Effects of Oleic Acid Addition Methods on the Metabolic Flux Distribution of Ganoderic Acids R, S and T’s Biosynthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Medium and Culture Conditions
2.3. Analysis Methods
2.4. Statistical Analysis
3. Results
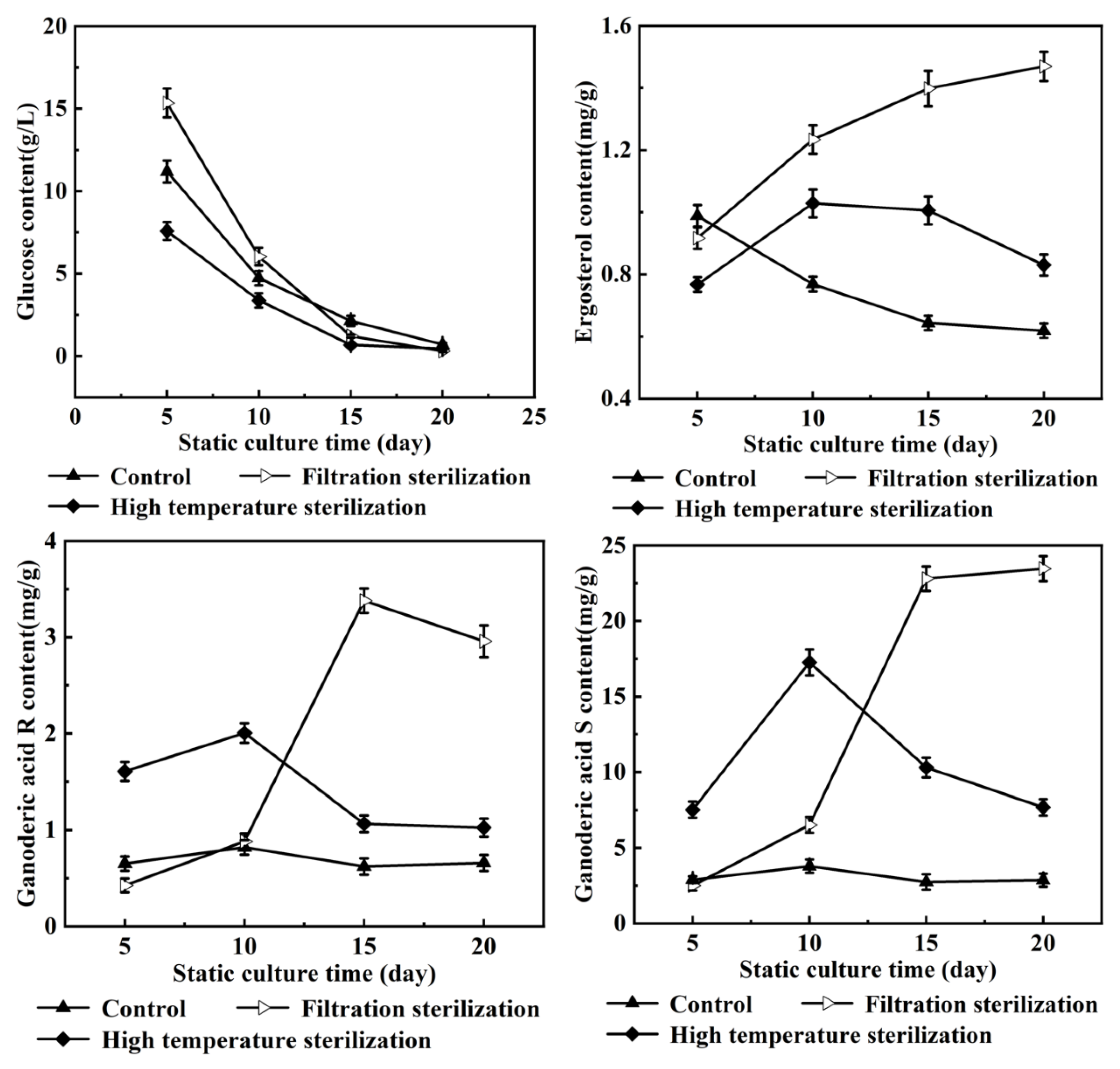
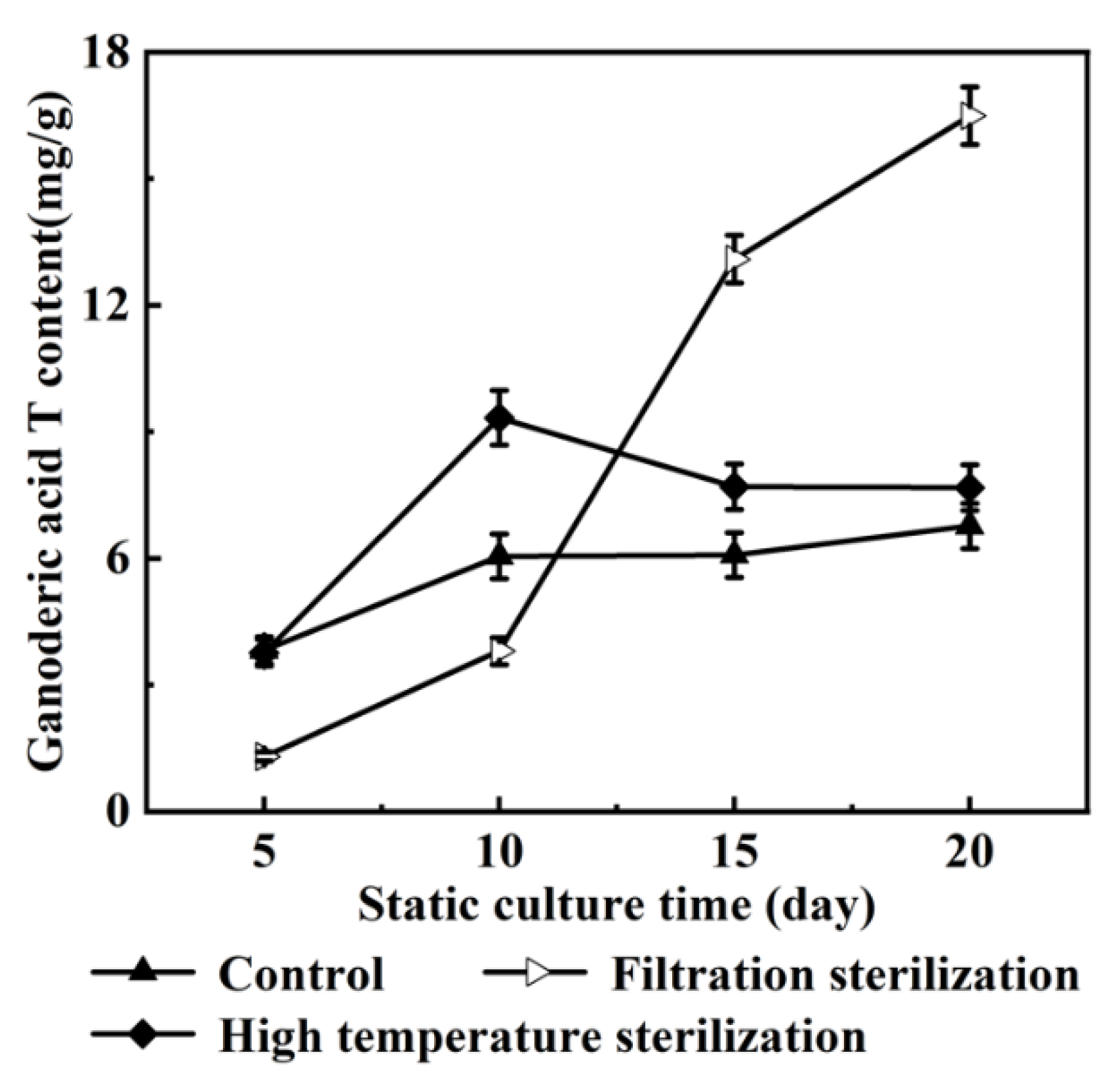
3.1. Effects of Oleic Acid Addition Methods on Ganoderic Acids R, S and T’s Biosynthesis
3.2. Metabolic Flux Balance Model of Ganoderic Acids R, S and T
3.3. Effects of Oleic Acid Addition Methods on Metabolic Flux Distribution of Ganoderic Acids R, S and T’s Biosynthesis
3.4. Effects of Oleic Acid Addition Methods on Metabolic Pathway Flux in Ganoderic Acids R, S and T’s Biosynthesis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Z.H.; Liu, L.; Guo, X.F.; Li, Y.J.; Hou, B.C.; Fan, Q.L.; Wang, K.X.; Luo, Y.; Zhong, J.J. Sucrose fed-batch strategy enhanced biomass, polysaccharide, and ganoderic acids production in fermentation of Ganoderma lucidum 5.26. Bioprocess Biosyst. Eng. 2016, 39, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Zhang, H.; Sun, X.; Zhao, H.; Wu, L.; Zhu, D.; Yang, G.; Shao, Y.; Zhang, X.; Mao, X.; et al. A comprehensive review of the structure elucidation and biological activity of triterpenoids from Ganoderma spp. Molecules 2014, 19, 17478–17535. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Zhong, J.J. Ganoderic acid Mf and S induce mitochondria mediated apoptosis in human cervical carcinoma HeLa cells. Phytomedicine 2011, 18, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Liu, J.W.; Zhao, W.M.; Wei, D.Z.; Zhong, J.J. Ganoderic acid T from Ganoderma lucidum mycelia induces