Diversity of Root-Associated Fungi of the Terrestrial Orchids Gavilea lutea and Chloraea collicensis in a Temperate Forest Soil of South-Central Chile
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Site Description
2.2. DNA Isolation and Processing
2.3. Sequence Analyses
2.4. Isolation of Peloton-Associated Mycorrhizal Fungi
2.5. Symbiotic Germination Tests
3. Results
3.1. Soil Characteristics
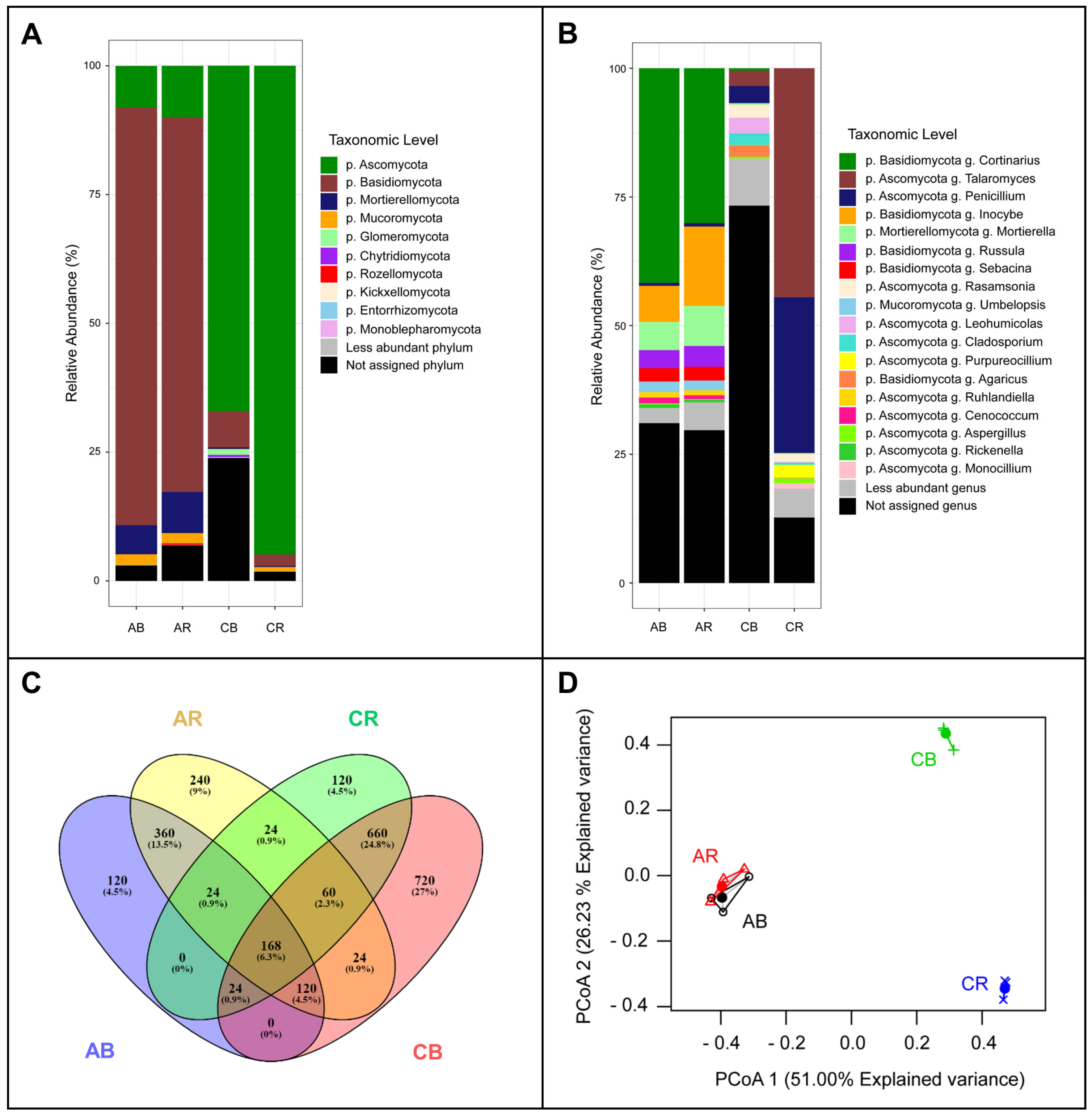
3.2. Sequence Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Dearnaley, J.; Perotto, S.; Selosse, M.A. Structure and development of orchid mycorrhizas. Mol. Mycorrhizal Symbiosis 2016, 2016, 63–86. [Google Scholar]
- Herrera, H.; García-Romera, I.; Meneses, C.; Pereira, G.; Arriagada, C. Orchid Mycorrhizal Interactions on the Pacific Side of the Andes from Chile. A Review. J. Soil Sci. Plant Nutr. 2019, 19, 187–202. [Google Scholar] [CrossRef]
- Kuga, Y.; Sakamoto, N.; Yurimoto, H. Stable isotope cellular imaging reveals that both live and degenerating fungal pelotons transfer carbon and nitrogen to orchid protocorms. New Phytol. 2014, 202, 594–605. [Google Scholar] [CrossRef]
- Pereira, G.; Herrera, H.; Arriagada, C.; Cid, H.; García, J.L.; Atala, C. Controlled mycorrhization of the endemic Chilean orchid Chloraea gavilu (Orchidaceae). Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 848–855. [Google Scholar] [CrossRef]
- Merckx, V.S. Mycoheterotrophy: An introduction. In Mycoheterotrophy; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–17. [Google Scholar]
- Sakamoto, Y.; Ogura-Tsujita, Y.; Ito, K.; Suetsugu, K.; Yokoyama, J.; Yamazaki, J.; Yukawa, T.; Maki, M. The tiny-leaved orchid Cephalanthera subaphylla obtains most of its carbon via mycoheterotrophy. J. Plant Res. 2016, 129, 1013–1020. [Google Scholar] [CrossRef]
- Pereira, G.; Romero, C.; Suz, L.M.; Atala, C. Essential mycorrhizal partners of the endemic Chilean orchids Chloraea collicensis and C. gavilu. Flora Morphol. Distrib. Funct. Ecol. Plants 2014, 209, 95–99. [Google Scholar] [CrossRef]
- Meng, Y.-Y.; Zhang, W.-L.; Selosse, M.-A.; Gao, J.-Y. Are fungi from adult orchid roots the best symbionts at germination? A case study. Mycorrhiza 2019, 29, 541–547. [Google Scholar] [CrossRef]
- Sisti, L.S.; Flores-Borges, D.N.A.; Andrade, S.A.L.D.; Koehler, S.; Bonatelli, M.L.; Mayer, J.L.S. The role of non-mycorrhizal fungi in germination of the mycoheterotrophic orchid Pogoniopsis schenckii Cogn. Front. Plant Sci. 2019, 10, 1589. [Google Scholar] [CrossRef]
