Scholar] [CrossRef]
- Nordbring-Hertz, B.; Jansson, H.B.; Tunlid, A. Nematophagous fungi. Encycl. Life Sci. 2006, 1–11. [Google Scholar] [CrossRef]
- Song, J.; Li, S.; Xu, Y.; Wei, W.; Yao, Q.; Pan, F. Diversity of parasitic fungi from soybean cyst nematode associated with long-term continuous cropping of soybean in black soil. Acta Agric. Scand. Sect. B Soil Plant Sci. 2016, 66, 432–442. [Google Scholar] [CrossRef]
- Liu, X.Z.; Chen, S.Y. Screening isolates of Hirsutella species for biocontrol of Heterodera glycines. Biocontrol. Sci. Technol. 2011, 11, 151–160. [Google Scholar] [CrossRef]
- Ma, R.; Liu, X.Z.; Jian, H.; Li, S.D. Detection of Hirsutella spp. And Pasteuria sp. parasitizing second-stage juveniles of Heterodera glycinesin soybean fields in China. Biol. Control. 2005, 33, 223–229. [Google Scholar] [CrossRef]
- Rajeswari, S.; Sivakumar, C.V. Nematophagous fungi associated with the potato cyst nematodes, Globodera spp. in the Nilgiris, Tamil Nadu. Biol. Control. 1999, 13, 137–139. [Google Scholar]
- Chen, S.Y.; Liu, X.Z. Control of soybean cyst nematode by the fungi Hirsutella rhossiliensis and Hirsutella minnesotensis in greenhouse studies. Biol. Control. 2005, 32, 208–219. [Google Scholar] [CrossRef]
- Borneman, J.; Becker, O. Identifying microorganisms involved in specific pathogen suppression in soil. Annu. Rev. Phytopathol. 2007, 45, 153–172. [Google Scholar]
- Alabouvette, C.; Olivain, C.; Migheli, Q.; Steinberg, C. Microbiological control of soil-borne phytopathogenic fungi with special emphasis on wilt-inducing Fusarium oxysporum. New Phytol. 2009, 184, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Silveira, M.F.; Herrera, J.O. An overview of nematological problems in Cuba. Nematropica 1998, 28, 151–163. [Google Scholar]
- Mittal, N.; Saxena, G.; Mukerji, K.G. Integrated control of root-knot disease in three crop plants using chitin and Paecilomyces lilacinus. Crop Prot. 1995, 14, 647–651. [Google Scholar] [CrossRef]
- Franco, J.; Jatala, P.; Bocangel, M. Efficiency of Paecilomyces lilacinus as a biological agent of Globodera pallida. J. Nematol. 1981, 13, 438–439. [Google Scholar]
- Westphal, A.; Becker, J.O. Components of soil suppressiveness against Heterodera schachtii. Soil Biol. Biochem. 2001, 33, 9–16. [Google Scholar] [CrossRef]
- Holland, R.J.; Williams, K.L.; Khan, A. Infection of Meloidogyne javanica by Paecilomyces lilacinus. Nematology 1999, 1, 131–139. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Mahmood, I. Biological control of plant parasitic nematodes by fungi: A review. Bioresour. Technol. 1996, 58, 229–239. [Google Scholar] [CrossRef]
- Dunn, M.T.; Sayre, R.M.; Carrell, A.; Wergin, W.P. Colonization of nematode eggs by Paecilomyces lilacinus (Thom) Samson as observed with scanning electron-microscopy. Scan. Electron Micros. 1984, 1982, 1351–1357. [Google Scholar]
- Dackman, C. Fungal parasites of the potato cyst nematode Globodera rostochiensis: Isolation and reinfection. J. Nematol. 1990, 22, 594–597. [Google Scholar] [PubMed]
- Crump, D.H.; Flynn, C.A. Isolation and screening of fungi for the biological control of potato cyst nematodes. Nematologica 1995, 41, 628–638. [Google Scholar] [CrossRef]
- Oro, V.; Boskovic, J.; Milenkovic, S.; Tosi, S. Taxonomic diversity of fungi associated with some PCN populations from Serbia. Pestic. Phytomed. 2012, 27, 41–47. [Google Scholar] [CrossRef]
