Antifungal Activity of Isavuconazole and Comparator Agents against Contemporaneous Mucorales Isolates from USA, Europe, and Asia-Pacific
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates
2.2. Antifungal Susceptibility Testing
3. Results
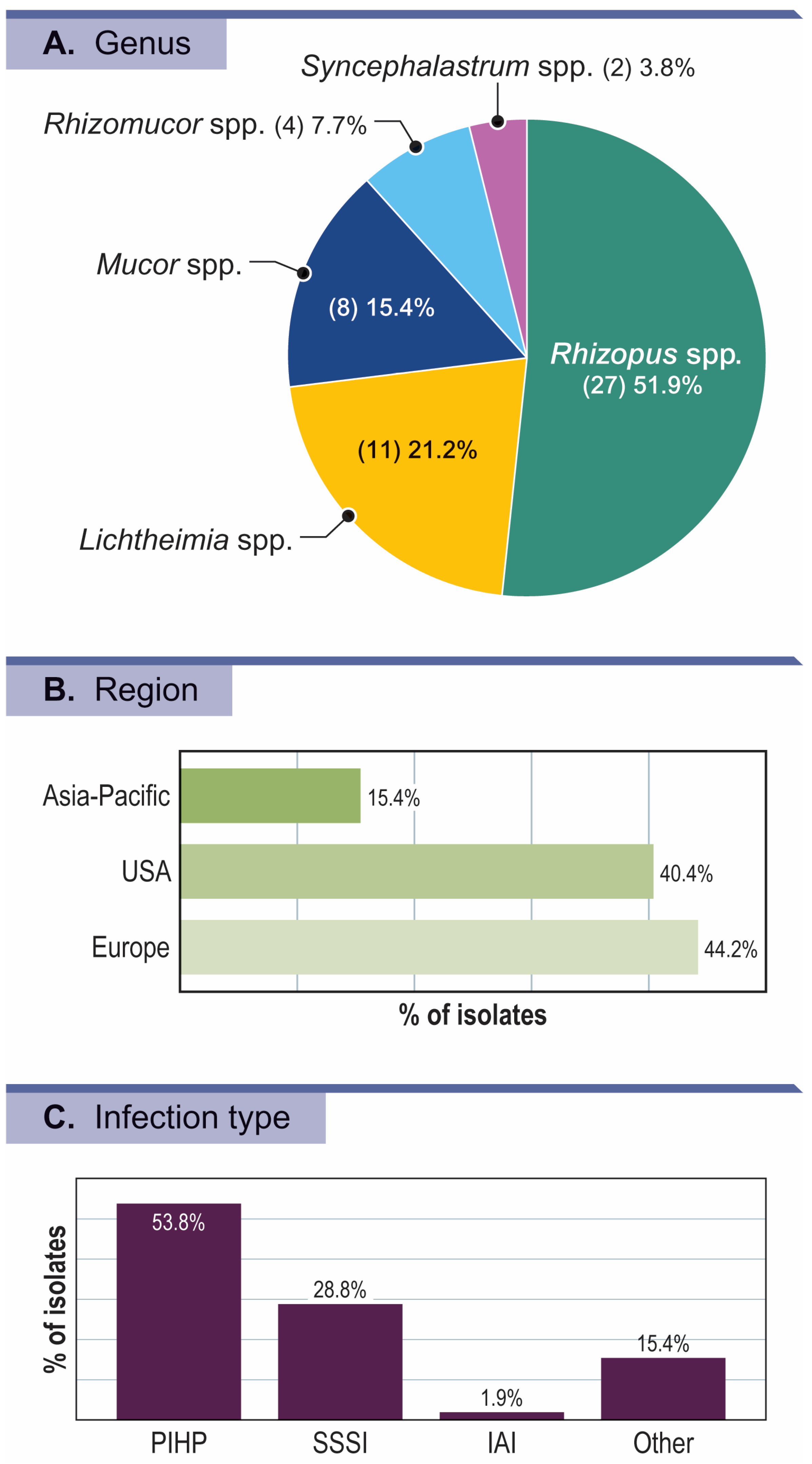
Activity of Isavuconazole and Comparators against Mucorales Isolates
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef] [PubMed]
- Jenks, J.D.; Cornely, O.A.; Chen, S.C.; Iii, G.R.T.; Hoenigl, M. Breakthrough invasive fungal infections: Who is at risk? Mycoses 2020, 63, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- E Corzo-León, D.; Chora-Hernández, L.D.; Rodríguez-Zulueta, A.P.; Walsh, T.J. Diabetes mellitus as the major risk factor for mucormycosis in Mexico: Epidemiology, diagnosis, and outcomes of reported cases. Med. Mycol. 2017, 56, 29–43. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Das, A.; Mandal, J.; Shivaprakash, M.R.; George, V.K.; Tarai, B.; Rao, P.; Panda, N.; Verma, S.C.; Sakhuja, V. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Med. Mycol. 2006, 44, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Bartoletti, M.; Ferrarese, A.; Franceschini, E.; Campoli, C.; Coladonato, S.; Pascale, R.; Tedeschi, S.; Gatti, M.; Cricca, M.; et al. Breakthrough invasive fungal infection after liver transplantation in patients on targeted antifungal prophylaxis: A prospective multicentre study. Transpl. Infect. Dis. 2021, 23, e13608. [Google Scholar] [CrossRef] [PubMed]
- Trifilio, S.; Singhal, S.; Williams, S.; Frankfurt, O.; Gordon, L.; Evens, A.; Winter, J.; Tallman, M.; Pi, J.; Mehta, J. Breakthrough fungal infections after allogeneic hematopoietic stem cell transplantation in patients on prophylactic voriconazole. Bone Marrow Transplant. 2007, 40, 451–456. [Google Scholar] [CrossRef]
