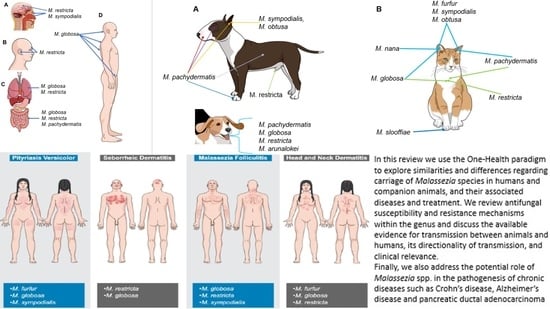
Malassezia: Zoonotic Implications, Parallels and Differences in Colonization and Disease in Humans and Animals
Abstract
1. Introduction
2. Classification of Malassezia Yeasts
3. Malassezia Species in the Environment and Possible Vectors
4. Malassezia Species and Their Role as Commensals in Humans
5. Malassezia Species and Their Role as Commensals in Companion Animals
6. Malassezia Species in Other Animals
7. Zoonotic and Reverse Zoonotic Transmission of Malassezia Species
8. Superficial Malassezia-Associated Diseases in Humans and Animals
8.1. Malassezia-Associated Dermatological Diseases in Humans
8.1.1. Pityriasis Versicolor
8.1.2. Seborrheic Dermatitis
8.1.3. Malassezia Folliculitis
8.1.4. Atopic Dermatitis (Head and Neck Dermatitis)
8.2. Malassezia Dermatitis and Otitis Externa in Animals
8.3. Miscellaneous Forms of Superficial Malassezia-Associated Diseases
9. Systemic Infections and Chronic Malassezia-Associated Diseases in Humans and Animals
9.1. Fungemia and Systemic Infections
9.2. Chronic Diseases in Humans and Animals
10. Antifungal Susceptibility Testing
10.1. Methodology
10.2. Patterns of Antifungal Susceptibility
11. Resistance Mechanisms
12. Treatment of Malassezia-Related Diseases
12.1. Treatment of Malassezia-Associated Skin Diseases
12.1.1. Treatment in Animals
12.1.2. Treatment in Humans
12.2. Treatment of Systemic Malassezia Infections
13. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, G.; Zhao, H.; Li, C.; Rajapakse, M.P.; Wong, W.C.; Xu, J.; Saunders, C.W.; Reeder, N.L.; Reilman, R.A.; Scheynius, A.; et al. Genus-Wide Comparative Genomics of Malassezia Delineates Its Phylogeny, Physiology, and Niche Adaptation on Human Skin. PLoS Genet. 2015, 11, e1005614. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.; Morris, D.O.; Guillot, J.; Bensignor, E.J.; Robson, D.; Mason, K.V.; Kano, R.; Hill, P.B. Biology, diagnosis and treatment of Malassezia dermatitis in dogs and cats Clinical Consensus Guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 31, 28–74. [Google Scholar] [CrossRef] [PubMed]
- Saadatzadeh, M.R. The Immunology of the Mycelial Phase of Malassezia; University of Leeds: Leeds, UK, 1998. [Google Scholar]
- Saadatzadeh, M.; Ashbee, H.; Holland, K.; Ingham, E. Production of the mycelial phase of Malassezia in vitro. Sabouraudia 2001, 39, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Saadatzadeh, M.; Ashbee, H.; Cunliffe, W.; Ingham, E. Cell-mediated immunity to the mycelial phase of Malassezia spp. in patients with pityriasis versicolor and controls. Br. J. Dermatol. 2001, 144, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Prohic, A.; Jovovic Sadikovic, T.; Krupalija-Fazlic, M.; Kuskunovic-Vlahovljak, S. Malassezia species in healthy skin and in dermatological conditions. Int. J. Dermatol. 2016, 55, 494–504. [Google Scholar] [CrossRef]
- Gioti, A.; Nystedt, B.R.; Li, W.; Xu, J.; Andersson, A.; Averette, A.F.; MŘnch, K.; Wang, X.; Kappauf, C.; Kingsbury, J.M. Genomic insights into the atopic eczema-associated skin commensal yeast Malassezia sympodialis. MBio 2013, 4, e00572-12. [Google Scholar] [CrossRef]
- Shifrine, M.; Marr, A. The requirement of fatty acids by Pityrosporum ovale. Microbiology 1963, 32, 263–270. [Google Scholar] [CrossRef]
- Brunke, S.; Hube, B. MfLIP1, a gene encoding an extracellular lipase of the lipid-dependent fungus Malassezia furfur. Microbiology 2006, 152, 547–554. [Google Scholar] [CrossRef]
- Juntachai, W.; Oura, T.; Murayama, S.Y.; Kajiwara, S. The lipolytic enzymes activities of Malassezia species. Sabouraudia 2009, 47, 477–484. [Google Scholar] [CrossRef]
- Puig, L.; Bragulat, M.R.; Castella, G.; Cabanes, F.J. Characterization of the species Malassezia pachydermatis and re-evaluation of its lipid dependence using a synthetic agar medium. PLoS ONE 2017, 12, e0179148. [Google Scholar] [CrossRef]
- Vest, B.E.; Krauland, K. Malassezia Furfur. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Saunte, D.M.L.; Gaitanis, G.; Hay, R.J. Malassezia-Associated Skin Diseases, the Use of Diagnostics and Treatment. Front. Cell. Infect. Microbiol. 2020, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, W.; Theelen, B.; Boekhout, T.; Otranto, D.; Cafarchia, C. Malassezia spp. Yeasts of Emerging Concern in Fungemia. Front. Cell Infect. Microbiol. 2020, 10, 370. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.H.; Díaz, J.A.; Lee, S.A. Emerging opportunistic yeast infections. Lancet Infect. Dis. 2011, 11, 142–151. [Google Scholar] [CrossRef]
- Iatta, R.; Cafarchia, C.; Cuna, T.; Montagna, O.; Laforgia, N.; Gentile, O.; Rizzo, A.; Boekhout, T.; Otranto, D.; Montagna, M.T. Bloodstream infections by Malassezia and Candida species in critical care patients. Med. Mycol. 2014, 52, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, A.; Hadrich, I.; Neji, S.; Trabelsi, H.; Makni, F.; Ayadi, A. Real-Time PCR Identification of Six Malassezia Species. Curr. Microbiol. 2017, 74, 671–677. [Google Scholar] [CrossRef]
- Pedrosa, A.F.; Lisboa, C.; Rodrigues, A.G. Malassezia infections with systemic involvement: Figures and facts. J. Dermatol. 2018, 45, 1278–1282. [Google Scholar] [CrossRef]
- Salkin, I.; Stone, W.; Gordon, M. Association of Malassezia (Pityrosporum) pachydermatis with sarcoptic mange in New York State. J. Wildl. Dis. 1980, 16, 509–514. [Google Scholar] [CrossRef]
- Breuer-Strosberg, R.; Hochleithner, M.; Kuttin, E. Malassezia pachydermatis isolation from a scarlet macaw. Mycoses 1990, 33, 247–250. [Google Scholar] [CrossRef]
- Guillot, J.; Petit, T.; Degorce-Rubiales, F.; Guého, E.; Chermette, R. Dermatitis caused by Malassezia pachydermatis in a California sea lion (Zalophus californianus). Vet. Rec. 1998, 142, 311–312. [Google Scholar] [CrossRef]
- Nakagaki, K.; Hata, K.; Iwata, E.; Takeo, K. Malassezia pachydermatis isolated from a South American sea lion (Otaria byronia) with dermatitis. J. Vet. Med. Sci. 2000, 62, 901–903. [Google Scholar] [CrossRef]
- Begerow, D.; Bauer, R.; Boekhout, T. Phylogenetic placements of ustilaginomycetous anamorphs as deduced from nuclear LSU rDNA sequences. Mycol. Res. 2000, 104, 53–60. [Google Scholar] [CrossRef]
- Fell, J.W.; Boekhout, T.; Fonseca, A.; Scorzetti, G.; Statzell-Tallman, A. Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int. J. Syst. Evol. Microbiol. 2000, 50, 1351–1371. [Google Scholar] [CrossRef] [PubMed]
- Hibbett, D.S.; Binder, M.; Bischoff, J.F.; Blackwell, M.; Cannon, P.F.; Eriksson, O.E.; Huhndorf, S.; James, T.; Kirk, P.M.; Lücking, R. A higher-level phylogenetic classification of the Fungi. Mycol. Res. 2007, 111, 509–547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.-M.; Theelen, B.; Groenewald, M.; Bai, F.-Y.; Boekhout, T. Moniliellomycetes and Malasseziomycetes, two new classes in Ustilaginomycotina. Pers. Mol. Phylogeny Evol. Fungi 2014, 33, 41. [Google Scholar] [CrossRef] [PubMed]
- Lorch, J.M.; Palmer, J.M.; Vanderwolf, K.J.; Schmidt, K.Z.; Verant, M.L.; Weller, T.J.; Blehert, D.S. Malassezia vespertilionis sp. nov.: A new cold-tolerant species of yeast isolated from bats. Persoonia 2018, 41, 56–70. [Google Scholar] [CrossRef]
- Castella, G.; Coutinho, S.D.; Cabanes, F.J. Phylogenetic relationships of Malassezia species based on multilocus sequence analysis. Med. Mycol. 2014, 52, 99–105. [Google Scholar] [PubMed]
- Cabanes, F.J.; Theelen, B.; Castella, G.; Boekhout, T. Two new lipid-dependent Malassezia species from domestic animals. FEMS Yeast Res. 2007, 7, 1064–1076. [Google Scholar] [PubMed]
- Cabañes, F.; Vega, S.; Castellá, G. Malassezia cuniculi sp. nov. a novel yeast species isolated from rabbit skin. Med. Mycol. 2011, 49, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Cabanes, F.J.; Coutinho, S.D.; Puig, L.; Bragulat, M.R.; Castella, G. New lipid-dependent Malassezia species from parrots. Rev. Iberoam. Micol. 2016, 33, 92–99. [Google Scholar] [CrossRef]
- Park, M.; Cho, Y.J.; Lee, Y.W.; Jung, W.H. Whole genome sequencing analysis of the cutaneous pathogenic yeast Malassezia restricta and identification of the major lipase expressed on the scalp of patients with dandruff. Mycoses 2017, 60, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.C.; Bertignac, M.; Iltis, A.; Kolder, I.; Pirovano, W.; Jourdain, R.; Clavaud, C. Complete Genome Sequence of Malassezia restricta CBS 7877, an Opportunist Pathogen Involved in Dandruff and Seborrheic Dermatitis. Microbiol. Resour. Announc. 2019, 8, e01543-18. [Google Scholar] [CrossRef] [PubMed]
- Chow, N.A.; Chinn, R.; Pong, A.; Schultz, K.; Kim, J.; Gade, L.; Jackson, B.R.; Beer, K.D.; Litvintseva, A.P. Use of whole-genome sequencing to detect an outbreak of Malassezia pachydermatis infection and colonization in a neonatal intensive care unit—California, 2015–2016. Infect. Control. Hosp. Epidemiol. 2020, 41, 851–853. [Google Scholar] [CrossRef] [PubMed]
- D’Andreano, S.; Viñes, J.; Francino, O. Whole-Genome Sequencing and De Novo Assembly of Malassezia pachydermatis Isolated from the Ear Canal of a Dog with Otitis. Microbiol. Resour. Announc. 2021, 10, e00205-21. [Google Scholar] [CrossRef] [PubMed]
- Amend, A. From dandruff to deep-sea vents: Malassezia-like fungi are ecologically hyper-diverse. PLoS Pathog. 2014, 10, e1004277. [Google Scholar] [CrossRef] [PubMed]
- Amend, A.; Burgaud, G.; Cunliffe, M.; Edgcomb, V.P.; Ettinger, C.L.; Gutiérrez, M.; Heitman, J.; Hom, E.F.; Ianiri, G.; Jones, A.C. Fungi in the marine environment: Open questions and unsolved problems. MBio 2019, 10, e01189-18. [Google Scholar] [CrossRef]
- Theelen, B.; Cafarchia, C.; Gaitanis, G.; Bassukas, I.D.; Boekhout, T.; Dawson, T.L., Jr. Malassezia ecology, pathophysiology, and treatment. Med. Mycol. 2018, 56 (Suppl. S1), S10–S25. [Google Scholar] [CrossRef]
- Arenz, B.E.; Held, B.W.; Jurgens, J.A.; Farrell, R.L.; Blanchette, R.A. Fungal diversity in soils and historic wood from the Ross Sea Region of Antarctica. Soil Biol. Biochem. 2006, 38, 3057–3064. [Google Scholar] [CrossRef]
- Fell, J.W.; Scorzetti, G.; Connell, L.; Craig, S. Biodiversity of micro-eukaryotes in Antarctic Dry Valley soils with<5% soil moisture. Soil Biol. Biochem. 2006, 38, 3107–3119. [Google Scholar]
- Bass, D.; Howe, A.; Brown, N.; Barton, H.; Demidova, M.; Michelle, H.; Li, L.; Sanders, H.; Watkinson, S.C.; Willcock, S.; et al. Yeast forms dominate fungal diversity in the deep oceans. Proc. Biol. Sci. 2007, 274, 3069–3077. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Cao, L.; Tan, H.; Fang, S.; Huang, Y.; Zhou, S. Fungal communities from methane hydrate-bearing deep-sea marine sediments in South China Sea. ISME J. 2007, 1, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Le Calvez, T.; Burgaud, G.; Mahe, S.; Barbier, G.; Vandenkoornhuyse, P. Fungal diversity in deep-sea hydrothermal ecosystems. Appl. Environ. Microbiol. 2009, 75, 6415–6421. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Johnson, Z.I.; Wang, G. Molecular characterization of the spatial diversity and novel lineages of mycoplankton in Hawaiian coastal waters. ISME J. 2010, 4, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Watthana, S.; Stier, A.; Richard, F.; Vessabutr, S.; Selosse, M.A. Two mycoheterotrophic orchids from Thailand tropical dipterocarpacean forests associate with a broad diversity of ectomycorrhizal fungi. BMC Biol. 2009, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Jebaraj, C.S.; Raghukumar, C.; Behnke, A.; Stoeck, T. Fungal diversity in oxygen-depleted regions of the Arabian Sea revealed by targeted environmental sequencing combined with cultivation. FEMS Microbiol. Ecol. 2010, 71, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Edgcomb, V.P.; Beaudoin, D.; Gast, R.; Biddle, J.F.; Teske, A. Marine subsurface eukaryotes: The fungal majority. Environ. Microbiol. 2011, 13, 172–183. [Google Scholar] [CrossRef]
- Singh, P.; Raghukumar, C.; Verma, P.; Shouche, Y. Fungal community analysis in the deep-sea sediments of the Central Indian Basin by culture-independent approach. Microb. Ecol. 2011, 61, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.A.; Jones, M.D.; Leonard, G.; Bass, D. Marine fungi: Their ecology and molecular diversity. Ann. Rev. Mar. Sci. 2012, 4, 495–522. [Google Scholar] [CrossRef]
- Orsi, W.; Biddle, J.F.; Edgcomb, V. Deep sequencing of subseafloor eukaryotic rRNA reveals active Fungi across marine subsurface provinces. PLoS ONE 2013, 8, e56335. [Google Scholar]
- Zhang, T.; Wang, N.F.; Zhang, Y.Q.; Liu, H.Y.; Yu, L.Y. Diversity and distribution of fungal communities in the marine sediments of Kongsfjorden, Svalbard (High Arctic). Sci. Rep. 2015, 5, 14524. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.L.; Guo, S.Y.; Chen, I.A.; Burgaud, G.; Luo, Z.H.; Dahms, H.U.; Hwang, J.S.; Lin, Y.L.; Huang, J.S.; Ho, T.W.; et al. Insights into fungal diversity of a shallow-water hydrothermal vent field at Kueishan Island, Taiwan by culture-based and metabarcoding analyses. PLoS ONE 2019, 14, e0226616. [Google Scholar] [CrossRef]
- Gao, Z.; Li, B.; Zheng, C.; Wang, G. Molecular detection of fungal communities in the Hawaiian marine sponges Suberites zeteki and Mycale armata. Appl. Environ. Microbiol. 2008, 74, 6091–6101. [Google Scholar] [CrossRef] [PubMed]
- Amend, A.S.; Barshis, D.J.; Oliver, T.A. Coral-associated marine fungi form novel lineages and heterogeneous assemblages. ISME J. 2012, 6, 1291–1301. [Google Scholar] [CrossRef]
