Invasive Pulmonary Aspergillosis
Abstract
1. Introduction
2. Patients
2.1. Acute Myeloid Leukemias and Myelodysplastic Syndromes
2.2. Other Hematological Malignancies
2.3. Allogeneic Hematopoietic Stem Cell Transplantations
2.4. Chimeric Antigen Receptor T-Cell Therapy
2.5. Acquired or Inherited Immunodeficiency
2.6. Solid Organ Transplantations
2.7. Solid Tumors
2.8. Critically Ill Patients
2.9. Viral Pneumonias
2.10. Inflammatory and Autoimmune Diseases
2.11. Environmental Factors
3. Clinical and Radiological Features
3.1. Clinical Features
3.2. Radiological Features
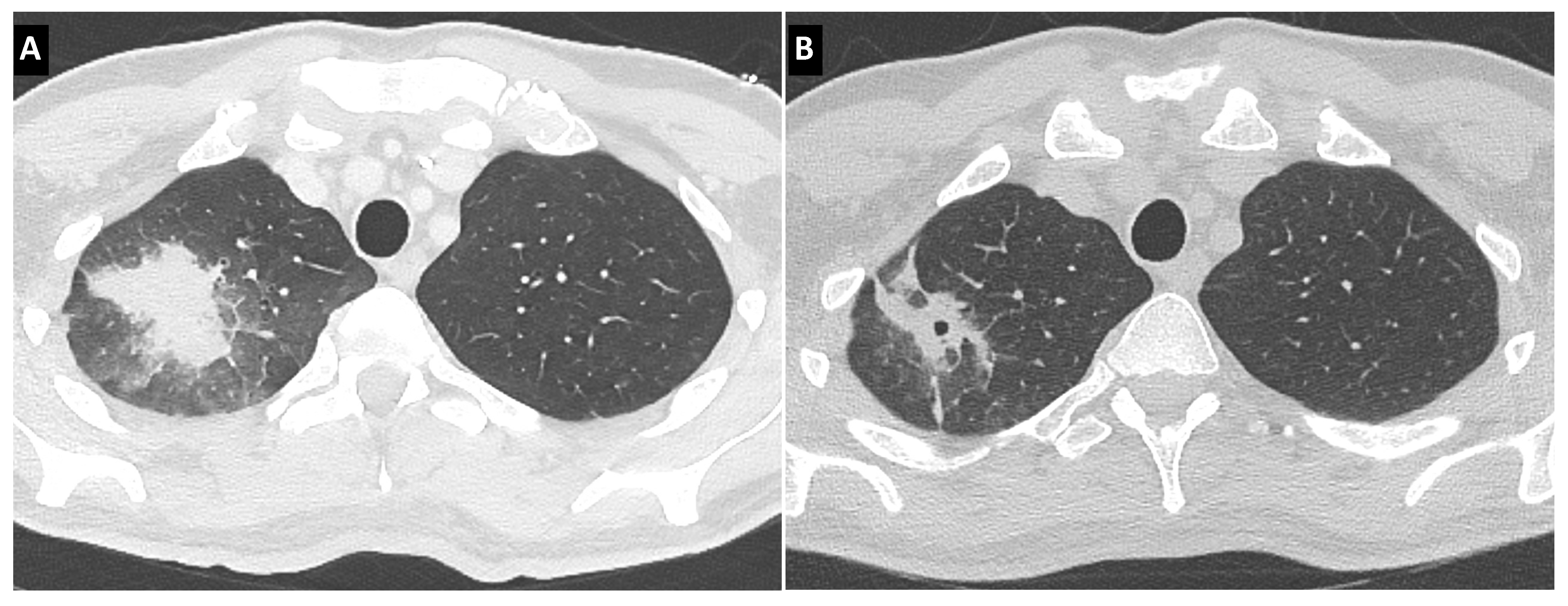
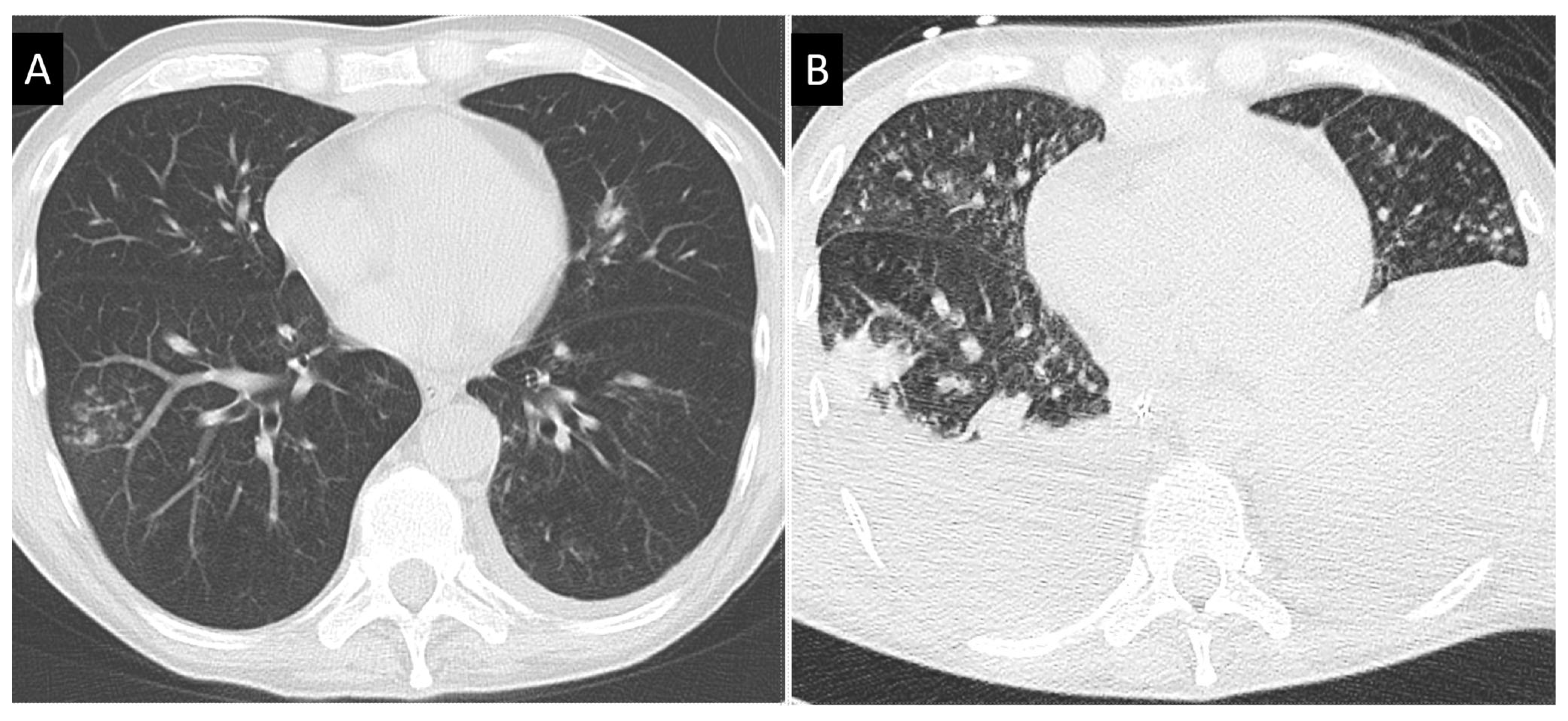
3.3. Bronchoscopic Features
4. Microbiological Findings
4.1. Samples
4.2. Direct Microscopy
4.3. Culture
4.4. Antibody
4.5. Galactomannan
4.6. (1,3)-β-D-Glucan
4.7. Polymerase Chain Reaction
4.8. Point of Care Tests
5. Diagnostic Criteria
5.1. EORTC-MSG Criteria
5.2. AspICU
5.3. Invasive Aspergillosis in Specific Conditions
6. Treatment
6.1. Amphotericin B and Lipid Formulations
6.2. Azoles
6.3. Echinocandins
6.4. Perspectives
6.5. Combination Therapies
6.6. Antifungal Strategies
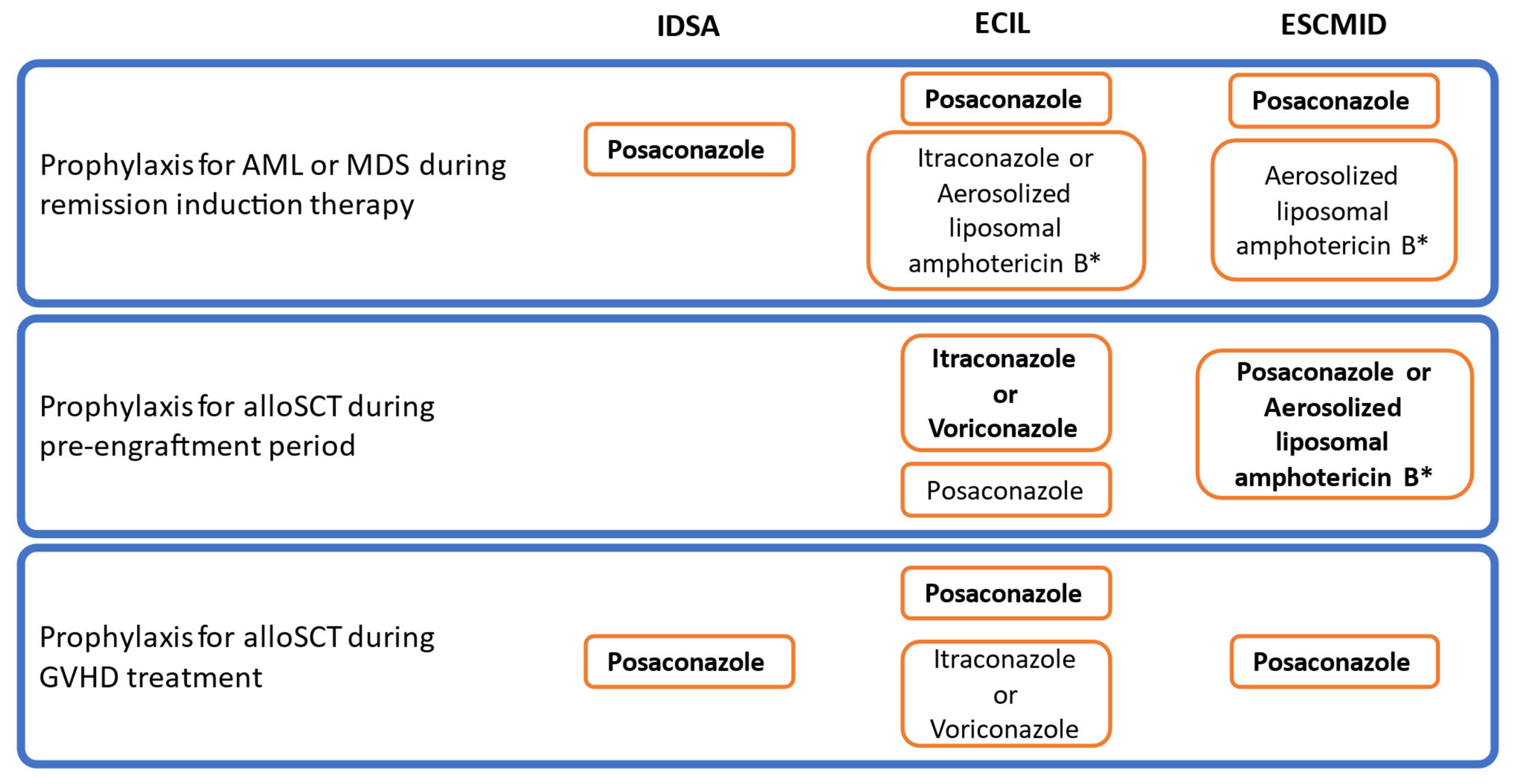

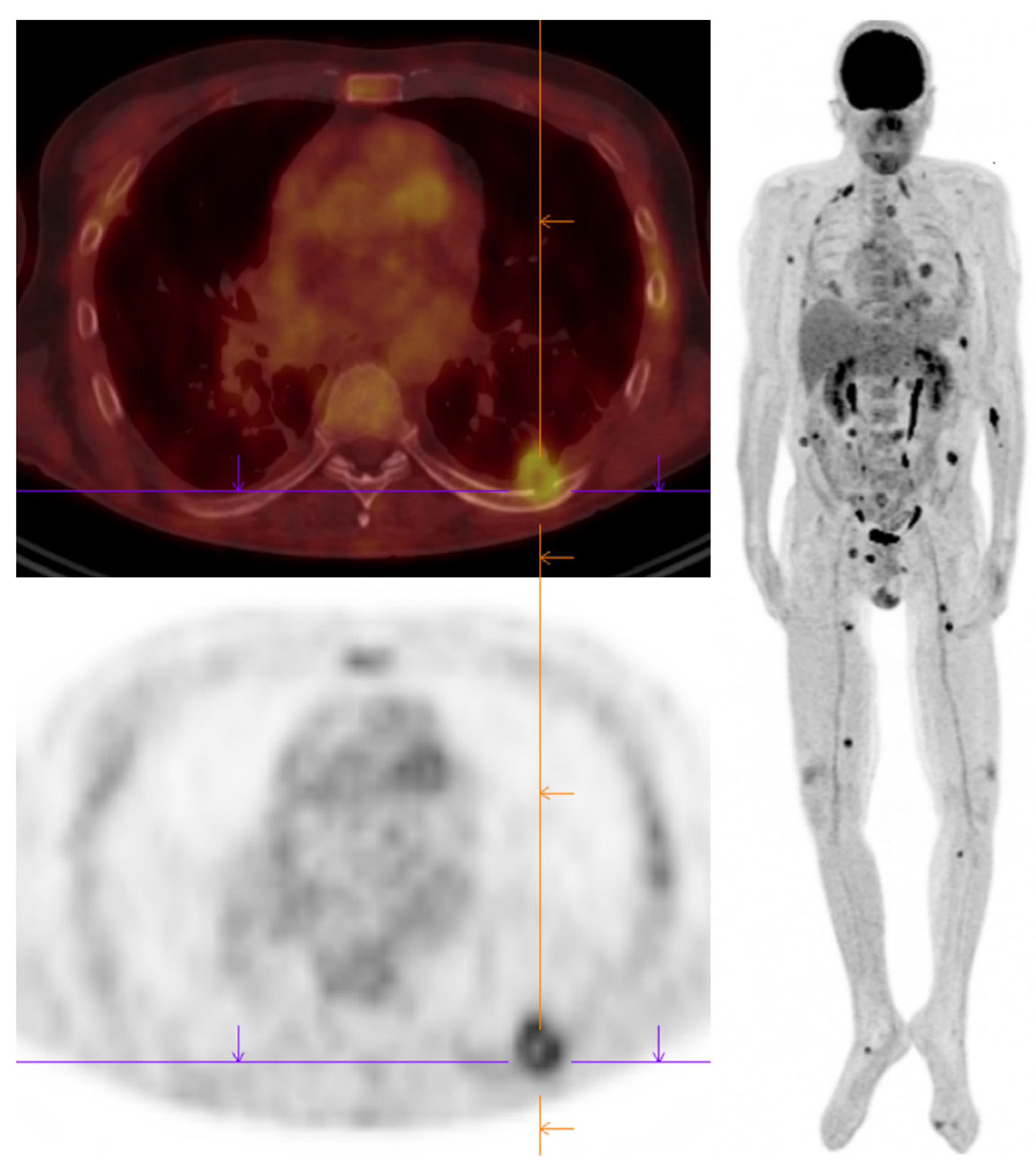
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hajjeh, R.A.; Warnock, D.W. Counterpoint: Invasive aspergillosis and the environment--rethinking our approach to prevention. Clin. Infect Dis. 2001, 33, 1549–1552. [Google Scholar] [CrossRef] [PubMed]
- Ledoux, M.P.; Guffroy, B.; Nivoix, Y.; Simand, C.; Herbrecht, R. Invasive pulmonary aspergillosis. Semin. Respir. Crit. Care Med. 2020, 41, 80–98. [Google Scholar] [CrossRef] [PubMed]
- Munoz, P.; Guinea, J.; Pelaez, T.; Duran, C.; Blanco, J.L.; Bouza, E. Nosocomial invasive aspergillosis in a heart transplant patient acquired during a break in the HEPA air filtration system. Transpl. Infect. Dis. 2004, 6, 50–54. [Google Scholar] [CrossRef]
- Panackal, A.A.; Li, H.; Kontoyiannis, D.P.; Mori, M.; Perego, C.A.; Boeckh, M.; Marr, K.A. Geoclimatic influences on invasive aspergillosis after hematopoietic stem cell transplantation. Clin. Infect. Dis. 2010, 50, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, T.; Munoz, P.; Guinea, J.; Valerio, M.; Giannella, M.; Klaassen, C.H.; Bouza, E. Outbreak of invasive aspergillosis after major heart surgery caused by spores in the air of the intensive care unit. Clin. Infect. Dis. 2012, 54, e24–e31. [Google Scholar] [CrossRef]
- Houbraken, J.; Kocsube, S.; Visagie, C.M.; Yilmaz, N.; Wang, X.C.; Meijer, M.; Kraak, B.; Hubka, V.; Bensch, K.; Samson, R.A.; et al. Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): An overview of families, genera, subgenera, sections, series and species. Stud. Mycol. 2020, 95, 5–169. [Google Scholar] [CrossRef]
- Lamoth, F. Aspergillus fumigatus-related species in clinical practice. Front. Microbiol. 2016, 7, 683. [Google Scholar] [CrossRef]
- Badiee, P.; Boekhout, T.; Mahmoudabadi, A.Z.; Mohammadi, R.; Mousavi, S.A.A.; Najafzadeh, M.J.; Soltani, J.; Hashemi, J.; Diba, K.; Ghadimi-Moghadam, A.; et al. Multicenter study of susceptibility of Aspergillus species isolated from Iranian university hospitals to seven antifungal agents. Microbiol. Spectr. 2022, 10, e0253921. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Chatterjee, S.S.; Das, A.; Shivaprakash, M.R. Invasive aspergillosis in developing countries. Med. Mycol. 2011, 49, S35–S47. [Google Scholar] [CrossRef]
- Walsh, T.J.; Wissel, M.C.; Grantham, K.J.; Petraitiene, R.; Petraitis, V.; Kasai, M.; Francesconi, A.; Cotton, M.P.; Hughes, J.E.; Greene, L.; et al. Molecular detection and species-specific identification of medically important Aspergillus species by real-time PCR in experimental invasive pulmonary aspergillosis. J. Clin. Microbiol. 2011, 49, 4150–4157. [Google Scholar] [CrossRef]
- Cui, L.; Morris, A.; Huang, L.; Beck, J.M.; Twigg, H.L., 3rd; von Mutius, E.; Ghedin, E. The microbiome and the lung. Ann Am Thorac. Soc. 2014, 11, S227–S232. [Google Scholar] [CrossRef] [PubMed]
- Lass-Florl, C.; Roilides, E.; Löffler, J.; Wilflingseder, D.; Romani, L. Minireview: Host defence in invasive aspergillosis. Mycoses 2013, 56, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Lu, R.-Y.; Wang, Y. Neutrophil extracellular traps in fungal infections: A seesaw battle in hosts. Front. Immunol. 2022, 13, 977493. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Lu, R.-Y.; Wang, Y. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef]
- Bongomin, F.; Lu, R.-Y.; Wang, Y. Global and multi-national prevalence of fungal diseases-estimate precision. J. Fungi. 2017, 3, 57. [Google Scholar] [CrossRef]
- Cornely, O.A.; Maertens, J.; Bresnik, M.; Ebrahimi, R.; Ullmann, A.J.; Bouza, E.; Heussel, C.P.; Lortholary, O.; Rieger, C.; Boehme, A.; et al. Liposomal amphotericin B as initial therapy for invasive mold infection: A randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin. Infect. Dis. 2007, 44, 1289–1297. [Google Scholar] [CrossRef]
- Herbrecht, R.; Denning, D.W.; Patterson, T.F.; Bennett, J.E.; Greene, R.E.; Oestmann, J.-W.; Kern, W.V.; Marr, K.A.; Ribaud, P.; Lortholary, O.; et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N. Engl. J. Med. 2002, 347, 408–415. [Google Scholar] [CrossRef]
- Cordonnier, C.; Pautas, C.; Maury, S.; Vekhoff, A.; Farhat, H.; Suarez, F.; Dhédin, N.; Isnard, F.; Ades, L.; Kuhnowski, F.; et al. Empirical versus preemptive antifungal therapy for high-risk, febrile, neutropenic patients: A randomized, controlled trial. Clin. Infect. Dis. 2009, 48, 1042–1051. [Google Scholar] [CrossRef]
- Leone, G.; Pagano, L. Infections in myelodysplastic syndrome in relation to stage and therapy. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018039. [Google Scholar] [CrossRef]
- On, S.; Rath, C.G.; Lan, M.; Wu, B.; Lau, K.M.; Cheung, E.; Alegria, W.; Young, R.; Tan, M.; Kim, C.; et al. Characterisation of infections in patients with acute myeloid leukaemia receiving venetoclax and a hypomethylating agent. Br. J. Haematol. 2022, 197, 63–70. [Google Scholar] [CrossRef]
- Maertens, J.A.; Girmenia, C.; Brüggemann, R.J.; Duarte, R.F.; Kibbler, C.C.; Ljungman, P.; Racil, Z.; Ribaud, P.; Slavin, M.; Cornely, A.O.; et al. European guidelines for primary antifungal prophylaxis in adult haematology patients: Summary of the updated recommendations from the European Conference on Infections in Leukaemia. J. Antimicrob. Chemother. 2018, 73, 3221–3230. [Google Scholar] [CrossRef]
