The Identification and Role of the Key Mycotoxin of Pestalotiopsis kenyana Causing Leaf Spot Disease of Zanthoxylum schinifolium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
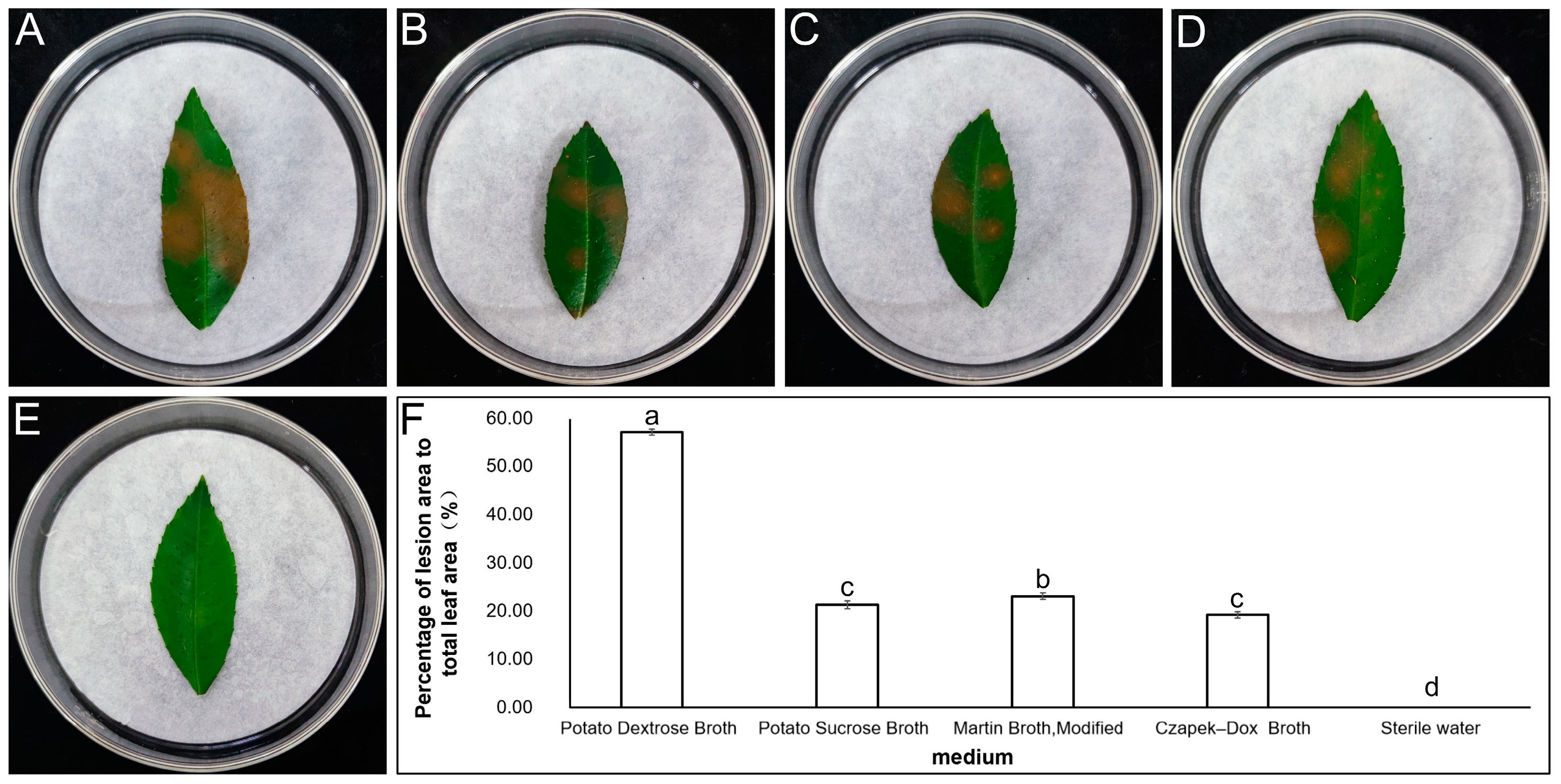
2.2. Screening of the Best Medium for P. kenyana to Produce Mycotoxin and Determination of the Pathogenic Components of the Mycotoxin
2.2.1. Preparation of Mycotoxin Stock Solution and Determination of Toxicity
2.2.2. Determination of Toxic Components of Fungal Secondary Metabolites
2.2.3. Determination of Biological Activity
2.3. Purification of Mycotoxins
2.3.1. Preparation of the Crude Mycotoxin Sample
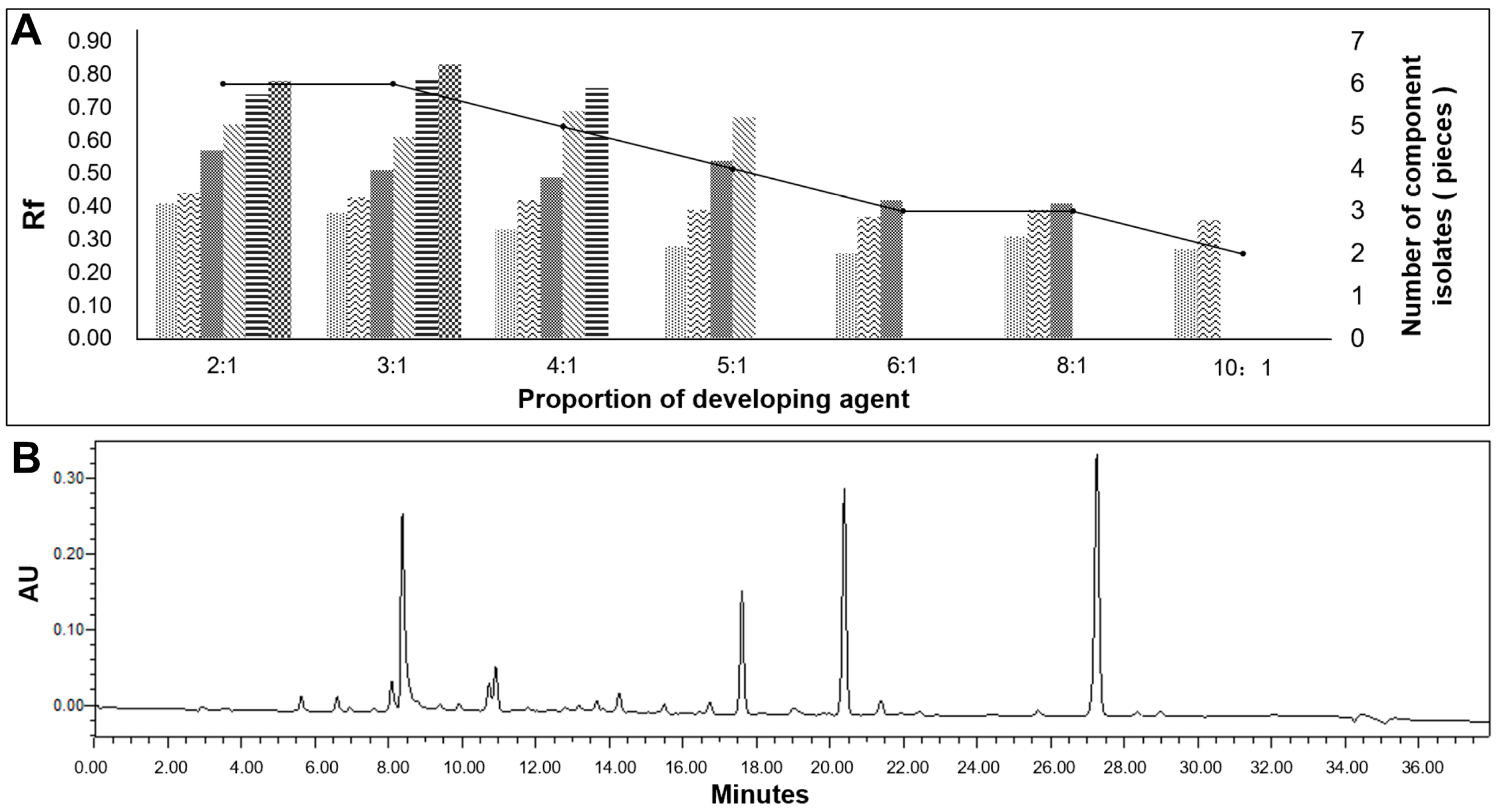
2.3.2. Separation and Purification by TLC and Silica Gel Column Chromatography
2.3.3. Preparation of HPLC
2.3.4. UV-Visible Spectrophotometer Scanning
2.3.5. Purity Detection of Active Components
2.3.6. Structure Identification and Biological Activity Determination of Active Components
2.4. Determination of Physiological and Biochemical Indexes of Z. schinifolium after Mycotoxin Treatment
2.4.1. Sample Pretreatment
2.4.2. Determination of Soluble Protein Content
2.4.3. Determination of Soluble Sugar Content
2.4.4. Determination of Chlorophyll Content
2.4.5. Determination of MDA Content
2.4.6. Effect of Mycotoxin on Plant Enzyme Activities
2.5. Statistical Analyses
3. Results
3.1. Screening of the Best Medium for P. kenyana to Produce Mycotoxin and Determination of the Toxin Components of Fungal Secondary Metabolites
3.2. Isolation and Purification of Mycotoxin
3.2.1. The Results of Thin Layer Chromatography, Silica Gel Column Chromatography, and Preparative High-Performance Liquid Chromatography
3.2.2. UV-Visible Spectrophotometer Scanning
3.2.3. Purity Detection of Active Components
3.2.4. Structure Identification and Biological Activity Determination
3.3. Determination of Physiological and Biochemical Indexes of Z. schinifolium after Mycotoxin Application
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staples, R.C.; Mayer, A.M. Suppression of host resistance by fungal plant pathogens REVIEW. Isr. J. Plant Sci. 2003, 51, 173–184. [Google Scholar] [CrossRef]
- Hof, H. Mycotoxins: Pathogenicity factors or virulence factors? Mycoses 2008, 51, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Neto, J.R.C.; dos Santos, M.S.N.; Mazutti, M.A.; Zabot, G.L.; Tres, M.V. Phoma dimorpha phytotoxic activity potentialization for bioherbicide production. Biocatal. Agric. Biotechnol. 2021, 33, 101986. [Google Scholar] [CrossRef]