- Pereira, G.; Roa, N.; Castillo-Novales, D.; Arriagada, C.; Herrera, H.; Molina-Montenegro, M.; Atala, C. Mycorrhizal fungi isolated from Chilean orchids as biocontrollers of the pathogen Rhizoctonia solani. Gayana Bot. 2021, 78, 113–120. [Google Scholar] [CrossRef]
- Ogura-Tsujita, Y.; Yokoyama, J.; Miyoshi, K.; Yukawa, T. Shifts in mycorrhizal fungi during the evolution of autotrophy to mycoheterotrophy in Cymbidium (Orchidaceae). Am. J. Bot. 2012, 99, 1158–1176. [Google Scholar]
- Yuan, Z.-L.; Chen, Y.-C.; Yang, Y. Diverse non-mycorrhizal fungal endophytes inhabiting an epiphytic, medicinal orchid (Dendrobium nobile): Estimation and characterization. World J. Microbiol. Biotechnol. 2009, 25, 295. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, K.; Cheng, S.; Nie, Q.; Zhou, S.-X.; Chen, Q.; Zhou, J.; Zhen, X.; Li, X.; Zhen, T.; et al. Fusarium oxysporum KB-3 from Bletilla striata: An orchid mycorrhizal fungus. Mycorrhiza 2019, 29, 531–540. [Google Scholar] [CrossRef]
- McCormick, M.K.; Whigham, D.F.; Sloan, D.; O’Malley, K.; Hodkinson, B. Orchid–fungus fidelity: A marriage meant to last? Ecology 2006, 87, 903–911. [Google Scholar] [CrossRef]
- Rasmussen, H.N.; Rasmussen, F.N. Orchid mycorrhiza: Implications of a mycophagous life style. Oikos 2009, 118, 334–345. [Google Scholar] [CrossRef]
- Kull, T. Population dynamics of north temperate orchids. Orchid Biol. Rev. Perspect. 2002, 8, 139–165. [Google Scholar]
- Wraith, J.; Pickering, C. A continental scale analysis of threats to orchids. Biol. Conserv. 2019, 234, 7–17. [Google Scholar]
- Diantina, S.; Kartikaningrum, S.; McCormick, A.C.; Millner, J.; McGill, C.; Pritchard, H.W.; Nadarajan, J. Comparative in vitro seed germination and seedling development in tropical and temperate epiphytic and temperate terrestrial orchids. Plant Cell Tissue Organ Cult. 2020, 143, 619–633. [Google Scholar] [CrossRef]
- Dearnaley, J.D.; Martos, F.; Selosse, M.-A. 12 Orchid mycorrhizas: Molecular ecology, physiology, evolution and conservation aspects. In Fungal Associations; Springer: Berlin/Heidelberg, Germany, 2012; pp. 207–230. [Google Scholar]
- Li, T.; Yang, W.; Wu, S.; Selosse, M.-A.; Gao, J. Progress and prospects of mycorrhizal fungal diversity in orchids. Front. Plant Sci. 2021, 12, 646325. [Google Scholar] [CrossRef] [PubMed]