- Maschmeyer, G.; Bullinger, L.; Garcia-Vidal, C.; Herbrecht, R.; Maertens, J.; Menna, P.; Pagano, L.; Thiebaut-Bertrand, A.; Calandra, T. Infectious complications of targeted drugs and biotherapies in acute leukemia. Clinical practice guidelines by the European Conference on Infections in Leukemia (ECIL), a joint venture of the European Group for Blood and Marrow Transplantation (EBMT), the European Organization for Research and Treatment of Cancer (EORTC), the International Immunocompromised Host Society (ICHS) and the European Leukemia Net (ELN). Leukemia 2022, 36, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Megias-Vericat, J.E.; Solana-Altabella, A.; Ballesta-López, O.; Martínez-Cuadrón, D.; Montesinos, P. Drug-drug interactions of newly approved small molecule inhibitors for acute myeloid leukemia. Ann. Hematol. 2020, 99, 1989–2007. [Google Scholar] [CrossRef]
- Stemler, J.; de Jonge, N.; Skoetz, N.; Sinkó, J.; Brüggemann, R.J.; Busca, A.; Ben-Ami, R.; Ráčil, Z.; Piechotta, V.; Lewis, R.; et al. Antifungal prophylaxis in adult patients with acute myeloid leukaemia treated with novel targeted therapies: A systematic review and expert consensus recommendation from the European Hematology Association. Lancet Haematol. 2022, 9, e361–e373. [Google Scholar] [CrossRef]
- Herbrecht, R.; Moghaddam, A.; Mahmal, L.; Natarajan-Ame, S.; Fornecker, L.-M.; Letscher-Bru, V. Invasive aspergillosis in the hematologic and immunologic patient: New findings and key questions in leukemia. Med. Mycol. 2005, 43, S239–S242. [Google Scholar] [CrossRef]
- Stafylidis, C.; Diamantopoulos, P.; Athanasoula, E.; Solomou, E.; Anastasopoulou, A. Acute lymphoblastic leukemia and invasive mold infections: A challenging field. J. Fungi. Basel 2022, 8, 1127. [Google Scholar] [CrossRef]
- Di Blasi, R.; Cattaneo, C.; Lewis, R.E.; Tumbarello, M.; Angelici, L.; Dragonetti, G.; Busca, A.; Cambo, B.; Candoni, A.; Cesarini, M.; et al. Febrile events in acute lymphoblastic leukemia: A prospective observational multicentric SEIFEM study (SEIFEM-2012/B ALL). Ann. Hematol. 2018, 97, 791–798. [Google Scholar] [CrossRef]
- Colombo, A.L.; Bergamasco, M.D.; Nouér, S.A.; Castro, P.D.T.O.E.; Pasqualotto, A.C.; de Queiroz-Telles, F.; Abdala, E.; Ramos, J.F.; Falci, D.R.; Nucci, M. Invasive aspergillosis in patients with acute leukemia: Comparison between acute myeloid and acute lymphoid leukemia. Mycopathologia 2022. [Google Scholar] [CrossRef]
- Wang, S.S.; Kotecha, R.S.; Bernard, A.; Blyth, C.C.; McMullan, B.J.; Cann, M.P.; Yeoh, D.; Bartlett, A.W.; Ryan, A.L.; Moore, A.; et al. Invasive fungal infections in children with acute lymphoblastic leukaemia: Results from four Australian centres, 2003–2013. Pediatr. Blood Cancer 2019, 66, e27915. [Google Scholar] [CrossRef]
- Kyriakidis, I.; Vasileiou, E.; Rossig, C.; Roilides, E.; Groll, A.; Tragiannidis, A. Invasive fungal diseases in children with hematological malignancies treated with therapies that target cell surface antigens: Monoclonal antibodies, immune checkpoint inhibitors and CAR T-cell therapies. J. Fungi Basel 2021, 7, 186. [Google Scholar] [CrossRef]
- Mikulska, M.; Lanini, S.; Gudiol, C.; Drgona, L.; Ippolito, G.; Fernández-Ruiz, M.; Salzberger, B. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) consensus document on the safety of targeted and biological therapies: An infectious diseases perspective (Agents targeting lymphoid cells surface antigens [I]: CD19, CD20 and CD52). Clin. Microbiol. Infect. 2018, 24, S71–S82. [Google Scholar] [CrossRef] [PubMed]
- So, W.; Pandya, S.; Quilitz, R.; Greene, J.N. Infectious risks and complications in adult leukemic patients receiving blinatumomab. Mediterr. J. Hematol. Infect. Dis. 2018, 10, e2018029. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Lionakis, M.S.; Kontoyiannis, D.P. Call for action: Invasive fungal infections associated with ibrutinib and other small molecule kinase inhibitors targeting immune signaling pathways. Clin. Infect. Dis. 2018, 66, 140–148. [Google Scholar] [CrossRef]
- Little, J.S.; Weiss, Z.F.; Hammond, S.P. Invasive fungal infections and targeted therapies in hematological malignancies. J. Fungi. 2021, 7, 58. [Google Scholar] [CrossRef]
- Blez, D.; Blaize, M.; Soussain, C.; Boissonnas, A.; Meghraoui-Kheddar, A.; Menezes, N.; Portalier, A.; Combadière, C.; Leblond, V.; Ghez, D.; et al. Ibrutinib induces multiple functional defects in the neutrophil response against Aspergillus fumigatus. Haematologica 2019, 105, 478–489. [Google Scholar] [CrossRef]
- Ghez, D.; Calleja, A.; Protin, C.; Baron, M.; LeDoux, M.-P.; Damaj, G.; Dupont, M.; Dreyfus, B.; Ferrant, E.; Herbaux, C.; et al. Early-onset invasive aspergillosis and other fungal infections in patients treated with ibrutinib. Blood 2018, 131, 1955–1959. [Google Scholar] [CrossRef]
- Varughese, T.; Taur, Y.; Cohen, N.; Palomba, M.L.; Seo, S.K.; Hohl, T.M.; Redelman-Sidi, G. Serious infections in patients receiving ibrutinib for treatment of lymphoid cancer. Clin. Infect. Dis. 2018, 67, 687–692. [Google Scholar] [CrossRef]
- Pagano, L.; Caira, M.; Candoni, A.; Offidani, M.; Fianchi, L.; Martino, B.; Pastore, D.; Picardi, M.; Bonini, A.; Chierichini, A.; et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 2006, 91, 1068–1075. [Google Scholar]
- Tisi, M.C.; Hohaus, S.; Cuccaro, A.; Innocenti, I.; de Carolis, E.; Za, T.; D’Alò, F.; Laurenti, L.; Fianchi, L.; Sica, S.; et al. Invasive fungal infections in chronic lymphoproliferative disorders: A monocentric retrospective study. Haematologica 2017, 102, e108–e111. [Google Scholar] [CrossRef]
- Lim, C.; Sinha, P.; Harrison, S.J.; Quach, H.; Slavin, M.A.; Teh, B.W. Low rates of invasive fungal disease in patients with multiple myeloma managed with new generation therapies: Results from a multi-centre cohort study. Mycoses 2021, 64, 30–34. [Google Scholar] [CrossRef]
- Tsai, C.K.; Liu, Y.-C.; Kuan, A.S.; Lee, K.-L.; Yeh, C.-M.; Lee, Y.-T.; Hsiao, L.-T.; Ko, P.-S.; Wang, H.-Y.; Chen, P.-M.; et al. Risk and impact of invasive fungal infections in patients with multiple myeloma. Ann. Hematol. 2020, 99, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Jantunen, E.; Ruutu, P.; Niskanen, L.; Volin, L.; Parkkali, T.; Koukila-Kähkölä, P.; Ruutu, T. Incidence and risk factors for invasive fungal infections in allogeneic BMT recipients. Bone Marrow Transplant. 1997, 19, 801–808. [Google Scholar] [CrossRef]