- Justyna, L.; Natalia, W.; Agnieszka, W.; Jan, B.; Łukasz, S. Plant Metabolites Affect Fusarium proliferatum Metabolism and In Vitro Fumonisin Biosynthesis. Int. J. Mol. Sci. 2023, 24, 3002. [Google Scholar] [CrossRef]
- Liu, C.; Luo, F.; Zhu, T.; Han, S.; Li, S. Leaf Spot Disease Caused by Pestalotiopsis kenyana on Zanthoxylum schinifolium in Sichuan Province, China. Plant Dis. 2021, 105, 3747. [Google Scholar] [CrossRef] [PubMed]
- Xun, W.; Wu, C.; Wu, X.; Bai, Q.; Sun, Y.; Shi, J.; Xie, D.; Jin, L. First Report of Bayberry Leaf Blight Caused by Pestalotiopsis kenyana in Zhejiang Province, China. Plant Dis. 2023, 107, 2860. [Google Scholar] [CrossRef] [PubMed]
- Maharachchikumbura, S.; Hyde, K.; Groenewald, J.; Xu, J.; Crous, P. Pestalotiopsis revisited. Stud. Mycol. 2014, 79, 121–186. [Google Scholar] [CrossRef]
- Morales-Mora, L.A.; Martínez-Salgado, S.J.; de Ita, M.A.V.; Andrade-Hoyos, P.; Silva-Rojas, H.V.; Romero-Arenas, O. First Report of Leaf Spot and Anthracnosis Caused by Pestalotiopsis sp. on Strawberry in Puebla, Mexico. Plant Dis. 2019, 103, 2668. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Guo, L.-D.; Chukeatirote, E.; Bahkali, A.H.; Hyde, K.D. Pestalotiopsis—Morphology, phylogeny, biochemistry and diversity. Fungal Divers. 2011, 50, 167–187. [Google Scholar] [CrossRef]
- Bhuiyan, A.B.; Sultana, N.; Mahmud, N.U.; Kader, A.; Hassan, O.; Chang, T.; Islam, T.; Akanda, A.M. Characterization of Pestalotiopsis sp. causing gray leaf spot in coconut (Cocos nucifera L.) in Bangladesh. J. Basic Microbiol. 2021, 61, 1085–1097. [Google Scholar] [CrossRef]
- Zhou, L.; Li, J.; Chen, F.; Chen, J.; Ye, J. First Report of Shoot Blight on Cryptomeria japonica Caused by Pestalotiopsis neglecta in China. Plant Dis. 2019, 103, 2140–2141. [Google Scholar] [CrossRef]
- Borrero, C.; Castaño, R.; Avilés, M. First Report of Pestalotiopsis clavispora (Neopestalotiopsis clavispora) Causing Canker and Twig Dieback on Blueberry Bushes in Spain. Plant Dis. 2018, 102, 1178. [Google Scholar] [CrossRef]
- Maharachchikumbura, S.S.N.; Guo, L.-D.; Cai, L.; Chukeatirote, E.; Wu, W.P.; Sun, X.; Crous, P.W.; Bhat, D.J.; McKenzie, E.H.C.; Bahkali, A.H.; et al. A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers. 2012, 56, 95–129. [Google Scholar] [CrossRef]
