Population Pharmacokinetics of Isavuconazole in Critical Care Patients with COVID-19-Associated Pulmonary Aspergillosis and Monte Carlo Simulations of High Off-Label Doses
Abstract
1. Introduction
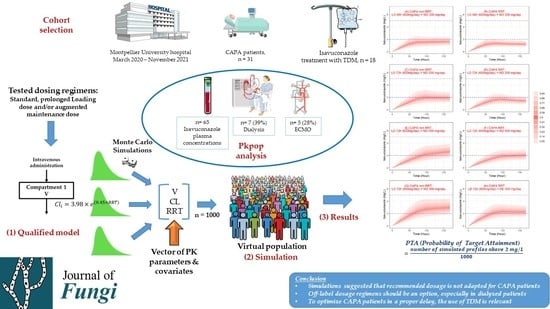
2. Materials and Methods
2.1. Study Design, Subjects, Sample Collection, and Assay Method
2.2. Population Pharmacokinetic Modeling
2.2.1. First Step—Basic Model Building
2.2.2. Second Step—Covariate Analysis
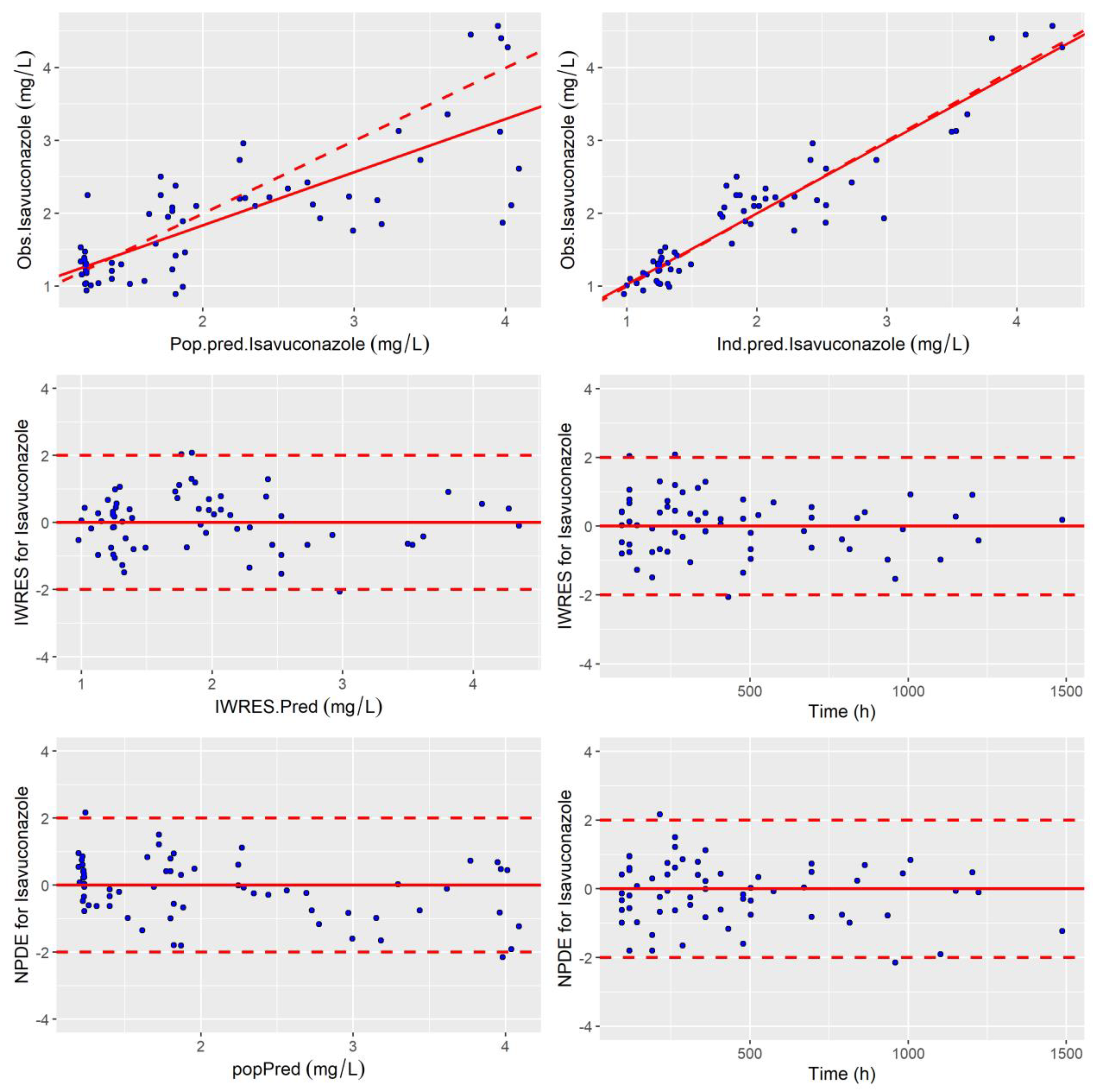
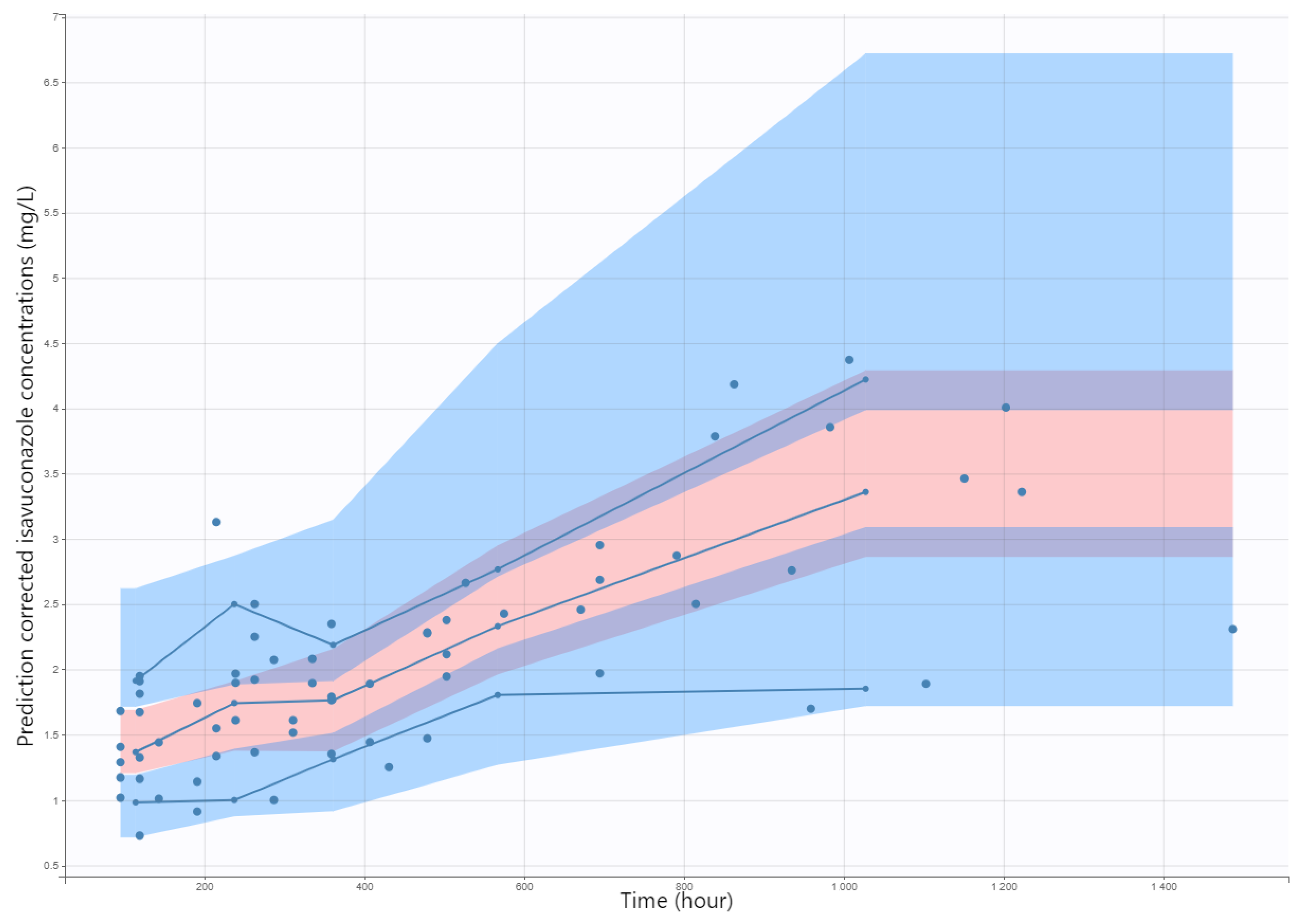
2.3. Internal Evaluation of the Model
2.4. Model Qualification Process
2.5. Monte Carlo Simulation Assessment for Maximum and Off-Label Dose Regimens
3. Results
3.1. Subject Characteristics
3.2. Basic and Covariate Model Building
3.3. Internal Evaluation and Validation of the Final Model
3.4. Monte Carlo Simulation of Maximum/Off-Label Dosing Regimens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van de Veerdonk, F.L.; Gommers, D.; et al. Invasive Aspergillosis in Patients Admitted to the Intensive Care Unit with Severe Influenza: A Retrospective Cohort Study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Rijnders, B.J.A.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T.; et al. Review of Influenza-Associated Pulmonary Aspergillosis in ICU Patients and Proposal for a Case Definition: An Expert Opinion. Intensive Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef]
- Gangneux, J.-P.; Dannaoui, E.; Fekkar, A.; Luyt, C.-E.; Botterel, F.; De Prost, N.; Tadié, J.-M.; Reizine, F.; Houzé, S.; Timsit, J.-F.; et al. Fungal Infections in Mechanically Ventilated Patients with COVID-19 during the First Wave: The French Multicentre MYCOVID Study. Lancet Respir. Med. 2022, 10, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Pascale, R.; Cricca, M.; Rinaldi, M.; Maccaro, A.; Bussini, L.; Fornaro, G.; Tonetti, T.; Pizzilli, G.; Francalanci, E.; et al. Epidemiology of Invasive Pulmonary Aspergillosis among COVID-19 Intubated Patients: A Prospective Study. Clin. Infect. Dis. 2020, 73, e3606–e3614. [Google Scholar] [CrossRef]
- Lahmer, T.; Kriescher, S.; Herner, A.; Rothe, K.; Spinner, C.D.; Schneider, J.; Mayer, U.; Neuenhahn, M.; Hoffmann, D.; Geisler, F.; et al. Invasive Pulmonary Aspergillosis in Critically Ill Patients with Severe COVID-19 Pneumonia: Results from the Prospective AspCOVID-19 Study. PLoS ONE 2021, 16, e0238825. [Google Scholar] [CrossRef]