- Robin, C.; Cordonnier, C.; Sitbon, K.; Raus, N.; Lortholary, O.; Maury, S.; de la Tour, R.P.; Bretagne, S.; Bastuji-Garin, S. Mainly post-transplant factors are associated with invasive aspergillosis after allogeneic stem cell transplantation: A study from the Surveillance des Aspergilloses Invasives en France and Société Francophone de Greffe de Moelle et de Thérapie Cellulaire. Biol. Blood Marrow Transplant. 2019, 25, 354–361. [Google Scholar] [CrossRef]
- Ballen, K.; Ahn, K.W.; Chen, M.; Abdel-Azim, H.; Ahmed, I.; Aljurf, M.; Antin, J.; Bhatt, A.S.; Boeckh, M.; Chen, G.; et al. Infection rates among acute leukemia patients receiving alternative donor hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2016, 22, 1636–1645. [Google Scholar] [CrossRef]
- Liberatore, C.; Farina, F.; Greco, R.; Giglio, F.; Clerici, D.; Oltolini, C.; Stanghellini, M.L.; Barzaghi, F.; Vezzulli, P.; Orsenigo, E.; et al. Breakthrough invasive fungal infections in allogeneic hematopoietic stem cell transplantation. J. Fungi. Basel 2021, 7, 347. [Google Scholar] [CrossRef]
- Gudiol, C.; Lewis, E.R.; Strati, P.; Kontoyiannis, D.P. Chimeric antigen receptor T-cell therapy for the treatment of lymphoid malignancies: Is there an excess risk for infection? Lancet Haematol. 2021, 8, e216–e228. [Google Scholar] [CrossRef]
- Garner, W.; Samanta, P.; Haidar, G. Invasive fungal infections after anti-CD19 chimeric antigen receptor-modified T-cell therapy: State of the evidence and future directions. J. Fungi. 2021, 7, 156. [Google Scholar] [CrossRef]
- Hill, J.A.; Li, D.; Hay, K.; Green, M.L.; Cherian, S.; Chen, X.; Riddell, S.R.; Maloney, D.G.; Boeckh, M.; Turtle, C.J. Turtle. Infectious complications of CD19-targeted chimeric antigen receptor-modified T-cell immunotherapy. Blood 2018, 131, 121–130. [Google Scholar] [CrossRef]
- Park, J.H.; Romero, F.A.; Taur, Y.; Sadelain, M.; Brentjens, R.J.; Hohl, T.M.; Seo, S.K. Cytokine release syndrome grade as a predictive marker for infections in patients with relapsed or refractory B-cell acute lymphoblastic leukemia treated with chimeric antigen receptor T cells. Clin. Infect. Dis. 2018, 67, 533–540. [Google Scholar] [CrossRef]
- Kaur, R.; Mehra, B.; Dhakad, M.S.; Goyal, R.; Dewan, R. Pulmonary aspergillosis as opportunistic mycoses in a cohort of human immunodeficiency virus-infected patients: Report from a tertiary care hospital in North India. Int. J. Health Sci. Qassim 2017, 11, 45–50. [Google Scholar]
- Holding, K.J.; Dworkin, M.S.; Wan, P.-C.T.; Hanson, D.L.; Klevens, R.M.; Jones, J.L.; Sullivan, P.S. Aspergillosis among people infected with human immunodeficiency virus: Incidence and survival. Adult and Adolescent Spectrum of HIV Disease Project. Clin. Infect. Dis. 2000, 31, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Barlam, T.F.; Flanigan, T.; Rich, J.D. Pulmonary aspergillosis and invasive disease in AIDS: Review of 342 cases. Chest 1998, 114, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Roos, D. Chronic granulomatous disease. Br. Med. Bull. 2016, 118, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Blumental, S.; Mouy, R.; Mahlaoui, N.; Bougnoux, M.-E.; Debré, M.; Beauté, J.; Lortholary, O.; Blanche, S.; Fischer, A. Invasive mold infections in chronic granulomatous disease: A 25-year retrospective survey. Clin. Infect. Dis. 2011, 53, e159–e169. [Google Scholar] [CrossRef] [PubMed]
- Bochud, P.Y.; Chien, J.W.; Marr, K.A.; Leisenring, W.M.; Upton, A.; Janer, M.; Rodrigues, S.D.; Li, S.; Hansen, J.A.; Zhao, L.P.; et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl. J. Med. 2008, 359, 1766–1777. [Google Scholar] [CrossRef]
- Sainz, J.; Lupiáñez, C.B.; Segura-Catena, J.; Vazquez, L.; Ríos, R.; Oyonarte, S.; Hemminki, K.; Försti, A.; Jurado, M. Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection. PLoS ONE 2012, 7, e32273. [Google Scholar] [CrossRef]
- Lambourne, J.; Agranoff, D.; Herbrecht, R.; Troke, P.F.; Buchbinder, A.; Willis, F.; Letscher-Bru, V.; Agrawal, S.; Doffman, S.; Johnson, E.; et al. Association of mannose-binding lectin deficiency with acute invasive aspergillosis in immunocompromised patients. Clin. Infect. Dis. 2009, 49, 1486–1491. [Google Scholar] [CrossRef]
- White, P.L.; Price, J.S. Incorporating the detection of single nucleotide polymorphisms associated with invasive aspergillosis into the clinic. Front Cell Infect Microbiol. 2022, 12, 860779. [Google Scholar] [CrossRef]
- Tang, T.; Dai, Y.; Zeng, Q.; Bu, S.; Huang, B.; Xiao, Y.; Wei, Z.; Lin, X.; Huang, L.; Jiang, S. Pentraxin-3 polymorphisms and pulmonary fungal disease in non-neutropenic patients. Ann. Transl. Med. 2020, 8, 1142. [Google Scholar] [CrossRef]
- Pappas, P.G.; Alexander, B.D.; Andes, D.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Luong, M.L.; Chaparro, C.; Stephenson, A.; Rotstein, C.; Singer, L.G.; Waters, V.; Azad, S.; Keshavjee, S.; Tullis, E.; Husain, S. Pretransplant Aspergillus colonization of cystic fibrosis patients and the incidence of post-lung transplant invasive aspergillosis. Transplantation 2014, 97, 351–357. [Google Scholar] [CrossRef]
- Husain, S.; Bhaskaran, A.; Rotstein, C.; Li, Y.; Bhimji, A.; Pavan, R.; Kumar, D.; Humar, A.; Keshavjee, S.; Singer, L.G. A strategy for prevention of fungal infections in lung transplantation: Role of bronchoalveolar lavage fluid galactomannan and fungal culture. J. Heart Lung Transplant. 2018, 37, 886–894. [Google Scholar] [CrossRef]
- Peghin, M.; Monforte, V.; Martin-Gomez, M.-T.; Ruiz-Camps, I.; Berastegui, C.; Saez, B.; Riera, J.; Ussetti, P.; Solé, J.; Gavaldá, J.; et al. 10 years of prophylaxis with nebulized liposomal amphotericin B and the changing epidemiology of Aspergillus spp. infection in lung transplantation. Transpl. Int. 2016, 29, 51–62. [Google Scholar] [CrossRef]
- Bhaskaran, A.; Mumtaz, K.; Husain, S. Anti-Aspergillus prophylaxis in lung transplantation: A systematic review and meta-analysis. Curr. Infect. Dis. Rep. 2013, 15, 514–525. [Google Scholar] [CrossRef]
- He, S.Y.; Makhzoumi, Z.; Singer, J.; Chin-Hong, P.; Arron, S. Practice variation in Aspergillus prophylaxis and treatment among lung transplant centers: A national survey. Transpl. Infect Dis. 2015, 17, 14–20. [Google Scholar] [CrossRef]
- Minari, A.; Husni, R.; Avery, R.; Longworth, D.; DeCamp, M.; Bertin, M.; Schilz, R.; Smedira, N.; Haug, M.; Mehta, A.; et al. The incidence of invasive aspergillosis among solid organ transplant recipients and implications for prophylaxis in lung transplants. Transpl. Infect Dis. 2002, 4, 195–200. [Google Scholar] [CrossRef]