- Ogura-Tsujita, Y.; Tetsuka, K.; Tagane, S.; Kubota, M.; Anan, S.; Yamashita, Y.; Tone, K.; Yukawa, T. Differing life-history strategies of two mycoheterotrophic orchid species associated with leaf litter-and wood-decaying fungi. Diversity 2021, 13, 161. [Google Scholar] [CrossRef]
- Pérez-Escobar, O.A.; Chomicki, G.; Condamine, F.L.; Karremans, A.P.; Bogarín, D.; Matzke, N.J.; Silvestro, D.; Antonelli, A. Recent origin and rapid speciation of Neotropical orchids in the world’s richest plant biodiversity hotspot. New Phytol. 2017, 215, 891–905. [Google Scholar] [CrossRef] [Green Version]
- Cartay, A. Ecotourism in the earthly paradise of South America: Orchids, butterflies and hummingbirds in this mega biodiversity zone. Tur. Soc. 2020, 27, 43–56. [Google Scholar]
- Novoa, P.; Espejo, J.; Cisternas, M.; Rubio, M.; Dominguez, E. Guía De Campo De Las Orquídeas Chilenas; Corporación Chilena De La Madera (Corma): Concepción, Chile, 2006. [Google Scholar]
- Rodriguez, R.; Marticorena, C.; Alarcón, D.; Baeza, C.; Cavieres, L.; Finot, V.L.; Fuentes, N.; Kiessling, A.; Mihoc, M.; Pauchard, A. Catálogo de las plantas vasculares de Chile. Gayana Bot. 2018, 75, 1–430. [Google Scholar] [CrossRef] [Green Version]
- Mujica, M.I.; Cisternas, M.; Claro, A.; Simunovic, M.; Pérez, F. Nutrients and fungal identity affect the outcome of symbiotic germination in Bipinnula fimbriata (Orchidaceae). Symbiosis 2021, 83, 91–101. [Google Scholar] [CrossRef]
- Mora, M.; Alfaro, M.; Jarvis, S.; Demanet, R.; Cartes, P.J.S.U. Soil aluminium availability in Andisols of southern Chile and its effect on forage production and animal metabolism. Soil Use Manag. 2006, 22, 95–101. [Google Scholar] [CrossRef]
- Aguayo, R.; León-Muñoz, J.; Garreaud, R.; Montecinos, A. Hydrological droughts in the southern Andes (40–45° S) from an ensemble experiment using CMIP5 and CMIP6 models. Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef]
- Masiokas, M.H.; Villalba, R.; Christie, D.; Betman, E.; Luckman, B.; Le Quesne, C.; Prieto, M.R.; Mauget, S. Snowpack variations since AD 1150 in the Andes of Chile and Argentina (30°–37°S) inferred from rainfall, tree-ring and documentary records. J. Geophys. Res. 2012, 117, D05112. [Google Scholar] [CrossRef]
- Valadares, R.; Perotto, S.; Lucheta, A.R.; Santos, E.C.; Oliveira, R.M.; Lambais, M.R. Proteomic and Transcriptomic Analyses Indicate Metabolic Changes and Reduced Defense Responses in Mycorrhizal Roots of Oeceoclades maculata (Orchidaceae) Collected in Nature. J. Fungi 2020, 6, 148. [Google Scholar] [CrossRef]