- Salmanton-García, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; Garcia-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19-Associated Pulmonary Aspergillosis, March-August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and Managing COVID-19-Associated Pulmonary Aspergillosis: The 2020 ECMM/ISHAM Consensus Criteria for Research and Clinical Guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Luong, M.-L.; Al-Dabbagh, M.; Groll, A.H.; Racil, Z.; Nannya, Y.; Mitsani, D.; Husain, S. Utility of Voriconazole Therapeutic Drug Monitoring: A Meta-Analysis. J. Antimicrob. Chemother. 2016, 71, 1786–1799. [Google Scholar] [CrossRef]
- Hussaini, T.; Rüping, M.J.G.T.; Farowski, F.; Vehreschild, J.J.; Cornely, O.A. Therapeutic Drug Monitoring of Voriconazole and Posaconazole. Pharmacotherapy 2011, 31, 214–225. [Google Scholar] [CrossRef]
- McCreary, E.K.; Bayless, M.; Van, A.P.; Lepak, A.J.; Wiebe, D.A.; Schulz, L.T.; Andes, D.R. Impact of Triazole Therapeutic Drug Monitoring Availability and Timing. Antimicrob. Agents Chemother. 2019, 63, e01245-19. [Google Scholar] [CrossRef] [PubMed]
- Vena, A.; Muñoz, P.; Mateos, M.; Guinea, J.; Galar, A.; Pea, F.; Alvarez-Uria, A.; Escribano, P.; Bouza, E. Therapeutic Drug Monitoring of Antifungal Drugs: Another Tool to Improve Patient Outcome? Infect. Dis. Ther. 2020, 9, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.; Gordon, M.; Villarreal, E.; Peruccioni, M.; Marqués, M.R.; Poveda-Andrés, J.L.; Castellanos-Ortega, Á.; Ramirez, P. Impact of Voriconazole Plasma Concentrations on Treatment Response in Critically Ill Patients. J. Clin. Pharm. Ther. 2019, 44, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, T.; Xie, J.; Yang, Q.; Zheng, X.; Dong, W.; Xing, J.; Wang, X.; Dong, Y. Risk Factors for Voriconazole-Associated Hepatotoxicity in Patients in the Intensive Care Unit. Pharmacotherapy 2016, 36, 757–765. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Flörl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 Guidelines for the Treatment of Invasive Candidiasis, Aspergillosis and Mucormycosis in Leukemia and Hematopoietic Stem Cell Transplant Patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef]
- Miceli, M.H.; Kauffman, C.A. Isavuconazole: A New Broad-Spectrum Triazole Antifungal Agent. Clin. Infect. Dis. 2015, 61, 1558–1565. [Google Scholar] [CrossRef]
- Maertens, J.A.; Raad, I.I.; Marr, K.A.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, O.A.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus Voriconazole for Primary Treatment of Invasive Mould Disease Caused by Aspergillus and Other Filamentous Fungi (SECURE): A Phase 3, Randomised-Controlled, Non-Inferiority Trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef]