mitochondria mediated apoptosis in lung cancer cells. Life Sci. 2006, 80, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Hirotani, M.; Ino, C.; Furuya, T.; Shiro, M. Ganoderic acids T, S and R, new triterpenoids from the cultured mycelia of Ganoderma lucidum. Chem. Pharm. Bull. 1986, 34, 2282–2285. [Google Scholar] [CrossRef] [Green Version]
- Xiao, H.; Zhong, J.J. Production of useful terpenoids by higher-fungus cell factory and synthetic biology approaches. Trends Biotechnol. 2016, 34, 242–255. [Google Scholar] [CrossRef]
- Ren, A.; Li, X.B.; Miao, Z.G.; Shi, L.; Jaing, A.L.; Zhao, M.W. Transcript and metabolite alterations increase ganoderic acid content in Ganoderma lucidum using acetic acid as an inducer. Biotechnol. Lett. 2014, 36, 2529–2536. [Google Scholar] [CrossRef]
- Ren, A.; Li, M.J.; Shi, L.; Mu, D.S.; Jiang, A.L.; Han, Q.; Zhao, M.W. Profiling and quantifying differential gene transcription provide insights into ganoderic acid biosynthesis in Ganoderma lucidum in response to methyl jasmonate. PLoS ONE 2013, 8, e65027. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Zhang, J.S.; Feng, N.; Yan, M.Q.; Yang, Y.; Jia, W.; Lin, C.C. A novel Ganoderma lucidum G0119 fermentation strategy for enhanced triterpenes production by statistical process optimization and addition of oleic acid. Eng. Life Sci. 2016, 17, 430–439. [Google Scholar] [CrossRef]
- Su, X.W.; Tang, Q.J.; Zhang, J.S.; Feng, N.; Wang, J.Y.; Zhou, S.; Feng, J.; Yu, L. Synthesis of triterpenes in liquid submerged fermentation of Ganoderma lingzhi promoted by oleic acid. Mycosystema 2021, 40, 2445–2460. (In Chinese) [Google Scholar]
