Identification and Biosynthesis of DHN-melanin Related Pigments in the Pathogenic Fungi Monilinia laxa, M. fructicola, and M. fructigena
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Material and Culture Conditions
2.2. Plant Material and Fruit Inoculations
2.3. Extraction and Quantification of Melanin-like Pigments Produced by Monilinia spp.
2.4. Identification of DHN Biosynthetic and Regulatory Genes in Monilinia spp.
2.5. Fungal RNA Extraction and qPCR Analysis
2.6. Determination of Fungal Biomass of Nectarines
2.7. Statistical Analysis
3. Results and Discussion
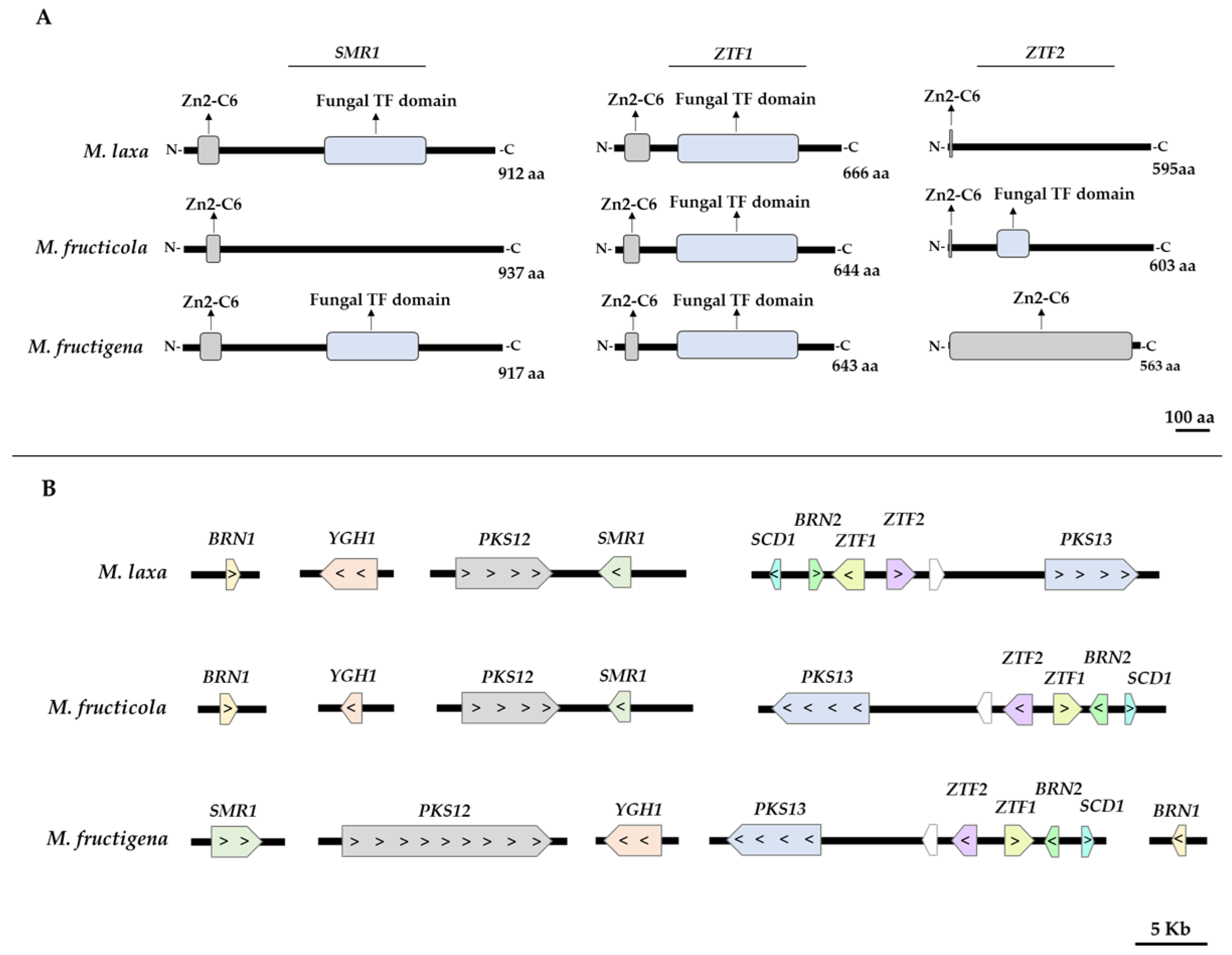
3.1. The Biosynthetic and Regulatory Genes of the DHN-melanin Pathway Are Present and Differently Clustered in the Genome of the Three Monilinia spp.