- Renker, C.; Alphei, J.; Buscot, F. Soil nematodes associated with the mammal pathogenic fungal genus Malassezia (Basidiomycota: Ustilaginomycetes) in Central European forests. Biol. Fertil. Soils 2003, 37, 70–72. [Google Scholar] [CrossRef]
- Tondello, A.; Vendramin, E.; Villani, M.; Baldan, B.; Squartini, A. Fungi associated with the southern Eurasian orchid Spiranthes spiralis (L.) Chevall. Fungal Biol. 2012, 116, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Quandt, A.; Glasco, A.; James, T. Intestinal Mycobiome Variation Across Geography and Phylogeny in the Snail Genus Conus. Genetics Society of America. In Proceedings of the 29th Fungal Genetics Conference Asilomar, Asilomar, CA, USA, 14–19 March 2017; Volume 20. [Google Scholar]
- Malacrinò, A.; Schena, L.; Campolo, O.; Laudani, F.; Mosca, S.; Giunti, G.; Strano, C.P.; Palmeri, V. A metabarcoding survey on the fungal microbiota associated to the olive fruit fly. Microb. Ecol. 2017, 73, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Duarte, E.; Resende, J.; Rosa, C.; Hamdan, J. Prevalence of yeasts and mycelial fungi in bovine parasitic otitis in the State of Minas Gerais, Brazil. J. Vet. Med. Ser. B 2001, 48, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Elhady, A.; Gine, A.; Topalovic, O.; Jacquiod, S.; Sorensen, S.J.; Sorribas, F.J.; Heuer, H. Microbiomes associated with infective stages of root-knot and lesion nematodes in soil. PLoS ONE 2017, 12, e0177145. [Google Scholar] [CrossRef] [PubMed]
- Simmons, R.B.; Gueho, E. A new species of Malassezia. Mycol. Res. 1990, 94, 1146–1149. [Google Scholar] [CrossRef]
- Nakabayashi, A.; Sei, Y.; Guillot, J. Identification of Malassezia species isolated from patients with seborrhoeic dermatitis, atopic dermatitis, pityriasis versicolor and normal subjects. Med. Mycol. 2000, 38, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Bernier, V.; Weill, F.X.; Hirigoyen, V.; Elleau, C.; Feyler, A.; Labrèze, C.; Sarlangue, J.; Chène, G.; Couprie, B.; Taïeb, A. Skin colonization by Malassezia species in neonates: A prospective study and relationship with neonatal cephalic pustulosis. Arch. Dermatol. 2002, 138, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Takashima, M.; Shinoda, T.; Suto, H.; Unno, T.; Tsuboi, R.; Ogawa, H.; Nishikawa, A. New yeast species, Malassezia dermatis, isolated from patients with atopic dermatitis. J. Clin. Microbiol. 2002, 40, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Takashima, M.; Kodama, M.; Tsuboi, R.; Nishikawa, A. Description of a new yeast species, Malassezia japonica, and its detection in patients with atopic dermatitis and healthy subjects. J. Clin. Microbiol. 2003, 41, 4695–4699. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Tajima, M.; Takashima, M.; Amaya, M.; Saito, M.; Tsuboi, R.; Nishikawa, A. A new yeast, Malassezia yamatoensis, isolated from a patient with seborrheic dermatitis, and its distribution in patients and healthy subjects. Microbiol. Immunol. 2004, 48, 579–583. [Google Scholar] [CrossRef]
- Gupta, A.K.; Boekhout, T.; Theelen, B.; Summerbell, R.; Batra, R. Identification and typing of Malassezia species by amplified fragment length polymorphism and sequence analyses of the internal transcribed spacer and large-subunit regions of ribosomal DNA. J. Clin. Microbiol. 2004, 42, 4253–4260. [Google Scholar] [CrossRef] [PubMed]
- Batra, R.; Boekhout, T.; Guého, E.; Cabanes, F.J.; Dawson, T.L., Jr.; Gupta, A.K. Malassezia Baillon, emerging clinical yeasts. FEMS Yeast Res. 2005, 5, 1101–1113. [Google Scholar] [CrossRef]
- Boekhout, T.; Guého-Kellermann, E.; Mayser, P.; Velegraki, A. Malassezia and the Skin: Science and Clinical Practice; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Gaitanis, G.; Velegraki, A.; Mayser, P.; Bassukas, I.D. Skin diseases associated with Malassezia yeasts: Facts and controversies. Clin. Dermatol. 2013, 31, 455–463. [Google Scholar] [CrossRef]
- Cabañes, F. Malassezia Yeasts: How Many Species Infect Humans and Animals? PLoS Pathog. 2014, 10, e1003892. [Google Scholar] [CrossRef]
- Aguirre, C.; Euliarte, C.; Finquelievich, J.; de los Ángeles Sosa, M.; Giusiano, G. Fungemia and interstitial lung compromise caused by Malassezia sympodialis in a pediatric patient. Rev. Iberoam. Micol. 2015, 32, 118–121. [Google Scholar] [CrossRef]
- Honnavar, P.; Chakrabarti, A.; Dogra, S.; Handa, S.; Rudramurthy, S.M. Phenotypic and molecular characterization of Malassezia japonica isolated from psoriasis vulgaris patients. J. Med. Microbiol. 2015, 64, 232–236. [Google Scholar] [CrossRef][Green Version]
- Honnavar, P.; Prasad, G.S.; Ghosh, A.; Dogra, S.; Handa, S.; Rudramurthy, S.M. Malassezia arunalokei sp. nov. a novel yeast species isolated from seborrheic dermatitis patients and healthy individuals from India. J. Clin. Microbiol. 2016, 54, 1826–1834. [Google Scholar] [CrossRef]
- Patron, R.L. A 34-year-old man with cough, lung nodules, fever, and eosinophilia. Clin. Infect. Dis. 2016, 63, 1525–1526. [Google Scholar] [PubMed]
- Niemiec, B.A.; Gawor, J.; Tang, S.; Prem, A.; Krumbeck, J.A. The mycobiome of the oral cavity in healthy dogs and dogs with periodontal disease. Am. J. Vet. Res. 2021, 83, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Nagata, R.; Nagano, H.; Ogishima, D.; Nakamura, Y.; Hiruma, M.; Sugita, T. Transmission of the major skin microbiota, Malassezia, from mother to neonate. Pediatrics Int. 2012, 54, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Jo, J.-H.; Deming, C.; Kennedy, E.A.; Conlan, S.; Polley, E.C.; Ng, W.-I.; Segre, J.A.; Kong, H.H.; Program, N.C.S. Diverse human skin fungal communities in children converge in adulthood. J. Investig. Dermatol. 2016, 136, 2356–2363. [Google Scholar] [CrossRef]
- Ward, T.L.; Dominguez-Bello, M.G.; Heisel, T.; Al-Ghalith, G.; Knights, D.; Gale, C.A. Development of the human mycobiome over the first month of life and across body sites. MSystems 2018, 3, e00140-17. [Google Scholar] [CrossRef] [PubMed]
- Gueho, E.; Midgley, G.; Guillot, J. The genus Malassezia with description of four new species. Antonie Van Leeuwenhoek 1996, 69, 337–355. [Google Scholar] [CrossRef]
- Aspiroz, C.; Moreno, L.-A.; Rezusta, A.; Rubio, C. Differentiation of three biotypes of Malassezia species on human normal skin. Correspondence with M. globosa, M. sympodialis and M. restricta. Mycopathologia 1999, 145, 69–74. [Google Scholar] [CrossRef]
- Gupta, A.; Kohli, Y.; Summerbell, R.; Faergemann, J. Quantitative culture of Malassezia species from different body sites of individuals with or without dermatoses. Med. Mycol. 2001, 39, 243–251. [Google Scholar] [CrossRef]
- Gemmer, C.M.; DeAngelis, Y.M.; Theelen, B.; Boekhout, T.; Dawson, T.L., Jr. Fast, noninvasive method for molecular detection and differentiation of Malassezia yeast species on human skin and application of the method to dandruff microbiology. J. Clin. Microbiol. 2002, 40, 3350–3357. [Google Scholar] [CrossRef]
- Gupta, A.K.; Batra, R.; Bluhm, R.; Boekhout, T.; Dawson, T.L., Jr. Skin diseases associated with Malassezia species. J. Am. Acad. Dermatol. 2004, 51, 785–798. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, G.; Velegraki, A.; Alexopoulos, E.; Chasapi, V.; Tsigonia, A.; Katsambas, A. Distribution of Malassezia species in pityriasis versicolor and seborrhoeic dermatitis in Greece. Typing of the major pityriasis versicolor isolate M. globosa. Br. J. Dermatol. 2006, 154, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Tajima, M.; Tsubuku, H.; Tsuboi, R.; Nishikawa, A. Quantitative analysis of cutaneous Malassezia in atopic dermatitis patients using real-time PCR. Microbiol. Immunol. 2006, 50, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Akaza, N.; Akamatsu, H.; Sasaki, Y.; Takeoka, S.; Kishi, M.; Mizutani, H.; Sano, A.; Hirokawa, K.; Nakata, S.; Matsunaga, K. Cutaneous Malassezia microbiota of healthy subjects differ by sex, body part and season. J. Dermatol. 2010, 37, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Clavaud, C.; Jourdain, R.; Bar-Hen, A.; Tichit, M.; Bouchier, C.; Pouradier, F.; El Rawadi, C.; Guillot, J.; Ménard-Szczebara, F.; Breton, L. Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp. PLoS ONE 2013, 8, e58203. [Google Scholar] [CrossRef]
- Tanaka, A.; Cho, O.; Saito, M.; Tsuboi, R.; Kurakado, S.; Sugita, T. Molecular characterization of the skin fungal microbiota in patients with seborrheic dermatitis. J. Clin. Exp. Dermatol. Res. 2014, 5, 239. [Google Scholar]
- Leung, M.H.; Chan, K.C.; Lee, P.K. Skin fungal community and its correlation with bacterial community of urban Chinese individuals. Microbiome 2016, 4, 1–15. [Google Scholar] [CrossRef]
- Aydogan, K.; Tore, O.; Akcaglar, S.; Oral, B.; Ener, B.; Tunalı, S.; Saricaoglu, H. Effects of Malassezia yeasts on serum Th1 and Th2 cytokines in patients with guttate psoriasis. Int. J. Dermatol. 2013, 52, 46–52. [Google Scholar]
- Gomez-Moyano, E.; Crespo-Erchiga, V.; Martínez-Pilar, L.; Diaz, D.G.; Martínez-García, S.; Navarro, M.L.; Casaño, A.V. Do Malassezia species play a role in exacerbation of scalp psoriasis? J. Mycol. Med. 2014, 24, 87–92. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, R.; Ran, Y.; Dai, Y.; Lu, Y.; Wang, P. Genetic polymorphism of Malassezia furfur isolates from Han and Tibetan ethnic groups in China using DNA fingerprinting. Med. Mycol. 2010, 48, 1034–1038. [Google Scholar] [CrossRef][Green Version]
- Leong, C.; Schmid, B.; Toi, M.J.; Wang, J.; Irudayaswamy, A.S.; Goh, J.P.Z.; Bosshard, P.P.; Glatz, M.; Dawson, T.L., Jr. Geographical and ethnic differences influence culturable commensal yeast diversity on healthy skin. Front. Microbiol. 2019, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Grice, E.A.; Dawson, T.L. Host–microbe interactions: Malassezia and human skin. Curr. Opin. Microbiol. 2017, 40, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Gaitanis, G.; Velegraki, A.; Alexopoulos, E.C.; Kapsanaki-Gotsi, E.; Zisova, L.; Ran, Y.; Zhang, H.; Arsenis, G.; Bassukas, I.D.; Faergemann, J. Malassezia furfur fingerprints as possible markers for human phylogeography. ISME J. 2009, 3, 498–502. [Google Scholar] [CrossRef]
- Giusiano, G.; de los Angeles Sosa, M.; Rojas, F.; Vanacore, S.T.; Mangiaterra, M. Prevalence of Malassezia species in pityriasis versicolor lesions in northeast Argentina. Rev. Iberoam. Micol. 2010, 27, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Sugita, T.; Suzuki, M.; Goto, S.; Nishikawa, A.; Hiruma, M.; Yamazaki, T.; Makimura, K. Quantitative analysis of the cutaneous Malassezia microbiota in 770 healthy Japanese by age and gender using a real-time PCR assay. Med. Mycol. 2010, 48, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Kong, H.H. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef]
- Oh, J.; Byrd, A.L.; Deming, C.; Conlan, S.; Kong, H.H.; Segre, J.A. Biogeography and individuality shape function in the human skin metagenome. Nature 2014, 514, 59–64. [Google Scholar] [CrossRef]
- Prohic, A.; Simic, D.; Sadikovic, T.J.; Krupalija-Fazlic, M. Distribution of Malassezia species on healthy human skin in Bosnia and Herzegovina: Correlation with body part, age and gender. Iran J. Microbiol. 2014, 6, 253–262. [Google Scholar]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef]
- Oh, K.J.; Lee, S.E.; Jung, H.; Kim, G.; Romero, R.; Yoon, B.H. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J. Perinat. Med. 2010, 38, 261–268. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.B.; Jordan, S.; Baker, B.J.; Carlson, N.S. The maternal infant microbiome: Considerations for labor and birth. MCN. Am. J. Matern. Child Nurs. 2017, 42, 318. [Google Scholar] [CrossRef] [PubMed]
- Georgountzou, A.; Papadopoulos, N.G. Postnatal innate immune development: From birth to adulthood. Front. Immunol. 2017, 8, 957. [Google Scholar] [CrossRef] [PubMed]
- Bellemain, E.; Carlsen, T.; Brochmann, C.; Coissac, E.; Taberlet, P.; Kauserud, H. ITS as an environmental DNA barcode for fungi: An in silico approach reveals potential PCR biases. BMC Microbiol. 2010, 10, 1–9. [Google Scholar] [CrossRef]
- Bazzicalupo, A.L.; Bálint, M.; Schmitt, I. Comparison of ITS1 and ITS2 rDNA in 454 sequencing of hyperdiverse fungal communities. Fungal Ecol. 2013, 6, 102–109. [Google Scholar] [CrossRef]
- Gaitanis, G.; Magiatis, P.; Hantschke, M.; Bassukas, I.D.; Velegraki, A. The Malassezia genus in skin and systemic diseases. Clin. Microbiol. Rev. 2012, 25, 106–141. [Google Scholar] [CrossRef]
- Dupuy, A.K.; David, M.S.; Li, L.; Heider, T.N.; Peterson, J.D.; Montano, E.A.; Dongari-Bagtzoglou, A.; Diaz, P.I.; Strausbaugh, L.D. Redefining the human oral mycobiome with improved practices in amplicon-based taxonomy: Discovery of Malassezia as a prominent commensal. PLoS ONE 2014, 9, e90899. [Google Scholar] [CrossRef]
- Nash, A.K.; Auchtung, T.A.; Wong, M.C.; Smith, D.P.; Gesell, J.R.; Ross, M.C.; Stewart, C.J.; Metcalf, G.A.; Muzny, D.M.; Gibbs, R.A. The gut mycobiome of the Human Microbiome Project healthy cohort. Microbiome 2017, 5, 1–13. [Google Scholar] [CrossRef]
- Hoggard, M.; Zoing, M.; Biswas, K.; Taylor, M.W.; Douglas, R.G. The sinonasal mycobiota in chronic rhinosinusitis and control patients. Rhinology 2019, 57, 190–199. [Google Scholar] [CrossRef]