- Abbasi, K.; Zafari, D.; Wick, R. Evaluation of chitinase enzyme in fungal isolates obtained from golden potato cyst nematode (Globodera rostochiensis). Zemdirbyste 2017, 104, 179–184. [Google Scholar] [CrossRef]
- Benttoumi, N.; Colagiero, M.; Sellami, S.; Boureghda, H.; Keddad, A.; Ciancio, A. Diversity of nematode microbial antagonists from Algeria shows occurrence of nematotoxic Trichoderma spp. Plants 2020, 9, 941. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Jiang, Z.; Bai, Q.; Wu, S.; Wang, Y.; Li, C.; Zeng, X.; Gan, X.; Xie, X.; et al. A new strain of Volutella citrinella with nematode predation and nematicidal activity, isolated from the cysts of potato cyst nematodes in China. BMC Microbiol. 2021, 21, 323. [Google Scholar] [CrossRef]
- Vieira Dos Santos, M.C.; Horta, J.; Moura, L.; Pires, D.; Conceicao, I.; Abrantes, I.; Costa, S.R. An integrative approach for the selection of Pochonia chlamydosporia isolates for biocontrol of potato cyst and root knot nematodes. Phytopathol. Mediterr 2019, 58, 187–199. [Google Scholar]
- Nagachandrabose, S. Management of potato cyst nematodes using liquid bioformulations of Pseudomonas fluorescens, Purpureocillium lilacinum and Trichoderma viride. Potato Res. 2020, 63, 479–496. [Google Scholar] [CrossRef]
- Duan, S.M.; Huang, X.F.; Hu, J.W.; Xia, P.H.; Wang, Y.; Liu, Y.Y. Study on Speciation of Phosphorus in Rhizosphere Sediments from Caohai Wetland in Guizhou Province. Res. Environ. Sci. 2013, 26, 743–749. [Google Scholar]
- Zhu, Z.J.; Chen, J.A.; Zeng, Y. Paleotemperature variations at Lake Caohai, southwestern China, during the past 500 years: Evidence from combined δ18O analysis of cellulose and carbonates. Sci. China Earth Sci. 2014, 44, 250–258. [Google Scholar] [CrossRef]
- Mo, M.H. Advance on Ecological Status of Nematophagous Fungi. Guizhou Agric. Sci. 1995, 23, 57–60. [Google Scholar]
- Southey, J.F. Methods for detection of potato cyst nematodes. Bull. OEPP 1974, 4, 463–473. [Google Scholar] [CrossRef]
- Been, T.H.; Schomaker, C.H. Development and evaluation of sampling methods for fields with infestation foci of potato cyst nematodes (Globodera rostochiensis and G. pallida). Phytopathology 2000, 90, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.; Pickup, J. Molecular characterization of a morphologically unusual potato cyst nematode. Bull. OEPP 2005, 35, 69–72. [Google Scholar] [CrossRef]
- Nurjanah Trisyono, Y.A.; Indarti, S.; Hartono, S. Identification, distribution and genetic diversity of the golden potato cyst nematode (Globodera rostochiensis) in Java Indonesia. AIP Conf. Proc. 2016, 1755, 130006. [Google Scholar]
- Baunacke, W. Investigations on biology and control of Beet nematodes, Heterodera schachtii Schmidt. Arb. Aus Der Biol. Reichsanst. 1922, 11, 185–288. [Google Scholar]
- Hallman, J.; Viaene, N. PM 7/119 (1) Nematode extraction. Bull. OEPP 2013, 43, 471–495. [Google Scholar]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Camargo, J.A. Can dominance influence stability in competitive interactions? Oikos 1992, 64, 605–609. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Indarti, S.; Widianto, D.; Kim, Y.H.; Mulyadi, M.; Suryanti, S. Survey of egg-and cyst-parasitic fungi of potato cyst nematode in Indonesia. Plant Pathol. J. 2010, 26, 32–36. [Google Scholar] [CrossRef]
- Liu, X.Z.; Zhang, K.Q.; Li, T.F. Biological Control of Plant Parasitic Nematodes; China Science and Technology Press: Beijing, China, 2004; pp. 1–345. [Google Scholar]
- Zhang, Y.; Li, G.H.; Zhang, K.Q. A review on the research of nematophagous fungal species. Mycosystema 2011, 30, 836–845. [Google Scholar]