- Fochi, V.; Chitarra, W.; Kohler, A.; Voyron, S.; Singan, V.R.; Lindquist, E.A.; Barry, K.W.; Girlanda, M.; Grigoriev, I.V.; Martin, F. Fungal and plant gene expression in the Tulasnella calospora–Serapias vomeracea symbiosis provides clues about nitrogen pathways in orchid mycorrhizas. New Phytol. 2017, 213, 365–379. [Google Scholar] [CrossRef] [Green Version]
- Herrera, H.; Sanhueza, T.; Martiarena, R.; Valadares, R.; Fuentes, A.; Arriagada, C. Mycorrhizal Fungi Isolated from Native Terrestrial Orchids from Region of La Araucanía, Southern Chile. Microorganisms 2020, 8, 1120. [Google Scholar] [CrossRef]
- Pereira, G.; Suz, L.M.; Albornoz, V.; Romero, C.; García, L.; Leiva, V.; Atala, C. Mycorrhizal fungi associated with Codonorchis lessonii (Brongn.) Lindl., a terrestrial orchid from Chile. Gayana Bot. 2018, 75, 447–458. [Google Scholar] [CrossRef] [Green Version]
- Herrera, H.; Sanhueza, T.; Novotná, A.; Charles, T.C.; Arriagada, C. Isolation and identification of endophytic bacteria from mycorrhizal tissues of terrestrial orchids from southern Chile. Diversity 2020, 12, 55. [Google Scholar] [CrossRef] [Green Version]
- Egidi, E.; May, T.W.; Franks, A.E. Seeking the needle in the haystack: Undetectability of mycorrhizal fungi outside of the plant rhizosphere associated with an endangered Australian orchid. Fungal Ecol. 2018, 33, 13–23. [Google Scholar] [CrossRef]
- Li, O.; Xiao, R.; Sun, L.; Guan, C.; Kong, D.; Hu, X. Bacterial and diazotrophic diversities of endophytes in Dendrobium catenatum determined through barcoded pyrosequencing. PLoS ONE 2017, 12, e0184717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujica, M.I.; Pérez, M.F.; Jakalski, M.; Martos, F.; Selosse, M.A. Soil P reduces mycorrhizal colonization while favors fungal pathogens: Observational and experimental evidence in Bipinnula (Orchidaceae). FEMS Microbiol. Ecol. 2020, 96, fiaa178. [Google Scholar] [CrossRef] [PubMed]
- Burt, R. Soil Survey Laboratory Methods Manual; Soil Survey Investigations Report No. 51, Version 2; US Department of Agriculture, Natural Resources Conservation Service: Lincoln, NE, USA, 2014.
- Blume, T.; Zehe, E.; Bronstert, A. Investigation of runoff generation in a pristine, poorly gauged catchment in the Chilean Andes II: Qualitative and quantitative use of tracers at three spatial scales. Hydrol. Processes 2008, 22, 3676–3688. [Google Scholar] [CrossRef]
- CIREN. Descripciones De Suelos Materiales y Símbolos: Estudio Agrológico IX Región; Publicación CIREN: Santiago, Chile, 2002; p. 122.