- Pisa, D.; Alonso, R.; Carrasco, L. Parkinson’s Disease: A Comprehensive Analysis of Fungi and Bacteria in Brain Tissue. Int. J. Biol. Sci. 2020, 16, 1135. [Google Scholar] [CrossRef]
- Abdillah, A.; Ranque, S. Chronic Diseases Associated with Malassezia Yeast. J. Fungi 2021, 7, 855. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zhang, I.; Kyman, S.; Kask, O.; Cope, E.K. Co-infection of Malassezia sympodialis with bacterial pathobionts Pseudomonas aeruginosa or Staphylococcus aureus leads to distinct sinonasal inflammatory responses in a murine acute sinusitis model. Front. Cell. Infect. Microbiol. 2020, 10, 472. [Google Scholar] [CrossRef] [PubMed]
- Hallen-Adams, H.E.; Suhr, M.J. Fungi in the healthy human gastrointestinal tract. Virulence 2017, 8, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Fraczek, M.G.; Chishimba, L.; Niven, R.M.; Bromley, M.; Simpson, A.; Smyth, L.; Denning, D.W.; Bowyer, P. Corticosteroid treatment is associated with increased filamentous fungal burden in allergic fungal disease. J. Allergy Clin. Immunol. 2018, 142, 407–414. [Google Scholar] [CrossRef]
- Martinsen, E.M.; Eagan, T.M.; Leiten, E.O.; Haaland, I.; Husebø, G.R.; Knudsen, K.S.; Drengenes, C.; Sanseverino, W.; Paytuví-Gallart, A.; Nielsen, R. The pulmonary mycobiome—A study of subjects with and without chronic obstructive pulmonary disease. PLoS ONE 2021, 16, e0248967. [Google Scholar] [CrossRef]
- Prado, M.R.; Brilhante, R.S.; Cordeiro, R.A.; Monteiro, A.J.; Sidrim, J.J.; Rocha, M.F. Frequency of yeasts and dermatophytes from healthy and diseased dogs. J. Vet. Diagn. Investig. 2008, 20, 197–202. [Google Scholar] [CrossRef]
- Brito, E.H.; Fontenelle, R.O.; Brilhante, R.S.; Cordeiro, R.A.; Monteiro, A.J.; Sidrim, J.J.; Rocha, M.F. The anatomical distribution and antimicrobial susceptibility of yeast species isolated from healthy dogs. Vet. J. 2009, 182, 320–326. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Diesel, A.; Patterson, A.P.; Older, C.E.; Mansell, J.M.; Suchodolski, J.S.; Rodrigues Hoffmann, A. What is living on your dog’s skin? Characterization of the canine cutaneous mycobiota and fungal dysbiosis in canine allergic dermatitis. FEMS Microbiol. Ecol. 2015, 91, fiv139. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Diesel, A.; Patterson, A.P.; Older, C.E.; Johnson, T.J.; Mansell, J.M.; Suchodolski, J.S.; Hoffmann, A.R. Characterization of the cutaneous mycobiota in healthy and allergic cats using next generation sequencing. Adv. Vet. Dermatol. 2017, 8, 84–94. [Google Scholar]
- Gustafson, B.A. Otitis Externa in the Dog. A Bacteriological and Experimental Study; Gernandts Boktryckeri: Stockholm, Sweden, 1955; p. 117. [Google Scholar]
- Dufait, R. Presence of Malassezia pachydermatis (syn. Pityrosporum canis) on the hair and feathers of domestic animals. Bull. Soc. Franc. Mycol. Med. 1985, 14, 19–22. [Google Scholar]
- Hajsig, M.; Tadic, V.; Lukman, P. Malassezia pachydermatis in dogs: Significance of its location. Vet Arhiv 1985, 55, 259–266. [Google Scholar]
- Bond, R.; Anthony, R.; Dodd, M.; Lloyd, D. Isolation of Malassezia sympodialis from feline skin. J. Med. Vet. Mycol. 1996, 34, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.; Howell, S.; Haywood, P.; Lloyd, D. Isolation of Malassezia sympodialis and Malassezia globosa from healthy pet cats. Vet. Rec. 1997, 141, 200–201. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.; Lloyd, D. Skin and mucosal populations of Malassezia pachydermatis in healthy and seborrhoeic basset hounds. Vet. Dermatol. 1997, 8, 101–106. [Google Scholar] [CrossRef]
- Raabe, P.; Mayser, P.; Weiss, R. Demonstration of Malassezia furfur and M. sympodialis together with M. pachydermatis in veterinary specimens: Nachweis von Malassezia furfur und M. sympodialis in veterinärmedizinischem Untersuchungsgut. Mycoses 1998, 41, 493–500. [Google Scholar] [CrossRef]
- Crespo, M.; Abarca, M.; Cabanes, F. Isolation of Malassezia furfur from a cat. J. Clin. Microbiol. 1999, 37, 1573–1574. [Google Scholar] [CrossRef]
- Crespo, M.; Abarca, M.; Cabanes, F. Atypical lipid-dependent Malassezia species isolated from dogs with otitis externa. J. Clin. Microbiol. 2000, 38, 2383–2385. [Google Scholar] [CrossRef]
- Crespo, M.; Abarca, M.; Cabanes, F. Otitis externa associated with Malassezia sympodialis in two cats. J. Clin. Microbiol. 2000, 38, 1263–1266. [Google Scholar] [CrossRef]
- Nardoni, S.; Mancianti, F.; Rum, A.; Corazza, M. Isolation of Malassezia species from healthy cats and cats with otitis. J. Feline Med. Surg. 2005, 7, 141–145. [Google Scholar] [CrossRef]
- Åhman, S.; Perrins, N.; Bond, R. Carriage of Malassezia spp. yeasts in healthy and seborrhoeic Devon Rex cats. Sabouraudia 2007, 45, 449–455. [Google Scholar] [CrossRef][Green Version]
- Bellis, F.D.; Castellá, G.; Cabañes, F.J.; Bond, R. Absence of DNA sequence diversity of the intergenic spacer 1 region in Malassezia nana isolates from cats. Med. Mycol. 2010, 48, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Castellá, G.; de Bellis, F.; Bond, R.; Cabañes, F.J. Molecular characterization of Malassezia nana isolates from cats. Vet. Microbiol. 2011, 148, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Sihelská, Z.; Čonková, E.; Váczi, P.; Harčárová, M.; Böhmová, E. Occurrence of Malassezia yeasts In dermatologically diseased dogs. Folia Vet. 2017, 61, 17–21. [Google Scholar] [CrossRef][Green Version]
- Older, C.E.; Diesel, A.B.; Lawhon, S.D.; Queiroz, C.R.; Henker, L.C.; Rodrigues Hoffmann, A. The feline cutaneous and oral microbiota are influenced by breed and environment. PLoS ONE 2019, 14, e0220463. [Google Scholar] [CrossRef]
- Meason-Smith, C.; Olivry, T.; Lawhon, S.D.; Hoffmann, A.R. Malassezia species dysbiosis in natural and allergen-induced atopic dermatitis in dogs. Med. Mycol. 2020, 58, 756–765. [Google Scholar] [CrossRef]
- Guillot, J.; Bond, R. Malassezia Yeasts in Veterinary Dermatology: An Updated Overview. Front. Cell Infect. Microbiol. 2020, 10, 79. [Google Scholar] [CrossRef]
- Crespo, M.; Abarca, M.; Cabañes, F.J. Occurrence of Malassezia spp. in the external ear canals of dogs and cats with and without otitis externa. Med. Mycol. 2002, 40, 115–121. [Google Scholar] [CrossRef]
- Hajsig, D.; Hajsig, M.; Svoboda Vukovic, D. Malassezia pachydermatis in healthy cats. Vet Arhiv 1990, 60, 69–73. [Google Scholar]
- Hirai, A.; Kano, R.; Makimura, K.; Duarte, E.R.; Hamdan, J.S.; Lachance, M.-A.; Yamaguchi, H.; Hasegawa, A. Malassezia nana sp. nov. a novel lipid-dependent yeast species isolated from animals. Int. J. Syst. Evol. Microbiol. 2004, 54, 623–627. [Google Scholar] [CrossRef]
- Ahman, S.E.; Bergstrom, K.E. Cutaneous carriage of Malassezia species in healthy and seborrhoeic Sphynx cats and a comparison to carriage in Devon Rex cats. J. Feline Med. Surg. 2009, 11, 970–976. [Google Scholar] [CrossRef]
- Volk, A.V.; Belyavin, C.E.; Varjonen, K.; Cadiergues, M.-C.; Stevens, K.B.; Bond, R. Malassezia pachydermatis and M nana predominate amongst the cutaneous mycobiota of Sphynx cats. J. Feline Med. Surg. 2010, 12, 917–922. [Google Scholar] [CrossRef] [PubMed]
- Bond, R.; Stevens, K.; Perrins, N.; Åhman, S. Carriage of Malassezia spp. yeasts in Cornish Rex, Devon Rex and Domestic short-haired cats: A cross-sectional survey. Vet. Dermatol. 2008, 19, 299–304. [Google Scholar] [CrossRef]
- Korbelik, J.; Singh, A.; Rousseau, J.; Weese, J.S. Analysis of the otic mycobiota in dogs with otitis externa compared to healthy individuals. Vet. Dermatol. 2018, 29, 417-e138. [Google Scholar] [CrossRef] [PubMed]
- Crespo, M.; Abarca, M.; Cabanes, F. Occurrence of Malassezia spp. in horses and domestic ruminants. Mycoses 2002, 45, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Cafarchia, C.; Gallo, S.; Capelli, G.; Otranto, D. Occurrence and population size of Malassezia spp. in the external ear canal of dogs and cats both healthy and with otitis. Mycopathologia 2005, 160, 143–149. [Google Scholar] [CrossRef]
- Dizotti, C.; Coutinho, S. Isolation of Malassezia pachydermatis and M. sympodialis from the external ear canal of cats with and without otitis externa. Acta Vet. Hung. 2007, 55, 471–477. [Google Scholar] [CrossRef]
- Shokri, H.; Khosravi, A.; Rad, M.; Jamshidi, S. Occurrence of Malassezia species in Persian and domestic short hair cats with and without otitis externa. J. Vet. Med. Sci. 2010, 72, 293–296. [Google Scholar] [CrossRef]
- Eidi, S.; Khosravi, A.R.; Jamshidi, S. A comparison of different kinds of Malassezia species in healthy dogs and dogs with otitis externa and skin lesions. Turk. J. Vet. Anim. Sci. 2011, 35, 345–350. [Google Scholar] [CrossRef]
- Bradley, C.W.; Lee, F.F.; Rankin, S.C.; Kalan, L.R.; Horwinski, J.; Morris, D.O.; Grice, E.A.; Cain, C.L. The otic microbiota and mycobiota in a referral population of dogs in eastern USA with otitis externa. Vet. Dermatol. 2020, 31, 225-e49. [Google Scholar] [CrossRef]
- Bradley, C.W.; Morris, D.O.; Rankin, S.C.; Cain, C.L.; Misic, A.M.; Houser, T.; Mauldin, E.A.; Grice, E.A. Longitudinal evaluation of the skin microbiome and association with microenvironment and treatment in canine atopic dermatitis. J. Investig. Dermatol. 2016, 136, 1182–1190. [Google Scholar] [CrossRef]
- Nardoni, S.; Mancianti, F.; Corazza, M.; Rum, A. Occurrence of Malassezia species in healthy and dermatologically diseased dogs. Mycopathologia 2004, 157, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Suchodolski, J.S.; Morris, E.K.; Allenspach, K.; Jergens, A.E.; Harmoinen, J.A.; Westermarck, E.; Steiner, J.M. Prevalence and identification of fungal DNA in the small intestine of healthy dogs and dogs with chronic enteropathies. Vet. Microbiol. 2008, 132, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Handl, S.; Dowd, S.E.; Garcia-Mazcorro, J.F.; Steiner, J.M.; Suchodolski, J.S. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol. Ecol. 2011, 76, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.S.; Dowd, S.E.; Suchodolski, J.S.; Middelbos, I.S.; Vester, B.M.; Barry, K.A.; Nelson, K.E.; Torralba, M.; Henrissat, B.; Coutinho, P.M. Phylogenetic and gene-centric metagenomics of the canine intestinal microbiome reveals similarities with humans and mice. ISME J. 2011, 5, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Foster, M.L.; Dowd, S.E.; Stephenson, C.; Steiner, J.M.; Suchodolski, J.S. Characterization of the fungal microbiome (mycobiome) in fecal samples from dogs. Vet. Med. Int. 2013, 2013, 658373. [Google Scholar] [CrossRef]
- Melgarejo, T.; Oakley, B.B.; Krumbeck, J.A.; Tang, S.; Krantz, A.; Linde, A. Assessment of bacterial and fungal populations in urine from clinically healthy dogs using next-generation sequencing. J. Vet. Intern. Med. 2021, 35, 1416–1426. [Google Scholar] [CrossRef]
- Garau, M.; del Palacio, A.; Garcia, J. Prevalence of Malassezia spp. in healthy pigs. Mycoses 2005, 48, 17–20. [Google Scholar] [CrossRef]
- Kuttin, E.; Glas, I. Mycotic otitis externa in animals. Mycoses 1985, 28, 61–68. [Google Scholar] [CrossRef]
- Pinter, L.; Anthony, R.M.; Glumac, N.; Hajsig, D.; Pogacnik, M.; Drobnic-Kosorok, M. Apparent cross-infection with a single strain of Malassezia pachydermatis on a pig farm. Acta Vet. Hung. 2002, 50, 151–156. [Google Scholar] [CrossRef]
- Nardoni, S.; Merildi, V.; Frangioni, S.; Ariti, G.; Verin, R.; Vannucci, P.; Mancianti, F. Isolation and characterization of Malassezia spp. in healthy swine of different breeds. Vet. Microbiol. 2010, 141, 155–158. [Google Scholar] [CrossRef]
- Colombo, S.; Nardoni, S.; Cornegliani, L.; Mancianti, F. Prevalence of Malassezia spp. yeasts in feline nail folds: A cytological and mycological study. Vet. Dermatol. 2007, 18, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Zia, M.; Mirhendi, H.; Toghyani, M. Detection and identification of Malassezia species in domestic animals and aquatic birds by PCR-RFLP. Iran J. Vet. Res. 2015, 16, 36–41. [Google Scholar] [PubMed]
- Duarte, E.; Batista, R.; Hahn, R.; Hamdan, J. Factors associated with the prevalence of Malassezia species in the external ears of cattle from the state of Minas Gerais, Brazil. Med. Mycol. 2003, 41, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Pin, D. Seborrhoeic dermatitis in a goat due to Malassezia pachydermatis. Vet. Dermatol. 2004, 15, 53–56. [Google Scholar] [CrossRef]
- Uzal, F.A.; Paulson, D.; Eigenheer, A.L.; Walker, R.L. Malassezia slooffiae-associated dermatitis in a goat. Vet. Dermatol. 2007, 18, 348–352. [Google Scholar] [CrossRef]
- Galuppi, R.; Morandi, B.; Agostini, S.; Dalla Torre, S.; Caffara, M. Survey on the Presence of Malassezia spp. in Healthy Rabbit Ear Canals. Pathogens 2020, 9, 696. [Google Scholar] [CrossRef]
- Mendes, J.F.; Albano, A.P.N.; Coimbra, M.A.A.; Ferreira, G.F.D.; Gonçalves, C.L.; Nascente, P.d.S.; Mello, J.R.B.d. Fungi isolated from the excreta of wild birds in screening centers in Pelotas, RS, Brazil. Rev. Do Inst. Med. Trop. São Paulo 2014, 56, 525–528. [Google Scholar] [CrossRef]
- Gründer, S.; Mayser, P.; Redmann, T.; Kaleta, E. Mycological examinations on the fungal flora of the chicken comb. Mycoses 2005, 48, 114–119. [Google Scholar] [CrossRef]