- Dandachi, D.; Dib, R.W.; Fernández-Cruz, A.; Jiang, Y.; Chaftari, A.-M.; Hachem, R.; Raad, I. Invasive pulmonary aspergillosis in patients with solid tumours: Risk factors and predictors of clinical outcomes. Ann. Med. 2018, 50, 713–720. [Google Scholar] [CrossRef]
- Yan, X.; Li, M.; Jiang, M.; Zou, L.-Q.; Luo, F.; Jiang, Y. Clinical characteristics of 45 patients with invasive pulmonary aspergillosis: Retrospective analysis of 1711 lung cancer cases. Cancer 2009, 115, 5018–5025. [Google Scholar] [CrossRef]
- Park, S.; Park, S.; Choi, H.; Park, J.Y.; Lim, H.S.; Park, M.J.; Kim, S.-Y. Invasive pulmonary aspergillosis in a patient with metastatic non-small cell lung cancer after treatment with gefitinib. Korean J. Int. Med. 2018, 33, 211–213. [Google Scholar] [CrossRef]
- Williams, J.; Lim, R.; Tambyah, P. Invasive aspergillosis associated with bevacizumab, a vascular endothelial growth factor inhibitor. Int. J. Infect. Dis. 2007, 11, 549–550. [Google Scholar] [CrossRef]
- Johnson, D.H.; Fehrenbacher, L.; Novotny, W.F.; Herbst, R.S.; Nemunaitis, J.J.; Jablons, D.M.; Langer, C.J.; Devore, R.F.; Gaudreault, J.; Damico, L.A.; et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol. 2004, 22, 2184–2191. [Google Scholar] [CrossRef] [PubMed]
- Iijima, Y.; Fujioka, N.; Uchida, Y.; Kobayashi, Y.; Tsutsui, T.; Kakizaki, Y.; Miyashita, Y. Invasive pulmonary aspergillosis mimicking organizing pneumonia after mTOR inhibitor therapy: A case report. Int. J. Infect. Dis. 2018, 69, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Inthasot, V.; Bruyneel, M.; Muylle, I.; Ninane, V. Severe pulmonary infections complicating nivolumab treatment for lung cancer: A report of two cases. Acta Clin. Belg. 2020, 75, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Oltolini, C.; Ripa, M.; Andolina, A.; Brioschi, E.; Cilla, M.; Petrella, G.; Gregorc, V.; Castiglioni, B.; Din, C.T.; Scarpellini, P. Invasive pulmonary aspergillosis complicated by carbapenem-resistant Pseudomonas aeruginosa infection during pembrolizumab immunotherapy for metastatic lung adenocarcinoma: Case report and review of the literature. Mycopathologia 2019, 184, 181–185. [Google Scholar] [CrossRef]
- Abers, M.S.; Lionakis, M.S.; Kontoyiannis, D.P. Checkpoint inhibition and infectious diseases: A good thing? Trends Mol. Med. 2019, 25, 1080–1093. [Google Scholar] [CrossRef]
- Del Castillo, M.; Romero, F.A.; Argüello, E.; Kyi, C.; Postow, M.A.; Redelman-Sidi, G. The spectrum of serious infections among patients receiving imune checkpoint blockade for the treatment of melanoma. Clin. Infect. Dis. 2016, 63, 1490–1493. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Frantzeskaki, F.; Poulakou, G.; Armaganidis, A. Invasive aspergillosis in the intensive care unit. Ann. N. Y. Acad. Sci. 2012, 1272, 31–39. [Google Scholar] [CrossRef]
- Meersseman, W.; Vandecasteele, S.J.; Wilmer, A.; Verbeken, E.; Peetermans, W.E.; Van Wijngaerden, E. Invasive aspergillosis in critically ill patients without malignancy. Am. J. Respir. Crit. Care Med. 2004, 170, 621–625. [Google Scholar] [CrossRef]
- Jenks, J.D.; Nam, H.H.; Hoenigl, M. Invasive aspergillosis in critically ill patients: Review of definitions and diagnostic approaches. Mycoses 2021, 64, 1002–1014. [Google Scholar] [CrossRef]
- Hartemink, K.J.; Paul, M.A.; Spijkstra, J.J.; Girbes, A.R.J.; Polderman, K.H. Immunoparalysis as a cause for invasive aspergillosis? Intensive Care Med. 2003, 29, 2068–2071. [Google Scholar] [CrossRef]
- Falcone, M.; Massetti, A.P.; Venditti, M.; Russo, A.; Vullo, V. Invasive aspergillosis in patients with liver disease. Med. Mycol. 2011, 49, 406–413. [Google Scholar] [CrossRef]
- Gustot, T.; Maillart, E.; Bocci, M.; Surin, R.; Trépo, E.; Degré, D.; Lucidi, V.; Taccone, F.S.; Delforge, M.-L.; Vincent, J.-L.; et al. Invasive aspergillosis in patients with severe alcoholic hepatitis. J. Hepatol. 2014, 60, 267–274. [Google Scholar] [CrossRef]
- Schauwvlieghe, A.F.A.D.; Rijnders, B.J.A.; Philips, N.; Verwijs, R.; Vanderbeke, L.; Van Tienen, C.; Lagrou, K.; Verweij, P.E.; Van De Veerdonk, F.L.; Gommers, D.; et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: A retrospective cohort study. Lancet Respir. Med. 2018, 6, 782–792. [Google Scholar] [CrossRef]
- Sun, K.; Metzger, D.W. Influenza infection suppresses NADPH oxidase-dependent phagocytic bacterial clearance and enhances susceptibility to secondary methicillin-resistant Staphylococcus aureus infection. J. Immunol. 2014, 192, 3301–3307. [Google Scholar] [CrossRef]
- Wauters, J.; Baar, I.; Meersseman, P.; Meersseman, W.; Dams, K.; De Paep, R.; Lagrou, K.; Wilmer, A.; Jorens, P.; Hermans, G. Invasive pulmonary aspergillosis is a frequent complication of critically ill H1N1 patients: A retrospective study. Intensive Care Med. 2012, 38, 1761–1768. [Google Scholar] [CrossRef]
- Koehler, P.; Bassetti, M.; Chakrabarti, A.; Chen, S.C.A.; Colombo, A.L.; Hoenigl, M.; Klimko, N.; Lass-Flörl, C.; Oladele, R.O.; Vinh, D.C.; et al. Defining and managing COVID-19-associated pulmonary aspergillosis: The 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect. Dis. 2021, 21, e149–e162. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Dannaoui, E.; Fekkar, A.; Luyt, C.-E.; Botterel, F.; De Prost, N.; Tadié, J.-M.; Reizine, F.; Houzé, S.; Timsit, J.-F.; et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: The French multicentre MYCOVID study. Lancet Respir. Med. 2022, 10, 180–190. [Google Scholar] [CrossRef]
- Fekkar, A.; Lampros, A.; Mayaux, J.; Poignon, C.; Demeret, S.; Constantin, J.-M.; Marcelin, A.-G.; Monsel, A.; Luyt, C.-E.; Blaize, M. Occurrence of invasive pulmonary fungal infections in patients with severe COVID-19 admitted to the ICU. Am. J. Respir. Crit. Care Med. 2021, 203, 307–317. [Google Scholar] [CrossRef]
- Pruthi, H.S. When to initiate antifungal treatment in COVID-19 patients with secondary fungal co-infection. Curr. Clin. Microbiol. Rep. 2022, 9, 60–68. [Google Scholar] [CrossRef]
- Nedel, W.L.; Kontoyiannis, D.P.; Pasqualotto, A.C. Aspergillosis in patients treated with monoclonal antibodies. Rev. Iberoam. Micol. 2009, 26, 175–183. [Google Scholar] [CrossRef]
- Salmon-Ceron, D.; Tubach, F.; Lortholary, O.; Chosidow, O.; Bretagne, S.; Nicolas, N.; Cuillerier, E.; Fautrel, B.; Michelet, C.; Morel, J.; et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann. Rheum. Dis. 2011, 70, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.L.; Liao, H.T.; Chen, W.S.; Chen, M.H.; Lai, C.C.; Tsai, C.Y.; Chang, D.M. Invasive aspergillosis in patients with systemic lupus erythematosus: A retrospective study on clinical characteristics and risk factors for mortality. Lupus 2018, 27, 1944–1952. [Google Scholar] [CrossRef] [PubMed]