- Wang, X.L.; Yang, H.; Liu, G.Q. Enhanced triterpene acid production by Ganoderma lucidum using a feeding stimulus integrated with a two-stage pH-control strategy. J. Chem. Technol. Biotechnol. 2016, 91, 974–982. [Google Scholar]
- Wang, Q.; Cao, R.; Zhang, Y.; Qi, P.; Wang, L.; Fang, S. Biosynthesis and regulation of terpenoids from basidiomycetes: Exploration of new research. AMB Express 2021, 11, 150. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, J.; Liu, C.; Zhu, Y.; Nelson, D.R.; Zhou, S.; Li, C.; Wang, L.; Guo, X.; Sun, Y.; et al. Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 2012, 3, 913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goel, A.; Ferrance, J.; Jeong, J.; Ataai, M.M. Analysis of metabolic fluxes in batch and continuous cultures of Bacillus subtilis. Biotechnol. Bioeng. 1993, 42, 686–696. [Google Scholar] [CrossRef]
- Feng, J.; Feng, N.; Tang, Q.J.; Liu, Y.F.; Tang, C.H.; Zhou, S.; Wang, J.Y.; Tan, Y.; Zhang, J.S.; Lin, C.C. Development and optimization of the triterpenoid and sterol production process with Lingzhi or Reishi medicinal mushroom, Ganoderma lucidum strain G0017 (agaricomycetes), in liquid submerged fermentation at large scale. Int. J. Med. Mushrooms 2021, 23, 43–53. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.; Sharma, A.; Tuli, H.S.; Batra, P.; Beniwal, V.; Gupta, G.K.; Sharma, A.K. Bioactive metabolites of Ganoderma lucidum: Factors, mechanism and broad spectrum therapeutic potential. J. Herb. Med. 2019, 17–18, 100268. [Google Scholar] [CrossRef]
- Wang, J.Y.; Feng, N.; Liu, Y.F.; Yan, P.L.; Tang, Q.J. Ergosterol and fingerprint of liposoluble constituent in the Ganoderma lingzhi basidiospore. Mycosystema 2018, 37, 1215–1223. (In Chinese) [Google Scholar]