- Nozawa, S.; Togawa, M.; Watanabe, K. Reidentification of Pestalotiopsis sensu lato causing gray blight of tea in Japan. J. Gen. Plant Pathol. 2022, 88, 293–299. [Google Scholar] [CrossRef]
- Mukhtar, I.; Li, H.; Quan, X.; Chou, T.; Jiang, S.; Chen, B.; Wen, Z.; Xie, B. First Report of Pestalotiopsis theae Causing Leaf Spot of Ixora chinensis in China. Plant Dis. 2019, 103, 370. [Google Scholar] [CrossRef]
- Suwannarach, N.; Sujarit, K.; Kumla, J.; Bussaban, B.; Lumyong, S. First report of leaf spot disease on oil palm caused by Pestalotiopsis theae in Thailand. J. Gen. Plant Pathol. 2013, 79, 277–279. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S.; Zhang, Y.; Chen, Y.; Zhou, H.; Zhang, G. Identification, Culture Characteristics and Whole-Genome Analysis of Pestalotiopsis neglecta Causing Black Spot Blight of Pinus sylvestris var. mongolica. J. Fungi 2023, 9, 564. [Google Scholar] [CrossRef]
- Suryanarayanand, T.; Kumaresan, V. Endophytic fungi of some halophytes from an estuarine mangrove forest. Mycol. Res. 2000, 104, 1465–1467. [Google Scholar] [CrossRef]
- Xing, J.-G.; Deng, H.-Y.; Luo, D.-Q. Two new compounds from an endophytic fungus Pestalotiopsis heterocornis. J. Asian Nat. Prod. Res. 2011, 13, 1069–1073. [Google Scholar] [CrossRef] [PubMed]
- Venkatasubbaiah, P.; Van Dyke, C. Phytotoxins produced by Pestalotiopsis oenotherae, a pathogen of evening primrose. Phytochemistry 1991, 30, 1471–1474. [Google Scholar] [CrossRef]
- Nagata, T.; Ando, Y.; Hirota, A. Phytotoxins from Tea Gray Blight Fungi, Pestalotiopsis longiseta and Pestalotiopsis theae. Biosci. Biotechnol. Biochem. 1992, 56, 810–811. [Google Scholar] [CrossRef] [PubMed]
- Tü, R.M.; Andolfi, A.; Zonno, M.C.; Erper, I.; Perrone, C.; Cimmino, A.; Vurro, M.; Evidente, A. Phytotoxins produced by Pestalotiopsis guepinii, the causal agent of hazelnut twig blight. Phytopathol. Mediterr. 2011, 50, 154–158. [Google Scholar]
- Yamada, K.; Sonoda, R.; Ishikawa, K. Population Genetic Structure of QoI-Resistant Pestalotiopsis longiseta Isolates Causing Tea Gray Blight. Plant Dis. 2016, 100, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Khambhati, V.H.; Abbas, H.K.; Sulyok, M.; Tomaso-Peterson, M.; Shier, W.T. First Report of the Production of Mycotoxins and Other Secondary Metabolites by Macrophomina phaseolina (Tassi) Goid. Isolates from Soybeans (Glycine max L.) Symptomatic with Charcoal Rot Disease. J. Fungi 2020, 6, 332. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Tan, Y.; Wang, S.; Gardiner, D.M.; De Saeger, S.; Liao, Y.; Wang, C.; Fan, Y.; Wang, Z.; Wu, A. Mycotoxigenic Potentials of Fusarium Species in Various Culture Matrices Revealed by Mycotoxin Profiling. Toxins 2016, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, B.-Y.; Yang, X.-L. Antifungal Monoterpene Derivatives from the Plant Endophytic Fungus Pestalotiopsis foedan. Chem. Biodivers. 2016, 13, 1422–1425. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, Z.; Guo, L.; Liu, L. New Cytotoxic Meroterpenoids from the Plant Endophytic Fungus Pestalotiopsis fici. Helv. Chim. Acta 2016, 99, 151–156. [Google Scholar] [CrossRef]
- Mshelia, L.P.; Selamat, J.; Samsudin, N.I.P.; Rafii, M.Y.; Mutalib, N.-A.A.; Nordin, N.; Berthiller, F. Effect of Temperature, Water Activity and Carbon Dioxide on Fungal Growth and Mycotoxin Production of Acclimatised Isolates of Fusarium verticillioides and F. graminearum. Toxins 2020, 12, 478. [Google Scholar] [CrossRef]
- Chou, G. Medium for Toxin Production by Clostridium perfringens in Continuous Culture. Appl. Microbiol. 1971, 21, 794–798. [Google Scholar] [CrossRef]
- Hou, R.L.; Liu, X.; Wang, D.T.; Chen, L.; Lin, W.X.; Zheng, M.F.; Fu, J.S. Optimization of culture conditions, and analysis of the antioxidant and antibacterial activities of the endophyte Pestalotiopsis vismiae N1 (Article). Chin. J. Appl. Environ. Biol. 2019, 25, 385–391. [Google Scholar] [CrossRef]
- Carvalho, D.D.C.; Oliveira, R.M.; Marques, M.G.; Milan, M.D.; Pinho, D.B.; Dianese, C. Molecular, morphophysiological and pathogenic characterization of eucalypt Pestalotiopsis grandis-urophylla isolates, a new species. Trop. Plant Pathol. 2019, 44, 132–139. [Google Scholar] [CrossRef]
- Qian, Y.-X.; Kang, J.-C.; Luo, Y.-K.; Zhao, J.-J.; He, J.; Geng, K. A Bilobalide-Producing Endophytic Fungus, Pestalotiopsis uvicola from Medicinal Plant Ginkgo biloba. Curr. Microbiol. 2016, 73, 280–286. [Google Scholar] [CrossRef]
- Majumdar, N.; Mandal, N.C. Effect of pH on Mycelial Growth and Sporulation of Postharvest Pathogen Colletotrichum gloeosporioides (Penz.) Penz & Sacc. and Pestalotiopsis mangiferae (Henn.) Steyaert. Int. J. Bio-Resour. Stress Manag. 2018, 9, 416–420. [Google Scholar] [CrossRef]