- Furfaro, E.; Signori, A.; Di Grazia, C.; Dominietto, A.; Raiola, A.M.; Aquino, S.; Ghiggi, C.; Ghiso, A.; Ungaro, R.; Angelucci, E.; et al. Serial Monitoring of Isavuconazole Blood Levels during Prolonged Antifungal Therapy. J. Antimicrob. Chemother. 2019, 74, 2341–2346. [Google Scholar] [CrossRef]
- Risum, M.; Vestergaard, M.-B.; Weinreich, U.M.; Helleberg, M.; Vissing, N.H.; Jørgensen, R. Therapeutic Drug Monitoring of Isavuconazole: Serum Concentration Variability and Success Rates for Reaching Target in Comparison with Voriconazole. Antibiotics 2021, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Andes, D.; Kovanda, L.; Desai, A.; Kitt, T.; Zhao, M.; Walsh, T.J. Isavuconazole Concentration in Real-World Practice: Consistency with Results from Clinical Trials. Antimicrob. Agents Chemother. 2018, 62, e00585-18. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Kovanda, L.L.; Hope, W.W.; Andes, D.; Mouton, J.W.; Kowalski, D.L.; Townsend, R.W.; Mujais, S.; Bonate, P.L. Exposure-Response Relationships for Isavuconazole in Patients with Invasive Aspergillosis and Other Filamentous Fungi. Antimicrob. Agents Chemother. 2017, 61, e01034-17. [Google Scholar] [CrossRef] [PubMed]
- Zurl, C.; Waller, M.; Schwameis, F.; Muhr, T.; Bauer, N.; Zollner-Schwetz, I.; Valentin, T.; Meinitzer, A.; Ullrich, E.; Wunsch, S.; et al. Isavuconazole Treatment in a Mixed Patient Cohort with Invasive Fungal Infections: Outcome, Tolerability and Clinical Implications of Isavuconazole Plasma Concentrations. J. Fungi 2020, 6, 90. [Google Scholar] [CrossRef]
- EMA Cresemba. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/cresemba (accessed on 5 September 2022).
- Blijlevens, N.M.A.; Brüggemann, R.J.M.; Janssen, J.J.W.M.; de Lange, D.W.; Meis, J.F.; Oude Lashof, A.M.L.; Reijers, M.H.E.; Rijnders, B.J.A.; Schouten, J.A.; van de Veerdonk, F.L.; et al. The Dutch Working Party on Antibiotic Policy (SWAB) Recommendations for the Diagnosis and Management of COVID-19 Associated Pulmonary Aspergillosis; Stichting Werkgroep Antibioticabeleid (SWAB): Leiden, The Netherland, 2021. [Google Scholar]
- Pfaller, M.A.; Carvalhaes, C.G.; Rhomberg, P.; Messer, S.A.; Castanheira, M. Antifungal Susceptibilities of Opportunistic Filamentous Fungal Pathogens from the Asia and Western Pacific Region: Data from the SENTRY Antifungal Surveillance Program (2011–2019). J. Antibiot. 2021, 74, 519–527. [Google Scholar] [CrossRef]