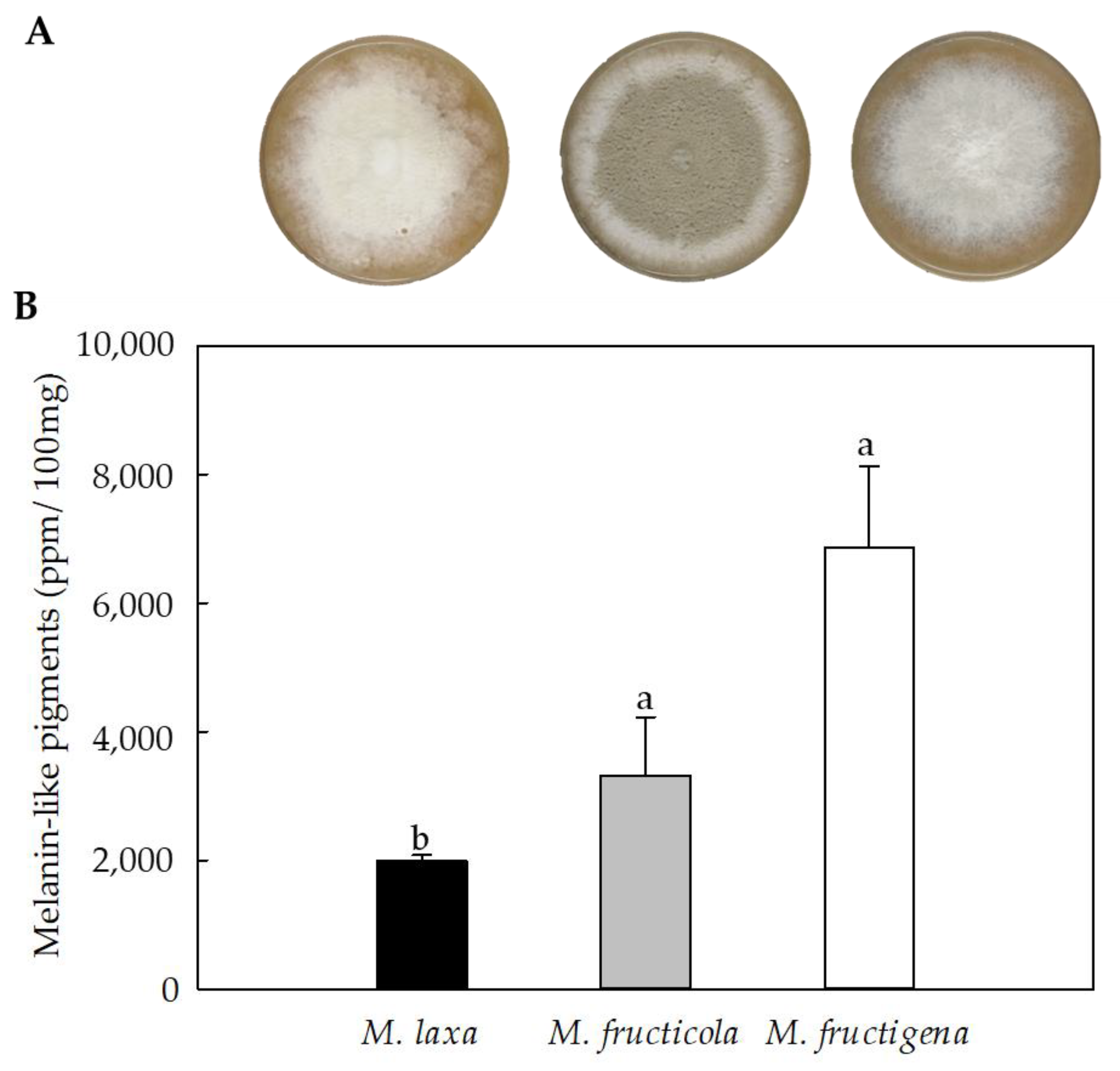
3.2. All Three Monilinia Species Produce Melanin-like Pigments
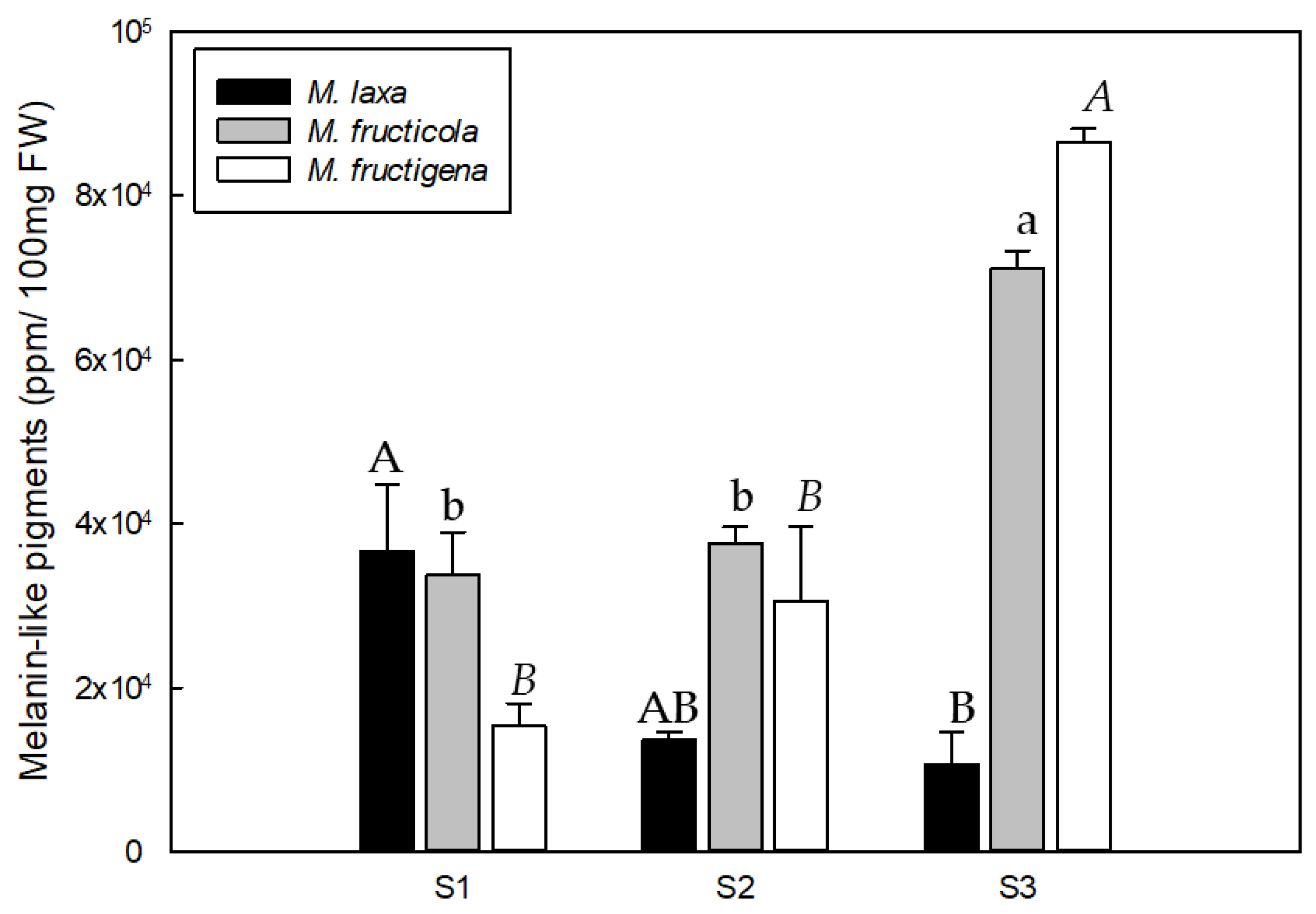
3.3. Melanin Content along the Nectarine Infection Process Is Different among Monilinia spp.
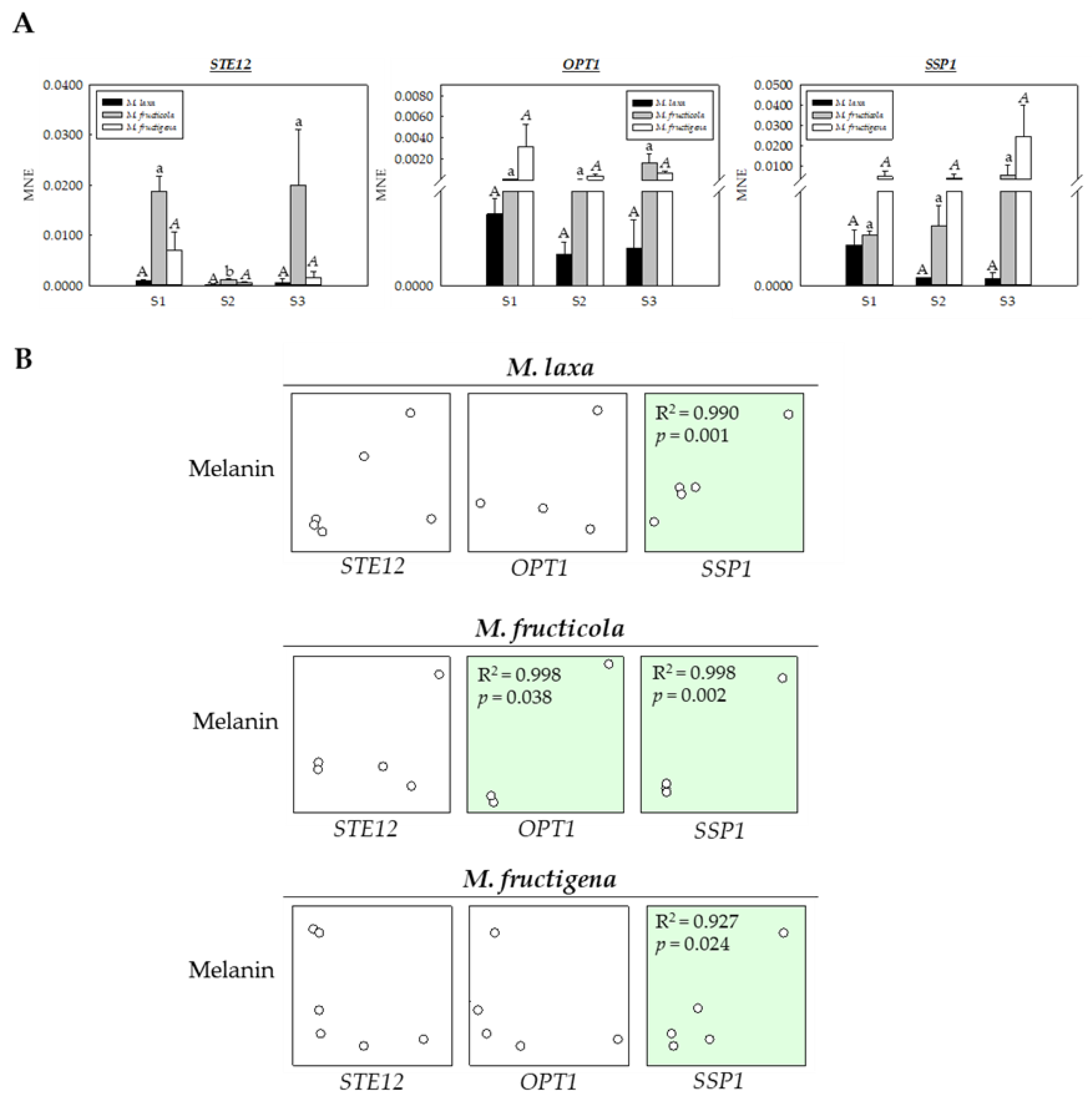
3.4. The DHN-melanin Biosynthetic Machinery Is Activated in Monilinia spp., Dependent on the Species and on the Disease Stage
3.5. Melanin Specific Genes Seem to Cooperate with Specific Genes to Induce Survival Mechanisms in Monilinia spp.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willetts, H.J.; Byrde, R.J.W.; Fielding, A.H.; Wong, A.L. The taxonomy of the brown rot fungi (Monilinia spp.) related to their extracellular cell wall-degrading enzymes. J. Gen. Microbiol. 1977, 103, 77–83. [Google Scholar] [CrossRef]
- Bernat, M.; Segarra, J.; Navas-Cortés, J.A.; Casals, C.; Torres, R.; Teixidó, N.; Usall, J. Influence of temperature and humidity on the survival of Monilinia fructicola conidia on stone fruits and inert surfaces. Ann. Appl. Biol. 2018, 173, 63–70. [Google Scholar] [CrossRef]
- Verde-Yáñez, L.; Vall-llaura, N.; Usall, J.; Teixidó, N.; Torres, R. Phenotypic plasticity of Monilinia spp. in response to light wavelengths: From in vitro development to virulence on nectarines. Int. J. Food Microbiol. 2022, 373, 109700. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Riley, P.A. Melanin. Int. J. Biochem. Cell Biol. 1997, 29, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Cordero, R.J.B.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal. Biol. Rev. 2017, 31, 99–112. [Google Scholar] [CrossRef]
- Bell, A.A.; Wheeler, M.H. Biosynthesis and Functions of Fungal Melanins. Annu. Rev. Phytopathol. 1986, 24, 411–451. [Google Scholar] [CrossRef]
- Plonka, P.M.; Grabacka, M. Melanin synthesis in microorganisms—Biotechnological and medical aspects. Acta Biochim. Pol. 2006, 53, 429–443. [Google Scholar] [CrossRef]