- Raffaelli, F.; Tanzarella, E.S.; De Pascale, G.; Tumbarello, M. Invasive Respiratory Fungal Infections in COVID-19 Critically Ill Patients. J. Fungi 2022, 8, 415. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Alhumaid, S.; Alshukairi, A.N.; Temsah, M.-H.; Barry, M.; Al Mutair, A.; Rabaan, A.A.; Al-Omari, A.; Tirupathi, R.; AlQahtani, M.; et al. COVID-19 and mucormycosis superinfection: The perfect storm. Infection 2021, 49, 833–853. [Google Scholar] [CrossRef]
- Rudramurthy, S.M.; Hoenigl, M.; Meis, J.F.; Cornely, O.A.; Muthu, V.; Gangneux, J.P.; Perfect, J.; Chakrabarti, A.; Isham, E.A. ECMM/ISHAM recommendations for clinical management of COVID-19 associated mucormycosis in low- and middle-income countries. Mycoses 2021, 64, 1028–1037. [Google Scholar] [CrossRef]
- Singh, N.K.; Hage, N.; Ramamourthy, B.; Kappagantu, K.M. COVID 19 associated Rhino- Orbital- Cerebral Mucormycosis: A proposed Classification and Treatment Strategies. Infect. Disord.–Drug Targets, 2022; ahead of print. [Google Scholar] [CrossRef]
- Rybak, J.; Marx, K.; Nishimoto, A.; Rogers, P. Isavuconazole: Pharmacology, Pharmacodynamics, and Current Clinical Experience with a New Triazole Antifungal Agent. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 1037–1051. [Google Scholar] [CrossRef]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef] [PubMed]
- FDA, U. CRESEMBA® (isavuconazonium sulfate) prescribing information. 2015. Available online: http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207500Orig1s000lbl.pdf (accessed on 15 November 2022).
- Pfaller, M.A.; Woosley, L.N.; Messer, S.A.; Jones, R.N.; Castanheira, M. Significance of molecular identification and antifungal susceptibility of clinically significant yeasts and moulds in a global antifungal surveillance program. Mycopathologia 2012, 174, 259–271. [Google Scholar] [CrossRef] [PubMed]
- CL.S.I. M18Ed2; Interpretive Criteria for Identification of Bacteria and Fungi by Targeted DNA Sequencing. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2018.