- Sugita, T.; Tajima, M.; Amaya, M.; Tsuboi, R.; Nishikawa, A. Genotype analysis of Malassezia restricta as the major cutaneous flora in patients with atopic dermatitis and healthy subjects. Microbiol. Immunol. 2004, 48, 755–759. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kano, R.; Nagata, M.; Hasegawa, A.; Kamata, H. Genotyping of Malassezia pachydermatis isolates from canine healthy skin and atopic dermatitis by internal spacer 1 (IGS1) region analysis. Vet. Dermatol. 2011, 22, 401–405. [Google Scholar] [CrossRef]
- Åkerstedt, J.; Vollset, I. Malassezia pachydermatis with special referenceto canine skin disease. Br. Vet. J. 1996, 152, 269–281. [Google Scholar] [CrossRef]
- Guillot, J.; Bond, R. Malassezia pachydermatis: A review. Med. Mycol. 1999, 37, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Outerbridge, C.A. Mycologic disorders of the skin. Clin. Tech. Small Anim. Pract. 2006, 21, 128–134. [Google Scholar] [CrossRef]
- Bajwa, J. Canine Malassezia dermatitis. Can. Vet. J. 2017, 58, 1119–1121. [Google Scholar] [PubMed]
- Dawson, T.L., Jr. Malassezia: The forbidden kingdom opens. Cell Host Microbe 2019, 25, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Bandhaya, M. The distribution of Malassezia furfur and Malassezia pachydermatis on normal human skin. Southeast Asian J. Trop. Med. Public Health 1993, 24, 343–346. [Google Scholar]
- Morris, D.O.; O’Shea, K.; Shofer, F.S.; Rankin, S. Malassezia pachydermatis carriage in dog owners. Emerg. Infect. Dis. 2005, 11, 83. [Google Scholar] [CrossRef]
- Welbel, S.F.; McNeil, M.M.; Pramanik, A.; Silberman, R.; Oberle, A.D.; Midgley, G.; Crow, S.; Jarvis, W.R. Nosocomial Malassezia pachydermatis bloodstream infections in a neonatal intensive care unit. Pediatric Infect. Dis. J. 1994, 13, 104–108. [Google Scholar] [CrossRef]
- Chang, H.J.; Miller, H.L.; Watkins, N.; Arduino, M.J.; Ashford, D.A.; Midgley, G.; Aguero, S.M.; Pinto-Powell, R.; von Reyn, C.F.; Edwards, W. An epidemic of Malassezia pachydermatis in an intensive care nursery associated with colonization of health care workers’ pet dogs. N. Engl. J. Med. 1998, 338, 706–711. [Google Scholar] [CrossRef]
- Chryssanthou, E.; Broberger, U.; Petrini, B. Malassezia pachydermatis fungaemia in a neonatal intensive care unit. Acta Paediatr. 2001, 90, 323–327. [Google Scholar] [CrossRef]
- Ayhan, M.; Sancak, B.; Karaduman, A.; Arıkan, S.; Şahin, S. Colonization of neonate skin by Malassezia species: Relationship with neonatal cephalic pustulosis. J. Am. Acad. Dermatol. 2007, 57, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Al-Sweih, N.; Ahmad, S.; Joseph, L.; Khan, S.; Khan, Z. Malassezia pachydermatis fungemia in a preterm neonate resistant to fluconazole and flucytosine. Med. Mycol. Case Rep. 2014, 5, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, S.; Marte, R.L. Malassezia pachydermatis fungaemia in an adult on posaconazole prophylaxis for acute myeloid leukaemia. Pathol. J. RCPA 2014, 46, 466–467. [Google Scholar] [CrossRef] [PubMed]
- Roman, J.; Bagla, P.; Ren, P.; Blanton, L.S.; Berman, M.A. Malassezia pachydermatis fungemia in an adult with multibacillary leprosy. Med. Mycol. Case Rep. 2016, 12, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cho, Y.G.; Im Choi, S.; Lee, H.S. First case of catheter-related Malassezia pachydermatis fungemia in an adult. Ann. Lab. Med. 2019, 39, 99–101. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Peng, C.-C.; Hsu, C.-H.; Chang, J.-H.; Chiu, N.-C.; Chi, H. Systemic infection caused by Malassezia pachydermatis in infants: Case series and review of the literature. Pediatric Infect. Dis. J. 2020, 39, 444–448. [Google Scholar] [CrossRef]
- Cafarchia, C.; Gallo, S.; Romito, D.; Capelli, G.; Chermette, R.; Guillot, J.; Otranto, D. Frequency, body distribution, and population size of Malassezia species in healthy dogs and in dogs with localized cutaneous lesions. J. Vet. Diagn. Investig. 2005, 17, 316–322. [Google Scholar] [CrossRef]
- Gueho, E.; Simmons, R.; Pruitt, W.; Meyer, S.; Ahearn, D. Association of Malassezia pachydermatis with systemic infections of humans. J. Clin. Microbiol. 1987, 25, 1789–1790. [Google Scholar] [CrossRef]
- Van Belkum, A.; Boekhout, T.; Bosboom, R. Monitoring spread of Malassezia infections in a neonatal intensive care unit by PCR-mediated genetic typing. J. Clin. Microbiol. 1994, 32, 2528–2532. [Google Scholar] [CrossRef]
- Iatta, R.; Figueredo, L.A.; Montagna, M.T.; Otranto, D.; Cafarchia, C. In vitro antifungal susceptibility of Malassezia furfur from bloodstream infections. J. Med. Microbiol. 2014, 63, 1467–1473. [Google Scholar] [CrossRef]
- Velegraki, A.; Cafarchia, C.; Gaitanis, G.; Iatta, R.; Boekhout, T. Malassezia infections in humans and animals: Pathophysiology, detection, and treatment. PLoS Pathog. 2015, 11, e1004523. [Google Scholar] [CrossRef] [PubMed]
- Archer-Dubon, C.; Icaza-Chivez, M.E.; Orozco-Topete, R.; Reyes, E.; Baez-Martinez, R.; Ponce de Leon, S. An epidemic outbreak of Malassezia folliculitis in three adult patients in an intensive care unit: A previously unrecognized nosocomial infection. Int. J. Dermatol. 1999, 38, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Ilahi, A.; Hadrich, I.; Goudjil, S.; Kongolo, G.; Chazal, C.; Léké, A.; Ayadi, A.; Chouaki, T.; Ranque, S. Molecular epidemiology of a Malassezia pachydermatis neonatal unit outbreak. Med. Mycol. 2018, 56, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Prohic, A.; Kasumagic-Halilovic, E. Identification of Malassezia pachydermatis from healthy and diseased human skin. Med. Arh. 2009, 63, 317–319. [Google Scholar]
- Fan, Y.-M.; Huang, W.-M.; Li, S.-F.; Wu, G.-F.; Lai, K.; Chen, R.-Y. Granulomatous skin infection caused by Malassezia pachydermatis in a dog owner. Arch. Dermatol. 2006, 142, 1181–1184. [Google Scholar] [CrossRef]
- Puig, L.; Bragulat, M.R.; Castella, G.; Cabanes, F.J. Phenotypic and genetic diversity of Malassezia furfur from domestic and zoo animals. Med. Mycol. 2018, 56, 941–949. [Google Scholar] [CrossRef]
- Rubenstein, R.M.; Malerich, S.A. Malassezia (pityrosporum) folliculitis. J. Clin. Aesthetic Dermatol. 2014, 7, 37. [Google Scholar]
- Tucker, D.; Masood, S. Seborrheic Dermatitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Karray, M.; McKinney, W.P. Tinea Versicolor. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Alvarado, Z.; Pereira, C. Fungal diseases in children and adolescents in a referral centre in Bogota, Colombia. Mycoses 2018, 61, 543–548. [Google Scholar] [CrossRef]
- De Luca, D.A.; Maianski, Z.; Averbukh, M. A study of skin disease spectrum occurring in Angola phototype V–VI population in Luanda. Int. J. Dermatol. 2018, 57, 849–855. [Google Scholar] [CrossRef]
- Diongue, K.; Kébé, O.; Faye, M.; Samb, D.; Diallo, M.; Ndiaye, M.; Seck, M.; Badiane, A.; Ranque, S.; Ndiaye, D. MALDI-TOF MS identification of Malassezia species isolated from patients with pityriasis versicolor at the Seafarers’ Medical Service in Dakar, Senegal. J. Mycol. Médicale 2018, 28, 590–593. [Google Scholar] [CrossRef]
- Brandi, N.; Starace, M.; Alessandrini, A.; Piraccini, B.M. Tinea versicolor of the neck as side effect of topical steroids for alopecia areata. J. Dermatol. Treat. 2019, 30, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Choi, F.D.; Juhasz, M.L.; Mesinkovska, N.A. Topical ketoconazole: A systematic review of current dermatological applications and future developments. J. Dermatol. Treat. 2019, 30, 760–771. [Google Scholar] [CrossRef] [PubMed]
- Errichetti, E.; Stinco, G. Dermoscopy in general dermatology: A practical overview. Dermatol. Ther. 2016, 6, 471–507. [Google Scholar] [CrossRef]
- Rosen, T. Mycological Considerations in the Topical Treatment of Superficial Fungal Infections. J. Drugs Dermatol. JDD 2016, 15 (Suppl. 2), s49–s55. [Google Scholar] [PubMed]
- Palamaras, I.; Kyriakis, K.; Stavrianeas, N. Seborrheic dermatitis: Lifetime detection rates. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 524–526. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, P.; Chiaradia, G.; de Carli, G.; Giuliani, M.; Mastroianni, C.M.; Barbacci, S.A.; Buonomini, A.R.; Grisetti, S.; Sampaolesi, A.; Corpolongo, A. The potential impact of routine testing of individuals with HIV indicator diseases in order to prevent late HIV diagnosis. BMC Infect. Dis. 2013, 13, 473. [Google Scholar] [CrossRef]
- Sanders, M.; Pardo, L.; Franco, O.; Ginger, R.; Nijsten, T. Prevalence and determinants of seborrhoeic dermatitis in a middle-aged and elderly population: The Rotterdam Study. Br. J. Dermatol. 2018, 178, 148–153. [Google Scholar] [CrossRef]
- Lally, A.; Casabonne, D.; Imko-Walczuk, B.; Newton, R.; Wojnarowska, F. Prevalence of benign cutaneous disease among Oxford renal transplant recipients. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 462–470. [Google Scholar] [CrossRef]
- Dessinioti, C.; Katsambas, A. Seborrheic dermatitis: Etiology, risk factors, and treatments: Facts and controversies. Clin. Dermatol. 2013, 31, 343–351. [Google Scholar] [CrossRef]
- Harada, K.; Saito, M.; Sugita, T.; Tsuboi, R. Malassezia species and their associated skin diseases. J. Dermatol. 2015, 42, 250–257. [Google Scholar] [CrossRef]
- Celis, A.; Wösten, H.; Triana, S.; Restrepo, S.; de Cock, H. Malassezia spp. beyond the mycobiota. SM Dermatol. J. 2017, 3, 1–10. [Google Scholar]
- Kamamoto, C.; Nishikaku, A.; Gompertz, O.; Melo, A.; Hassun, K.; Bagatin, E. Cutaneous fungal microbiome: Malassezia yeasts in seborrheic dermatitis scalp in a randomized, comparative and therapeutic trial. Derm. Endocrinol. 2017, 9, e1361573. [Google Scholar] [CrossRef] [PubMed]
- Peyri, J.; Lleonart, M. Clinical and therapeutic profile and quality of life of patients with seborrheic dermatitis. Actas Dermo-Sifiliográficas 2007, 98, 476–482. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Messenger, A.G.; Tosti, A.; Todd, G.; Hordinsky, M.; Hay, R.; Wang, X.; Zachariae, C.; Kerr, K.M.; Henry, J.P. A comprehensive pathophysiology of dandruff and seborrheic dermatitis: Towards a more precise definition of scalp health. Acta Derm. Venereol. 2013, 93, 131–137. [Google Scholar] [CrossRef]
- Parsad, D.; Saini, R.; Negi, K. Short-term treatment of pityrosporum folliculitis: A double blind placebo-controlled study. J. Eur. Acad. Dermatol. Venereol. 1998, 11, 188–190. [Google Scholar] [CrossRef]
- Poli, F. Differential diagnosis of facial acne on black skin. Int. J. Dermatol. 2012, 51, 24–26. [Google Scholar] [CrossRef]
- Durdu, M.; Güran, M.; Ilkit, M. Epidemiological characteristics of Malassezia folliculitis and use of the May-Grünwald-Giemsa stain to diagnose the infection. Diagn. Microbiol. Infect. Dis. 2013, 76, 450–457. [Google Scholar] [CrossRef]
- Hill, M.K.; Goodfield, M.J.; Rodgers, F.G.; Crowley, J.L.; Saihan, E.M. Skin surface electron microscopy in Pityrosporum folliculitis: The role of follicular occlusion in disease and the response to oral ketoconazole. Arch. Dermatol. 1990, 126, 1071–1074. [Google Scholar] [CrossRef]
- Erchiga, V.C.; Florencio, V.D. Malassezia species in skin diseases. Curr. Opin. Infect. Dis. 2002, 15, 133–142. [Google Scholar] [CrossRef]
- Akaza, N.; Akamatsu, H.; Sasaki, Y.; Kishi, M.; Mizutani, H.; Sano, A.; Hirokawa, K.; Nakata, S.; Nishijima, S.; Matsunaga, K. Malassezia folliculitis is caused by cutaneous resident Malassezia species. Med. Mycol. 2009, 47, 618–624. [Google Scholar] [CrossRef]
- Ko, J.H.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Epidemiologic study of Malassezia yeasts in patients with Malassezia folliculitis by 26S rDNA PCR-RFLP analysis. Ann. Dermatol. 2011, 23, 177–184. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tragiannidis, A.; Bisping, G.; Koehler, G.; Groll, A.H. Minireview: Malassezia infections in immunocompromised patients. Mycoses 2010, 53, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.V.; Martins, J.E.C.; de Ribeiro, E.B.O.; Sotto, M.N. Pityrosporum folliculitis: Renal transplantation case report. J. Dermatol. 2000, 27, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Potter, B.S.; Burgoon, C.F.; Johnson, W.C. Pityrosporum folliculitis: Report of seven cases and review of the Pityrosporum organism relative to cutaneous disease. Arch. Dermatol. 1973, 107, 388–391. [Google Scholar] [CrossRef]
- Hald, M.; Arendrup, M.C.; Svejgaard, E.L.; Lindskov, R.; Foged, E.K.; Saunte, D.M.L. Evidence-based Danish guidelines for the treatment of Malassezia-related skin diseases. Acta Derm. Venereol. 2015, 95, 12–19. [Google Scholar] [CrossRef]
- Bieber, T. Atopic Dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]
- Katsarou, A.; Armenaka, M. Atopic dermatitis in older patients: Particular points. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 12–18. [Google Scholar] [CrossRef]
- Baron, S.; Cohen, S.; Archer, C. British Association of Dermatologists and Royal College of General Practitioners. Guidance on the diagnosis and clinical management of atopic eczema. Clin. Exp. Dermatol. 2012, 37 (Suppl. S1), 7–12. [Google Scholar] [CrossRef]
- Weidinger, S.; Novak, N. Atopic dermatitis. Lancet 2016, 387, 1109–1122. [Google Scholar] [CrossRef]
- Kim, T.; Jang, I.; Park, Y.; Kim, H.; Kim, C. Head and neck dermatitis: The role of Malassezia furfur, topical steroid use and environmental factors in its causation. Clin. Exp. Dermatol. 1999, 24, 226–231. [Google Scholar] [CrossRef]
- Brehler, R.; Luger, T. Atopic dermatitis: The role of Pityrosporum ovale. J. Eur. Acad. Dermatol. Venereol. 2001, 15, 5–6. [Google Scholar] [CrossRef] [PubMed]