- Matus, F.J.; Escudey, M.; Förster, J.E.; Gutiérrez, M.; Chang, A.C. Is the Walkley–Black method suitable for organic carbon determination in chilean volcanic soils? Commun. Soil Sci. Plant Anal. 2009, 40, 1862–1872. [Google Scholar] [CrossRef]
- Herrera, H.; Palma, G.; Almonacid, L.; Campos, R.; Fuentes, A.; Garcia-Romera, I.; Arriagada, C. Improving Soil Simazine Dissipation Through an Organic Amendment Inoculated with Trametes versicolor. J. Soil Sci. Plant Nutr. 2019, 19, 262–269. [Google Scholar] [CrossRef]
- Fuentes, A.; Herrera, H.; Charles, T.C.; Arriagada, C. Fungal and Bacterial Microbiome Associated with the Rhizosphere of Native Plants from the Atacama Desert. Microorganisms 2020, 8, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; Volume 18, pp. 315–322. [Google Scholar]
- Nguyen, N.H.; Song, Z.; Bates, S.T.; Branco, S.; Tedersoo, L.; Menke, J.; Schilling, J.S.; Kennedy, P.G. FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 2016, 20, 241–248. [Google Scholar] [CrossRef]
- Valadares, R.B.; Pereira, M.C.; Otero, J.T.; Cardoso, E.J. Narrow fungal mycorrhizal diversity in a population of the orchid Coppensia doniana. Biotropica 2012, 44, 114–122. [Google Scholar] [CrossRef]
- Herrera, H.; Fuentes, A.; Ortiz, J.; Soto, J.; da Silva Valadares, R.B.; Salas-Eljatib, C.; Arriagada, C. Root-associated endophytes isolated from juvenile Ulex europaeus L. (Fabaceae) plants colonizing rural areas in South-Central Chile. Plant Soil 2022, 474, 181–193. [Google Scholar] [CrossRef]
- Vasudevan, R.; van Staden, J. Fruit harvesting time and corresponding morphological changes of seed integuments influence in vitro seed germination of Dendrobium nobile Lindl. Plant Growth Regul. 2010, 60, 237–246. [Google Scholar] [CrossRef]
- Calevo, J.; Voyron, S.; Adamo, M.; Alibrandi, P.; Perotto, S.; Girlanda, M. Can orchid mycorrhizal fungi be persistently harbored by the plant host? Fungal Ecol. 2021, 53, 101071. [Google Scholar] [CrossRef]
- Li, Z.T.; Janisiewicz, W.J.; Liu, Z.; Callahan, A.M.; Evans, B.E.; Jurick, W.M.; Dardick, C. Exposure in vitro to an environmentally isolated strain TC09 of Cladosporium sphaerospermum triggers plant growth promotion, early flowering, and fruit yield increase. Front. Plant Sci. 2019, 9, 1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baron, N.C.; de Souza Pollo, A.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 2020, 8, e9005. [Google Scholar] [CrossRef] [PubMed]
- Ozimek, E.; Hanaka, A. Mortierella species as the plant growth-promoting fungi present in the agricultural soils. Agriculture 2021, 11, 7. [Google Scholar] [CrossRef]
- Huang, C.-L.; Jian, F.-Y.; Huang, H.-J.; Chang, W.-C.; Wu, W.-L.; Hwang, C.-C.; Lee, R.-H.; Chiang, T.-Y. Deciphering mycorrhizal fungi in cultivated Phalaenopsis microbiome with next-generation sequencing of multiple barcodes. Fungal Divers. 2014, 66, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Deslippe, J.R.; Hartmann, M.; Grayston, S.J.; Simard, S.W.; Mohn, W.W. Stable isotope probing implicates a species of Cortinarius in carbon transfer through ectomycorrhizal fungal mycelial networks in Arctic tundra. New Phytol. 2016, 210, 383–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyamsanjaa, K.; Oyuntsetseg, B.; Takashima, Y.; Sakagami, N.; Watanabe, M. Characteristics of Cenococcum geophilum sclerotia found in steppe forest soil in Mongolia. J. For. Res. 2022, 27, 76–82. [Google Scholar] [CrossRef]