- Aguilar-Pérez, M.M.; Torres-Mendoza, D.; Vásquez, R.; Rios, N.; Cubilla-Rios, L. Exploring the Antibacterial Activity of Pestalotiopsis spp. under Different Culture Conditions and Their Chemical Diversity Using LC–ESI–Q–TOF–MS. J. Fungi 2020, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterization and toxicological potential of Alternaria-mycotoxins (TeA, AOH and AME) in different Alternaria species from various regions of India. Sci. Rep. 2017, 7, 8777. [Google Scholar] [CrossRef]
- De Souza, E.M.; Da Silva, E.L.; Marinho, A.M.; Marinho, P.S. (4S)-4,8-dihydroxy-1-tetralone and other chemical constituents from Pestalotiopsis sp. EJC07, endophytic from Bauhinia guianensis. An. Acad. Bras. Ciênc. 2016, 88, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, X.; Sun, M.; Li, M. Two Novel Tyrosinase Inhibitory Sesquiterpenes Induced by CuCl2 from a Marine-Derived Fungus Pestalotiopsis sp. Z233. Mar. Drugs 2013, 11, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, X.; Ding, G.; Feng, Y.; Jiang, X.; Guo, L.; Che, Y. α-Pyrones and Pyranes from the Plant Pathogenic Fungus Pestalotiopsis scirpina. Eur. J. Org. Chem. 2012, 2012, 2445–2452. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, J.-Q.; Jiang, W.-K.; Yuan, Q.-S.; Wang, Y.-H.; Guo, L.-P.; Yang, Y.; Yang, Y.; Zhou, T. [Isolation, identification, and pathogenicity research of brown rot pathogens from Gastrodia elata]. China J. Chin. Mater. Medica 2022, 47, 2288–2295. [Google Scholar] [CrossRef]
- Scheffer, J.; Tudzynski, P. In vitro pathogenicity assay for the ergot fungus Claviceps purpurea. Mycol. Res. 2006, 110, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Atik, W.; Nanung, A.F.; Ambar, P.; Zaenal, B.; Yudi, P.; Yuny, E. Optimizing of Protease Purification from Bacillus cereus TD5B by Ammonium Sulfate Precipitation. Chem. Eng. Trans. 2018, 63, 709–714. [Google Scholar] [CrossRef]
- Li, X.; Hou, H.; Liu, H.; Wang, H.; Cai, L.; An, M.; Zhang, C.; Wu, Y. Identification of 3-Methoxyphenylacetic Acid as a Phytotoxin, Produced by Rhizoctonia solani AG-3 TB. Molecules 2023, 28, 790. [Google Scholar] [CrossRef] [PubMed]
- Kankam, F.; Qiu, H.; Pu, L.; Long, H.; Zhang, C.; He, J.; Zhang, H. Isolation, Purification and Characterization of Phytotoxins Produced by Rhizoctonia solani AG-3, the Cause Agent of Potato Stem Canker. Am. J. Potato Res. 2016, 93, 321–330. [Google Scholar] [CrossRef]
- Ruan, J.; Yan, J.; Zheng, D.; Sun, F.; Wang, J.; Han, L.; Zhang, Y.; Wang, T. Comprehensive Chemical Profiling in the Ethanol Extract of Pluchea indica Aerial Parts by Liquid Chromatography/Mass Spectrometry Analysis of Its Silica Gel Column Chromatography Fractions. Molecules 2019, 24, 2784. [Google Scholar] [CrossRef]