- EUCAST. Overview of Antifungal ECOFFs and Clinical Breakpoints for Yeasts, Moulds and Dermatophytes Using the EUCAST E.Def 7.3, E.Def 9.4 and E.Def 11.0 Procedures; EUCAST: Växjö, Sweden, 2022. [Google Scholar]
- Lahmer, T.; Batres Baires, G.; Heilmaier, M.; Schmid, R.M.; Sörgel, F.; Kinzig, M.; Huber, W.; Mayr, U.; Rasch, S. Influence of Sustained Low-Efficiency Dialysis Treatment on Isavuconazole Plasma Levels in Critically Ill Patients. Antimicrob. Agents Chemother. 2019, 63, e01162-19. [Google Scholar] [CrossRef]
- Eiden, C.; Mathieu, O.; Peyrière, H.; Hillaire-Buys, D.; Cociglio, M. Simultaneous Quantification of Voriconazole and Its N-Oxide Metabolite in Human Plasma by an Easy and Rapid Isocratic LC Method with UV Detection. Chroma 2008, 67, 275–280. [Google Scholar] [CrossRef]
- Lavielle, M.; Mentré, F. Estimation of Population Pharmacokinetic Parameters of Saquinavir in HIV Patients with the MONOLIX Software. J. Pharmacokinet. Pharmacodyn. 2007, 34, 229–249. [Google Scholar] [CrossRef]
- Allard, Q.; Djerada, Z.; Pouplard, C.; Repessé, Y.; Desprez, D.; Galinat, H.; Frotscher, B.; Berger, C.; Harroche, A.; Ryman, A.; et al. Real Life Population Pharmacokinetics Modelling of Eight Factors VIII in Patients with Severe Haemophilia A: Is It Always Relevant to Switch to an Extended Half-Life? Pharmaceutics 2020, 12, 380. [Google Scholar] [CrossRef]
- Comets, E.; Brendel, K.; Mentré, F. Computing Normalised Prediction Distribution Errors to Evaluate Nonlinear Mixed-Effect Models: The Npde Add-on Package for R. Comput. Methods Programs Biomed. 2008, 90, 154–166. [Google Scholar] [CrossRef]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-Corrected Visual Predictive Checks for Diagnosing Nonlinear Mixed-Effects Models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Bonate, P.L.; Strougo, A.; Desai, A.; Roy, M.; Yassen, A.; van der Walt, J.S.; Kaibara, A.; Tannenbaum, S. Guidelines for the Quality Control of Population Pharmacokinetic-Pharmacodynamic Analyses: An Industry Perspective. AAPS J. 2012, 14, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Thai, H.-T.; Mentré, F.; Holford, N.H.G.; Veyrat-Follet, C.; Comets, E. Evaluation of Bootstrap Methods for Estimating Uncertainty of Parameters in Nonlinear Mixed-Effects Models: A Simulation Study in Population Pharmacokinetics. J. Pharmacokinet. Pharmacodyn. 2014, 41, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Lavielle, M. Rsmlx: R Speaks ‘Monolix’. R Package Version 5.0.1. 2022. Available online: https://CRAN.R-project.org/package=Rsmlx (accessed on 1 January 2023).