- Savolainen, J.; Lintu, P.; Kosonen, J.; Kortekangas-Savolainen, O.; Viander, M.; Pene, J.; Kalimo, K.; Terho, E.; Bousquet, J. Pityrosporum and Candida specific and non-specific humoral, cellular and cytokine responses in atopic dermatitis patients. Clin. Exp. Allergy 2001, 31, 125–134. [Google Scholar] [PubMed]
- Faergemann, J. Atopic dermatitis and fungi. Clin. Microbiol. Rev. 2002, 15, 545–563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Tanaka, T.; Tajima, M.; Tsuboi, R.; Kato, H.; Nishikawa, A.; Sugita, T. Anti-Malassezia-specific IgE antibodies production in Japanese patients with head and neck atopic dermatitis: Relationship between the level of specific IgE antibody and the colonization frequency of cutaneous Malassezia species and clinical severity. J. Allergy 2011, 2011, 645670. [Google Scholar] [CrossRef]
- Reguiaï, Z. In Atopic dermatitis of the adult: Clinical presentation, complications and comorbidities. Ann. Dermatol. Venereol. 2017, 144, VS15–VS22. [Google Scholar] [CrossRef]
- Silvestre Salvador, J.; Romero-Pérez, D.; Encabo-Durán, B. Atopic dermatitis in adults: A diagnostic challenge. J. Investig. Allergol. Clin. Immunol. 2017, 27, 78–88. [Google Scholar] [CrossRef]
- Hiragun, T.; Ishii, K.; Hiragun, M.; Suzuki, H.; Kan, T.; Mihara, S.; Yanase, Y.; Bartels, J.; Schröder, J.-M.; Hide, M. Fungal protein MGL_1304 in sweat is an allergen for atopic dermatitis patients. J. Allergy Clin. Immunol. 2013, 132, 608–615.e4. [Google Scholar] [CrossRef]
- Maarouf, M.; Saberian, C.; Lio, P.A.; Shi, V.Y. Head-and-neck dermatitis: Diagnostic difficulties and management pearls. Pediatric Dermatol. 2018, 35, 748–753. [Google Scholar] [CrossRef]
- Kohsaka, T.; Hiragun, T.; Ishii, K.; Hiragun, M.; Kamegashira, A.; Hide, M. Different hypersensitivities against homologous proteins of MGL_1304 in patients with atopic dermatitis. Allergol. Int. 2018, 67, 103–108. [Google Scholar] [CrossRef]
- Sugita, T.; Suto, H.; Unno, T.; Tsuboi, R.; Ogawa, H.; Shinoda, T.; Nishikawa, A. Molecular analysis of Malassezia microflora on the skin of atopic dermatitis patients and healthy subjects. J. Clin. Microbiol. 2001, 39, 3486–3490. [Google Scholar] [CrossRef]
- Guglielmo, A.; Sechi, A.; Patrizi, A.; Gurioli, C.; Neri, I. Head and neck dermatitis, a subtype of atopic dermatitis induced by Malassezia spp: Clinical aspects and treatment outcomes in adolescent and adult patients. Pediatric Dermatol. 2021, 38, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Moraru, R.; Chermette, R.; Guillot, J. Superficial mycoses in dogs and cats. In Recent Trends in Human and Animal Mycology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 27–45. [Google Scholar]
- Guillot, J.; Guého, E.; Lesourd, M.; Midgley, G.; Chévrier, G.; Dupont, B. Identification of Malassezia species. A practical approach. J. Mycol. Med. 1996, 6, 103–110. [Google Scholar]
- Guillot, J.; Poujade, A.; Boulouha, L.; Chermette, R. Could Malassezia Dermatitis be Diagnosed in Animals Other than Pet Carnivores. In Advances in Vetinary Dermatology, 4th ed.; Kusochka, K.W., Willemse, T., von Tscharner, C., Eds.; Butterworth Heinemann: Oxford, UK, 2000. [Google Scholar]
- Duarte, E.; Melo, M.; Hahn, R.; Hamdan, J. Prevalence of Malassezia spp. in the ears of asymptomatic cattle and cattle with otitis in Brazil. Med. Mycol. 1999, 37, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Nell, A.; Herrtage, M.; James, S.; Bond, C.; Hunt, B. Identification and distribution of a novel Malassezia species yeast on normal equine skin. Vet. Rec. 2002, 150, 395–398. [Google Scholar] [CrossRef] [PubMed]
- White, S.D.; Vandenabeele, S.; Drazenovich, N.; Foley, J.E. Malassezia species isolated from the intermammary and preputial fossa areas of horses. J. Vet. Intern. Med. 2006, 20, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Eguchi-Coe, Y.; Valentine, B.A.; Gorman, E.; Villarroel, A. Putative Malassezia dermatitis in six goats. Vet. Dermatol. 2011, 22, 497–501. [Google Scholar] [CrossRef]
- Kim, D.Y.; Johnson, P.J.; Senter, D. Severe bilaterally symmetrical alopecia in a horse. Vet. Pathol. 2011, 48, 1216–1220. [Google Scholar] [CrossRef]
- Weidman, F. Exfoliative Dermatitis in the Indian Rhinoceros (Rhinoceros unicornis), with Description of a New Species: Pityrosporum pachydermatis; Zoological Society of Philadelphia: Philadelphia, PA, USA, 1925; pp. 36–44. [Google Scholar]
- Midgley, G.; Clayton, Y. The yeast flora of birds and mammals in captivity. Antonie Van Leeuwenhoek 1969, 35, E23–E24. [Google Scholar]
- Guillot, J.; Gueho, E. The diversity of Malassezia yeasts confirmed by rRNA sequence and nuclear DNA comparisons. Antonie Van Leeuwenhoek 1995, 67, 297–314. [Google Scholar] [CrossRef]
- Dinsdale, J.; Rest, J. Yeast infection in ferrets. Vet. Rec. 1995, 137, 647–648. [Google Scholar]
- Pier, A.; Cabañes, F.; Chermette, R.; Ferreiro, L.; Guillot, J.; Jensen, H.; Santurio, J. Prominent animal mycoses from various regions of the world. Sabouraudia 2000, 38 (Suppl. S1), 47–58. [Google Scholar] [CrossRef]
- Pollock, C.G.; Rohrbach, B.; Ramsay, E.C. Fungal dermatitis in captive pinnipeds. J. Zoo Wildl. Med. 2000, 31, 374–378. [Google Scholar]
- Radi, Z.A. Outbreak of sarcoptic mange and malasseziasis in rabbits (Oryctolagus cuniculus). Comp. Med. 2004, 54, 434–437. [Google Scholar]
- Tani, K.; Iwanaga, T.; Sonoda, K.; Hayashiya, S.; Hayashiya, M.; Taura, Y. Ivermectin treatment of demodicosis in 56 hamsters. J. Vet. Med. Sci. 2001, 63, 1245–1247. [Google Scholar] [CrossRef] [PubMed]
- Shaheena, A.S. Combined infection of malasseziosis and demodicosis in golden hamster: A case report. J. Entomol. Zool. Stud. 2020, 8, 947–948. [Google Scholar]
- Mason, K.; Evans, A. Dermatitis associated with Malassezia pachydermatis in 11 dogs. J. Am. Anim. Hosp. Assoc. 1991, 27, 1–13. [Google Scholar]
- Plant, J.; Rosenkrantz, W.; Griffin, C. Factors associated with and prevalence of high Malassezia pachydermatis numbers on dog skin. J. Am. Vet. Med. Assoc. 1992, 201, 879–882. [Google Scholar]
- Bond, R.; Ferguson, E.; Curtis, C.; Craig, J.; Lloyd, D. Factors associated with elevated cutaneous Malassezia pachydermatis populations in dogs with Pruritic skin isease. J. Small Anim. Pract. 1996, 37, 103–107. [Google Scholar] [CrossRef]
- Mauldin, E.A.; Scott, D.W.; Miller, W.H.; Smith, C.A. Malassezia dermatitis in the dog: A retrospective histopathological and immunopathological study of 86 cases (1990–1995). Vet. Dermatol. 1997, 8, 191–202. [Google Scholar] [CrossRef]
- Bond, R.; Guillot, J.; Cabañes, F.J. Malassezia Yeasts in Animal Disease. In Malassezia and the Skin; Springer: Berlin/Heidelberg, Germany, 2010; pp. 271–299. [Google Scholar]
- Seemanthini, R.; Tresamol, P.; Vinodkumar, K.; Suchithra, S.; Sreenesh, S. Malassezial dermatitis in a guinea pig–a case report. Indian J. Vet. Sci. Biotechnol. 2016, 11, 55–56. [Google Scholar]
- Bond, R. Malassezia pachydermatis Colonisation and Infection of Canine Skin; Royal Veterinary College (University of London): London, UK, 1996. [Google Scholar]
- Morris, D.O. Malassezia dermatitis and otitis. Vet. Clin. N. Am. Small Anim. Pract. 1999, 29, 1303–1310. [Google Scholar] [CrossRef]
- Matousek, J.L.; Campbell, K.L. Malassezia dermatitis. Compend. Contin. Educ. Pract. Vet. 2002, 24, 224–232. [Google Scholar]
- CHEN, T.; Hill, P.B. The biology of Malassezia organisms and their ability to induce immune responses and skin disease. Vet. Dermatol. 2005, 16, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Nardoni, S.; Dini, M.; Taccini, F.; Mancianti, F. Occurrence, distribution and population size of Malassezia pachydermatis on skin and mucosae of atopic dogs. Vet. Microbiol. 2007, 122, 172–177. [Google Scholar] [CrossRef]
- Nuttall, T.J.; Halliwell, R.E. Serum antibodies to Malassezia yeasts in canine atopic dermatitis. Vet. Dermatol. 2001, 12, 327–332. [Google Scholar] [CrossRef]
- Morris, D.; Olivier, N.; Rosser, E. Type-1 hypersensitivity reactions to Malassezia pachydermatis extracts in atopic dogs. Am. J. Vet. Res. 1998, 59, 836–841. [Google Scholar]
- Morris, D.O.; DeBoer, D.J. Evaluation of serum obtained from atopic dogs with dermatitis attributable to Malassezia pachydermatis for passive transfer of immediate hypersensitivity to that organism. Am. J. Vet. Res. 2003, 64, 262–266. [Google Scholar] [CrossRef]
- Chen, T.A.; Halliwell, R.E.; Pemberton, A.D.; Hill, P.B. Identification of major allergens of Malassezia pachydermatis in dogs with atopic dermatitis and Malassezia overgrowth. Vet. Dermatol. 2002, 13, 141–150. [Google Scholar] [CrossRef]
- Bensignor, E. Malassezia dermatitis in cats: 15 cases treated with itraconazole. Vet. Rec. 2010, 167, 1011–1012. [Google Scholar] [CrossRef]
- Ordeix, L.; Galeotti, F.; Scarampella, F.; Dedola, C.; Bardagi, M.; Romano, E.; Fondati, A. Malassezia spp. overgrowth in allergic cats. Vet. Dermatol. 2007, 18, 316–323. [Google Scholar] [CrossRef]
- Bond, R.; Curtis, C.; Ferguson, E.; Mason, I.; Rest, J. An idiopathic facial dermatitis of Persian cats. Vet. Dermatol. 2000, 11, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, J.; Heimann, M. P-70 Idiopathic facial dermatitis of the Persian cat: Three cases controlled with cyclosporine. Vet. Dermatol. 2004, 15, 64. [Google Scholar] [CrossRef]
- Chung, T.H.; Ryu, M.H.; Kim, D.Y.; Yoon, H.Y.; Hwang, C.Y. Topical tacrolimus (FK506) for the treatment of feline idiopathic facial dermatitis. Aust. Vet. J. 2009, 87, 417–420. [Google Scholar] [CrossRef] [PubMed]
- White, S.D.; Bordeau, P.B.; Blumstein, P.; Ibisch, C.; GuaguÈre, E.; Denerolle, P.; Carlotti, D.N.; Scott, K.V. Feline acne and results of treatment with mupirocin in an open clinical trial: 25 cases (1994–1996). Vet. Dermatol. 1997, 8, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Jazic, E.; Coyner, K.; Loeffler, D.; Lewis, T. An evaluation of the clinical, cytological, infectious and histopathological features of feline acne. Vet. Dermatol. 2006, 17, 134–140. [Google Scholar] [CrossRef]
- Pascal-Tenorio, A.; Olivry, T.; Gross, T.L.; Atlee, B.A.; Ihrke, P.J. Paraneoplastic alopecia associated with internal malignancies in the cat. Vet. Dermatol. 1997, 8, 47–52. [Google Scholar] [CrossRef]
- Godfrey, D. A case of feline paraneoplastic alopecia with secondary Malassezia-associated dermatitis. J. Small Anim. Pract. 1998, 39, 394–396. [Google Scholar] [CrossRef]
- Tasker, S.; Griffon, D.; Nuttall, T.; Hill, P. Resolution of paraneoplastic alopecia following surgical removal of a pancreatic carcinoma in a cat. J. Small Anim. Pract. 1999, 40, 16–19. [Google Scholar] [CrossRef]
- Roccabianca, P.; Rondena, M.; Paltrinieri, S.; Pocacqua, V.; Scarpa, P.; Faverzani, S.; Scanziani, E.; Caniatti, M. Multiple endocrine neoplasia type-I-like syndrome in two cats. Vet. Pathol. 2006, 43, 345–352. [Google Scholar] [CrossRef]
- Marconato, L.; Albanese, F.; Viacava, P.; Marchetti, V.; Abramo, F. Paraneoplastic alopecia associated with hepatocellular carcinoma in a cat. Vet. Dermatol. 2007, 18, 267–271. [Google Scholar] [CrossRef]
- Grandt, L.-M.; Roethig, A.; Schroeder, S.; Koehler, K.; Langenstein, J.; Thom, N.; Neiger, R. Feline paraneoplastic alopecia associated with metastasising intestinal carcinoma. J. Feline Med. Surg. Open Rep. 2015, 1, 2055116915621582. [Google Scholar] [CrossRef] [PubMed]
- Caporali, C.; Albanese, F.; Binanti, D.; Abramo, F. Two cases of feline paraneoplastic alopecia associated with a neuroendocrine pancreatic neoplasia and a hepatosplenic plasma cell tumour. Vet. Dermatol. 2016, 27, 508-e137. [Google Scholar] [CrossRef] [PubMed]
- HLJJFTE, M.F.V.; Curtis, C.; White, R. Resolution of exfoliative dermatitis and Malassezia pachydermatis overgrowth in a cat after surgical thymoma resection. J. Small Anim. Pract. 1997, 38, 451–454. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Von Tscharner, C.; Roosje, P. Thymoma-associated exfoliative dermatitis in cats. Vet. Pathol. 2004, 41, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Reche, A., Jr.; Daniel, A.G.; Strauss, T.C.L.; Taborda, C.P.; Vieira Marques, S.A.; Haipek, K.; Oliveira, L.J.; Monteiro, J.M.; Kfoury, J.R., Jr. Cutaneous mycoflora and CD4: CD8 ratio of cats infected with feline immunodeficiency virus. J. Feline Med. Surg. 2010, 12, 355–358. [Google Scholar] [CrossRef]
- Sierra, P.; Guillot, J.; Jacob, H.; Bussiéras, S.; Chermette, R. Fungal flora on cutaneous and mucosal surfaces of cats infected with feline immunodeficiency virus or feline leukemia virus. Am. J. Vet. Res. 2000, 61, 158–161. [Google Scholar] [CrossRef]
- Mauldin, E.A.; Morris, D.O.; Goldschmidt, M.H. Retrospective study: The presence of Malassezia in feline skin biopsies. A clinicopathological study. Vet. Dermatol. 2002, 13, 7–14. [Google Scholar] [CrossRef]
- Han, J.-I.; Na, K.-J. Otitis externa caused by Malassezia furfur in a miniature pig. J. Vet. Clin. 2009, 26, 303–305. [Google Scholar]
- Nunes Rodrigues, T.C.; Vandenabeele, S.I. Pilot study of dogs with suppurative and non-suppurative Malassezia otitis: A case series. BMC Vet. Res. 2021, 17, 353. [Google Scholar] [CrossRef]
- Shiota, R.; Kaneko, T.; Yano, H.; Takeshita, K.; Nishioka, K.; Makimura, K. A Study of Otitis Externa Associated with Malassezia. Jpn. J. Med. Mycol. 2009, 50, 109–116. [Google Scholar] [CrossRef][Green Version]