- Zhao, N.; Feng, N.; Jia, W.; Feng, J.; Liu, Y.F.; Zhang, J.S. Quantitative analysis of ganoderic acid T, S and R in Ganoderma mycelium by HPLC. Sci. Technol. Food Ind. 2014, 35, 71–75. (In Chinese) [Google Scholar]
- Krömer, J.O.; Wittmann, C.; Schröder, H.; Heinzle, E. Metabolic pathway analysis for rational design of L-methionine production by Escherichia coli and Corynebacterium glutamicum. Metab. Eng. 2006, 8, 353–369. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.Q.; Baker, P.J.; Yi, M.; Wu, H.; Xu, F.; Teng, Y.; Zheng, Y.G. Enhancement of cordyceps polysaccharide production via biosynthetic pathway analysis in Hirsutella sinensis. Int. J. Biol. Macromol. 2016, 92, 872–880. [Google Scholar] [CrossRef]
- Wushensky, J.A.; Youngster, T.; Mendonca, C.M.; Aristilde, L. Flux connections between gluconate pathway, glycolysis, and pentose-phosphate pathway during carbohydrate metabolism in Bacillus megaterium QM B1551. Front. Microbiol. 2018, 9, 2789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sacco, S.A.; Young, J.D. 13C metabolic flux analysis in cell line and bioprocess development. Curr. Opin. Chem. Eng. 2021, 34, 100718. [Google Scholar] [CrossRef]
- Vallino, J.J.; Stephanopoulos, G. Metabolic flux distributions in Corynebacterium glutamicum during growth and lysine overproduction. Biotechnol. Bioeng. 1993, 41, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Wang, Z.; Wang, Y.; Ma, Y.; Su, Z. Metabolic flux analysis of simultaneous production of vitamin B12 and propionic acid in a coupled fermentation process by Propionibacterium freudenreichii. Appl. Biochem. Biotechnol. 2021, 193, 3045–3061. [Google Scholar] [CrossRef]