- Taniguchi, T.; Kitajima, K.; Douhan, G.W.; Yamanaka, N.; Allen, M.F. A pulse of summer precipitation after the dry season triggers changes in ectomycorrhizal formation, diversity, and community composition in a Mediterranean forest in California, USA. Mycorrhiza 2018, 28, 665–677. [Google Scholar] [CrossRef] [Green Version]
- Costa, P.H.D.O.; Nascimento, S.V.D.; Herrera, H.; Gastauer, M.; Ramos, S.J.; Caldeira, C.F.; Oliveira, G.; Valadares, R.B.D.S. Non-Specific Interactions of Rhizospheric Microbial Communities Support the Establishment of Mimosa acutistipula var. ferrea in an Amazon Rehabilitating Mineland. Processes 2021, 9, 2079. [Google Scholar] [CrossRef]
- Böhmer, M.; Ozdín, D.; Račko, M.; Lichvár, M.; Budiš, J.; Szemes, T. Identification of Bacterial and Fungal Communities in the Roots of Orchids and Surrounding Soil in Heavy Metal Contaminated Area of Mining Heaps. Appl. Sci. 2020, 10, 7367. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, Y.; Shi, A.; Cai, Z.; Nian, H.; Hartmann, M.; Lian, T. Rhizosphere soil fungal communities of aluminum-tolerant and-sensitive soybean genotypes respond differently to aluminum stress in an acid soil. Front. Microbiol. 2020, 11, 1177. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, T.; Wen, X.; Liu, Y.; Han, J.; Liao, Y.; DeBruyn, J.M. Fungal communities in rhizosphere soil under conservation tillage shift in response to plant growth. Front. Microbiol. 2017, 8, 1301. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant growth-promoting fungi (PGPF): Phytostimulation and induced systemic resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Springer: Singapore, 2017; pp. 135–191. [Google Scholar]
- Wardle, D.A. Communities and ecosystems. In Communities and Ecosystems; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Almonacid-Muñoz, L.; Herrera, H.; Fuentes-Ramírez, A.; Vargas-Gaete, R.; Larama, G.; Jara, R.; Fernández-Urrutia, C.; da Silva Valadares, R.B. Tree Cover Species Modify the Diversity of Rhizosphere-Associated Microorganisms in Nothofagus obliqua (Mirb.) Oerst Temperate Forests in South-Central Chile. Forests 2022, 13, 756. [Google Scholar]
- Li, T.; Wu, S.; Yang, W.; Selosse, M.-A.; Gao, J. How mycorrhizal associations influence orchid distribution and population dynamics. Front. Plant Sci. 2021, 12, 647114. [Google Scholar] [CrossRef] [PubMed]
- Djordjević, V.; Tsiftsis, S. The role of ecological factors in distribution and abundance of terrestrial orchids. In Orchids Phytochemistry, Biology and Horticulture; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–72. [Google Scholar]
- Duran-Lopez, M.; Caroca-Cáceres, R.; Jahreis, K.; Narváez-Vera, M.; Ansaloni, R.; Cazar, M. The micorryzal fungi Ceratobasidium sp. and Sebacina vermifera promote seed germination and seedling development of the terrestrial orchid Epidendrum secundum Jacq. S. Afr. J. Bot. 2019, 125, 54–61. [Google Scholar] [CrossRef]