- Cao, W.; Zhou, X.; McCallum, N.C.; Hu, Z.; Ni, Q.Z.; Kapoor, U.; Heil, C.M.; Cay, K.S.; Zand, T.; Mantanona, A.J.; et al. Unraveling the structure and function of melanin through synthesis. J. Am. Chem. Soc. 2021, 143, 2622–2637. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, S.; Wang, G.; An, Z.; Liu, X.; Li, K.; Yin, W.B. Two transcription factors cooperatively regulate DHN melanin biosynthesis and development in Pestalotiopsis fici. Mol. Microbiol. 2019, 112, 649–666. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Watanabe, B.; Matsui, K.; Lumyong, S. Characterization of melanin and optimal conditions for pigment production by an endophytic fungus, Spissiomyces endophytica SDBR-CMU319. PLoS ONE 2019, 14, e0222187. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J. DHN melanin biosynthesis in the plant pathogenic fungus Botrytis cinerea is based on two developmentally regulated key enzyme (PKS)-encoding genes. Mol. Microbiol. 2016, 99, 729–748. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.J.; Gardiner, R.B.; Day, A.W. Melanin synthesis by Sclerotinia sclerotiorum. Mycologia 2009, 101, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Breitenbach, R.; Gerrits, R.; Dementyeva, P.; Knabe, N.; Schumacher, J.; Feldmann, I.; Jörg, R.; Masahiro, R.; Anna, A.G. The role of extracellular polymeric substances of fungal biofilms in mineral attachment and weathering. npj Mater Degrad. 2022, 6, 42. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Casadevall, A. Synthesis and assembly of fungal melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef]
- Rehnstrom, A.L.; Free, S.J. The isolation and characterization of melanin-deficient mutants of Monilinia fructicola. Physiol. Mol. Plant Pathol. 1996, 49, 321–330. [Google Scholar] [CrossRef]