- Schmitt-Hoffmann, A.; Desai, A.; Kowalski, D.; Pearlman, H.; Yamazaki, T.; Townsend, R. Isavuconazole Absorption Following Oral Administration in Healthy Subjects Is Comparable to Intravenous Dosing, and Is Not Affected by Food, or Drugs That Alter Stomach PH. Int. J. Clin. Pharmacol. Ther. 2016, 54, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Garnacho-Montero, J.; Calandra, T.; Kullberg, B.; Dimopoulos, G.; Azoulay, E.; Chakrabarti, A.; Kett, D.; Leon, C.; Ostrosky-Zeichner, L.; et al. Intensive Care Medicine Research Agenda on Invasive Fungal Infection in Critically Ill Patients. Intensive Care Med. 2017, 43, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Aziz, M.H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: A Position Paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Cusumano, J.A.; Klinker, K.P.; Huttner, A.; Luther, M.K.; Roberts, J.A.; LaPlante, K.L. Towards Precision Medicine: Therapeutic Drug Monitoring–Guided Dosing of Vancomycin and β-Lactam Antibiotics to Maximize Effectiveness and Minimize Toxicity. Am. J. Health-Syst. Pharm. 2020, 77, 1104–1112. [Google Scholar] [CrossRef]
- Matusik, E.; Boidin, C.; Friggeri, A.; Richard, J.-C.; Bitker, L.; Roberts, J.A.; Goutelle, S. Therapeutic Drug Monitoring of Antibiotic Drugs in Patients Receiving Continuous Renal Replacement Therapy or Intermittent Hemodialysis: A Critical Review. Ther. Drug Monit. 2022, 44, 86–102. [Google Scholar] [CrossRef]
- Buil, J.B.; Brüggemann, R.J.M.; Wasmann, R.E.; Zoll, J.; Meis, J.F.; Melchers, W.J.G.; Mouton, J.W.; Verweij, P.E. Isavuconazole Susceptibility of Clinical Aspergillus fumigatus Isolates and Feasibility of Isavuconazole Dose Escalation to Treat Isolates with Elevated MICs. J. Antimicrob. Chemother. 2018, 73, 134–142. [Google Scholar] [CrossRef]
- Petraitis, V.; Petraitiene, R.; Moradi, P.W.; Strauss, G.E.; Katragkou, A.; Kovanda, L.L.; Hope, W.W.; Walsh, T.J. Pharmacokinetics and Concentration-Dependent Efficacy of Isavuconazole for Treatment of Experimental Invasive Pulmonary Aspergillosis. Antimicrob. Agents Chemother. 2016, 60, 2718–2726. [Google Scholar] [CrossRef]