- Cheng, J.; Hou, X.; Cui, Q.; Shen, G.; Li, S.; Luo, Q.; Zhou, M.; Chen, H.; Zhang, Z. Separation and Purification of Hydroxyl-α-Sanshool from Zanthoxylum armatum DC. by Silica Gel Column Chromatography. Int. J. Mol. Sci. 2023, 24, 3156. [Google Scholar] [CrossRef]
- Hao, Z.; Yan, L.; Liu, J.; Song, F.; Zhang, J.; Li, X. Extraction of antibiotic zwittermicin A from Bacillus thuringiensis by macroporous resin and silica gel column chromatography. Biotechnol. Appl. Biochem. 2015, 62, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Biju, C.N.; Krishnamurthy, K.S.; Bhat, A.I. Physiological and biochemical response of ginger varieties to virus infection. Plant Physiol. Rep. 2022, 27, 171–179. [Google Scholar] [CrossRef]
- Lin, L.; Wang, Y.; Wang, F.X.; He, J.Y.; Zhang, H.L. Determination of polysaccharides content of Gentiana farreri from different producing areas based on anthrone-sulfuric acid method. China J. Chin. Mater. Medica 2014, 39, 2774–2776. [Google Scholar] [CrossRef]
- Wang, K.; Xu, C.; Li, D.; Gu, Z. Physiological and Biochemical Responses of Sagittaria trifolia L. to Phytotoxic Ethyl Acetate Fungal Extract from Curvularia lunata Strain CLST-01. Plants 2023, 12, 1758. [Google Scholar] [CrossRef]
- Kovács, G.E.; Szőke, L.; Tóth, B.; Kovács, B.; Bojtor, C.; Illés, Á.; Radócz, L.; Moloi, M.J.; Radócz, L. The Physiological and Biochemical Responses of European Chestnut (Castanea sativa L.) to Blight Fungus (Cryphonectria parasitica (Murill) Barr). Plants 2021, 10, 2136. [Google Scholar] [CrossRef]
- Ayvacı, H.; Güldür, M.E.; Dikilitas, M. Physiological and Biochemical Changes in Lucerne (Medicago sativa) Plants Infected with ‘Candidatus Phytoplasma australasia’-Related Strain (16SrII-D Subgroup). Plant Pathol. J. 2022, 38, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.Y.; Li, G.; Liang, S.M.; Yang, G.L.; Zheng, X.L.; Wei, J.G.; Qi, X.J. Effects of culture media, carbon and nitrogen sources and environmental factors on mycelial growth and sporulation of Pestalotiopsis microspora strains, the agent of bayberry twig blight in southern China. Asia Life Sci. 2013, 22, 713–727. [Google Scholar]