- Yu, S.; Ramkumar, G.; Lee, Y.H. Light quality influences the virulence and physiological responses of Colletotrichum acutatum causing anthracnose in pepper plants. J. Appl. Microbiol. 2013, 115, 509–516. [Google Scholar] [CrossRef]
- Villarino, M.; Sandín-España, P.; Melgarejo, P.; De Cal, A. High chlorogenic and neochlorogenic acid levels in immature peaches reduce Monilinia laxa infection by interfering with fungal melanin biosynthesis. J. Agric. Food Chem. 2011, 59, 3205–3213. [Google Scholar] [CrossRef]
- Yu, F.Y.; Chiu, C.M.; Lee, Y.Z.; Lee, S.J.; Chou, C.M.; You, B.J.; Hsieh, D.K.; Lee, M.R.; Lee, M.H.; Bostock, R.M. Polyketide synthase gene expression in relation to chloromonilicin and melanin production in Monilinia fructicola. Phytopatholog 2020, 110, 1465–1475. [Google Scholar] [CrossRef]
- Baró-Montel, N.; Vall-llaura, N.; Usall, J.; Teixidó, N.; Naranjo-Ortíz, M.A.; Gabaldón, T.; Rosario, T. Pectin methyl esterases and rhamnogalacturonan hydrolases: Weapons for successful Monilinia laxa infection in stone fruit? Plant Pathol. 2019, 68, 1381–1393. [Google Scholar] [CrossRef]
- Naranjo-Ortíz, M.A.; Rodríguez-Píres, S.; Torres, R.; De Cal, A.; Usall, J.; Gabaldón, T. Genome sequence of the brown rot fungal pathogen Monilinia laxa. Genome Announc. 2018, 6, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Vilanova, L.; Valero-Jiménez, C.A.; van Kan, J.A.L. Deciphering the Monilinia fructicola genome to discover effector genes possibly involved in virulence. Genes 2021, 12, 568. [Google Scholar] [CrossRef] [PubMed]
- Marcet-Houben, M.; Villarino, M.; Vilanova, L.; De Cal, A.; van Kan, J.A.L.; Usall, J.; Gabaldon, T.; Torres, R. Comparative genomics used to predict virulence factors and metabolic genes among Monilinia species. J. Fungi. 2021, 7, 464. [Google Scholar] [CrossRef] [PubMed]