- CL.S.I. M27Ed4; Reference Method for Broth Dilution Antifungal Susceptbility Testing of Yeasts. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2017.
- CL.S.I. M38Ed3; Reference Method for Broth Dilution Antifungal Susceptibilty Testing of Filamentous Fungi. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2018.
- CL.S.I. M61Ed2; Performance Standards for Antifungal Susceptibility Testing of Filamentous Fungi. Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2020.
- WHO. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. 2022. Available online: https://www.who.int/publications/i/item/9789240060241 (accessed on 15 November 2022).
- Roden, M.M.; Zaoutis, T.E.; Buchanan, W.L.; Knudsen, T.A.; Sarkisova, T.A.; Schaufele, R.L.; Sein, M.; Sein, T.; Chiou, C.C.; Chu, J.H.; et al. Epidemiology and Outcome of Zygomycosis: A Review of 929 Reported Cases. Clin. Infect. Dis. 2005, 41, 634–653. [Google Scholar] [CrossRef] [PubMed]
- Cornely, O.; Arikan-Akdagli, S.; Dannaoui, E.; Groll, A.; Lagrou, K.; Chakrabarti, A.; Lanternier, F.; Pagano, L.; Skiada, A.; Akova, M.; et al. ESCMID† and ECMM‡ joint clinical guidelines for the diagnosis and management of mucormycosis 2013. Clin. Microbiol. Infect. 2014, 20, 5–26. [Google Scholar] [CrossRef]
- Petrikkos, G.; Skiada, A.; Drogari-Apiranthitou, M. Epidemiology of mucormycosis in Europe. Clin. Microbiol. Infect. 2014, 20, 67–73. [Google Scholar] [CrossRef]
- Walther, G.; Pawłowska, J.; Alastruey-Izquierdo, A.; Wrzosek, M.; Rodriguez-Tudela, J.; Dolatabadi, S.; Chakrabarti, A.; de Hoog, G. DNA barcoding in Mucorales: An inventory of biodiversity. Persoonia—Mol. Phylogeny Evol. Fungi 2013, 30, 11–47. [Google Scholar] [CrossRef]
- Patel, A.; Agarwal, R.; Rudramurthy, S.M.; Shevkani, M.; Xess, I.; Sharma, R.; Savio, J.; Sethuraman, N.; Madan, S.; Shastri, P.; et al. Multicenter Epidemiologic Study of Coronavirus Disease–Associated Mucormycosis, India. Emerg. Infect. Dis. 2021, 27, 2349–2359. [Google Scholar] [CrossRef]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef]
- Imbert, S.; Portejoie, L.; Pfister, E.; Tauzin, B.; Revers, M.; Uthurriague, J.; Hernandez-Grande, M.; Lafon, M.-E.; Jubert, C.; Issa, N.; et al. A Multiplex PCR and DNA-Sequencing Workflow on Serum for the Diagnosis and Species Identification for Invasive Aspergillosis and Mucormycosis. J. Clin. Microbiol. 2023, 61. [Google Scholar] [CrossRef]
- Millon, L.; Caillot, D.; Berceanu, A.; Bretagne, S.; Lanternier, F.; Morio, F.; Letscher-Bru, V.; Dalle, F.; Denis, B.; Alanio, A.; et al. Evaluation of Serum Mucorales Polymerase Chain Reaction (PCR) for the Diagnosis of Mucormycoses: The MODIMUCOR Prospective Trial. Clin. Infect. Dis. 2022, 75, 777–785. [Google Scholar] [CrossRef]