- Herrera, H.; Valadares, R.; Contreras, D.; Bashan, Y.; Arriagada, C. Mycorrhizal compatibility and symbiotic seed germination of orchids from the Coastal Range and Andes in south central Chile. Mycorrhiza 2017, 27, 175–188. [Google Scholar]
- Gónzalez, D.; Rodriguez-Carres, M.; Boekhout, T.; Stalpers, J.; Kuramae, E.E.; Nakatani, A.K.; Vilgalys, R.; Cubeta, M.A. Phylogenetic relationships of Rhizoctonia fungi within the Cantharellales. Fungal Biol. 2016, 120, 603–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moncalvo, J.-M.; Nilsson, R.H.; Koster, B.; Dunham, S.M.; Bernauer, T.; Matheny, P.B.; Porter, T.M.; Margaritescu, S.; Weiß, M.; Garnica, S. The cantharelloid clade: Dealing with incongruent gene trees and phylogenetic reconstruction methods. Mycologia 2006, 98, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Petrolli, R.; Vieira, C.A.; Jakalski, M.; Bocayuva, M.F.; Valle, C.; Cruz, E.D.S.; Selosse, M.A.; Martos, F.; Kasuya, M.C.M. A fine-scale spatial analysis of fungal communities on tropical tree bark unveils the epiphytic rhizosphere in orchids. New Phytol. 2021, 231, 2002–2014. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.K.; Taylor, D.L.; Whigham, D.F.; Burnett, R.K., Jr. Germination patterns in three terrestrial orchids relate to abundance of mycorrhizal fungi. J. Ecol. 2016, 104, 744–754. [Google Scholar] [CrossRef] [Green Version]
- Roy, M.; Yagame, T.; Yamato, M.; Iwase, K.; Heinz, C.; Faccio, A.; Bonfante, P.; Selosse, M.-A. Ectomycorrhizal Inocybe species associate with the mycoheterotrophic orchid Epipogium aphyllum but not its asexual propagules. Ann. Bot. 2009, 104, 595–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suetsugu, K.; Haraguchi, T.F.; Tanabe, A.S.; Tayasu, I. Specialized mycorrhizal association between a partially mycoheterotrophic orchid Oreorchis indica and a Tomentella taxon. Mycorrhiza 2021, 31, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.D.; Barrett, M.D.; Dalziell, E.L.; Dixon, K.W.; Swarts, N.D. Geographical range and host breadth of Sebacina orchid mycorrhizal fungi associating with Caladenia in south-western Australia. Bot. J. Linn. Soc. 2016, 182, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Freestone, M.W.; Swarts, N.D.; Reiter, N.; Tomlinson, S.; Sussmilch, F.C.; Wright, M.M.; Holmes, G.D.; Phillips, R.D.; Linde, C.C. Continental-scale distribution and diversity of Ceratobasidium orchid mycorrhizal fungi in Australia. Ann. Bot. 2021, 128, 329–343. [Google Scholar] [CrossRef]
- Davis, B.J.; Phillips, R.D.; Wright, M.; Linde, C.C.; Dixon, K.W. Continent-wide distribution in mycorrhizal fungi: Implications for the biogeography of specialized orchids. Ann. Bot. 2015, 116, 413–421. [Google Scholar] [CrossRef]
- Linde, C.C.; May, T.W.; Phillips, R.D.; Ruibal, M.; Smith, L.M.; Peakall, R. New species of Tulasnella associated with terrestrial orchids in Australia. IMA Fungus 2017, 8, 28–47. [Google Scholar] [CrossRef] [Green Version]
- Mujica, M.I.; Saez, N.; Cisternas, M.; Manzano, M.; Armesto, J.J.; Pérez, F. Relationship between soil nutrients and mycorrhizal associations of two Bipinnula species (Orchidaceae) from central Chile. Ann. Bot. 2016, 118, 149–158. [Google Scholar] [CrossRef] [Green Version]

| Sites | Malalcahuello (Andes Cordillera Piedmont) | Cholchol (Coastal Cordillera Piedmont) |