- Vallabhaneni, S.; Chiller, T.M. Fungal infections and new biologic therapies. Curr. Rheumatol. Rep. 2016, 18, 29. [Google Scholar] [CrossRef] [PubMed]
- Akan, H.; Antia, V.P.; Kouba, M.; Sinko, J.; Tănase, A.D.; Vrhovac, R.; Herbrecht, R. Preventing invasive fungal disease in patients with haematological malignancies and the recipients of haematopoietic stem cell transplantation: Practical aspects. J. Antimicrob. Chemother. 2013, 68, iii5–iii16. [Google Scholar] [CrossRef]
- Benedict, K.; Thompson, G.R., 3rd; Jackson, B.R. Cannabis use and fungal infections in a commercially insured population, United States, 2016. Emerg Infect Dis. 2020, 26, 1308–1310. [Google Scholar] [CrossRef]
- Herbrecht, R.; Bories, P.; Moulin, J.C.; Ledoux, M.P.; Letscher-Bru, V. Risk stratification for invasive aspergillosis in immunocompromised patients. Ann. N. Y. Acad. Sci. 2012, 1272, 23–30. [Google Scholar] [CrossRef]
- Pagano, L.; Akova, M.; Dimopoulos, G.; Herbrecht, R.; Drgona, L.; Blijlevens, N. Risk assessment and prognostic factors for mould-related diseases in immunocompromised patients. J. Antimicrob. Chemother. 2011, 66, i5–i14. [Google Scholar] [CrossRef]
- Patterson, T.F.; Thompson, G.R., III.; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24, e1–e38. [Google Scholar] [CrossRef]
- Bergeron, A.; Porcher, R.; Sulahian, A.; De Bazelaire, C.; Chagnon, K.; Raffoux, E.; Vekhoff, A.; Cornet, M.; Isnard, F.; Brethon, B.; et al. The strategy for the diagnosis of invasive pulmonary aspergillosis should depend on both the underlying condition and the leukocyte count of patients with hematologic malignancies. Blood 2012, 119, 1831–1837. [Google Scholar] [CrossRef]
- Greene, R.E.; Schlamm, H.T.; Oestmann, J.-W.; Stark, P.; Durand, C.; Lortholary, O.; Wingard, J.R.; Herbrecht, R.; Ribaud, P.; Patterson, T.F.; et al. Imaging findings in acute invasive pulmonary aspergillosis: Clinical significance of the halo sign. Clin. Infect. Dis. 2007, 44, 373–379. [Google Scholar] [CrossRef]
- Prasad, A.; Agarwal, K.; Deepak, D.; Atwal, S.S. Pulmonary aspergillosis: What CT can offer before it is too late! J. Clin. Diagn. Res. 2016, 10, TE01–TE05. [Google Scholar] [CrossRef]
- Munoz, P.; Vena, A.; Cerón, I.; Valerio, M.; Palomo, J.; Guinea, J.; Escribano, P.; Martínez-Sellés, M.; Bouza, E.; Promulga Project Group. Invasive pulmonary aspergillosis in heart transplant recipients: Two radiologic patterns with a different prognosis. J. Heart Lung Transplant. 2014, 33, 1034–1040. [Google Scholar] [CrossRef]
- Herbrecht, R.; Guffroy, B.; Danion, F.; Venkatasamy, A.; Simand, C.; LeDoux, M.-P. Validation by real-life data of the new radiological criteria of the revised and updated consensus definition for invasive fungal diseases. Clin. Infect. Dis. 2020, 71, 2773–2774. [Google Scholar] [CrossRef]
- Jin, J.; Wu, D.; Liu, Y.; Pan, S.; Yan, J.L.; Aram, J.A.; Lou, Y.-J.; Meng, H.; Chen, X.; Zhang, X.; et al. Utility of CT assessment in hematology patients with invasive aspergillosis: A post-hoc analysis of phase 3 data. BMC Infect. Dis. 2019, 19, 471. [Google Scholar] [CrossRef]
- Chamilos, G.; Macapinlac, H.A.; Kontoyiannis, D.P. The use of 18F-fluorodeoxyglucose positron emission tomography for the diagnosis and management of invasive mould infections. Med. Mycol. 2008, 46, 23–29. [Google Scholar] [CrossRef]
- Leroy-Freschini, B.; Treglia, G.; Argemi, X.; Bund, C.; Kessler, R.; Herbrecht, R.; Imperiale, A. 18F-FDG PET/CT for invasive fungal infection in immunocompromised patients. QJM 2018, 111, 613–622. [Google Scholar] [CrossRef]
- Fernandez-Cruz, A.; Lewis, E.R.; Kontoyiannis, D.P. How long do we need to treat an invasive mold disease in hematology patients? Factors influencing duration of therapy and future questions. Clin. Infect Dis. 2020, 71, 685–692. [Google Scholar] [CrossRef]
- Shannon, V.R.; Andersson, B.S.; Lei, X.; Champlin, E.R.; Kontoyiannis, D.P. Utility of early versus late fiberoptic bronchoscopy in the evaluation of new pulmonary infiltrates following hematopoietic stem cell transplantation. Bone Marrow. Transplant. 2010, 45, 647–655. [Google Scholar] [CrossRef]
- Danion, F.; Duval, C.; Séverac, F.; Bachellier, P.; Candolfi, E.; Castelain, V.; Clere-Jehl, R.; Denis, J.; Dillenseger, L.; Epailly, E.; et al. Factors associated with coinfections in invasive aspergillosis: A retrospective cohort study. Clin. Microbiol. Infect. 2021, 27, 1644–1651. [Google Scholar] [CrossRef]
- Hardak, E.; Avivi, I.; Berkun, L.; Raz-Pasteur, A.; Lavi, N.; Geffen, Y.; Yigla, M.; Oren, I. Polymicrobial pulmonary infection in patients with hematological malignancies: Prevalence, co-pathogens, course and outcome. Infection 2016, 44, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Krenke, R.; Kołkowska-Leśniak, A.; Pałynyczko, G.; Prochorec-Sobieszek, M.; Konopka, L. Ulcerative and pseudomembranous Aspergillus tracheobronchitis in a patient with acute myeloid leukemia. Int. J. Hematol. 2009, 89, 257–258. [Google Scholar] [CrossRef] [PubMed]
- Helmi, M.; Love, R.B.; Welter, D.; Cornwell, R.D.; Meyer, K.C. Aspergillus infection in lung transplant recipients with cystic fibrosis: Risk factors and outcomes comparison to other types of transplant recipients. Chest 2003, 123, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Kontoyiannis, D.P.; Sumoza, D.; Tarrand, J.; Bodey, G.P.; Storey, R.; Raad, I.I. Significance of aspergillemia in patients with cancer: A 10-year study. Clin. Infect. Dis. 2000, 31, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Lass-Florl, C. How to make a fast diagnosis in invasive aspergillosis. Med. Mycol. 2019, 57, S155–S160. [Google Scholar] [CrossRef]
- Chalupova, J.; Raus, M.; Sedlářová, M.; Šebela, M. Identification of fungal microorganisms by MALDI-TOF mass spectrometry. Biotechnol. Adv. 2014, 32, 230–241. [Google Scholar] [CrossRef]
- Richardson, M.D.; Page, I.D. Aspergillus serology: Have we arrived yet? Med. Mycol. 2017, 55, 48–55. [Google Scholar] [CrossRef]
- Herbrecht, R.; Letscher-Bru, V.; Oprea, C.; Lioure, B.; Waller, J.; Campos, F.; Villard, O.; Liu, K.-L.; Natarajan-Amé, S.; Lutz, P.; et al. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J. Clin. Oncol. 2002, 20, 1898–1906. [Google Scholar] [CrossRef]
- Marchetti, O.; Lamoth, F.; Mikulska, M.; Viscoli, C.; Verweij, P.; Bretagne, S. ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow. Transplant. 2012, 47, 846–854. [Google Scholar] [CrossRef]