- Walther, G.; Wagner, L.; Kurzai, O. Updates on the Taxonomy of Mucorales with an Emphasis on Clinically Important Taxa. J. Fungi 2019, 5, 106. [Google Scholar] [CrossRef]
- Shao, J.; Wan, Z.; Li, R.; Yu, J. Species Identification and Delineation of Pathogenic Mucorales by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2018, 56, e01886-17. [Google Scholar] [CrossRef]
- Vitale, R.G.; de Hoog, G.S.; Schwarz, P.; Dannaoui, E.; Deng, S.; Machouart, M.; Voigt, K.; van de Sande, W.W.J.; Dolatabadi, S.; Meis, J.F.; et al. Antifungal Susceptibility and Phylogeny of Opportunistic Members of the Order Mucorales. J. Clin. Microbiol. 2012, 50, 66–75. [Google Scholar] [CrossRef]
- Wagner, L.; Stielow, J.; de Hoog, G.; Bensch, K.; Schwartze, V.; Voigt, K.; Alastruey-Izquierdo, A.; Kurzai, O.; Walther, G. A new species concept for the clinically relevant Mucor circinelloides complex. Persoonia—Mol. Phylogeny Evol. Fungi 2020, 44, 67–97. [Google Scholar] [CrossRef]
- EUCAST. Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts European Committee on Antimicrobial Susceptibility Testing Definitive Document E.DEF 7.3.2; April 2020. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Files/EUCAST_E_Def_7.3.2_Yeast_testing_definitive_revised_2020.pdf (accessed on 1 November 2022).
- Spellberg, B.; Ibrahim, A.S.; Chin-Hong, P.V.; Kontoyiannis, D.P.; Morris, M.I.; Perfect, J.R.; Fredricks, D.; Brass, E.P. The Deferasirox–AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: A randomized, double-blinded, placebo-controlled trial. J. Antimicrob. Chemother. 2011, 67, 715–722. [Google Scholar] [CrossRef]
- Lanternier, F.; Lortholary, O. AMBIZYGO: étude de phase II de l’efficacité d’une posologie initiale élevée d’amphotéricine B liposomale (AmBisome®) [10 mg/kg/j] dans le traitement des zygomycoses. Med. Mal. Infect. 2008, 38, S90–S91. [Google Scholar] [CrossRef]
- A Maertens, J.; I Raad, I.; A Marr, K.; Patterson, T.F.; Kontoyiannis, D.P.; A Cornely, O.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2015, 387, 760–769. [Google Scholar] [CrossRef]
- Gmb H, B.P.D. Cresemba (isavuconazole). 2015. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cresemba (accessed on 1 November 2022).
- Verweij, P.; González, G.; Wiederhold, N.; Lass-Flörl, C.; Warn, P.; Heep, M.; Ghannoum, M.; Guinea, J. In Vitro Antifungal Activity of Isavuconazole against 345 Mucorales Isolates Collected at Study Centers in Eight Countries. J. Chemother. 2009, 21, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, K.; Astvad, K.; Hare, R.; Arendrup, M. EUCAST Susceptibility Testing of Isavuconazole: MIC Data for Contemporary Clinical Mold and Yeast Isolates. Antimicrob. Agents Chemother. 2019, 63, e00073-19. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.; Fraser, M.; Patterson, Z.; Palmer, M.; Johnson, E. In Vitro Antifungal Drug Resistance Profiles of Clinically Relevant Members of the Mucorales (Mucoromycota) Especially with the Newer Triazoles. J. Fungi 2021, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Singh, P.K.; Kathuria, S.; Hagen, F.; Meis, J.F. Comparison of the EUCAST and CLSI Broth Microdilution Methods for Testing Isavuconazole, Posaconazole, and Amphotericin B against Molecularly Identified Mucorales Species. Antimicrob. Agents Chemother. 2015, 59, 7882–7887. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Hoffmann, A.; Roos, B.; Maares, J.; Heep, M.; Spickerman, J.; Weidekamm, E.; Brown, T.; Roehrle, M. Multiple-Dose Pharmacokinetics and Safety of the New Antifungal Triazole BAL4815 after Intravenous Infusion and Oral Administration of Its Prodrug, BAL8557, in Healthy Volunteers. Antimicrob. Agents Chemother. 2006, 50, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Bellmann, R.; Smuszkiewicz, P. Pharmacokinetics of antifungal drugs: Practical implications for optimized treatment of patients. Infection 2017, 45, 737–779. [Google Scholar] [CrossRef]
- Bupha-Intr, O.; Butters, C.; Reynolds, G.; Kennedy, K.; Meyer, W.; Patil, S.; Bryant, P.; Morrissey, C.O.; Slavin, M.A.; Thursky, K.A.; et al. Consensus guidelines for the diagnosis and management of invasive fungal disease due to moulds other than Aspergillus in the haematology/oncology setting, 2021. Intern. Med. J. 2021, 51, 177–219. [Google Scholar] [CrossRef]
- Lamoth, F.; Kontoyiannis, D.P. Therapeutic Challenges of Non- Aspergillus Invasive Mold Infections in Immunosuppressed Patients. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
| Organism/Organism Group (No. of Isolates) | No. and Cumulative % of Isolates Inhibited at MIC (mg/L) of: | MIC50 | MIC90 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | > a | |||
| Mucorales order | ||||||||||
| Isavuconazole (52) | 0 0.0 | 14 26.9 | 17 59.6 | 6 71.2 | 4 78.8 | 11 100.0 | 2 | >8 | ||
| Itraconazole (52) | 0 0.0 | 3 5.8 | 20 44.2 | 15 73.1 | 4 80.8 | 7 94.2 | 3 100.0 | 2 | 8 | |
| Voriconazole (52) | 0 0.0 | 4 7.7 | 15 36.5 | 33 100.0 | >8 | >8 | ||||
| Posaconazole (52) | 0 0.0 | 1 1.9 | 28 55.8 | 10 75.0 | 5 84.6 | 2 88.5 | 2 92.3 | 4 100.0 | 0.5 | 8 |
| Amphotericin B (52) | 0 0.0 | 5 9.6 | 31 69.2 | 15 98.1 | 1 100.0 | 0.5 | 1 | |||
| Lichtheimia spp. | ||||||||||
| Isavuconazole (11) | 0 0.0 | 5 45.5 | 3 72.7 | 2 90.9 | 1 100.0 | 4 | 8 | |||
| Itraconazole (11) | 0 0.0 | 7 63.6 | 4 100.0 | 1 | 2 | |||||
| Voriconazole (11) | 0 0.0 | 11 100.0 | >8 | >8 | ||||||
| Posaconazole (11) | 0 0.0 | 8 72.7 | 3 100.0 | 0.5 | 1 | |||||
| Amphotericin B (11) | 0 0.0 | 9 81.8 | 2 100.0 | 0.5 | 1 | |||||