- Baró-Montel, N.; Vall-llaura, N.; Giné-Bordonaba, J.; Usall, J.; Serrano-Prieto, S.; Teixidó, N.; Torres, R. Double-sided battle: The role of ethylene during Monilinia spp. infection in peach at different phenological stages. Plant Physiol. Biochem. 2019, 144, 324–333. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Muller, P.Y.; Janovjak, H.; Miserez, A.R.; Dobbie, Z. Processing of Gene Expression Data Generated by Quantitative Real-Time RT-PCR. Gene Expr. 2002, 32, 1372–1379. [Google Scholar]
- Tsuji, G.; Kenmochi, Y.; Takano, Y.; Sweigard, J.; Farrall, L.; Furusawa, I.; Osamu, H.; Yasuyuki, K. Novel fungal transcriptional activators, Cmr1p of Colletotrichum lagenarium and Pig1p of Magnaporthe grisea, contain Cys2His2 zinc finger and Zn(II)2Cys6 binuclear cluster DNA-binding motifs and regulate transcription of melanin biosynthesis genes in a developmentally specific manner. Mol. Microbiol. 2000, 38, 940–954. [Google Scholar]
- Tsai, H.F.; Wheeler, M.H.; Chang, Y.C.; Kwon-Chung, K.J. A developmentally regulated gene cluster involved in conidial pigment biosynthesis in Aspergillus fumigatus. J. Bacteriol. 1999, 181, 6469–6477. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Greer, E.M.; McGrail, C.W. The role of melanins in melanotic fungi for pathogenesis and environmental survival. Appl. Microbiol. Biotechnol. 2020, 104, 4247–4257. [Google Scholar] [CrossRef]
- Pal, A.K.; Gajjar, D.U.; Vasavada, A.R. DOPA and DHN pathway orchestrate melanin synthesis in Aspergillus species. Med. Mycol. 2014, 52, 10–18. [Google Scholar]
- Hamada, T.; Asanagi, M.; Satozawa, T.; Araki, N.; Banba, S.; Higashimura, N.; Tomohisa, A.; Kangetsu, H. Action mechanism of the novel rice blast fungicide tolprocarb distinct from that of conventional melanin biosynthesis inhibitors. J. Pestic. Sci. 2014, 39, 152–158. [Google Scholar] [CrossRef]
- Liu, D.; Wei, L.; Guo, T.; Tan, W. Detection of DOPA-melanin in the dimorphic fungal pathogen Penicillium marneffei and its effect on macrophage phagocytosis in vitro. PLoS ONE 2014, 9, e92610. [Google Scholar] [CrossRef] [PubMed]