- Latha, R.; Sasikala, R.; Muruganandam, N. Chronic otomycosis due to Malassezia spp. J. Glob. Infect. Dis. 2010, 2, 189. [Google Scholar] [CrossRef] [PubMed]
- Crosier, W.J.; Wise, K.A. Onychomycosis due to Pityrosporum. Australas. J. Dermatol. 1993, 34, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Moreno, G.A.; Zaror, L.; de-Oliveira, E.; Fischman, O. Isolation of Malassezia furfur from patients with onychomycosis. J. Med. Vet. Mycol. 1997, 35, 73–74. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Randhawa, H.; Sharma, S.; Brandt, M.E.; Kumar, S. Malassezia furfur in a case of onychomycosis: Colonizer or etiologic agent? Med. Mycol. 2005, 43, 87–90. [Google Scholar] [CrossRef]
- Roodhooft, J.; van Rens, G.; Bogaerts, M.; Vermander, J. Infectious crystalline keratopathy: A case report. Bull. Société Belg. D’Ophtalmologie 1998, 268, 121–126. [Google Scholar]
- Suzuki, T.; Hori, N.; Miyake, T.; Hori, Y.; Mochizuki, K. Keratitis caused by a rare fungus, Malassezia restricta. Jpn. J. Ophthalmol. 2007, 51, 292–294. [Google Scholar] [CrossRef]
- Ledbetter, E.C.; Starr, J.K. Malassezia pachydermatis keratomycosis in a dog. Med. Mycol. Case Rep. 2015, 10, 24–26. [Google Scholar] [CrossRef]
- Prado, M.; Brito, E.; Girão, M.; Monteiro, A.; Sidrim, J.; Rocha, M. Higher incidence of Malassezia pachydermatis in the eyes of dogs with corneal ulcer than in healthy dogs. Vet. Microbiol. 2004, 100, 115–120. [Google Scholar] [CrossRef]
- Theelen, B.; Silvestri, M.; Guého, E.; van Belkum, A.; Boekhout, T. Identification and typing of Malassezia yeasts using amplified fragment length polymorphism (AFLPTm), random amplified polymorphic DNA (RAPD) and denaturing gradient gel electrophoresis (DGGE). FEMS Yeast Res. 2001, 1, 79–86. [Google Scholar] [CrossRef]
- Kaneko, T.; Murotani, M.; Ohkusu, K.; Sugita, T.; Makimura, K. Genetic and biological features of catheter-associated Malassezia furfur from hospitalized adults. Med. Mycol. 2012, 50, 74–80. [Google Scholar] [CrossRef]
- Iatta, R.; Battista, M.; Miragliotta, G.; Boekhout, T.; Otranto, D.; Cafarchia, C. Blood culture procedures and diagnosis of Malassezia furfur bloodstream infections: Strength and weakness. Med. Mycol. 2018, 56, 828–833. [Google Scholar] [CrossRef]
- Wallace, M.; Bagnall, H.; Glen, D.; Averill, S. Isolation of lipophilic yeast in “sterile” peritonitis. Lancet 1979, 2, 956. [Google Scholar] [CrossRef]
- Oliveri, S.; Trovato, L.; Betta, P.; Romeo, M.; Nicoletti, G. Malassezia furfur fungaemia in a neonatal patient detected by lysis-centrifugation blood culture method: First case reported in Italy. Mycoses 2011, 54, e638–e640. [Google Scholar] [CrossRef] [PubMed]
- Marcon, M.J.; Powell, D.A. Human infections due to Malassezia spp. Clin. Microbiol. Rev. 1992, 5, 101–119. [Google Scholar] [CrossRef] [PubMed]
- Ashbee, H.R.; Evans, E.G.V. Immunology of diseases associated with Malassezia species. Clin. Microbiol. Rev. 2002, 15, 21–57. [Google Scholar] [CrossRef]
- Cafarchia, C.; Otranto, D. Association between phospholipase production by Malassezia pachydermatis and skin lesions. J. Clin. Microbiol. 2004, 42, 4868–4869. [Google Scholar] [CrossRef]
- Cafarchia, C.; Dell’Aquila, M.; Traversa, D.; Albrizio, M.; Guaricci, A.; de Santis, T.; Otranto, D. Expression of the μ-opioid receptor on Malassezia pachydermatis and its effect in modulating phospholipase production. Med. Mycol. 2010, 48, 73–78. [Google Scholar] [CrossRef][Green Version]
- Gaitanis, G.; Velegraki, A.; Magiatis, P.; Pappas, P.; Bassukas, I. Could Malassezia yeasts be implicated in skin carcinogenesis through the production of aryl-hydrocarbon receptor ligands? Med. Hypotheses 2011, 77, 47–51. [Google Scholar] [CrossRef]
- Figueredo, L.A.; Cafarchia, C.; Otranto, D. Antifungal susceptibility of Malassezia pachydermatis biofilm. Med. Mycol. 2013, 51, 863–867. [Google Scholar] [CrossRef]
- Vlachos, C.; Gaitanis, G.; Alexopoulos, E.; Papadopoulou, C.; Bassukas, I. Phospholipase activity after β-endorphin exposure discriminates Malassezia strains isolated from healthy and seborrhoeic dermatitis skin. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1575–1578. [Google Scholar] [CrossRef]
- Angiolella, L.; Carradori, S.; Maccallini, C.; Giusiano, G.; Supuran, C.T. Targeting Malassezia species for novel synthetic and natural antidandruff agents. Curr. Med. Chem. 2017, 24, 2392–2412. [Google Scholar] [CrossRef] [PubMed]
- Johansson, H.J.; Vallhov, H.; Holm, T.; Gehrmann, U.; Andersson, A.; Johansson, C.; Blom, H.; Carroni, M.; Lehtiö, J.; Scheynius, A. Extracellular nanovesicles released from the commensal yeast Malassezia sympodialis are enriched in allergens and interact with cells in human skin. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Han, Y.; Sun, Y.-Z.; Jiang, H.-H.; Liu, M.; Qi, R.-Q.; Gao, X.-H. Extracellular vesicles derived from Malassezia furfur stimulate IL-6 production in keratinocytes as demonstrated in in vitro and in vivo models. J. Dermatol. Sci. 2019, 93, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, A.F.; Lisboa, C.; Faria-Ramos, I.; Silva, R.; Ricardo, E.; Teixeira-Santos, R.; Miranda, I.; Rodrigues, A.G. Epidemiology and susceptibility profile to classic antifungals and over-the-counter products of Malassezia clinical isolates from a Portuguese University Hospital: A prospective study. J. Med. Microbiol. 2019, 68, 778–784. [Google Scholar] [CrossRef]
- Findley, K.; Grice, E.A. The skin microbiome: A focus on pathogens and their association with skin disease. PLoS Pathog. 2014, 10, e1004436. [Google Scholar] [CrossRef]
- Thayikkannu, A.B.; Kindo, A.J.; Veeraraghavan, M. Malassezia—Can it be ignored? Indian J. Dermatol. 2015, 60, 332. [Google Scholar]
- Marcon, M.J.; Powell, D.A. Epidemiology, diagnosis, and management of Malassezia furfur systemic infection. Diagn. Microbiol. Infect. Dis. 1987, 7, 161–175. [Google Scholar] [CrossRef]
- Powell, D.A.; Marcon, M.J.; Durrell, D.E.; Pfister, R.M. Scanning electron microscopy of Malassezia furfur attachment to Broviac catheters. Hum. Pathol. 1987, 18, 740–745. [Google Scholar] [CrossRef]
- Cannizzo, F.T.; Eraso, E.; Ezkurra, P.A.; Villar-Vidal, M.; Bollo, E.; Castellá, G.; Javier Cabañes, F.; Vidotto, V.; Quindós, G. Biofilm development by clinical isolates of Malassezia pachydermatis. Med. Mycol. 2007, 45, 357–361. [Google Scholar] [CrossRef]
- Aoba, S.; Komiyama, A.; Hasegawa, O. Fungal meningitis caused by a Malassezia species masquerading as painful ophthalmoplegia. Rinsho Shinkeigaku Clin. Neurol. 1993, 33, 462–464. [Google Scholar]
- Rosales, C.M.; Jackson, M.A.; Zwick, D. Malassezia furfur meningitis associated with total parenteral nutrition subdural effusion. Pediatric Dev. Pathol. 2004, 7, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Schleman, K.A.; Tullis, G.; Blum, R. Intracardiac mass complicating Malassezia furfur fungemia. Chest 2000, 118, 1828–1829. [Google Scholar] [CrossRef] [PubMed]
- Shparago, C.N.I.; Bruno, L.P.P. Systemic Malassezia furfur infection in an adult receiving total parenteral nutrition. J. Osteopath. Med. 1995, 95, 375. [Google Scholar] [CrossRef]
- Chu, C.; Lai, R. Malassezia furfur fungaemia in a ventilator-dependent patient without known risk factors. Hong Kong Med. J. 2002, 8, 212–215. [Google Scholar]
- Fine, A.; Churchill, D.; Gault, H.; Fardy, P. Pityrosporum Pachydermatis Peritonitis in a CAPD Patient on Longterm Intraperitoneal Antibiotics. Perit. Dial. Int. 1983, 3, 108. [Google Scholar] [CrossRef]
- Johnson, A.; Bailey, E.; Wright, P.; Solomon, L. Malassezia furfur: A possible cause of culture-negative CAPD peritonitis. Perit. Dial. Int. 1996, 16, 187. [Google Scholar] [CrossRef]
- Wurtz, R.M.; Knospe, W.N. Malassezia furfur fungemia in a patient without the usual risk factors. Ann. Intern. Med. 1988, 109, 432–433. [Google Scholar] [CrossRef]
- Oberle, A.D.; Fowler, M.; Grafton, W.D. Pityrosporum isolate from the upper respiratory tract. Am. J. Clin. Pathol. 1981, 76, 112–116. [Google Scholar] [CrossRef]
- Bertini, B.; Kuttin, E.; Beemer, A. Cytopathology of nipple discharge due to Pityrosporum orbiculare and cocci in an elderly woman. Acta Cytol. 1975, 19, 38–42. [Google Scholar]
- Alpert, G.; Bell, L.M.; Campos, J.M. Malassezia furfur fungemia in infancy. Clin. Pediatrics 1987, 26, 528–531. [Google Scholar] [CrossRef]
- Shek, Y.H.; Tucker, M.C.; Viciana, A.L.; Manz, H.J.; Connor, D.H. Malassezia furfur—disseminated infection in premature infants. Am. J. Clin. Pathol. 1989, 92, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Devlin, R.K. Invasive fungal infections caused by Candida and Malassezia species in the neonatal intensive care unit. Adv. Neonatal Care 2006, 6, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Zomorodain, K.; Mirhendi, H.; Tarazooie, B.; Kordbacheh, P.; Zeraati, H.; Nayeri, F. Molecular analysis of Malassezia species isolated from hospitalized neonates. Pediatric Dermatol. 2008, 25, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Dankner, W.M.; Spector, S.A.; Fierer, J.; Davis, C.E. Malassezia fungemia in neonates and adults: Complication of hyperalimentation. Rev. Infect. Dis. 1987, 9, 743–753. [Google Scholar] [CrossRef]
- Barber, G.R.; Brown, A.E.; Kiehn, T.E.; Edwards, F.F.; Armstrong, D. Catheter-related Malassezia furfur fungemia in immunocompromised patients. Am. J. Med. 1993, 95, 365–370. [Google Scholar] [CrossRef]
- Morrison, V.; Weisdorf, D. The spectrum of Malassezia infections in the bone marrow transplant population. Bone Marrow Transplant. 2000, 26, 645–648. [Google Scholar] [CrossRef]
- Campigotto, A.; Richardson, S.E.; Sebert, M.; McElvania TeKippe, E.; Chakravarty, A.; Doern, C.D. Low utility of pediatric isolator blood culture system for detection of fungemia in children: A 10-year review. J. Clin. Microbiol. 2016, 54, 2284–2287. [Google Scholar] [CrossRef]
- Nelson, S.C.; Yau, Y.; Richardson, S.E.; Matlow, A.G. Improved detection of Malassezia species in lipid-supplemented Peds Plus blood culture bottles. J. Clin. Microbiol. 1995, 33, 1005–1007. [Google Scholar] [CrossRef]
- Schechtman, R.; Midgley, G.; Hay, R. HIV disease and Malassezia yeasts: A quantitative study of patients presenting with seborrhoeic dermatitis. Br. J. Dermatol. 1995, 133, 694–698. [Google Scholar] [CrossRef]
- Moreno-Coutiño, G.; Sánchez-Cárdenas, C.D.; Bello-Hernández, Y.; Fernández-Martínez, R.; Arroyo-Escalante, S.; Arenas, R. Isolation of Malassezia spp. in HIV-positive patients with and without seborrheic dermatitis. An. Bras. Dermatol. 2019, 94, 527–531. [Google Scholar] [CrossRef]
- Krzyściak, P.; Bakuła, Z.; Gniadek, A.; Garlicki, A.; Tarnowski, M.; Wichowski, M.; Jagielski, T. Prevalence of Malassezia species on the skin of HIV-seropositive patients. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Liguori, G.; Lamas, B.; Richard, M.L.; Brandi, G.; Da Costa, G.; Hoffmann, T.W.; Di Simone, M.P.; Calabrese, C.; Poggioli, G.; Langella, P. Fungal dysbiosis in mucosa-associated microbiota of Crohn’s disease patients. J. Crohn’s Colitis 2016, 10, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.-P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I. Fungal microbiota dysbiosis in IBD. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Limon, J.J.; Tang, J.; Li, D.; Wolf, A.J.; Michelsen, K.S.; Funari, V.; Gargus, M.; Nguyen, C.; Sharma, P.; Maymi, V.I. Malassezia is associated with Crohn’s disease and exacerbates colitis in mouse models. Cell Host Microbe 2019, 25, 377–388.e6. [Google Scholar] [CrossRef] [PubMed]
- Jawhara, S.; Thuru, X.; Standaert-Vitse, A.; Jouault, T.; Mordon, S.; Sendid, B.; Desreumaux, P.; Poulain, D. Colonization of mice by Candida albicans is promoted by chemically induced colitis and augments inflammatory responses through galectin-3. J. Infect. Dis. 2008, 197, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Chiaro, T.R.; Soto, R.; Zac Stephens, W.; Kubinak, J.L.; Petersen, C.; Gogokhia, L.; Bell, R.; Delgado, J.C.; Cox, J.; Voth, W. A member of the gut mycobiota modulates host purine metabolism exacerbating colitis in mice. Sci. Transl. Med. 2017, 9, eaaf9044. [Google Scholar] [CrossRef]
- Drummond, R.A.; Franco, L.M.; Lionakis, M.S. Human CARD9: A critical molecule of fungal immune surveillance. Front. Immunol. 2018, 9, 1836. [Google Scholar] [CrossRef]
- Gao, R.; Kong, C.; Li, H.; Huang, L.; Qu, X.; Qin, N.; Qin, H. Dysbiosis signature of mycobiota in colon polyp and colorectal cancer. Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 2457–2468. [Google Scholar] [CrossRef]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Coker, O.O.; Nakatsu, G.; Dai, R.Z.; Wu, W.K.K.; Wong, S.H.; Ng, S.C.; Chan, F.K.L.; Sung, J.J.Y.; Yu, J. Enteric fungal microbiota dysbiosis and ecological alterations in colorectal cancer. Gut 2019, 68, 654–662. [Google Scholar] [CrossRef]
- Alonso, R.; Pisa, D.; Fernández-Fernández, A.M.; Rábano, A.; Carrasco, L. Fungal infection in neural tissue of patients with amyotrophic lateral sclerosis. Neurobiol. Dis. 2017, 108, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Pisa, D.; Aguado, B.; Carrasco, L. Identification of fungal species in brain tissue from Alzheimer’s disease by next-generation sequencing. J. Alzheimer’s Dis. 2017, 58, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Pisa, D.; Fernández-Fernández, A.M.; Carrasco, L. Infection of fungi and bacteria in brain tissue from elderly persons and patients with Alzheimer’s disease. Front. Aging Neurosci. 2018, 10, 159. [Google Scholar] [CrossRef]