- Li, C.; Sun, L.; Liu, Y.; Zhou, H.; Chen, J.; She, M.; Wang, Y. Diagnostic value of bronchoalveolar lavage fluid galactomannan assay for invasive pulmonary aspergillosis in adults: A meta-analysis. J. Clin. Pharm. Ther. 2022, 47, 1913–1922. [Google Scholar] [CrossRef]
- Pfeiffer, C.D.; Fine, J.P.; Safdar, N. Diagnosis of invasive aspergillosis using a galactomannan assay: A meta-analysis. Clin. Infect. Dis. 2006, 42, 1417–1427. [Google Scholar] [CrossRef]
- Miceli, M.H.; Maertens, J. Role of non-culture-based tests, with an emphasis on galactomannan testing for the diagnosis of invasive aspergillosis. Semin. Respir. Crit. Care Med. 2015, 36, 650–661. [Google Scholar] [CrossRef]
- Chai, L.Y.; Kullberg, B.-J.; Johnson, E.M.; Teerenstra, S.; Khin, L.W.; Vonk, A.G.; Maertens, J.; Lortholary, O.; Donnelly, P.J.; Schlamm, H.T.; et al. Early serum galactomannan trend as a predictor of outcome of invasive aspergillosis. J. Clin. Microbiol. 2012, 50, 2330–2336. [Google Scholar] [CrossRef]
- Lamoth, F. Galactomannan and 1,3-beta-d-glucan testing for the diagnosis of invasive aspergillosis. J. Fungi. Basel 2016, 2, 22. [Google Scholar] [CrossRef]
- Dichtl, K.; Forster, J.; Ormanns, S.; Horns, H.; Suerbaum, S.; Seybold, U.; Wagener, J. Comparison of beta-D-glucan and galactomannan in serum for detection of invasive aspergillosis: Retrospective analysis with focus on early diagnosis. J. Fungi. Basel 2020, 6, 253. [Google Scholar] [CrossRef]
- Rose, S.R.; Vallabhajosyula, S.; Velez, M.G.; Fedorko, D.P.; VanRaden, M.J.; Gea-Banacloche, J.C.; Lionakis, M.S. The utility of bronchoalveolar lavage beta-D-glucan testing for the diagnosis of invasive fungal infections. J. Infect. 2014, 69, 278–283. [Google Scholar] [CrossRef]
- Barnes, R.A.; White, P.L.; Morton, C.O.; Rogers, T.R.; Cruciani, M.; Loeffler, J.; Donnelly, J.P. Diagnosis of aspergillosis by PCR: Clinical considerations and technical tips. Med. Mycol. 2018, 56, 60–72. [Google Scholar] [CrossRef]
- Patterson, T.F.; Donnelly, J.P. New concepts in diagnostics for invasive mycoses: Non-culture-based methodologies. J. Fungi. Basel 2019, 5, 9. [Google Scholar] [CrossRef]
- White, P.L.; Barnes, R.A.; Springer, J.; Klingspor, L.; Cuenca-Estrella, M.; Morton, C.O.; Lagrou, K.; Bretagne, S.; Melchers, W.J.G.; Mengoli, C.; et al. Clinical performance of Aspergillus PCR for testing serum and plasma: A study by the European Aspergillus PCR Initiative. J. Clin. Microbiol. 2015, 53, 2832–2837. [Google Scholar] [CrossRef]
- Chong, G.L.; Van De Sande, W.W.J.; Dingemans, G.J.H.; Gaajetaan, G.R.; Vonk, A.G.; Hayette, M.-P.; Van Tegelen, D.W.E.; Simons, G.F.M.; Rijnders, B.J.A. Validation of a new Aspergillus real-time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J. Clin. Microbiol. 2015, 53, 868–874. [Google Scholar] [CrossRef]
- Denis, J.; Forouzanfar, F.; Herbrecht, R.; Toussaint, E.; Kessler, R.; Sabou, M.; Candolfi, E.; Letsher-Bru, V. Evaluation of two commercial real-time PCR kits for Aspergillus DNA detection in bronchoalveolar lavage fluid in patients with invasive pulmonary aspergillosis. J. Mol. Diagn. 2018, 20, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Paterson, P.J.; Seaton, S.; McLaughlin, J.; Kibbler, C.C. Development of molecular methods for the identification of Aspergillus and emerging moulds in paraffin wax embedded tissue sections. Mol. Pathol. MP 2003, 56, 368–370. [Google Scholar] [CrossRef] [PubMed]
- Rath, P.M.; Steinmann, J. Overview of commercially available PCR assays for the detection of Aspergillus spp. DNA in patient samples. Front. Microbiol. 2018, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Pelzer, B.W.; Seufert, R.; Koldehoff, M.; Liebregts, T.; Schmidt, D.; Buer, J.; Rath, P.-M.; Steinmann, J. Performance of the AsperGenius(R) PCR assay for detecting azole resistant Aspergillus fumigatus in BAL fluids from allogeneic HSCT recipients: A prospective cohort study from Essen, West Germany. Med. Mycol. 2020, 58, 268–271. [Google Scholar] [CrossRef]
- Cruciani, M.; Mengoli, C.; Barnes, R.; Donnelly, J.P.; Loeffler, J.; Jones, B.L.; Klingspor, L.; Maertens, J.; Morton, O.C.; White, L.P. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database Syst. Rev. 2019, 9, CD009551. [Google Scholar] [CrossRef]
- Moura, S.; Cerqueira, L.; Almeida, A. Invasive pulmonary aspergillosis: Current diagnostic methodologies and a new molecular approach. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1393–1403. [Google Scholar] [CrossRef]
- Resendiz-Sharpe, A.; Van Holm, W.; Merckx, R.; Pauwels, M.; Teughels, W.; Lagrou, K.; Velde, G.V. Quantitative PCR effectively quantifies triazole-susceptible and triazole-resistant Aspergillus fumigatus in mixed infections. J. Fungi. Basel 2022, 8, 1120. [Google Scholar] [CrossRef]
- Thornton, C.R. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin. Vaccine Immunol. 2008, 15, 1095–1105. [Google Scholar] [CrossRef]
- Jenks, J.D.; Mehta, S.R.; Taplitz, R.; Aslam, S.; Reed, S.L.; Hoenigl, M. Point-of-care diagnosis of invasive aspergillosis in non-neutropenic patients: Aspergillus Galactomannan Lateral Flow Assay versus Aspergillus-specific Lateral Flow Device test in bronchoalveolar lavage. Mycoses 2019, 62, 230–236. [Google Scholar] [CrossRef]
- Mercier, T.; Schauwvlieghe, A.; de Kort, E.; Dunbar, A.; Reynders, M.; Guldentops, E.; Rijnders, B.; Verweij, P.; Lagrou, K.; Maertens, J. Diagnosing invasive pulmonary aspergillosis in hematology patients: A retrospective multicenter evaluation of a novel lateral flow device. J. Clin. Microbiol. 2019, 57, e01913–e1918. [Google Scholar] [CrossRef]
- Hoenigl, M.; Eigl, S.; Heldt, S.; Duettmann, W.; Thornton, C.; Prattes, J. Clinical evaluation of the newly formatted lateral-flow device for invasive pulmonary aspergillosis. Mycoses 2018, 61, 40–43. [Google Scholar] [CrossRef]
- Ascioglu, S.; Rex, J.H.; De Pauw, B.; Bennett, J.E.; Bille, J.; Crokaert, F.; Denning, D.W.; Donnelly, J.P.; Edwards, J.E.; Erjavec, Z.; et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: An international consensus. Clin. Infect. Dis. 2002, 34, 7–14. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A.; et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Blot, S.I.; Taccone, F.S.; Van den Abeele, A.-M.; Bulpa, P.; Meersseman, W.; Brusselaers, N.; Dimopoulos, G.; Paiva, J.A.; Misset, B.; Rello, J.; et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 2012, 186, 56–64. [Google Scholar] [CrossRef]