- Qi, M.; Xie, C.X.; Chen, Q.W.; Yu, Z.D. Pestalotiopsis trachicarpicola, a novel pathogen causes twig blight of Pinus bungeana (Pinaceae: Pinoideae) in China. Anton. Leeuw. Int. J. G. 2021, 114, 1–9. [Google Scholar] [CrossRef]
- Nozawa, S.; Seto, Y.; Watanabe, K. First report of leaf blight caused by Pestalotiopsis chamaeropis and Neopestalotiopsis sp. in Japanese andromeda. J. Gen. Plant Pathol. 2019, 85, 449–452. [Google Scholar] [CrossRef]
- Šafránková, I. Volutella leaf blight and stem canker on Japanese pachysandra in the Czech Republic. Plant Protect. Sci. 2007, 43, 10–12. [Google Scholar] [CrossRef]
- Shi, F.; Hsiang, T. Pseudonectria buxi causing leaf and stem blight on Buxus in Canada. Eur. J. Plant Pathol. 2014, 138, 763–773. [Google Scholar] [CrossRef]
- Cui, Y.; Peng, A.; Song, X.; Cheng, B.; Ling, J.; Chen, X. First report of chick peach (Prunus persica L.) leaf spot disease caused by Didymella glomerata in China. Plant Pathol. 2021, 103, 1015. [Google Scholar] [CrossRef]
- Kousalya, S.; Kamalakannan, A.; Chowdappa, A.; Gopalakrishnan, C.; Rajamani, K.; Venkatesh, A. First report of Xylaria sp. causing tuber rot on glory lily in India. New Dis. Rep. 2019, 39, 21. [Google Scholar] [CrossRef]
- Cui, B.K. A species of lignicolous Fungi New to China—Coriolopsis glabro-rigens. J. For. Res. 2008, 21, 734–736. [Google Scholar]
- Marco, J.L.D.; Valadares-Inglis, M.C.; Felix, C.R. Production of hydrolytic enzymes by Trichoderma isolates with antagonistic activity against Crinipellis perniciosa, the causal agent of witches’ broom of cocoa. Braz. Microbiol. 2003, 34, 33–38. [Google Scholar] [CrossRef]
- Phookamsak, R.; Hyde, K.D.; Jeewon, R.; Bhat, D.J.; Jones, E.G.; Maharachchikumbura, S.S.; Xu, J. Fungal diversity notes 929–1035: Taxonomic and phylogenetic contributions on genus and species of fungi. Fungal Divers. 2019, 95, 1–273. [Google Scholar]
- De Errasti, A.; Novas, M.V.; Carmarán, C.C. Plant-fungal association in trees: Insights into changes in ecological strategies of Peroneutypa scoparia (Diatrypaceae). Flora 2014, 209, 704–710. [Google Scholar] [CrossRef]
- Santos, T.F.B.; dos Santos Carvalho, C.; de Almeida, M.A.; Delforno, T.P.; Duarte, I.C.S. Endophytic fungi isolated from Brazilian medicinal plants as potential producers of antioxidants and their relations with anti-inflammatory activity. 3 Biotech 2020, 10, 223. [Google Scholar] [CrossRef]
- Cordeiro, L.; Lee, H.B.; Nguyen, T.T.T.; Gurgel, L.M.S.; de Azevedo, A.L.C.M. Absidia bonitoensis (Mucorales, Mucoromycota), a new species isolated from the soil of an upland Atlantic forest in Northeastern Brazil. Nova Hedwigia. 2021, 112, 241–251. [Google Scholar]
- Albert, Q.; Leleyter, L.; Lemoine, M.; Heutte, N.; Rioult, J.P.; Sage, L.; Baraud, F.; Garon, D. Comparison of tolerance and biosorption of three trace metals (Cd, Cu, Pb) by the soil fungus Absidia cylindrospora. Chemosphere 2018, 196, 386–392. [Google Scholar] [CrossRef]
- Sebastian, K.K.; Alzayer, H.; Abraham, E.; Roche, D.; Reddan, D.; Lappin, D. Cutaneous Absidia corymbifera in a Lupus Nephritis Patient. Cureus 2021, 13, e19512. [Google Scholar] [CrossRef]
- Cloughley, R.; Kelehan, J.; Corbett-Feeney, G.; Murray, M.; Callaghan, J.; Regan, P.; Cormican, M. Soft tissue infection with Absidia corymbifera in a patient with idiopathic aplastic anemia. J. Clin. Microbiol. 2002, 40, 725–727. [Google Scholar] [CrossRef]