|---|---|---|
| Coordinates | 38°26′12″ S 71°33′01″ W | 38°35′27.6″ S 72°54′57.9″ W |
| Elevation | 1616 m.a.s.l. | 29 m.a.s.l. |
| Mean annual precipitation | 1225 mm (18.8 to 341 mm) a | 464 mm (1.9 to 118 mm) a |
| Mean annual temperature | 7.4 °C (3.5 to 16.5 °C) a | 12.7 °C (7 to 23 °C) a |
| Vegetation details/understory | Nothofagus dombeyii, Araucaria araucana, Nothofagus antarctica, Alstroemeria spp. | Pinus radiata, Eucalyptus globulus, Ulex europaeus, and a mixture of native grasses |
| Soil order | Andisol | Ultisol |
| Forest | Native humid temperate rainforest | Exotic tree plantations surrounded by native grassland |
| Andes | Coastal | |
|---|---|---|
| N a | 16.75 ± 0.96 | 12.00 ± 1.41 |
| P a | 29.00 ± 8.83 | 4.25 ± 0.50 |
| K a | 81.13 ± 9.24 | 109.48 ± 43.54 |
| pH b | 5.53 ± 0.17 | 5.62 ± 0.15 |
| SOM | 8.50 ± 0.58 | 4.25 ± 0.50 |
| K d | 0.21 ± 0.02 | 0.28 ± 0.11 |
| Na d | 0.04 ± 0.01 | 0.10 ± 0.01 |
| Ca d | 2.11 ± 0.81 | 4.32 ± 0.48 |
| Mg d | 0.45 ± 0.18 | 1.50 ± 0.19 |
| Al d | 0.40 ± 0.18 | 0.52 ± 0.31 |
| Al saturation c | 13.42 ± 7.39 | 7.95 ± 4.92 |
| Cation exchange capacity d | 3.20 ± 0.95 | 6.71 ± 0.44 |
| Base saturation d | 2.81 ± 1.01 | 6.19 ± 0.74 |
| Fe a | 74.75 ± 13.84 | 30.00 ± 5.10 |
| Alextractable a | 722.75 ± 29.00 | 264.50 ± 17.00 |
| Soil texture | Sandy loam | Clay loam |
| Isolate | Close Relative (% Identity) | GenBank Accession (Close Relative) | Isolation Source (Close Relative) | Germination Index |
|---|---|---|---|---|
| CCC2 | Ceratobasidium sp. (99%) | MK792996 | Chloraea gavilu | 2.02 ± 0.54 |
| GLM3 | Ceratobasidiaceae sp. (99%) | MK876128 | Bipinnula sp. | 0.75 ± 0.14 |
| GLM5 | Tulasnella sp. (100%) | MK793004 | Chloraea lechleri | 0.58 ± 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera, H.; Sanhueza, T.; da Silva Valadares, R.B.; Matus, F.; Pereira, G.; Atala, C.; Mora, M.d.l.L.; Arriagada, C. Diversity of Root-Associated Fungi of the Terrestrial Orchids Gavilea lutea and Chloraea collicensis in a Temperate Forest Soil of South-Central Chile. J. Fungi 2022, 8, 794. https://doi.org/10.3390/jof8080794
Herrera H, Sanhueza T, da Silva Valadares RB, Matus F, Pereira G, Atala C, Mora MdlL, Arriagada C. Diversity of Root-Associated Fungi of the Terrestrial Orchids Gavilea lutea and Chloraea collicensis in a Temperate Forest Soil of South-Central Chile. Journal of Fungi. 2022; 8(8):794. https://doi.org/10.3390/jof8080794
Chicago/Turabian StyleHerrera, Héctor, Tedy Sanhueza, Rafael Borges da Silva Valadares, Francisco Matus, Guillermo Pereira, Cristian Atala, María de la Luz Mora, and Cesar Arriagada. 2022. "Diversity of Root-Associated Fungi of the Terrestrial Orchids Gavilea lutea and Chloraea collicensis in a Temperate Forest Soil of South-Central Chile" Journal of Fungi 8, no. 8: 794. https://doi.org/10.3390/jof8080794
APA StyleHerrera, H., Sanhueza, T., da Silva Valadares, R. B., Matus, F., Pereira, G., Atala, C., Mora, M. d. l. L., & Arriagada, C. (2022). Diversity of Root-Associated Fungi of the Terrestrial Orchids Gavilea lutea and Chloraea collicensis in a Temperate Forest Soil of South-Central Chile. Journal of Fungi, 8(8), 794. https://doi.org/10.3390/jof8080794