- Hamam, J.; Navellou, J.C.; Bellanger, A.P.; Bretagne, S.; Winiszewski, H.; Scherer, E.; Piton, G.; Millon, L.; Ressif group Collaborative. New clinical algorithm including fungal biomarkers to better diagnose probable invasive pulmonary aspergillosis in ICU. Ann. Intensive. Care 2021, 11, 41. [Google Scholar] [CrossRef]
- Verweij, P.E.; Rijnders, B.J.A.; Brüggemann, R.J.M.; Azoulay, E.; Bassetti, M.; Blot, S.; Calandra, T.; Clancy, C.J.; Cornely, O.A.; Chiller, T.; et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: An expert opinion. Intensive. Care Med. 2020, 46, 1524–1535. [Google Scholar] [CrossRef]
- Tissot, F.; Agrawal, S.; Pagano, L.; Petrikkos, G.; Groll, A.H.; Skiada, A.; Lass-Flörl, C.; Calandra, T.; Viscoli, C.; Herbrecht, R. ECIL-6 guidelines for the treatment of invasive candidiasis, aspergillosis and mucormycosis in leukemia and hematopoietic stem cell transplant patients. Haematologica 2017, 102, 433–444. [Google Scholar] [CrossRef]
- Driemeyer, C.; Falci, D.R.; Oladele, O.R.; Bongomin, F.; Ocansey, B.K.; Govender, N.P.; Hoenigl, M.; Gangneux, J.P.; Lass-Flörl, C.; Cornely, A.O.; et al. The current state of clinical mycology in Africa: A European Confederation of Medical Mycology and International Society for Human and Animal Mycology survey. Lancet Microbe. 2022, 3, e464–e470. [Google Scholar] [CrossRef]
- Tiphine, M.; Letscher-Bru, V.; Herbrecht, R. Amphotericin B and its new formulations: Pharmacologic characteristics, clinical efficacy, and tolerability. Transpl. Infect Dis. 1999, 1, 273–283. [Google Scholar] [CrossRef]
- Herbrecht, R.; Natarajan-Amé, S.; Nivoix, Y.; Letscher-Bru, V. The lipid formulations of amphotericin B. Expert Opin. Pharmacother. 2003, 4, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.; Behre, G.; Heinemann, V.; Wandt, H.; Schilling, E.; Arning, M.; Trittin, A.; Kern, W.V.; Boenisch, O.; Bosse, D.; et al. Aerosolized amphotericin B inhalations as prophylaxis of invasive Aspergillus infections during prolonged neutropenia: Results of a prospective randomized multicenter trial. Blood 1999, 93, 3654–3661. [Google Scholar] [PubMed]
- Rijnders, B.J.; Cornelissen, J.J.; Slobbe, L.; Becker, M.J.; Doorduijn, J.K.; Hop, W.C.; Ruijgrok, E.J.; Lowenberg, B.; Vulto, A.; Lugtenburg, P.J.; et al. Aerosolized liposomal amphotericin B for the prevention of invasive pulmonary aspergillosis during prolonged neutropenia: A randomized, placebo-controlled trial. Clin. Infect Dis. 2008, 46, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Sun, W.-K.; Tan, M.-M.; Zhang, M.; Ding, Y.; Liu, Z.-C.; Su, X.; Shi, Y. Aerosolized amphotericin B as prophylaxis for invasive pulmonary aspergillosis: A meta-analysis. Int. J. Infect Dis. 2015, 30, 78–84. [Google Scholar] [CrossRef]
- Ramos, E.R.; Jiang, Y.; Hachem, R.; Kassis, C.; Kontoyiannis, D.P.; Raad, I. Outcome analysis of invasive aspergillosis in hematologic malignancy and hematopoietic stem cell transplant patients: The role of novel antimold azoles. Oncologist 2011, 16, 1049–1060. [Google Scholar] [CrossRef]
- Denning, D.W.; Lee, J.Y.; Hostetler, J.S.; Pappas, P.; Kauffman, C.A.; Dewsnup, D.H.; Galgiani, J.N.; Graybill, J.R.; Sugar, A.M.; Catanzaro, A. NIAID Mycoses Study Group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am. J. Med. 1994, 97, 135–144. [Google Scholar] [CrossRef]
- Herbrecht, R.; Patterson, T.F.; Slavin, M.A.; Marchetti, O.; Maertens, J.; Johnson, E.M.; Schlamm, H.T.; Donnelly, J.P.; Pappas, P.G. Application of the 2008 definitions for invasive fungal diseases to the trial comparing voriconazole versus amphotericin B for therapy of invasive aspergillosis: A collaborative study of the Mycoses Study Group (MSG 05) and the European Organization for Research and Treatment of Cancer Infectious Diseases Group. Clin. Infect Dis. 2015, 60, 713–720. [Google Scholar] [CrossRef]
- Benitez, L.L.; Carver, P.L. Adverse effects associated with long-term administration of azole antifungal agents. Drugs 2019, 79, 833–853. [Google Scholar] [CrossRef]
- Walsh, T.J.; Raad, I.; Patterson, T.F.; Chandrasekar, P.; Donowitz, G.R.; Graybill, R.; Greene, R.E.; Hachem, R.; Hadley, S.; Herbrecht, R.; et al. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: An externally controlled trial. Clin. Infect Dis. 2007, 44, 2–12. [Google Scholar] [CrossRef]
- Maertens, J.A.; Rahav, G.; Lee, D.-G.; Ponce-De-León, A.; Sánchez, I.C.R.; Klimko, N.; Sonet, A.; Haider, S.; Vélez, J.D.; Raad, I.; et al. Posaconazole versus voriconazole for primary treatment of invasive aspergillosis: A phase 3, randomised, controlled, non-inferiority trial. Lancet 2021, 397, 499–509. [Google Scholar] [CrossRef]
- Leclerc, E.; Combarel, D.; Uzunov, M.; Leblond, V.; Funck-Brentano, C.; Zahr, N. Prevention of invasive Aspergillus fungal infections with the suspension and delayed-release tablet formulations of posaconazole in patients with haematologic malignancies. Sci. Rep. 2018, 8, 1681. [Google Scholar] [CrossRef]
- Greenberg, R.N.; Mullane, K.; van Burik, J.-A.H.; Raad, I.; Abzug, M.J.; Anstead, G.; Herbrecht, R.; Langston, A.; Marr, K.A.; Schiller, G.; et al. Posaconazole as salvage therapy for zygomycosis. Antimicrob. Agents Chemother. 2006, 50, 126–133. [Google Scholar] [CrossRef]
- van Burik, J.A.; Hare, R.S.; Solomon, H.F.; Corrado, M.L.; Kontoyiannis, D.P. Posaconazole is effective as salvage therapy in zygomycosis: A retrospective summary of 91 cases. Clin. Infect Dis. 2006, 42, e61–e65. [Google Scholar] [CrossRef]
- Maertens, J.A.; Raad, I.I.; Marr, A.K.; Patterson, T.F.; Kontoyiannis, D.P.; Cornely, A.O.; Bow, E.J.; Rahav, G.; Neofytos, D.; Aoun, M.; et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A phase 3, randomised-controlled, non-inferiority trial. Lancet 2016, 387, 760–769. [Google Scholar] [CrossRef]
- Herbrecht, R.; Kuessner, D.; Pooley, N.; Posthumus, J.; Escrig, C. Systematic review and network meta-analysis of clinical outcomes associated with isavuconazole versus relevant comparators for patients with invasive aspergillosis. Curr. Med. Res. Opin. 2018, 34, 2187–2195. [Google Scholar] [CrossRef]
- Marty, F.M.; Ostrosky-Zeichner, L.; Cornely, O.A.; Mullane, K.M.; Perfect, J.R.; Thompson, G.R.; Alangaden, G.J.; Brown, J.M.; Fredricks, D.N.; Heinz, W.J.; et al. Isavuconazole treatment for mucormycosis: A single-arm open-label trial and case-control analysis. Lancet Infect. Dis. 2016, 16, 828–837. [Google Scholar] [CrossRef]
- Aruanno, M.; Glampedakis, E.; Lamoth, F. Echinocandins for the treatment of invasive aspergillosis: From laboratory to bedside. Antimicrob. Agents Chemother. 2019, 63, e00399-19. [Google Scholar] [CrossRef]