- Niu, K.; Xu, Y.Y.; Wu, W.J.; Zhou, H.Y.; Liu, Z.Q.; Zheng, Y.G. Effect of dissolved oxygen on L-methionine production from glycerol by Escherichia coli W3110BL using metabolic flux analysis method. J. Ind. Microbiol. Biotechnol. 2020, 47, 287–297. [Google Scholar] [CrossRef]
- Tomàs-Gamisans, M.; Ødum, A.S.R.; Workman, M.; Ferrer, P.; Albiol, J. Glycerol metabolism of Pichia pastoris (Komagataella spp.) characterised by 13C-based metabolic flux analysis. New Biotechnol. 2019, 50, 52–59. [Google Scholar] [CrossRef]
- Hayakawa, K.; Matsuda, F.; Shimizu, H. 13C-metabolic flux analysis of ethanol-assimilating Saccharomyces cerevisiae for S-adenosyl-L-methionine production. Microb. Cell Fact. 2018, 17, 82. [Google Scholar] [CrossRef] [Green Version]



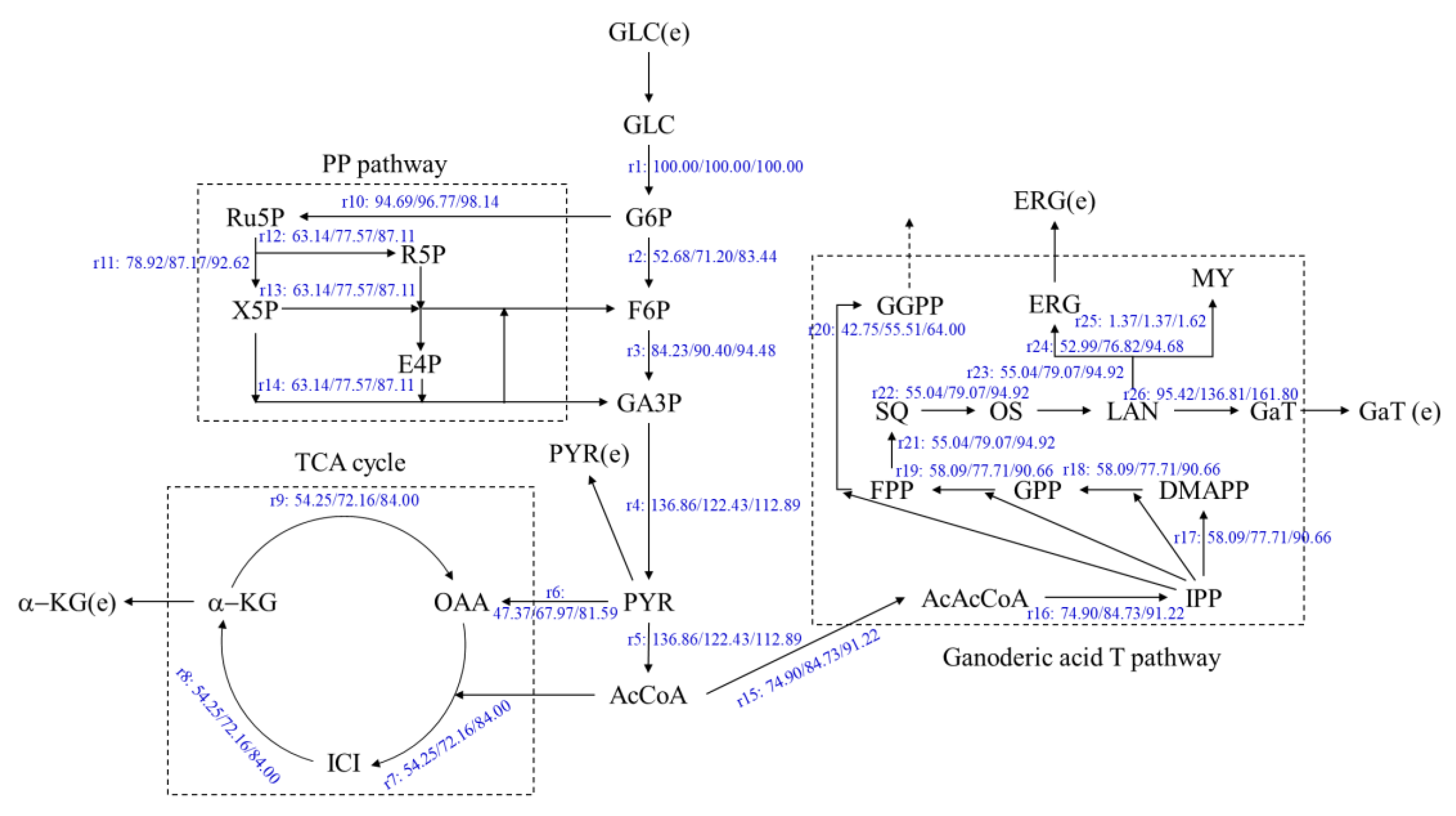
| Metabolic Reaction Equations | Metabolic Node Reaction Rate Equations |
|---|---|
| Glycolysis pathway (EMP): | (1) GLC: GLC(e) − r1 = 0 |
| r1: GLC + ATP → G6P + ADP | (2) G6P: r1 − r2 − r10 = 0 |
| r2: G6P → F6P | (3) F6P: r2 − r3 + r13 + r14 = 0 |
| r3: F6P + ATP → 2GA3P + ADP | (4) GA3P: 2r3 − r4 + r14 = 0 |
| r4: GA3P + NAD + PI + 2ADP → PYR + NADH + 2ATP | (5) PYR: r4 − r5 − r6 = 0 |
| Tricarboxylic acid cycle (TCA): | (6) AcCoA: r5 − r7 − 2r15 − r16 = 0 |
| r5: PYR + CoA + NAD → AcCoA + NADH + CO2 | (7) ICI: r7 − r8 = 0 |
| r6: PYR + ATP + CO2 → OAA + ADP + PI | (8) α-KG: r8 − r9 = 0 |
| r7: OAA + AcCoA → ICI + CoA | (9) OAA: r6 − r7 + r9 = 0 |
| r8: ICI + NADP → α − KG + NADPH + CO2 | (10) Ru5P: r10 − r11 − r12 = 0 |
| r9: α − KG + 2NAD + FAD + ADP + PI → OAA + 2NADH + FADH2 + ATP + CO2 | (11) X5P: r11 − r13 − r14 = 0 |