- Willeman, T.; Tonini, J.; Garnaud, C.; Bailly, S.; Gandia, P.; Stanke-Labesque, F.; Maubon, D.; Gautier-Veyret, E. Refining the Therapeutic Range of Posaconazole and Isavuconazole for Efficient Therapeutic Drug Monitoring Using a Bioassay Approach. Fundam. Clin. Pharmacol. 2020, 34, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Schmitt-Hoffmann, A.; Roos, B.; Maares, J.; Heep, M.; Spickerman, J.; Weidekamm, E.; Brown, T.; Roehrle, M. Multiple-Dose Pharmacokinetics and Safety of the New Antifungal Triazole BAL4815 after Intravenous Infusion and Oral Administration of Its Prodrug, BAL8557, in Healthy Volunteers. Antimicrob. Agents Chemother. 2006, 50, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ullah, N.; Sepulcri, C.; Mikulska, M. Isavuconazole for COVID-19-Associated Invasive Mold Infections. J. Fungi 2022, 8, 674. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and Management of Aspergillus Diseases: Executive Summary of the 2017 ESCMID-ECMM-ERS Guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef]
- Macleod, A.M.; Campbell, M.; Cody, J.D.; Daly, C.; Donaldson, C.; Grant, A.; Khan, I.; Rabindranath, K.S.; Vale, L.; Wallace, S. Cellulose, Modified Cellulose and Synthetic Membranes in the Haemodialysis of Patients with End-Stage Renal Disease. Cochrane Database Syst. Rev. 2005, 3, CD003234. [Google Scholar] [CrossRef]
- Honore, P.M.; Jacobs, R.; De Waele, E.; Spapen, H.D. Anidulafungin Dosing during CRRT: Do Not Underestimate Adsorption! Crit. Care 2014, 18, 618. [Google Scholar] [CrossRef]
- Biagi, M.; Butler, D.; Tan, X.; Qasmieh, S.; Tejani, K.; Patel, S.; Rivosecchi, R.M.; Nguyen, M.H.; Clancy, C.J.; Shields, R.K.; et al. Pharmacokinetics and Dialytic Clearance of Isavuconazole During In Vitro and In Vivo Continuous Renal Replacement Therapy. Antimicrob. Agents Chemother. 2019, 63, e01085-19. [Google Scholar] [CrossRef]
- Pascussi, J.M.; Drocourt, L.; Gerbal-Chaloin, S.; Fabre, J.M.; Maurel, P.; Vilarem, M.J. Dual Effect of Dexamethasone on CYP3A4 Gene Expression in Human Hepatocytes. Sequential Role of Glucocorticoid Receptor and Pregnane X Receptor. Eur. J. Biochem. 2001, 268, 6346–6358. [Google Scholar] [CrossRef]
- Yumoto, R.; Murakami, T.; Sanemasa, M.; Nasu, R.; Nagai, J.; Takano, M. Pharmacokinetic Interaction of Cytochrome P450 3A-Related Compounds with Rhodamine 123, a P-Glycoprotein Substrate, in Rats Pretreated with Dexamethasone. Drug Metab. Dispos. 2001, 29, 145–151. [Google Scholar]
- PharmGKB CYP3A5 Frequency Table. Available online: Https://Files.Cpicpgx.Org/Data/Report/Current/Frequency/CYP3A5_frequency_table.Xlsx (accessed on 1 January 2023).