- Nwe, N.; Furuike, T.; Osaka, I.; Fujimori, H.; Kawasaki, H.; Arakawa, R.; Tokura, S.; Stevens, W.F.; Kurozumi, S.; Takamori, Y. Laboratory scale production of 13c labeled chitosan by fungi Absidia coerulea and Gongronella butleri grown in solid substrate and submerged fermentation. Carbohydr. Polym. 2011, 84, 743–750. [Google Scholar] [CrossRef]
- Wang, J.; Suzuki, T.; Mori, T.; Yin, R.; Dohra, H.; Kawagishi, H.; Hirai, H. Transcriptomics analysis reveals the high biodegradation efficiency of white-rot fungus Phanerochaete sordida YK-624 on native lignin. J. Biosci. Bioeng. 2021, 132, 253–257. [Google Scholar] [CrossRef]
- Xalxo, P.C.; Karkun, D.; Poddar, A.N. Rhizospheric fungal associations of root knot nematode infested cucurbits: In vitro assessment of their nematicidal potential. Res. J. Microbiol. 2013, 8, 81. [Google Scholar]
- Devi, G.; Bora, L. Effect of some biocontrol agents against root-knot nematode (Meloidogyne incognita race2). Int. J. Environ. Agric. Biotechnol. 2018, 3, 1748–1755. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.; Wang, Y.; Zhu, X.; Liu, X.; Fan, H.; Duan, Y. In vitro evaluation of Penicillium chrysogenum Snef1216 against Meloidogyne incognita (root-knot nematode). Sci. Rep. 2020, 10, 8342. [Google Scholar] [CrossRef]
- Gogoi, D.; Mahanta, B. Comparative efficacy of Glomus fasciculatum, Trichoderma harzianum, carbofuran and carbendazim in management of Meloidogyne incognita and Rhizoctonia solani disease complex on French bean. Ann. Plant Prot. Sci. 2013, 21, 172–175. [Google Scholar]
- Baheti, B.L.; Dodwadiya, M.; Bhati, S.S. Eco-friendly management of maize cyst nematode, Heterodera zeae on sweet corn (Zea mays L. saccharata). J. Entomol. Zool. Stud. 2017, 5, 989–993. [Google Scholar]
- Nitao, J.K.; Meyer, S.L.; Oliver, J.E.; Schmidt, W.F.; Chitwood, D.J. Isolation of flavipin, a fungus compound antagonistic to plant-parasitic nematodes. Nematology 2002, 4, 55–63. [Google Scholar] [CrossRef]
- Gortari, C.; Cazau, C.; Hours, R. Hongos nematófagos de huevos de Toxocara canis en un paseo público de La Plata, Argentina. Rev. Iberoam. Micol. 2007, 24, 24–28. [Google Scholar] [CrossRef]
- Amin, N. Nematicidal activity of root exudates of sengon plant inoculated with endophytic fungi Nigrospora sp. to control of root-knot nematode Meloidogyne spp. J. Chem. Pharm. Res. 2015, 7, 307–310. [Google Scholar]
- Zhao, M.L.; Huang, J.S.; Mo, M.H.; Zhang, K.Q. A potential virulence factor involved in fungal pathogenicity: Serine-like protease activity of nematophagous fungus Clonostachys rosea. Fungal Divers. 2005, 19, 217–234. [Google Scholar]
- Tribe, H.T. Extent of disease in populations of Heterodera, with special reference to H. schachtii. Ann. Appl. Biol. 1980, 92, 61–72. [Google Scholar] [CrossRef]
- Clovis, C.J.; Nolan, R.A. Fungi associated with cysts, eggs and juveniles of the golden nematode (Globodera rostochiensis) in Newfoundland. Nematologica 1983, 29, 345–356. [Google Scholar] [CrossRef]
- Coosemans, J. Methods for introducing Verticillium chlamydosporium into soil. IOBC/WPRS Bull. 1991, 14, 39–46. [Google Scholar]
- Spiegel, Y.; Chet, I.; Cohn, E. Use of chitin for controlling plant-parasitic nematodes. II. Mode of action. Plant Soil 1987, 98, 337–345. [Google Scholar] [CrossRef]
- Schlang, J.W.; Stendel, J.W.; Muller, J. Influence of resistant green manure crops on the population dynamics of Heterodera schachtii and its fungal egg parasites. In Proceedings of the European Society of Nematologists 19th International Nematology Symposium, Uppsala, Sweden, 7–13 August 1988. [Google Scholar]