- Raman, N.M.; Shah, P.H.; Mohan, M.; Ramasamy, S. Improved production of melanin from Aspergillus fumigatus AFGRD105 by optimization of media factors. AMB Express. 2015, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Moon, K.M.; Kwon, E.B.; Lee, B.; Kim, C.Y. Recent Trends in Controlling the Enzymatic Browning of Fruit and Vegetable Products. Molecules 2020, 25, 2754. [Google Scholar] [CrossRef] [PubMed]
- Park, G.; Xue, C.; Zheng, L.; Lam, S.; Xu, J. MST12 Regulates Infectious Growth But Not Appressorium Formation in the Rice Blast Fungus Magnaporthe grisea. Am. Phytopathol. Soc. 2002, 15, 183–192. [Google Scholar] [CrossRef]
- Chagué, V.; Maor, R.; Sharon, A. CgOpt1, a putative oligopeptide transporter from Colletotrichum gloeosporioides that is involved in responses to auxin and pathogenicity. BMC Med. 2009, 9, 173. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Abate, D.; Rotolo, C.; Gerin, D.; Pollastro, S.; Faretra, F. De novo assembly and comparative transcriptome analysis of Monilinia fructicola, Monilinia laxa and Monilinia fructigena, the causal agents of brown rot on stone fruits. BMC Genomics 2018, 19, 436. [Google Scholar] [CrossRef]


| M. laxa | M. fructicola | M. fructigena | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Gene ID | Identity (%) | Coverage (%) | Gene ID | Identity (%) | Coverage (%) | Gene ID | Identity (%) | Coverage (%) |
| PKS12 | Monilinia__049320 | 87.49 | 99.67 | MFRU_042g00180.1 | 87.82 | 99.67 | g3016.t1 | 87.82 | 99.44 |
| PKS13 | Monilinia__009450 | 87.98 | 99.91 | MFRU_053g00290.1 | 87.93 | 99.95 | g3467.t1 | 87.42 | 99.91 |
| SCD1 | Monilinia__009400 | 90.42 | 100.00 | MFRU_053g00340.1 | 91.62 | 100.00 | g3472.t1 | 90.42 | 100.00 |