| Mucor spp. | ||||||||||
| Isavuconazole (8) | 0 0.0 | 1 12.5 | 1 25.0 | 1 37.5 | 5 100.0 | >8 | - | |||
| Itraconazole (8) | 0 0.0 | 2 25.0 | 2 50.0 | 4 100.0 | 4 | - | ||||
| Voriconazole (8) | 0 0.0 | 8 100.0 | >8 | - | ||||||
| Posaconazole (8) | 0 0.0 | 1 12.5 | 2 37.5 | 2 62.5 | 0 62.5 | 1 75.0 | 2 100.0 | 2 | - | |
| Amphotericin B (8) | 0 0.0 | 3 37.5 | 5 100.0 | 0.5 | - | |||||
| Rhizomucor spp. | ||||||||||
| Isavuconazole (4) | 0 0.0 | 3 75.0 | 0 75.0 | 0 75.0 | 1 100.0 | 2 | - | |||
| Itraconazole (4) | 0 0.0 | 1 25.0 | 2 75.0 | 0 75.0 | 1 100.0 | 1 | - | |||
| Voriconazole (4) | 0 0.0 | 1 25.0 | 3 100.0 | >8 | - | |||||
| Posaconazole (4) | 0 0.0 | 3 75.0 | 1 100.0 | 0.5 | - | |||||
| Amphotericin B (4) | 0 0.0 | 1 25.0 | 3 100.0 | 0.5 | - | |||||
| Rhizopus spp. | ||||||||||
| Isavuconazole (27) | 0 0.0 | 14 51.9 | 7 77.8 | 2 85.2 | 1 88.9 | 3 100.0 | 1 | >8 | ||
| Itraconazole (27) | 0 0.0 | 2 7.4 | 10 44.4 | 8 74.1 | 1 77.8 | 3 88.9 | 3 100.0 | 2 | >8 | |
| Voriconazole (27) | 0 0.0 | 4 14.8 | 14 66.7 | 9 100.0 | 8 | >8 | ||||
| Posaconazole (27) | 0 0.0 | 1 3.7 | 14 55.6 | 4 70.4 | 3 81.5 | 2 88.9 | 1 92.6 | 2 100.0 | 0.5 | 8 |
| Amphotericin B (27) | 0 0.0 | 13 48.1 | 13 96.3 | 1 100.0 | 1 | 1 | ||||
| Syncephalastrum spp. | ||||||||||
| Isavuconazole (2) | 0 0.0 | 1 50.0 | 0 50.0 | 0 50.0 | 1 100.0 | 2 | - | |||
| Itraconazole (2) | 0 0.0 | 1 50.0 | 1 100.0 | 1 | - | |||||
| Voriconazole (2) | 0 0.0 | 2 100.0 | >8 | - | ||||||
| Posaconazole (2) | 0 0.0 | 2 100.0 | 0.5 | - | ||||||
| Amphotericin B (2) | 0 0.0 | 1 50.0 | 1 100.0 | 0.25 | - | |||||
| Organism | Antifungal Agent | No. of Isolates | MIC (mg/L) | ||
|---|---|---|---|---|---|
| 50% | 90% | Range | |||
| Mucorales | |||||
| Isavuconazole | 52 | 2 | >8 | 1–>8 | |
| Itraconazole | 52 | 2 | 8 | 0.5->8 | |
| Voriconazole | 52 | >8 | >8 | 4–>8 | |
| Posaconazole | 52 | 0.5 | 8 | 0.25–>8 | |
| Amphotericin B | 52 | 0.5 | 1 | 0.25–2 | |
| All Rhizopus spp. | |||||
| Isavuconazole | 27 | 1 | >8 | 1–>8 | |
| Itraconazole | 27 | 2 | >8 | 0.5–>8 | |
| Voriconazole | 27 | 8 | >8 | 4–>8 | |
| Posaconazole | 27 | 0.5 | 8 | 0.25–>8 | |
| Amphotericin B | 27 | 1 | 1 | 0.5–2 | |
| R. microsporus group | |||||
| Isavuconazole | 16 | 1 | 2 | 1–4 | |
| Itraconazole | 16 | 2 | >8 | 0.5–>8 | |
| Voriconazole | 16 | 8 | >8 | 4–>8 | |
| Posaconazole | 16 | 0.5 | >8 | 0.25–>8 | |
| Amphotericin B | 16 | 1 | 1 | 0.5–1 | |
| R. arrhizus species complex | |||||
| Isavuconazole | 10 | 2 | >8 | 1–>8 | |
| Itraconazole | 10 | 1 | 8 | 0.5–8 | |
| Voriconazole | 10 | 8 | >8 | 4–>8 | |
| Posaconazole | 10 | 0.5 | 4 | 0.5–4 | |
| Amphotericin B | 10 | 0.5 | 1 | 0.5–1 | |
| All Lichtheimia spp. | |||||
| Isavuconazole | 11 | 4 | 8 | 2–>8 | |
| Itraconazole | 11 | 1 | 2 | 1–2 | |