| Pentose phosphate pathway (PP): | (12) R5P: r12 − r13 = 0 |
| r10: G6P + 2NADP → Ru5P + 2NADPH + CO2 | (13) E4P: r13 − r14 = 0 |
| r11: Ru5P → X5P | (14) CoA: -r5 + r7 + r15 + 2r16 = 0 |
| r12: Ru5P → R5P | (15) AcAcCoA: r15 − r16 = 0 |
| r13: X5P + R5P → F6P + E4P | (16) IPP: r16 − r17 − r18 − r19 − r20 = 0 |
| r14: X5P + E4P → F6P + GA3P | (17) DMAPP: r17 − r18 = 0 |
| Ganoderic acids R, S, T synthesis pathway (GAP): | (18) GPP: r18 − r19 = 0 |
| r15: 2AcCoA → AcAcCoA + CoA | (19) FPP: r19 − r20 − 2r21 = 0 |
| r16: AcAcCoA + AcCoA + 2NADPH + 2H+ + 3ATP + H2O → IPP + 2CoA + 2NADP+ + 3ADP + PI + CO2 | (20) SQ: r21 − r22 = 0 |
| r17: IPP → DMAPP | (21) OS: r22 − r23 = 0 |
| r18: DMAPP + IPP → GPP + PPI | (22) LAN: r23 − r24 − r25 − r26 = 0 |
| r19: GPP + IPP → FPP + PPI | (23) ERG: r24 − ERG(e) = 0 |
| r20: FPP + IPP → GGPP + PPI | (24) GaR: r26 − GaR(e) = 0 |
| r21: 2FPP + NADPH + H+ → SQ + NADP+ + 2PPI | GaS: r26 − GaS(e) = 0 |
| r22: SQ + O2 + NADPH + H+ → OS + NADP+ + H2O | GaT: r26 − GaT(e) = 0 |
| r23: OS → LAN | |
| r24: LAN → ERG | |
| r25: LAN → MY | |
| r26: LAN → GaR/GaS/GaT |
| Metabolic Pathway | Control | Filter-Sterilized Oleic Acid | High-Temperature-Sterilized Oleic Acid | ||
|---|---|---|---|---|---|
| Flow | Increasing Rate (%) | Flow | Increasing Rate (%) | ||
| Ganoderic acid R | |||||
| EMP | 362.17 | 370.75 | 2.37 | 379.93 | 4.91 |
| TCA | 279.42 | 329.46 | 17.91 | 383.00 | 37.07 |
| PP | 302.57 | 347.36 | 14.80 | 395.28 | 30.64 |
| Ganoderic acid R synthesis pathway (GaRP) | 423.70 | 614.77 | 45.10 | 819.22 | 93.35 |
| Ganoderic acid S | |||||
| EMP | 373.41 | 388.75 | 4.11 | 395.02 | 5.79 |
| TCA | 344.94 | 434.39 | 25.93 | 470.98 | 36.54 |