- Alonso, R.; Fernández-Fernández, A.M.; Pisa, D.; Carrasco, L. Multiple sclerosis and mixed microbial infections. Direct identification of fungi and bacteria in nervous tissue. Neurobiol. Dis. 2018, 117, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Pfaller, M.A. Has antifungal susceptibility testing come of age? Clin. Infect. Dis. 2002, 35, 982–989. [Google Scholar] [CrossRef]
- Eschenauer, G.A.; Carver, P.L. The evolving role of antifungal susceptibility testing. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013, 33, 465–475. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef]
- Olivry, T.; DeBoer, D.J.; Favrot, C.; Jackson, H.A.; Mueller, R.S.; Nuttall, T.; Prélaud, P. Treatment of canine atopic dermatitis: 2010 clinical practice guidelines from the International Task Force on Canine Atopic Dermatitis. Vet. Dermatol. 2010, 21, 233–248. [Google Scholar] [CrossRef]
- Ray, P.; Singh, S.; Gupta, S. Topical antimicrobial therapy: Current status and challenges. Indian J. Med. Microbiol. 2019, 37, 299–308. [Google Scholar] [CrossRef]
- Wayne, P. Reference Method for Broth Dilution Antifungal Susceptibility Testing of filamentous Fungi; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Peano, A.; Pasquetti, M.; Tizzani, P.; Chiavassa, E.; Guillot, J.; Johnson, E. Methodological issues in antifungal susceptibility testing of Malassezia pachydermatis. J. Fungi 2017, 3, 37. [Google Scholar] [CrossRef]
- Rhimi, W.; Theelen, B.; Boekhout, T.; Aneke, C.I.; Otranto, D.; Cafarchia, C. Conventional therapy and new antifungal drugs against Malassezia infections. Med. Mycol. 2021, 59, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Romald, P.N.; Kindo, A.J.; Mahalakshmi, V.; Umadevi, U. Epidemiological pattern of Malassezia, its phenotypic identification and antifungal susceptibility profile to azoles by broth microdilution method. Indian J. Med. Microbiol. 2020, 38, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Leong, C.; Buttafuoco, A.; Glatz, M.; Bosshard, P.P. Antifungal susceptibility testing of Malassezia spp. with an optimized colorimetric broth microdilution method. J. Clin. Microbiol. 2017, 55, 1883–1893. [Google Scholar] [CrossRef]
- Sharma, A. In Vitro Susceptibility of Malassezia Furfur to Azoles. Int. J. Health Sci. Res. 2015, 5, 158–164. [Google Scholar]
- Rojas, F.D.; Sosa, M.d.l.A.; Fernandez, M.S.; Cattana, M.E.; Cordoba, S.B.; Giusiano, G.E. Antifungal susceptibility of Malassezia furfur, Malassezia sympodialis, and Malassezia globosa to azole drugs and amphotericin B evaluated using a broth microdilution method. Sabouraudia 2014, 52, 641–646. [Google Scholar] [CrossRef]
- Gupta, A.K.; Kohli, Y.; Summerbell, R.C. Molecular differentiation of seven Malassezia species. J. Clin. Microbiol. 2000, 38, 1869–1875. [Google Scholar] [CrossRef]
- Brito, E.H.; Fontenelle, R.O.; Brilhante, R.S.; Cordeiro, R.A.; Júnior, F.A.S.; Monteiro, A.J.; Sidrim, J.J.; Rocha, M.F. Phenotypic characterization and in vitro antifungal sensitivity of Candida spp. and Malassezia pachydermatis strains from dogs. Vet. J. 2007, 174, 147–153. [Google Scholar] [CrossRef]
- Sugita, T.; Tajima, M.; Ito, T.; Saito, M.; Tsuboi, R.; Nishikawa, A. Antifungal activities of tacrolimus and azole agents against the eleven currently accepted Malassezia species. J. Clin. Microbiol. 2005, 43, 2824–2829. [Google Scholar] [CrossRef]
- Pasquetti, M.; Chiavassa, E.; Tizzani, P.; Danesi, P.; Peano, A. Agar diffusion procedures for susceptibility testing of Malassezia pachydermatis: Evaluation of Mueller-Hinton Agar Plus 2% glucose and 0.5 µg/ml methylene blue as the test medium. Mycopathologia 2015, 180, 153–158. [Google Scholar] [CrossRef]
- Muslimin, V.R.C.; Yogiswara, W.D.; Septiningrum, A.; Budiastuti, A.; Kustarini, S.I. In vitro antifungal susceptibility of Malassezia spp. to azole drugs. JPAD-J. Pak. Assoc. Dermatol. 2018, 28, 502–506. [Google Scholar]
- Arrese, J.; Fogouang, L.; Piérard-Franchimont, C.; Piérard, G. Euclidean and fractal computer-assisted corneofungimetry: A comparison of 2% ketoconazole and 1% terbinafine topical formulations. Dermatology 2002, 204, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Piérard-Franchimont, C.; Vroome, V.; Cauwenbergh, G.; Piérard, G. Corneofungimetry bioassay on Malassezia spp. under ketoconazole and desonide influences. Ski. Pharmacol. Physiol. 2005, 18, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Piérard-Franchimont, C.; Ausma, J.; Wouters, L.; Vroome, V.; Vandeplassche, L.; Borgers, M.; Cauwenbergh, G.; Piérard, G. Activity of the triazole antifungal R126638 as assessed by corneofungimetry. Ski. Pharmacol. Physiol. 2006, 19, 50–56. [Google Scholar] [CrossRef]
- Johnson, E.M. Issues in antifungal susceptibility testing. J. Antimicrob. Chemother. 2008, 61 (Suppl. S1), i13–i18. [Google Scholar] [CrossRef]
- Cantón, E.; Espinel-Ingroff, A.; Pemán, J. Trends in antifungal susceptibility testing using CLSI reference and commercial methods. Expert Rev. Anti-Infect. Ther. 2009, 7, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A. Antifungal drug resistance: Mechanisms, epidemiology, and consequences for treatment. Am. J. Med. 2012, 125, S3–S13. [Google Scholar] [CrossRef]
- Rex, J.H.; Pfaller, M.A.; Galgiani, J.N.; Bartlett, M.S.; Espinel-Ingroff, A.; Ghannoum, M.A.; Lancaster, M.; Odds, F.C.; Rinaldi, M.G.; Walsh, T.J. Development of interpretive breakpoints for antifungal susceptibility testing: Conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 1997, 24, 235–247. [Google Scholar] [CrossRef]
- Willinger, B.; Apfalter, P.; Hirschl, A.M.; Makristathis, A.; Rotter, M.; Seibold, M. Susceptibility testing of Candida species: Comparison of NCCLS microdilution method with Fungitest®. Diagn. Microbiol. Infect. Dis. 2000, 38, 11–15. [Google Scholar] [CrossRef]
- Carrillo-Muñoz, A.J.; Rojas, F.; Tur-Tur, C.; de los Ángeles Sosa, M.; Diez, G.O.; Espada, C.M.; Payá, M.J.; Giusiano, G. In vitro antifungal activity of topical and systemic antifungal drugs against Malassezia species. Mycoses 2013, 56, 571–575. [Google Scholar] [CrossRef]
- Cafarchia, C.; Iatta, R.; Immediato, D.; Puttilli, M.R.; Otranto, D. Azole susceptibility of Malassezia pachydermatis and Malassezia furfur and tentative epidemiological cut-off values. Med. Mycol. 2015, 53, 743–748. [Google Scholar] [CrossRef]
- Peano, A.; Johnson, E.; Chiavassa, E.; Tizzani, P.; Guillot, J.; Pasquetti, M. Antifungal resistance regarding Malassezia pachydermatis: Where are we now? J. Fungi 2020, 6, 93. [Google Scholar] [CrossRef] [PubMed]
- Rojas, F.D.; Córdoba, S.B.; de los Ángeles Sosa, M.; Zalazar, L.C.; Fernández, M.S.; Cattana, M.E.; Alegre, L.R.; Carrillo-Muñoz, A.J.; Giusiano, G.E. Antifungal susceptibility testing of Malassezia yeast: Comparison of two different methodologies. Mycoses 2017, 60, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, S.; García, M.E.; Peláez, T.; Blanco, J.L. Genotyping and antifungal susceptibility testing of multiple Malassezia pachydermatis isolates from otitis and dermatitis cases in pets: Is it really worth the effort? Med. Mycol. 2016, 54, 72–79. [Google Scholar] [PubMed]
- Velegraki, A.; Alexopoulos, E.C.; Kritikou, S.; Gaitanis, G. Use of fatty acid RPMI 1640 media for testing susceptibilities of eight Malassezia species to the new triazole posaconazole and to six established antifungal agents by a modified NCCLS M27-A2 microdilution method and Etest. J. Clin. Microbiol. 2004, 42, 3589–3593. [Google Scholar] [CrossRef]
- Rincón, S.; Cepero de García, M.; Espinel-Ingroff, A. A modified Christensen’s urea and CLSI broth microdilution method for testing susceptibilities of six Malassezia species to voriconazole, itraconazole, and ketoconazole. J. Clin. Microbiol. 2006, 44, 3429–3431. [Google Scholar] [CrossRef]
- Miranda, K.C.; de Araujo, C.R.; Costa, C.R.; Passos, X.S.; Fernandes, O.d.F.L.; Silva, M.d.R.R. Antifungal activities of azole agents against the Malassezia species. Int. J. Antimicrob. Agents 2007, 29, 281–284. [Google Scholar] [CrossRef]
- Yurayart, C.; Nuchnoul, N.; Moolkum, P.; Jirasuksiri, S.; Niyomtham, W.; Chindamporn, A.; Kajiwara, S.; Prapasarakul, N. Antifungal agent susceptibilities and interpretation of Malassezia pachydermatis and Candida parapsilosis isolated from dogs with and without seborrheic dermatitis skin. Med. Mycol. 2013, 51, 721–730. [Google Scholar] [CrossRef]
- Cafarchia, C.; Figueredo, L.A.; Iatta, R.; Montagna, M.T.; Otranto, D. In vitro antifungal susceptibility of Malassezia pachydermatis from dogs with and without skin lesions. Vet. Microbiol. 2012, 155, 395–398. [Google Scholar] [CrossRef]
- Weiler, C.B.; Jesus, F.P.K.D.; Nardi, G.H.; Loreto, É.S.; Santurio, J.M.; Coutinho, S.D.A.; Alves, S.H. Susceptibility variation of Malassezia pachydermatis to antifungal agents according to isolate source. Braz. J. Microbiol. 2013, 44, 175–178. [Google Scholar] [CrossRef]
- Watanabe, S.; Koike, A.; Kano, R.; Nagata, M.; Chen, C.; Hwang, C.-Y.; Hasegawa, A.; Kamata, H. In vitro susceptibility of Malassezia pachydermatis isolates from canine skin with atopic dermatitis to ketoconazole and itraconazole in East Asia. J. Vet. Med. Sci. 2014, 76, 579–581. [Google Scholar] [CrossRef]
- Cafarchia, C.; Figueredo, L.A.; Iatta, R.; Colao, V.; Montagna, M.T.; Otranto, D. In vitro evaluation of Malassezia pachydermatis susceptibility to azole compounds using E-test and CLSI microdilution methods. Med. Mycol. 2012, 50, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Chiavassa, E.; Tizzani, P.; Peano, A. In vitro antifungal susceptibility of Malassezia pachydermatis strains isolated from dogs with chronic and acute otitis externa. Mycopathologia 2014, 178, 315–319. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Pérez, S.; Blanco, J.L.; Peláez, T.; Cutuli, M.; García, M.E. In vitro amphotericin B susceptibility of Malassezia pachydermatis determined by the CLSI broth microdilution method and Etest using lipid-enriched media. Antimicrob. Agents Chemother. 2014, 58, 4203–4206. [Google Scholar] [CrossRef]
- Iatta, R.; Immediato, D.; Montagna, M.T.; Otranto, D.; Cafarchia, C. In vitro activity of two amphotericin B formulations against Malassezia furfur strains recovered from patients with bloodstream infections. Med. Mycol. 2015, 53, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Brilhante, R.S.N.; da Rocha, M.G.; de Melo Guedes, G.M.; de Oliveira, J.S.; dos Santos Araújo, G.; España, J.D.A.; Sales, J.A.; de Aguiar, L.; Paiva, M.d.A.N.; de Aguiar Cordeiro, R. Malassezia pachydermatis from animals: Planktonic and biofilm antifungal susceptibility and its virulence arsenal. Vet. Microbiol. 2018, 220, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Bumroongthai, K.; Chetanachan, P.; Niyomtham, W.; Yurayart, C.; Prapasarakul, N. Biofilm production and antifungal susceptibility of co-cultured Malassezia pachydermatis and Candida parapsilosis isolated from canine seborrheic dermatitis. Sabouraudia 2016, 54, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Kanafani, Z.A.; Perfect, J.R. Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef]
- Uchida, Y.; Onodera, S.; Nakade, T.; Otomo, K. Sterol composition in polyene antibiotic-sensitive and resistant strains of Malassezia pachydermatis. Vet. Res. Commun. 1994, 18, 183–187. [Google Scholar] [CrossRef]
- Kim, D.; Lim, Y.-R.; Ohk, S.O.; Kim, B.J.; Chun, Y.-J. Functional expression and characterization of CYP51 from dandruff-causing Malassezia globosa. FEMS Yeast Res. 2011, 11, 80–87. [Google Scholar] [CrossRef]
- Kim, M.; Cho, Y.-J.; Park, M.; Choi, Y.; Hwang, S.Y.; Jung, W.H. Genomic tandem quadruplication is associated with ketoconazole resistance in Malassezia pachydermatis. J. Microbiol. Biotechnol. 2018, 28, 1937–1945. [Google Scholar] [CrossRef]
- Park, M.; Cho, Y.J.; Lee, Y.W.; Jung, W.H. Genomic Multiplication and Drug Efflux Influence Ketoconazole Resistance in Malassezia restricta. Front. Cell. Infect. Microbiol. 2020, 10, 191. [Google Scholar] [CrossRef]
- Iatta, R.; Puttilli, M.R.; Immediato, D.; Otranto, D.; Cafarchia, C. The role of drug efflux pumps in Malassezia pachydermatis and Malassezia furfur defence against azoles. Mycoses 2017, 60, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Ianiri, G.; Dagotto, G.; Sun, S.; Heitman, J. Advancing functional genetics through Agrobacterium-mediated insertional mutagenesis and CRISPR/Cas9 in the commensal and pathogenic yeast Malassezia. Genetics 2019, 212, 1163–1179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Singh, K.; Sharma, A. Treatment of dermatitis in dogs associated with Malassezia pachydermatis. Indian Vet. J. 2002, 79, 730–732. [Google Scholar]
- Pinchbeck, L.R.; Hillier, A.; Kowalski, J.J.; Kwochka, K.W. Comparison of pulse administration versus once daily administration of itraconazole for the treatment of Malassezia pachydermatis dermatitis and otitis in dogs. J. Am. Vet. Med. Assoc. 2002, 220, 1807–1812. [Google Scholar] [CrossRef]
- Bensignor, E. Oral itraconazole as a pulse therapy for the treatment of canine Malassezia dermatitis: A randomised, blinded, comparative trial. Eur. J. Companion Anim. Pract. 2008, 18, 69–72. [Google Scholar]