- Mahapatra, S.; Banerjee, D. Production and structural elucidation of exopolysaccharide from endophytic Pestalotiopsis sp. BC55. Int. J. Biol. Macromol. 2016, 82, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Kim, S.; Liu, C.; Shim, S.H. Secondary Metabolites Produced by an Endophytic Fungus Pestalotiopsis sydowiana and Their 20S Proteasome Inhibitory Activities. Molecules 2016, 21, 944. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.Q.; Zhang, L.; Shi, B.Z.; Song, X.M. Two New Oxysporone Derivatives from the Fermentation Broth of the Endophytic Plant Fungus Pestalotiopsis karstenii Isolated from Stems of Camellia sasanqua. Molecules 2012, 17, 8554–8560. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Jiang, L.; Guo, L.; Chen, X.; Zhang, H.; Che, Y. Pestalazines and Pestalamides, Bioactive Metabolites from the Plant Pathogenic Fungus Pestalotiopsis theae. J. Nat. Prod. 2008, 71, 1861–1865. [Google Scholar] [CrossRef]
- Ola, A.R.B.; Lapailaka, T.; Wogo, H.E.; Henuk, J.B.D.; Simamora, A.; Mukkun, L.; Proksch, P.; Pham, C.D. Bioactive Secondary Metabolites from the Mangrove Endophytic Fungi Nigrospora oryzae. Indones. J. Chem. 2021, 21, 1016–1022. [Google Scholar] [CrossRef]
- Tiwari, R.K.; Bashyal, B.M.; Shanmugam, V.; Lal, M.K.; Kumar, R.; Sharma, S.; Vinod; Gaikwad, K.; Singh, B.; Aggarwal, R. Impact of Fusarium dry rot on physicochemical attributes of potato tubers during postharvest storage. Postharvest Biol. Technol. 2021, 181, 111638. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.-T.; Wang, F.; Shao, Y.-L.; Zhang, A.-M.; Chang, W. The Effects of Chilling Stress on Antioxidant Enzymes Activities and Proline, Malondialdehyde, Soluble Sugar Contents in Three Paphiopedilum Species. Russ. J. Plant Physiol. 2023, 70, 61. [Google Scholar] [CrossRef]
- Mantz, G.M.; Rossi, F.R.; Viretto, P.E.; Noelting, M.C.; Maiale, S.J. Stem canker caused by Phomopsis spp. Induces changes in polyamine levels and chlorophyll fluorescence parameters in pecan leaves. Plant Physiol. Biochem. 2021, 166, 761–769. [Google Scholar] [CrossRef]
- Ashraf, M.; O’Leary, J. Changes in Soluble Proteins in Spring Wheat Stressed with Sodium Chloride. Biol. Plant. 1999, 42, 113–117. [Google Scholar] [CrossRef]