| PP | 361.22 | 441.27 | 22.16 | 474.03 | 31.23 |
| Ganoderic acid S synthesis pathway (GaSP) | 673.91 | 1015.16 | 50.64 | 1154.66 | 71.34 |
| Ganoderic acid T | |||||
| EMP | 373.76 | 384.03 | 2.75 | 390.82 | 4.57 |
| TCA | 346.98 | 406.87 | 17.26 | 446.47 | 28.67 |
| PP | 363.05 | 416.64 | 14.76 | 452.09 | 24.53 |
| Ganoderic acid T synthesis pathway (GaTP) | 681.70 | 910.28 | 33.53 | 1061.26 | 55.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.-Q.; Su, X.-W.; Liu, Y.-F.; Tang, C.-H.; Tang, Q.-J.; Zhou, S.; Tan, Y.; Liu, L.-P.; Zhang, J.-S.; Feng, J. Effects of Oleic Acid Addition Methods on the Metabolic Flux Distribution of Ganoderic Acids R, S and T’s Biosynthesis. J. Fungi 2022, 8, 615. https://doi.org/10.3390/jof8060615
Yan M-Q, Su X-W, Liu Y-F, Tang C-H, Tang Q-J, Zhou S, Tan Y, Liu L-P, Zhang J-S, Feng J. Effects of Oleic Acid Addition Methods on the Metabolic Flux Distribution of Ganoderic Acids R, S and T’s Biosynthesis. Journal of Fungi. 2022; 8(6):615. https://doi.org/10.3390/jof8060615
Chicago/Turabian StyleYan, Meng-Qiu, Xiao-Wei Su, Yan-Fang Liu, Chuan-Hong Tang, Qing-Jiu Tang, Shuai Zhou, Yi Tan, Li-Ping Liu, Jing-Song Zhang, and Jie Feng. 2022. "Effects of Oleic Acid Addition Methods on the Metabolic Flux Distribution of Ganoderic Acids R, S and T’s Biosynthesis" Journal of Fungi 8, no. 6: 615. https://doi.org/10.3390/jof8060615
APA StyleYan, M.-Q., Su, X.-W., Liu, Y.-F., Tang, C.-H., Tang, Q.-J., Zhou, S., Tan, Y., Liu, L.-P., Zhang, J.-S., & Feng, J. (2022). Effects of Oleic Acid Addition Methods on the Metabolic Flux Distribution of Ganoderic Acids R, S and T’s Biosynthesis. Journal of Fungi, 8(6), 615. https://doi.org/10.3390/jof8060615