- Negre, A.; Bensignor, E.; Guillot, J. Evidence-based veterinary dermatology: A systematic review of interventions for Malassezia dermatitis in dogs. Vet. Dermatol. 2009, 20, 1–12. [Google Scholar] [CrossRef]
- Åhman, S.; Perrins, N.; Bond, R. Treatment of Malassezia pachydermatis-associated seborrhoeic dermatitis in Devon Rex cats with itraconazole–a pilot study. Vet. Dermatol. 2007, 18, 171–174. [Google Scholar] [CrossRef]
- Bensignor, E. Treatment of Malassezia overgrowth with itraconazole in 15 cats. Vet. Rec. 2010, 167, 1011. [Google Scholar] [CrossRef]
- Berger, D.J.; Lewis, T.P.; Schick, A.E.; Stone, R.T. Comparison of once-daily versus twice-weekly terbinafine administration for the treatment of canine Malassezia dermatitis–a pilot study. Vet. Dermatol. 2012, 23, 418-e79. [Google Scholar] [CrossRef]
- Guillot, J.; Bensignor, E.; Jankowski, F.; Seewald, W.; Chermette, R.; Steffan, J. Comparative efficacies of oral ketoconazole and terbinafine for reducing Malassezia population sizes on the skin of Basset Hounds. Vet. Dermatol. 2003, 14, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Rosales, M.S.; Marsella, R.; Kunkle, G.; Harris, B.L.; Nicklin, C.F.; Lopez, J. Comparison of the clinical efficacy of oral terbinafine and ketoconazole combined with cephalexin in the treatment of Malassezia dermatitis in dogs–a pilot study. Vet. Dermatol. 2005, 16, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Gimmler, J.R.; White, A.G.; Kennis, R.A.; Cruz-Espindola, C.; Boothe, D.M. Determining canine skin concentrations of terbinafine to guide the treatment of Malassezia dermatitis. Vet. Dermatol. 2015, 26, 411-e96. [Google Scholar] [CrossRef] [PubMed]
- Sickafoose, L.; Hosgood, G.; Snook, T.; Westermeyer, R.; Merchant, S. A noninferiority clinical trial comparing fluconazole and ketoconazole in combination with cephalexin for the treatment of dogs with Malassezia dermatitis. Vet. Ther. Res. Appl. Vet. Med. 2010, 11, E1–E13. [Google Scholar]
- Villars, V.; Jones, T. Clinical efficacy and tolerability of terbinafine (Lamisil)—a new topical and systemic fungicidal drug for treatment of dermatomycoses. Clin. Exp. Dermatol. 1989, 14, 124–127. [Google Scholar] [CrossRef]
- Gupta, A.K.; Foley, K.A. Antifungal treatment for pityriasis versicolor. J. Fungi 2015, 1, 13–29. [Google Scholar] [CrossRef]
- Gupta, A.K.; Lyons, D.C. Pityriasis versicolor: An update on pharmacological treatment options. Expert Opin. Pharmacother. 2014, 15, 1707–1713. [Google Scholar] [CrossRef]
- Hawkins, D.M.; Smidt, A.C. Superficial fungal infections in children. Pediatric Clin. 2014, 61, 443–455. [Google Scholar] [CrossRef]
- Cheong, W.K.; Yeung, C.K.; Torsekar, R.G.; Suh, D.H.; Ungpakorn, R.; Widaty, S.; Azizan, N.Z.; Gabriel, M.T.; Tran, H.K.; Chong, W.S. Treatment of seborrhoeic dermatitis in Asia: A consensus guide. Ski. Appendage Disord. 2015, 1, 187–196. [Google Scholar] [CrossRef]
- Das, A.; Panda, S. Use of topical corticosteroids in dermatology: An evidence-based approach. Indian J. Dermatol. 2017, 62, 237. [Google Scholar]
- Skorvanek, M.; Bhatia, K.P. The skin and Parkinson’s disease: Review of clinical, diagnostic, and therapeutic issues. Mov. Disord. Clin. Pract. 2017, 4, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.; Kirsten, N.; Körber, A.; Wilsmann-Theis, D.; Itschert, G.; Staubach-Renz, P.; Maul, J.T.; Zander, N. Prevalence, predictors and comorbidity of dry skin in the general population. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Victoire, A.; Magin, P.; Coughlan, J.; van Driel, M.L. Interventions for infantile seborrhoeic dermatitis (including cradle cap). In Cochrane Database of Systematic Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Abbas, Z.; Ghodsi, S.Z.; Abedeni, R. Effect of itraconazole on the quality of life in patients with moderate to severe seborrheic dermatitis: A randomized, placebo-controlled trial. Dermatol. Pract. Concept. 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Lévy, A.; Feuilhade de Chauvin, M.; Dubertret, L.; Morel, P.; Flageul, B. In Malassezia folliculitis: Characteristics and therapeutic response in 26 patients. Ann. Dermatol. Vénéréologie 2007, 11, 823–828. [Google Scholar] [CrossRef]
- Suzuki, C.; Hase, M.; Shimoyama, H.; Sei, Y. Treatment outcomes for malassezia folliculitis in theDermatology department of a university hospital in Japan. Med. Mycol. J. 2016, 57, E63–E66. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tsai, Y.-C.; Wang, J.-Y.; Wu, Y.-H.; Wang, Y.-J. Clinical differences in pediatric and adult Malassezia folliculitis: Retrospective analysis of 321 cases over 9 years. J. Am. Acad. Dermatol. 2019, 81, 278–280. [Google Scholar] [CrossRef]
- Rhie, S.; Turcios, R.; Buckley, H.; Suh, B. Clinical features and treatment of Malassezia folliculitis with fluconazole in orthotopic heart transplant recipients. J. Heart Lung Transplant. 2000, 19, 215–219. [Google Scholar] [CrossRef]
- Prindaville, B.; Belazarian, L.; Levin, N.A.; Wiss, K. Pityrosporum folliculitis: A retrospective review of 110 cases. J. Am. Acad. Dermatol. 2018, 78, 511–514. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, B.J.; Kim, M.N. Photodynamic therapy: New treatment for recalcitrant Malassezia folliculitis. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 2010, 42, 192–196. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, H.I.; Kim, M.N.; Kim, B.J.; Chun, Y.J.; Kim, D. Topical photodynamic therapy with methyl aminolevulinate may be an alternative therapeutic option for the recalcitrant Malassezia folliculitis. Int. J. Dermatol. 2011, 50, 488–490. [Google Scholar] [CrossRef]
- Buentke, E.; Zargari, A.; Heffler, L.; Avila-Carino, J.; Savolainen, J.; Scheynius, A. Uptake of the yeast Malassezia furfur and its allergenic components by human immature CD1a+ dendritic cells. Clin. Exp. Allergy 2000, 30, 1759–1770. [Google Scholar] [CrossRef]
- Selander, C.; Zargari, A.; Möllby, R.; Rasool, O.; Scheynius, A. Higher pH level, corresponding to that on the skin of patients with atopic eczema, stimulates the release of Malassezia sympodialis allergens. Allergy 2006, 61, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Vickery, B.P. Skin barrier function in atopic dermatitis. Curr. Opin. Pediatrics 2007, 19, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Darabi, K.; Hostetler, S.G.; Bechtel, M.A.; Zirwas, M. The role of Malassezia in atopic dermatitis affecting the head and neck of adults. J. Am. Acad. Dermatol. 2009, 60, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Brodská, P.; Panzner, P.; Pizinger, K.; Schmid-Grendelmeier, P. IgE-mediated sensitization to Malassezia in atopic dermatitis: More common in male patients and in head and neck type. Dermatitis 2014, 25, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Kaffenberger, B.H.; Mathis, J.; Zirwas, M.J. A retrospective descriptive study of oral azole antifungal agents in patients with patch test–negative head and neck predominant atopic dermatitis. J. Am. Acad. Dermatol. 2014, 71, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Kolmer, H.L.; Taketomi, E.A.; Hazen, K.C.; Hughs, E.; Wilson, B.B.; Platts-Mills, T.A. Effect of combined antibacterial and antifungal treatment in severe atopic dermatitis. J. Allergy Clin. Immunol. 1996, 98, 702–707. [Google Scholar] [CrossRef]
- Ruiz-Villaverde, R.; Sanchez-Cano, D.; Lopez-Delgado, D. In Head and neck dermatitis: Successful response to terbinafine. J. Am. Acad. Dermatol. 2018, 79, AB150. [Google Scholar]
- Mayser, P.; Kupfer, J.; Nemetz, D.; Schäfer, U.; Nilles, M.; Hort, W.; Gieler, U. Treatment of head and neck dermatitis with ciclopiroxolamine cream–results of a double-blind, placebo-controlled study. Ski. Pharmacol. Physiol. 2006, 19, 153–158. [Google Scholar] [CrossRef]
- Mehra, T.; Köberle, M.; Braunsdorf, C.; Mailänder-Sanchez, D.; Borelli, C.; Schaller, M. Alternative approaches to antifungal therapies. Exp. Dermatol. 2012, 21, 778–782. [Google Scholar] [CrossRef]
- Khosravi, A.; Shokri, H.; Fahimirad, S. Efficacy of medicinal essential oils against pathogenic Malassezia sp. isolates. J. Mycol. Médicale 2016, 26, 28–34. [Google Scholar] [CrossRef]
- Gupta, A.K.; Versteeg, S.G. Topical treatment of facial seborrheic dermatitis: A systematic review. Am. J. Clin. Dermatol. 2017, 18, 193–213. [Google Scholar] [CrossRef]
- Oliveira, A.M.; Devesa, J.S.; Hill, P.B. In vitro efficacy of a honey-based gel against canine clinical isolates of Staphylococcus pseudintermedius and Malassezia pachydermatis. Vet. Dermatol. 2018, 29, 180-e65. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, Y.; Mellor, P.; Bergvall, K. A novel non-azole topical treatment reduces Malassezia numbers and associated dermatitis: A short term prospective, randomized, blinded and placebo-controlled trial in naturally infected dogs. Vet. Dermatol. 2018, 29, 14-e7. [Google Scholar] [CrossRef] [PubMed]
- Sastoque, A.; Triana, S.; Ehemann, K.; Suarez, L.; Restrepo, S.; Wösten, H.; de Cock, H.; Fernández-Niño, M.; González Barrios, A.F.; Celis Ramírez, A.M. New Therapeutic Candidates for the Treatment of Malassezia pachydermatis -Associated Infections. Sci. Rep. 2020, 10, 4860. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Boekhout, T.; Akova, M.; Meis, J.F.; Cornely, O.A.; Lortholary, O.; Group, E.E.S. ECMM, ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin. Microbiol. Infect. 2014, 20, 76–98. [Google Scholar] [CrossRef] [PubMed]
- Mickelsen, P.A.; Viano-Paulson, M.C.; Stevens, D.A.; Diaz, P.S. Clinical and microbiological features of infection with Malassezia pachydermatis in high-risk infants. J. Infect. Dis. 1988, 157, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Jesus, F.; Lautert, C.; Zanette, R.; Mahl, D.; Azevedo, M.; Machado, M.; Dutra, V.; Botton, S.; Alves, S.; Santurio, J. In vitro susceptibility of fluconazole-susceptible and-resistant isolates of Malassezia pachydermatis against azoles. Vet. Microbiol. 2011, 152, 161–164. [Google Scholar] [CrossRef]
- Chen, I.-T.; Chen, C.-C.; Huang, H.-C.; Kuo, K.-C. Malassezia furfur emergence and candidemia trends in a neonatal intensive care unit during 10 years: The experience of fluconazole prophylaxis in a single hospital. Adv. Neonatal Care 2020, 20, E3. [Google Scholar] [CrossRef] [PubMed]
- Ashbee, H. Update on the genus Malassezia. Med. Mycol. 2007, 45, 287–303. [Google Scholar] [CrossRef]
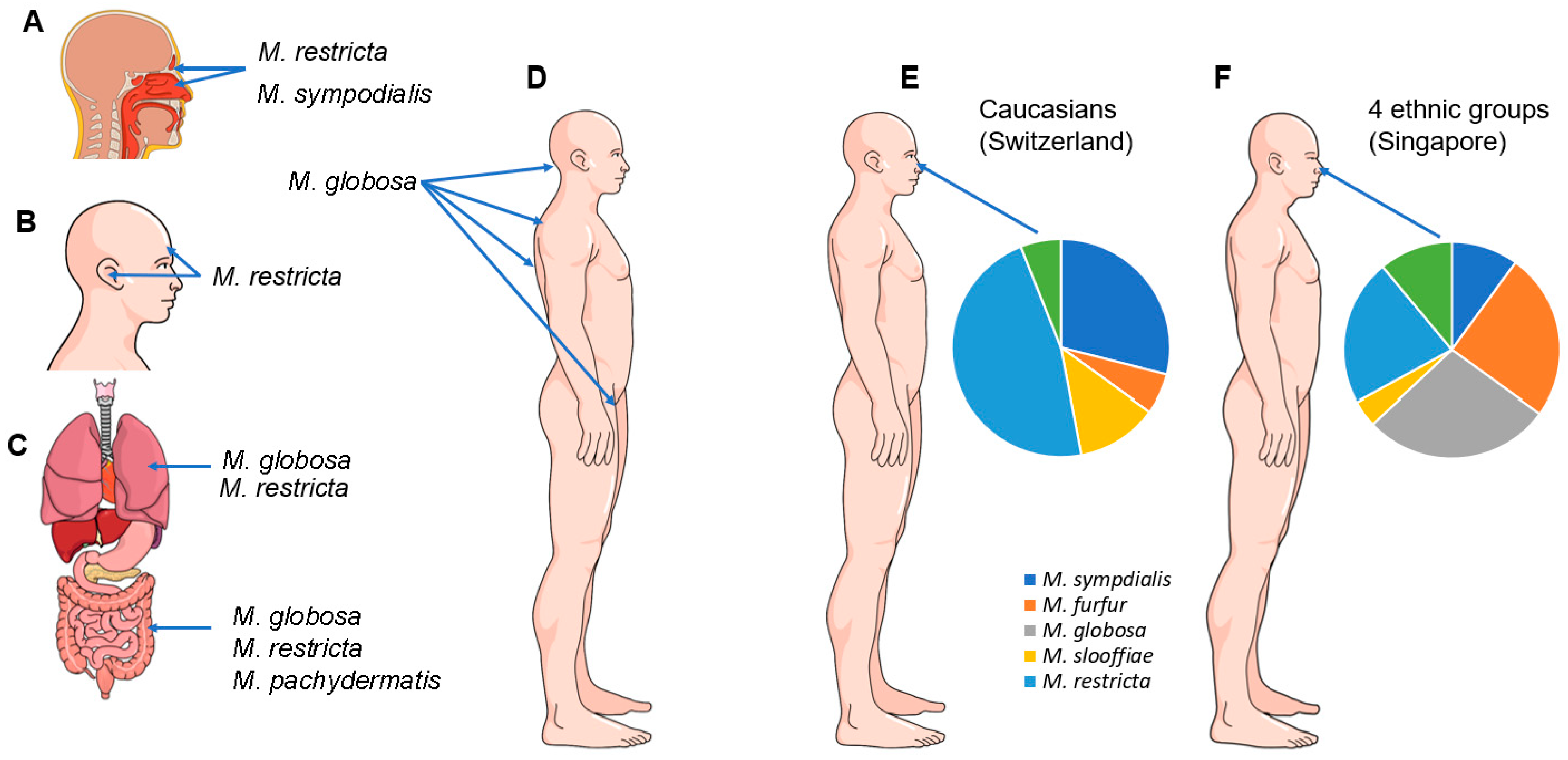
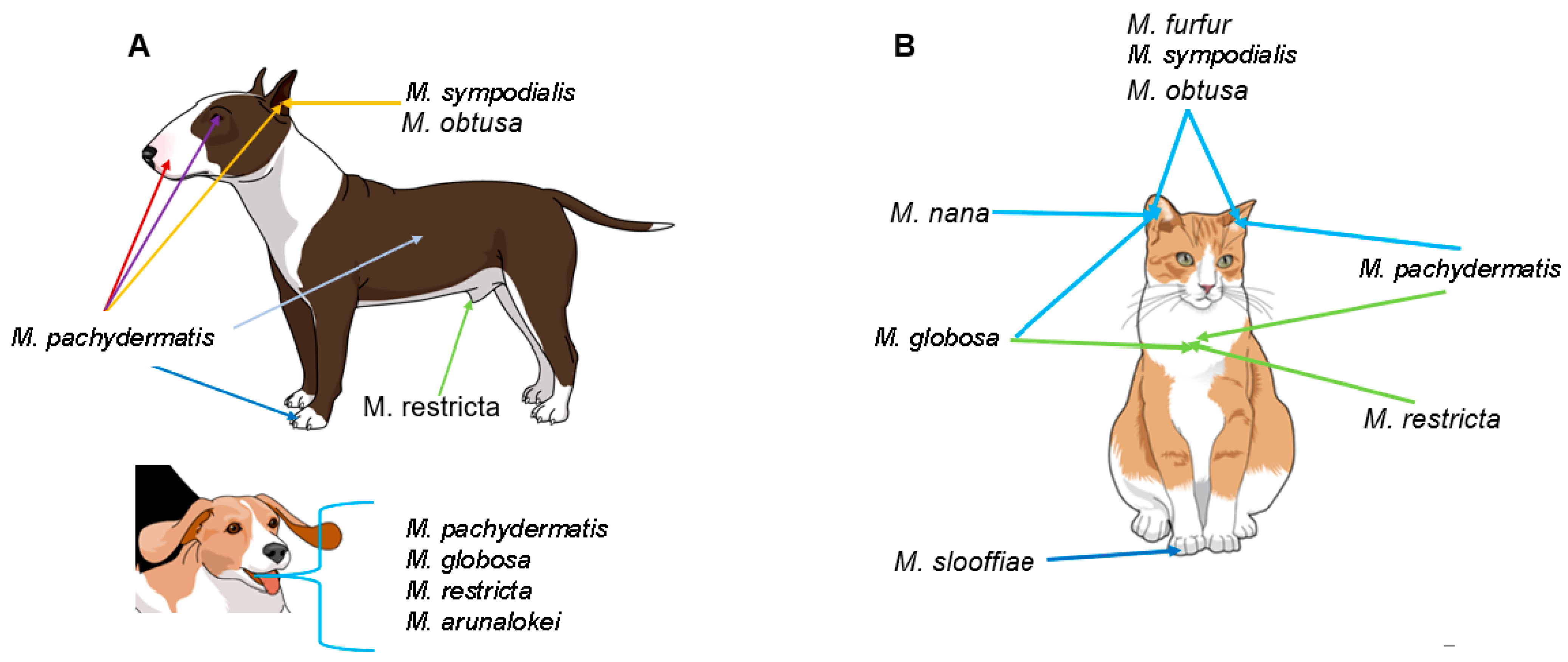
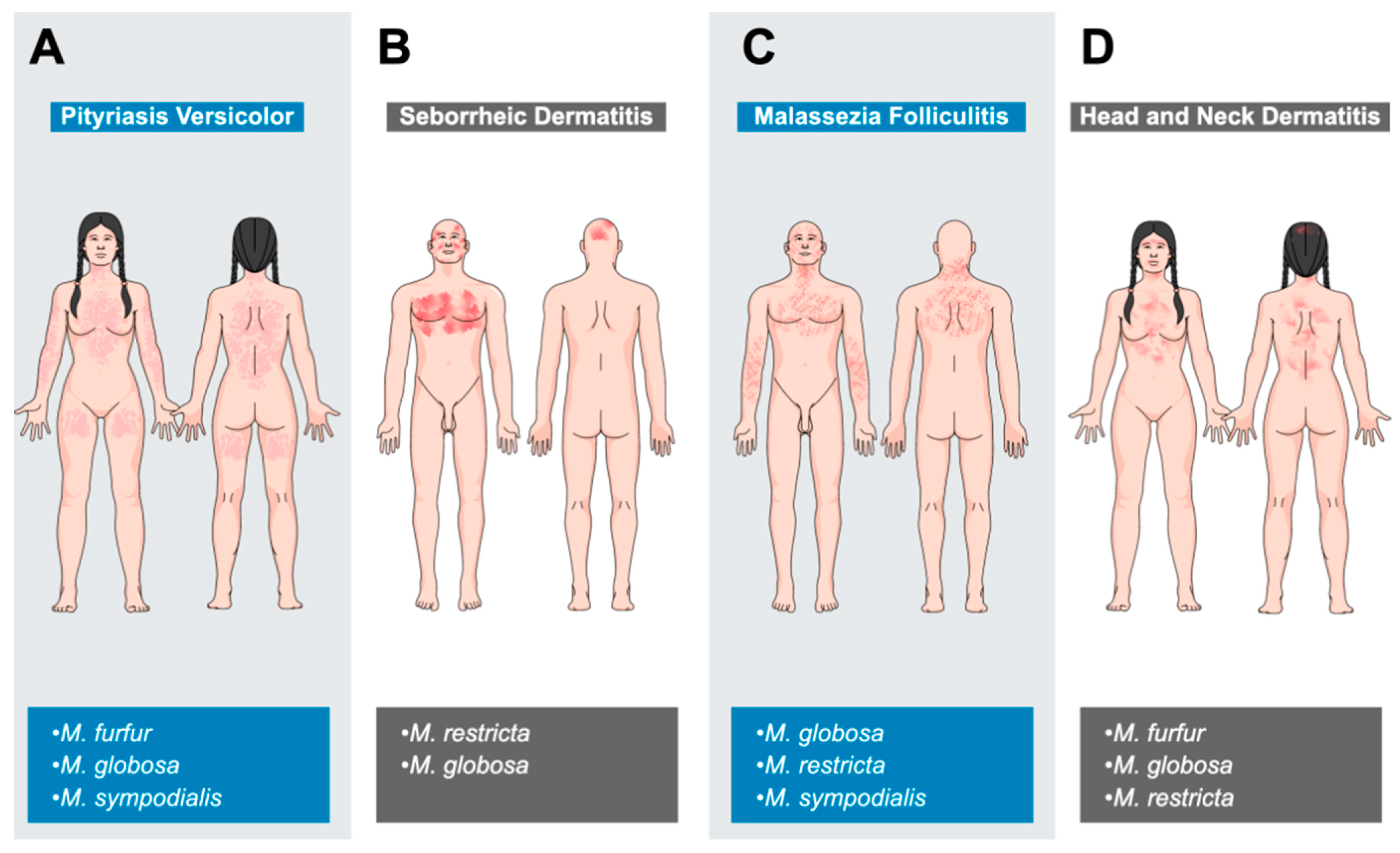
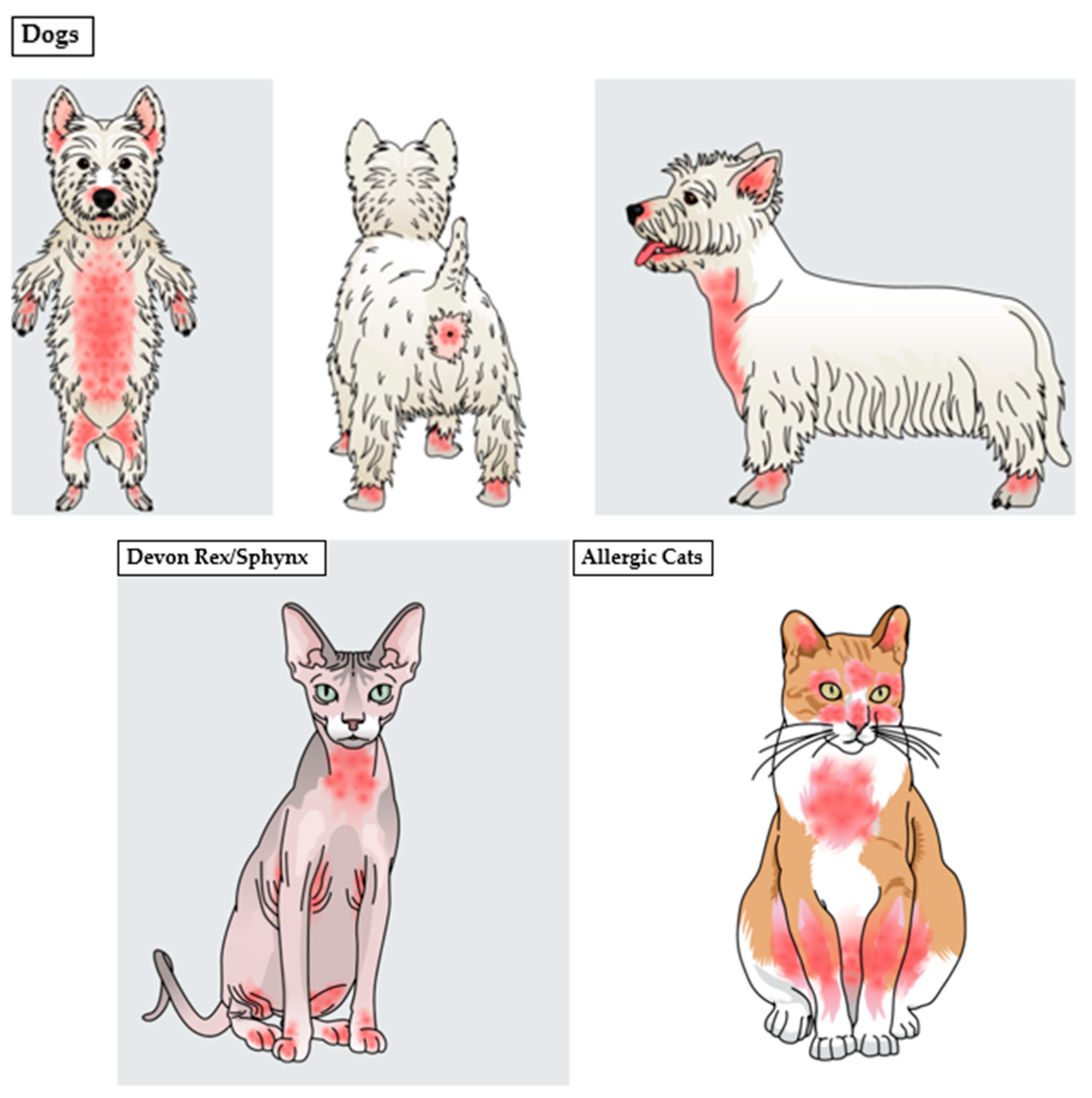
| Species | Reference Strain/GenBank Accession Genome Number | Described Hosts | Clade |
|---|---|---|---|
| M. furfur | CBS 14141, GCA_009938135 | Human, Cat, Dog, Cattle, Pig, Goat, Elk, Horse, Sheep, Elephant, Monkey, Ostrich, Pelican | A |
| M. brasiliensis * | MA 1455 | Parrot | A |
| M. yamatoensis | MY9725, GCA_001264885 | Human, Cat | A |
| M. psittaci * | MA 1454 | Parrot | A |
| M. obtusa | CBS 7876, GCA_001264985 | Human, Cat, Dog, Goat, Horse | A |
| M. japonica | CBS 9431, GCA_001264785 | Human, Cat | A |
| M. vespertilionis | CBS 15041, GCA_002818225 | Bat | A |
| M. globosa | CBS 7966, GCA_001264805 | Human, Cat, Dog, Cattle, Goat, Horse, Sheep, Cheetah | B |
| M. restricta | CBS 7877, GCA_001264765 | Human, Cat, Dog, Cattle, Goat, Horse, Sheep | B |
| M. arunalokei | CBS 13387, GCA_020085095 | Human, Dog | B |
| M. sympodialis | ATCC 42132, GCA_001264925 | Human, Dog, Cat, Pig, Cattle, Goat, Horse, Sheep, Chicken | B |
| M. dermatis | CBS 9169, GCA_001264665 | Human, Cat | B |
| M. caprae | CBS 10434, GCA_001264625 | Goat, Horse, Human | B |
| M. equina | CBS 9969, GCA_001264685 | Horse, Cattle | B |
| M. nana | JCM 12085, GCA_001600835 | Cat, Dog, Cattle, Horse | B |
| M. pachydermatis | CBS 1879, GCA_001264975 | Human, Dog, Cat, Pig, Goat, Rabbit, Various exotic and wild mammals, Birds (Thraupidae, Macaw) | B |
| M. cuniculi | CBS 11721, GCA_001264635 | Rabbit | C |
| M. slooffiae | CBS 7956, GCA_001264965 | Human, Cat Cattle, Sheep, Pig, Goat, Horse | C |
| Dog Breeds | Cat Breeds |
|---|---|
| West Highland White Terrier | Devon Rex |
| English Setter | Sphynx |
| Basset Hound | |
| Boxer | |
| American Cocker Spaniel | |
| Poodle | |
| Dachshund | |
| Australian Silky Terrier | |
| Shih Tzu |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobi, S.; Cafarchia, C.; Romano, V.; Barrs, V.R. Malassezia: Zoonotic Implications, Parallels and Differences in Colonization and Disease in Humans and Animals. J. Fungi 2022, 8, 708. https://doi.org/10.3390/jof8070708
Hobi S, Cafarchia C, Romano V, Barrs VR. Malassezia: Zoonotic Implications, Parallels and Differences in Colonization and Disease in Humans and Animals. Journal of Fungi. 2022; 8(7):708. https://doi.org/10.3390/jof8070708
Chicago/Turabian StyleHobi, Stefan, Claudia Cafarchia, Valentina Romano, and Vanessa R. Barrs. 2022. "Malassezia: Zoonotic Implications, Parallels and Differences in Colonization and Disease in Humans and Animals" Journal of Fungi 8, no. 7: 708. https://doi.org/10.3390/jof8070708
APA StyleHobi, S., Cafarchia, C., Romano, V., & Barrs, V. R. (2022). Malassezia: Zoonotic Implications, Parallels and Differences in Colonization and Disease in Humans and Animals. Journal of Fungi, 8(7), 708. https://doi.org/10.3390/jof8070708