- Maertens, J.; Raad, I.; Petrikkos, G.; Boogaerts, M.; Selleslag, D.; Petersen, F.B.; Sable, C.A.; Kartsonis, N.A.; Ngai, A.; Taylor, A.; et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin. Infect. Dis. 2004, 39, 1563–1571. [Google Scholar] [CrossRef]
- Viscoli, C.; Herbrecht, R.; Akan, H.; Baila, L.; Sonet, A.; Gallamini, A.; Giagounidis, A.; Marchetti, O.; Martino, R.; Meert, L.; et al. An EORTC Phase II study of caspofungin as first-line therapy of invasive aspergillosis in haematological patients. J. Antimicrob. Chemother. 2009, 64, 1274–1281. [Google Scholar] [CrossRef]
- Herbrecht, R.; Maertens, J.; Baila, L.; Aoun, M.; Heinz, W.; Martino, R.; Schwartz, S.; Ullmann, A.J.; Meert, L.; Paesmans, M.; et al. Caspofungin first-line therapy for invasive aspergillosis in allogeneic hematopoietic stem cell transplant patients: An European Organisation for Research and Treatment of Cancer study. Bone Marrow. Transplant. 2010, 45, 1227–1233. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Najvar, L.K.; Jaramillo, R.; Olivo, M.; Wickes, B.; Catano, G.; Patterson, T.F. Extended-interval dosing of rezafungin against azole-resistant Aspergillus fumigatus. Antimicrob. Agents Chemother. 2019, 63, e01165-19. [Google Scholar] [CrossRef] [PubMed]
- Hoenigl, M.; Sprute, R.; Egger, M.; Arastehfar, A.; Cornely, O.A.; Krause, R.; Lass-Flörl, C.; Prattes, J.; Spec, A.; Thompson, G.R., 3rd; et al. The antifungal pipeline: Fosmanogepix, ibrexafungerp, olorofim, opelconazole, and rezafungin. Drugs 2021, 81, 1703–1729. [Google Scholar] [CrossRef] [PubMed]
- Angulo, D.A.; Alexander, B.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A.; Hoenigl, M.; Ibrahim, A.S.; Ghannoum, M.A.; King, T.R.; Azie, N.E.; Walsh, T.J. Ibrexafungerp, a novel triterpenoid antifungal in development for the treatment of mold infections. J. Fungi. 2022, 8, 1121. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Menendez, O.; Soto-Debran, J.; Cuenca-Estrella, M.; Alastruey-Izquierdo, A. In vitro activity of ibrexafungerp against a collection of clinical isolates of Aspergillus, including cryptic species and Cyp51A mutants, using EUCAST and CLSI methodologies. J. Fungi. Basel 2021, 7, 232. [Google Scholar] [CrossRef]
- Rivero-Menendez, O.; Cuenca-Estrella, M.; Alastruey-Izquierdo, A. In vitro activity of olorofim (F901318) against clinical isolates of cryptic species of Aspergillus by EUCAST and CLSI methodologies. J. Antimicrob. Chemother. 2019, 74, 1586–1590. [Google Scholar] [CrossRef]
- Seyedmousavi, S.; Chang, Y.C.; Law, D.; Birch, M.; Rex, J.H.; Kwon-Chung, K.J. Efficacy of olorofim (F901318) against Aspergillus fumigatus, A. nidulans, and A. tanneri in murine models of profound neutropenia and chronic granulomatous disease. Antimicrob. Agents Chemother. 2019, 63, e00129-19. [Google Scholar] [CrossRef]
- Shaw, K.J.; Ibrahim, A.S. Fosmanogepix: A review of the first-in-class broad spectrum agent for the treatment of invasive fungal infections. J. Fungi. Basel 2020, 6, 239. [Google Scholar] [CrossRef]
- Aliff, T.B.; Maslak, P.; Jurcic, J.G.; Heaney, M.L.; Cathcart, K.N.; Sepkowitz, K.A.; Weiss, M.A. Refractory Aspergillus pneumonia in patients with acute leukemia: Successful therapy with combination caspofungin and liposomal amphotericin. Cancer 2003, 97, 1025–1032. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Hachem, R.; Lewis, R.E.; Rivero, G.A.; Torres, H.A.; Thornby, J.; Champlin, R.; Kantarjian, H.; Bodey, G.P.; Raad, I.I. Efficacy and toxicity of caspofungin in combination with liposomal amphotericin B as primary or salvage treatment of invasive aspergillosis in patients with hematologic malignancies. Cancer 2003, 98, 292–299. [Google Scholar] [CrossRef]
- Caillot, D.; Thiébaut, A.; Herbrecht, R.; de Botton, S.; Pigneux, A.; Bernard, F.; Larché, J.; Monchecourt, F.; Alfandari, S.; Mahi, L. Liposomal amphotericin B in combination with caspofungin for invasive aspergillosis in patients with hematologic malignancies: A randomized pilot study (Combistrat trial). Cancer 2007, 110, 2740–2746. [Google Scholar] [CrossRef]
- Singh, N.; Limaye, A.P.; Forrest, G.; Safdar, N.; Muñoz, P.; Pursell, K.; Houston, S.; Rosso, F.; Montoya, J.G.; Patton, P.; et al. Combination of voriconazole and caspofungin as primary therapy for invasive aspergillosis in solid organ transplant recipients: A prospective, multicenter, observational study. Transplantation 2006, 81, 320–326. [Google Scholar] [CrossRef]
- Marr, K.A.; Schlamm, H.T.; Herbrecht, R.; Rottinghaus, S.T.; Bow, E.J.; Cornely, O.A.; Heinz, W.J.; Jagannatha, S.; Koh, L.P.; Kontoyiannis, D.P.; et al. Combination antifungal therapy for invasive aspergillosis: A randomized trial. Ann. Int. Med. 2015, 162, 81–89. [Google Scholar] [CrossRef]
- Lagrou, K.; Duarte, R.F.; Maertens, J. Standards of CARE: What is considered ‘best practice’ for the management of invasive fungal infections? A haematologist’s and a mycologist’s perspective. J. Antimicrob. Chemother. 2019, 74, ii3–ii8. [Google Scholar] [CrossRef]
- Kanj, S.S.; Omrani, A.S.; Al-Abdely, H.M.; Subhi, A.; El Fakih, R.; Abosoudah, I.; Kanj, H.; Dimopoulos, G. Survival outcome of empirical antifungal therapy and the value of early initiation: A review of the last decade. J. Fungi. 2022, 8, 1146. [Google Scholar] [CrossRef]
- Pizzo, P.A.; Robichaud, K.; Gill, F.A.; Witebsky, F.G. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. Am. J. Med. 1982, 72, 101–111. [Google Scholar] [CrossRef]
- Maertens, J.; Lodewyck, T.; Donnelly, J.P.; Chantepie, S.; Robin, C.; Blijlevens, N.; Turlure, P.; Selleslag, D.; Baron, F.; Aoun, M.; et al. Empiric versus pre-emptive antifungal strategy in high-risk neutropenic patients on fluconazole prophylaxis: A randomized trial of the European organization for Research and Treatment of cancer (EORTC 65091). Clin. Infect. Dis. 2022. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledoux, M.-P.; Herbrecht, R. Invasive Pulmonary Aspergillosis. J. Fungi 2023, 9, 131. https://doi.org/10.3390/jof9020131
Ledoux M-P, Herbrecht R. Invasive Pulmonary Aspergillosis. Journal of Fungi. 2023; 9(2):131. https://doi.org/10.3390/jof9020131
Chicago/Turabian StyleLedoux, Marie-Pierre, and Raoul Herbrecht. 2023. "Invasive Pulmonary Aspergillosis" Journal of Fungi 9, no. 2: 131. https://doi.org/10.3390/jof9020131
APA StyleLedoux, M.-P., & Herbrecht, R. (2023). Invasive Pulmonary Aspergillosis. Journal of Fungi, 9(2), 131. https://doi.org/10.3390/jof9020131