- Anke, H.; Stadler, M.; Mayer, A.; Sterner, O. Secondary metabolites with nematicidal and antimicrobial activity from nematophagous fungi and Ascomycetes. Can. J. Bot. 1995, 73, S932–S939. [Google Scholar] [CrossRef]
| Genus | Culturable Strains | |
|---|---|---|
| Number | Percentage (%) | |
| Absidia | 4 | 2.88 |
| Arxotrichum | 2 | 1.44 |
| Aspergillus | 2 | 1.44 |
| Chaetomium | 4 | 2.88 |
| Clonostachys | 1 | 0.72 |
| Coriolopsis | 1 | 0.72 |
| Crinipellis | 1 | 0.72 |
| Didymella | 2 | 1.44 |
| Edenia | 5 | 3.60 |
| Fusarium | 82 | 58.99 |
| Gongronella | 1 | 0.72 |
| Mortierella | 2 | 1.44 |
| Nigrospora | 1 | 0.72 |
| Paecilomyces | 1 | 0.72 |
| Paraphaeosphaeria | 5 | 3.60 |
| Penicillium | 15 | 10.79 |
| Peroneutypa | 1 | 0.72 |
| Pestalotiopsis | 1 | 0.72 |
| Phaeophlebiopsis | 1 | 0.72 |
| Phanerochaete | 2 | 1.44 |
| Trichoderma | 3 | 2.16 |
| Volutella | 1 | 0.72 |
| Xylaria | 1 | 0.72 |
| Strain Number | Relative Colonization Rate (%) ± Standard Error | Strain Number | Relative Colonization Rate (%) ± Standard Error |
|---|---|---|---|
| GUCC2225 | 16.67 ± 5.77 | GUCC2208 | 100.00 ± 0 |
| GUCC2226 | 20.00 ± 0 | GUCC2209 | 100.00 ± 0 |
| GUCC2221 | 33.33 ± 5.77 | GUCC2211 | 100.00 ± 0 |
| GUCC2222 | 33.33 ± 5.77 | GUCC2212 | 100.00 ± 0 |
| GUCC2201 | 66.67 ± 5.77 | GUCC2213 | 100.00 ± 0 |
| GUCC2218 | 69.23 ± 5.77 | GUCC2215 | 100.00 ± 0 |
| GUCC2205 | 78.57 ± 5.77 | GUCC2216 | 100.00 ± 0 |
| GUCC2207 | 78.57 ± 5.77 | GUCC2217 | 100.00 ± 0 |
| GUCC2239 | 80.00 ± 10 | GUCC2224 | 100.00 ± 0 |
| GUCC2219 | 80.00 ± 0 | GUCC2227 | 100.00 ± 0 |
| GUCC2236 | 85.71 ± 5.77 | GUCC2228 | 100.00 ± 0 |
| GUCC2214 | 86.67 ± 5.77 | GUCC2229 | 100.00 ± 0 |
| GUCC2220 | 87.50 ± 5.77 | GUCC2230 | 100.00 ± 0 |
| GUCC220041 | 91.67 ± 0 | GUCC2231 | 100.00 ± 0 |
| GUCC2223 | 93.33 ± 5.77 | GUCC2232 | 100.00 ± 0 |
| GUCC220043 | 93.33 ± 5.77 | GUCC2233 | 100.00 ± 0 |
| GUCC2210 | 93.33 ± 5.77 | GUCC2234 | 100.00 ± 0 |
| GUCC2200 | 100.00 ± 0 | GUCC2235 | 100.00 ± 0 |
| GUCC2202 | 100.00 ± 0 | GUCC2237 | 100.00 ± 0 |
| GUCC2203 | 100.00 ± 0 | GUCC2238 | 100.00 ± 0 |
| GUCC2204 | 100.00 ± 0 | GUCC220040 | 100.00 ± 0 |
| GUCC2206 | 100.00 ± 0 | GUCC220042 | 100.00 ± 0 |
| Relative Colonization Rate (R) | Percentage |
|---|---|
| <50% | 9.09% |
| 50% ≤ R < 80% | 9.09% |
| 80% ≤ R < 100% | 20.45% |
| =100% | 61.36% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yang, Z.; Jiang, Z.; Zhang, X.; Nizamani, M.M.; Wu, Y.; Wei, S.; Wang, Y.; Xie, X. Diversity of Fungi Isolated from Potato Nematode Cysts in Guizhou Province, China. J. Fungi 2023, 9, 247. https://doi.org/10.3390/jof9020247
Zhang H, Yang Z, Jiang Z, Zhang X, Nizamani MM, Wu Y, Wei S, Wang Y, Xie X. Diversity of Fungi Isolated from Potato Nematode Cysts in Guizhou Province, China. Journal of Fungi. 2023; 9(2):247. https://doi.org/10.3390/jof9020247
Chicago/Turabian StyleZhang, Hui, Zaifu Yang, Zhaochun Jiang, Xinyue Zhang, Mir Muhammad Nizamani, Yan Wu, Shan Wei, Yong Wang, and Xin Xie. 2023. "Diversity of Fungi Isolated from Potato Nematode Cysts in Guizhou Province, China" Journal of Fungi 9, no. 2: 247. https://doi.org/10.3390/jof9020247
APA StyleZhang, H., Yang, Z., Jiang, Z., Zhang, X., Nizamani, M. M., Wu, Y., Wei, S., Wang, Y., & Xie, X. (2023). Diversity of Fungi Isolated from Potato Nematode Cysts in Guizhou Province, China. Journal of Fungi, 9(2), 247. https://doi.org/10.3390/jof9020247