- Cojutti, P.G.; Carnelutti, A.; Lazzarotto, D.; Sozio, E.; Candoni, A.; Fanin, R.; Tascini, C.; Pea, F. Population Pharmacokinetics and Pharmacodynamic Target Attainment of Isavuconazole against Aspergillus fumigatus and Aspergillus flavus in Adult Patients with Invasive Fungal Diseases: Should Therapeutic Drug Monitoring for Isavuconazole Be Considered as Mandatory as for the Other Mold-Active Azoles? Pharmaceutics 2021, 13, 2099. [Google Scholar] [CrossRef]
- Desai, A.; Kovanda, L.; Kowalski, D.; Lu, Q.; Townsend, R.; Bonate, P.L. Population Pharmacokinetics of Isavuconazole from Phase 1 and Phase 3 (SECURE) Trials in Adults and Target Attainment in Patients with Invasive Infections Due to Aspergillus and Other Filamentous Fungi. Antimicrob. Agents Chemother. 2016, 60, 5483–5491. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, L.L.; Desai, A.V.; Lu, Q.; Townsend, R.W.; Akhtar, S.; Bonate, P.; Hope, W.W. Isavuconazole Population Pharmacokinetic Analysis Using Nonparametric Estimation in Patients with Invasive Fungal Disease (Results from the VITAL Study). Antimicrob. Agents Chemother. 2016, 60, 4568–4576. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Clancy, C.J.; Rivosecchi, R.M.; Zhao, W.; Shields, R.K.; Marini, R.V.; Venkataramanan, R.; Nguyen, M.H. Pharmacokinetics of Intravenous Isavuconazole in Solid-Organ Transplant Recipients. Antimicrob. Agents Chemother. 2018, 62, e01643-18. [Google Scholar] [CrossRef] [PubMed]
- Shekar, K.; Fraser, J.F.; Smith, M.T.; Roberts, J.A. Pharmacokinetic Changes in Patients Receiving Extracorporeal Membrane Oxygenation. J. Crit. Care 2012, 27, 741.e9–741.e18. [Google Scholar] [CrossRef]
- Kriegl, L.; Hatzl, S.; Zurl, C.; Reisinger, A.C.; Schilcher, G.; Eller, P.; Gringschl, Y.; Muhr, T.; Meinitzer, A.; Prattes, J.; et al. Isavuconazole Plasma Concentrations in Critically Ill Patients during Extracorporeal Membrane Oxygenation. J. Antimicrob. Chemother. 2022, 77, 2500–2505. [Google Scholar] [CrossRef]
- Zhao, Y.; Seelhammer, T.G.; Barreto, E.F.; Wilson, J.W. Altered Pharmacokinetics and Dosing of Liposomal Amphotericin B and Isavuconazole during Extracorporeal Membrane Oxygenation. Pharmacotherapy 2020, 40, 89–95. [Google Scholar] [CrossRef]
- Höhl, R.; Bertram, R.; Kinzig, M.; Haarmeyer, G.-S.; Baumgärtel, M.; Geise, A.; Muschner, D.; Prosch, D.; Reger, M.; Naumann, H.-T.; et al. Isavuconazole Therapeutic Drug Monitoring in Critically Ill ICU Patients: A Monocentric Retrospective Analysis. Mycoses 2022, 65, 747–752. [Google Scholar] [CrossRef]


| Characteristics | Patients (n = 18) |
|---|---|
| Clinical features | |
| Sex, M/F | 14/4 |
| Age (years), Median (IQR) | 65 (56–70) |
| Weight (kg)/BMI (kg/m2), Median (IQR) | 85 (79–92)/29.2 (25.6–31.8) |
| Underlying disease | |
| Diabetes mellitus, n (%) | 6 (33.3) |
| Hypertension, n (%) | 8 (44.4) |
| Heart failure, n (%) | 4 (22.2) |
| COPD, n (%) | 2 (11.1) |
| Solid organ transplant, n (%) | 3 (16.7) |
| SOFA score at admission, Median (IQR) | 5.5 (3.3–8.8) |
| Mechanical ventilation, n (%) | 18 (100) |
| RRT/RTT during isavuconazole therapy, n (%) | 7 (38.9)/6 (33.3%) |
| CVVHD/CVVHDF/SLED, n (%) | 3 (50%)/1 (16.7%)/2 (33.3%) |
| ECMO, n (%) | 5 (27.8) |
| Biological parameters | |
| C reactive protein (mg/L), Median (IQR) | 101 (53–159) |
| White blood cells (G/L), Median (IQR) | 13.1 (9.5–14.6) |
| Platelets (G/L), Median (IQR) | 283 (174–362) |
| Hemoglobin (g/dL), Median (IQR) | 9.4 (9–10.9) |