- Debona, D.; Rodrigues, F.; Rios, J.A.; Nascimento, K.J.T.; Araújo, M.U.P.; Silva, E.T.; Fortunato, A.A.; Bernardeli, A.M.A.; Rahman, A.; Wallis, C.M.; et al. Biochemical Changes in the Leaves of Wheat Plants Infected by Pyricularia oryzae. Phytopathology 2012, 102, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Yahya, M.; Saeed, N.A.; Nadeem, S.; Hamed, M.; Saleem, K. Effect of leaf rust disease on photosynthetic rate, chlorophyll contents and grain yield of wheat. Arch. Phytopathol. Plant Prot. 2020, 53, 425–439. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Sinha, K.K. Influence of aflatoxin B1 on seed germination, seedling growth, chlorophyll and carotenoid contents of mustard (Brassica juncea L. var. pusa bold) seeds. Mycotoxin Res. 2002, 18, 2–6. [Google Scholar] [CrossRef]
- Chauhan, R.S.; Singh, B.M.; Develash, R.K. Effect of Toxic Compounds of Exserohilum turcicum on Chlorophyll Content, Callus Growth and Cell Viability of Susceptible and Resistant Inbred Lines of Maize. J. Phytopathol. 1997, 145, 435–440. [Google Scholar] [CrossRef]
- Gimenez, E.; Salinas, M.; Manzano-Agugliaro, F. Worldwide Research on Plant Defense against Biotic Stresses as Improvement for Sustainable Agriculture. Sustainability 2018, 10, 391. [Google Scholar] [CrossRef]
- Sheteiwy, M.S.; Ali, D.F.I.; Xiong, Y.-C.; Brestic, M.; Skalicky, M.; Hamoud, Y.A.; Ulhassan, Z.; Shaghaleh, H.; AbdElgawad, H.; Farooq, M.; et al. Physiological and biochemical responses of soybean plants inoculated with Arbuscular mycorrhizal fungi and Bradyrhizobium under drought stress. BMC Plant Biol. 2021, 21, 195. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Li, Y.; Chen, H.; Li, S.; Han, S.; Zhu, T.; Liu, Y.; Li, S. The Identification and Role of the Key Mycotoxin of Pestalotiopsis kenyana Causing Leaf Spot Disease of Zanthoxylum schinifolium. J. Fungi 2023, 9, 1194. https://doi.org/10.3390/jof9121194
Liu C, Li Y, Chen H, Li S, Han S, Zhu T, Liu Y, Li S. The Identification and Role of the Key Mycotoxin of Pestalotiopsis kenyana Causing Leaf Spot Disease of Zanthoxylum schinifolium. Journal of Fungi. 2023; 9(12):1194. https://doi.org/10.3390/jof9121194
Chicago/Turabian StyleLiu, Chang, Yiling Li, Hang Chen, Shuying Li, Shan Han, Tianhui Zhu, Yinggao Liu, and Shujiang Li. 2023. "The Identification and Role of the Key Mycotoxin of Pestalotiopsis kenyana Causing Leaf Spot Disease of Zanthoxylum schinifolium" Journal of Fungi 9, no. 12: 1194. https://doi.org/10.3390/jof9121194
APA StyleLiu, C., Li, Y., Chen, H., Li, S., Han, S., Zhu, T., Liu, Y., & Li, S. (2023). The Identification and Role of the Key Mycotoxin of Pestalotiopsis kenyana Causing Leaf Spot Disease of Zanthoxylum schinifolium. Journal of Fungi, 9(12), 1194. https://doi.org/10.3390/jof9121194