| YGH1 | Monilinia__012010 | 86.49 | 93.90 | MFRU_030g00600.1 | 87.78 | 99.76 | g3325.t1 | 83.46 | 96.10 |
| SMR1 | Monilinia__049330 | 85.75 | 96.16 | MFRU_042g00190.1 | 86.65 | 99.57 | g712.t1 | 86.80 | 95.10 |
| BRN1 | Monilinia__025870 | 27.15 | 89.62 | MFRU_016g00660.1 | 96.20 | 100.00 | g4002.t1 | 93.77 | 100.00 |
| BRN2 | Monilinia__009410 | 96.62 | 100.00 | MFRU_053g00330.1 | 96.99 | 100.00 | g3471.t1 | 93.99 | 100.00 |
| ZTF1 | Monilinia__009420 | 73.59 | 94.77 | MFRU_053g00320.1 | 72.76 | 96.92 | g3470.t1 | 71.38 | 96.92 |
| ZTF2 | Monilinia__009430 | 70.92 | 96.56 | MFRU_053g00310.1 | 71.64 | 96.23 | g3469.t1 | 70.45 | 89.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verde-Yáñez, L.; Vall-llaura, N.; Usall, J.; Teixidó, N.; Torreblanca-Bravo, È.; Torres, R. Identification and Biosynthesis of DHN-melanin Related Pigments in the Pathogenic Fungi Monilinia laxa, M. fructicola, and M. fructigena. J. Fungi 2023, 9, 138. https://doi.org/10.3390/jof9020138
Verde-Yáñez L, Vall-llaura N, Usall J, Teixidó N, Torreblanca-Bravo È, Torres R. Identification and Biosynthesis of DHN-melanin Related Pigments in the Pathogenic Fungi Monilinia laxa, M. fructicola, and M. fructigena. Journal of Fungi. 2023; 9(2):138. https://doi.org/10.3390/jof9020138
Chicago/Turabian StyleVerde-Yáñez, Lucía, Núria Vall-llaura, Josep Usall, Neus Teixidó, Èlia Torreblanca-Bravo, and Rosario Torres. 2023. "Identification and Biosynthesis of DHN-melanin Related Pigments in the Pathogenic Fungi Monilinia laxa, M. fructicola, and M. fructigena" Journal of Fungi 9, no. 2: 138. https://doi.org/10.3390/jof9020138
APA StyleVerde-Yáñez, L., Vall-llaura, N., Usall, J., Teixidó, N., Torreblanca-Bravo, È., & Torres, R. (2023). Identification and Biosynthesis of DHN-melanin Related Pigments in the Pathogenic Fungi Monilinia laxa, M. fructicola, and M. fructigena. Journal of Fungi, 9(2), 138. https://doi.org/10.3390/jof9020138