| Voriconazole | 11 | >8 | >8 | >8 | |
| Posaconazole | 11 | 0.5 | 1 | 0.5–1 | |
| Amphotericin B | 11 | 0.5 | 1 | 0.5–1 | |
| L. corymbifera | |||||
| Isavuconazole | 7 | 2 | - | 2–>8 | |
| Itraconazole | 7 | 1 | - | 1–2 | |
| Voriconazole | 7 | >8 | - | >8 | |
| Posaconazole | 7 | 0.5 | - | 0.5–1 | |
| Amphotericin B | 7 | 0.5 | - | 0.5–1 | |
| All Mucor spp. | |||||
| Isavuconazole | 8 | >8 | - | 2–>8 | |
| Itraconazole | 8 | 4 | - | 2–8 | |
| Voriconazole | 8 | >8 | - | >8 | |
| Posaconazole | 8 | 2 | - | 0.5–>8 | |
| Amphotericin B | 8 | 0.5 | - | 0.25–0.5 | |
| M. circinelloides | |||||
| Isavuconazole | 3 | >8 | - | 8–>8 | |
| Itraconazole | 3 | 8 | - | 2–8 | |
| Voriconazole | 3 | >8 | - | >8 | |
| Posaconazole | 3 | 2 | - | 0.5–>8 | |
| Amphotericin B | 3 | 0.5 | - | 0.5 | |
| M. circinelloides/M. ramosissimus | |||||
| Isavuconazole | 2 | 4 | - | 4–>8 | |
| Itraconazole | 2 | 2 | - | 2–4 | |
| Voriconazole | 2 | >8 | - | >8 | |
| Posaconazole | 2 | 2 | - | 2–8 | |
| Amphotericin B | 2 | 0.25 | - | 0.25 | |
| Rhizomucor pusillus | |||||
| Isavuconazole | 4 | 2 | - | 2–>8 | |
| Itraconazole | 4 | 1 | - | 0.5–4 | |
| Voriconazole | 4 | >8 | - | 8–>8 | |
| Posaconazole | 4 | 0.5 | - | 0.5–1 | |
| Amphotericin B | 4 | 0.5 | - | 0.25–0.5 | |
| Syncephalastrum spp. | |||||
| Isavuconazole | 2 | 2 | - | 2–>8 | |
| Itraconazole | 2 | 1 | - | 1–2 | |
| Voriconazole | 2 | >8 | - | >8 | |
| Posaconazole | 2 | 0.5 | - | 0.5 | |
| Amphotericin B | 2 | 0.25 | - | 0.25–0.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalhaes, C.G.; Rhomberg, P.R.; Huband, M.D.; Pfaller, M.A.; Castanheira, M. Antifungal Activity of Isavuconazole and Comparator Agents against Contemporaneous Mucorales Isolates from USA, Europe, and Asia-Pacific. J. Fungi 2023, 9, 241. https://doi.org/10.3390/jof9020241
Carvalhaes CG, Rhomberg PR, Huband MD, Pfaller MA, Castanheira M. Antifungal Activity of Isavuconazole and Comparator Agents against Contemporaneous Mucorales Isolates from USA, Europe, and Asia-Pacific. Journal of Fungi. 2023; 9(2):241. https://doi.org/10.3390/jof9020241
Chicago/Turabian StyleCarvalhaes, Cecilia G., Paul R. Rhomberg, Michael D. Huband, Michael A. Pfaller, and Mariana Castanheira. 2023. "Antifungal Activity of Isavuconazole and Comparator Agents against Contemporaneous Mucorales Isolates from USA, Europe, and Asia-Pacific" Journal of Fungi 9, no. 2: 241. https://doi.org/10.3390/jof9020241
APA StyleCarvalhaes, C. G., Rhomberg, P. R., Huband, M. D., Pfaller, M. A., & Castanheira, M. (2023). Antifungal Activity of Isavuconazole and Comparator Agents against Contemporaneous Mucorales Isolates from USA, Europe, and Asia-Pacific. Journal of Fungi, 9(2), 241. https://doi.org/10.3390/jof9020241