| Hemotocrit (%), Median (IQR) | 28.2 (26.7–32) |
| Albumin (g/L), Median (IQR) | 28.5 (26–31.8) |
| eGFR (mL/min/1.73 m2) estimated by CKD-EPI, Median (IQR) | 89.3 (60.5–98.9) |
| ASAT (U/L)/ALAT (U/L), Median (IQR) | 34 (25.8–51.8)/44.8 (30.3–70.3) |
| GGT (U/L), Median (IQR) | 198.3 (64–435) |
| Conjugated/total bilirubin (μmol/L), Median (IQR) | 5 (4–6.4)/7.8 (6–10.8) |
| Mycological diagnosis | |
| Aspergillus galactomannan antigen in BAL, n (%) | 15 (83.3) |
| BAL positive culture | 13 (72.2) |
| Aspergillus fumigatus/Aspergillus flavus/others, n (%) | 6 (46.2)/3 (23)/4 (30.8) |
| Treatments | |
| Concomitant cytochrome P450 inhibitor, n (%) | 3 (16) |
| Concomitant cytochrome P450 inducer: dexamethasone 6 mg per day, n (%) | 18 (100) |
| Isavuconazole 72 h LD, n (%)/MD (mg/day), Mean ± SD | 6 (33)/264 ± 79 |
| Intravenous route/per os switch during MD, n (%) | 18 (100)/4 (22.2) |
| Time after last dose (h), Mean ± SD/Median (IQR) | 18.6 ± 5.9/24 (12–24) |
| Isavuconazole trough concentration, Median (IQR) | 1.87 (1.29–2.25) |
| Plasma concentrations: 2–5 mg/L, n (%) | 28 (43) |
| Plasma concentrations < 2 mg/L, n (%) | 37 (57) |
| Plasma concentrations > 5 mg/L, n (%) | 0 (0) |
| Basic Model | Final Model | ||||
|---|---|---|---|---|---|
| Value (RSE %) | Value (RSE %) | Shrinkage (%) | Bootstrap Mean (95% CI) | Jackknife (95% CI) | |
| Fixed effect | |||||
| Cl (L.h−1) | 4.75 (11.5) | 3.98 (9.9) | – | 3.97 (3.11–5.02) | 4.04 (3.76–4.32) |
| βCl (log L.h−1)-RRT | – | 0.45 (22.6) * | – | 0.44 (0.15–0.73) | 0.43 (0.33–0.51) |
| V (L) | 838 (15) | 850 (13.5) | – | 837 (572–1102) | 817 (745–889) |
| Random effect | |||||
| ωCl (%) | 32 (25.9) | 25.8 (28.8) | 2.9 | 20.6 (4.2–37) | 23.2 (18.4–28) |
| ωV (%) | 38 (49) | 39.9 (33.2) | 5.1 | 37.4 (12–63) | 42.5 (35–50) |
| residual | |||||
| b (proportional) | 0.2 (12.9) | 0.17 (12.2) | – | 0.17 (0.11–0.23) | 0.17 (0.15–0.18) |
| BIC | 105.8 | 91.7 | – | – | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez, L.; Corne, P.; Pasquier, G.; Konecki, C.; Sadek, M.; Le Bihan, C.; Klouche, K.; Mathieu, O.; Reynes, J.; Cazaubon, Y. Population Pharmacokinetics of Isavuconazole in Critical Care Patients with COVID-19-Associated Pulmonary Aspergillosis and Monte Carlo Simulations of High Off-Label Doses. J. Fungi 2023, 9, 211. https://doi.org/10.3390/jof9020211
Perez L, Corne P, Pasquier G, Konecki C, Sadek M, Le Bihan C, Klouche K, Mathieu O, Reynes J, Cazaubon Y. Population Pharmacokinetics of Isavuconazole in Critical Care Patients with COVID-19-Associated Pulmonary Aspergillosis and Monte Carlo Simulations of High Off-Label Doses. Journal of Fungi. 2023; 9(2):211. https://doi.org/10.3390/jof9020211
Chicago/Turabian StylePerez, Lucas, Philippe Corne, Grégoire Pasquier, Céline Konecki, Meriem Sadek, Clément Le Bihan, Kada Klouche, Olivier Mathieu, Jacques Reynes, and Yoann Cazaubon. 2023. "Population Pharmacokinetics of Isavuconazole in Critical Care Patients with COVID-19-Associated Pulmonary Aspergillosis and Monte Carlo Simulations of High Off-Label Doses" Journal of Fungi 9, no. 2: 211. https://doi.org/10.3390/jof9020211
APA StylePerez, L., Corne, P., Pasquier, G., Konecki, C., Sadek, M., Le Bihan, C., Klouche, K., Mathieu, O., Reynes, J., & Cazaubon, Y. (2023). Population Pharmacokinetics of Isavuconazole in Critical Care Patients with COVID-19-Associated Pulmonary Aspergillosis and Monte Carlo Simulations of High Off-Label Doses. Journal of Fungi, 9(2), 211. https://doi.org/10.3390/jof9020211