Epidemiology of Clinical Sporotrichosis in the Americas in the Last Ten Years
Abstract
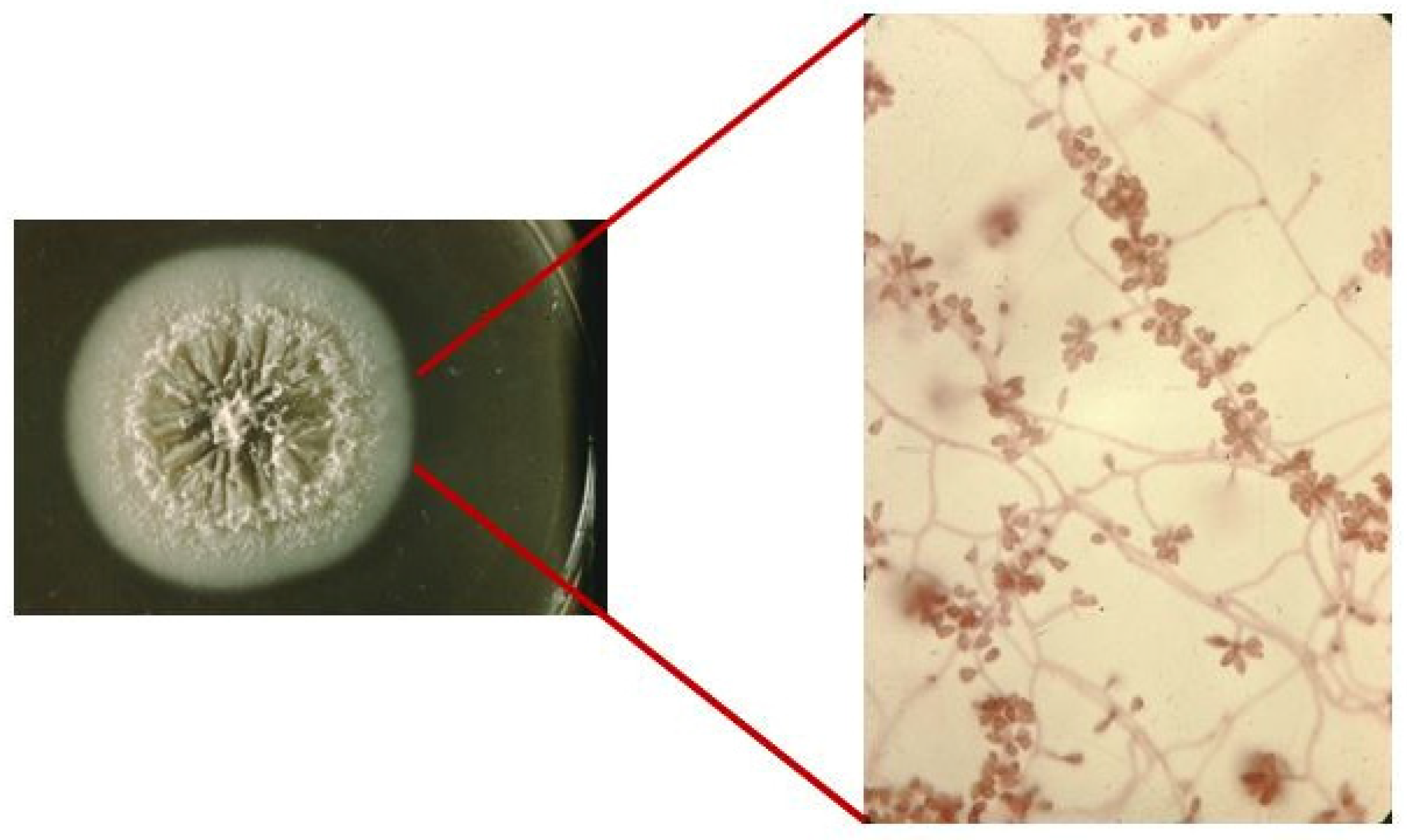
1. Introduction
2. Materials and Methods
3. Epidemiology of Sporotrichosis in North America
4. Epidemiology of Sporotrichosis in Central America and the Caribbean
5. Epidemiology of Sporotrichosis in South America
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopes-Bezerra, L.M.; Mora-Montes, H.M.; Zhang, Y.; Nino-Vega, G.; Rodrigues, A.M.; de Camargo, Z.P.; de Hoog, S. Sporotrichosis between 1898 and 2017: The evolution of knowledge on a changeable disease and on emerging etiological agents. Med. Mycol. 2018, 56, S126–S143. [Google Scholar] [CrossRef] [PubMed]
- Rabello, V.B.S.; Almeida, M.A.; Bernardes-Engemann, A.R.; Almeida-Paes, R.; de Macedo, P.M.; Zancopé-Oliveira, R.M. The Historical Burden of Sporotrichosis in Brazil: A Systematic Review of Cases Reported from 1907 to 2020. Braz. J. Microbiol. 2022, 53, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Della Terra, P.P.; Gremião, I.D.; Pereira, S.A.; Orofino-Costa, R.; de Camargo, Z.P. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia 2020, 185, 813–842. [Google Scholar] [CrossRef] [PubMed]
- Gremião, I.D.F.; Evangelista Oliveira, M.M.; Monteiro de Miranda, L.H.; Saraiva Freitas, D.F.; Peraira, S.A. Geographic Expansion of Sporotrichosis, Brazil. Emergy Infect. Dis. 2020, 26, 621–662. [Google Scholar] [CrossRef]
- Bunce, P.E.; Yang, L.; Chun, S.; Zhang, S.X.; Trinkaus, M.A.; Matukas, L.M. Disseminated sporotrichosis in a patient with hairy cell leukemia treated with amphotericin B and posaconazole. Med. Mycol. 2012, 50, 197–201. [Google Scholar] [CrossRef]
- Tai, F.; Jakubovic, H.; Alabdulrazzaq, S.; Alavi, A. A case of sporotrichosis infection mimicking pyoderma gangrenosum and the role of tissue culture in diagnosis: A case report. SAGE Open Med. Case Rep. 2020, 8, 2050313X20919600. [Google Scholar] [CrossRef]
- Hayfron, K.; Wiedeman, J.A. A 7-year-old girl with ulcerative lesion after a rodent bite. Pediatr. Infect. Dis. J. 2010, 29, 185–193. [Google Scholar] [CrossRef]
- Kamal, A.; Orenstein, R. Disseminated sporotrichosis. J. Hosp. Med. 2010, 5, E29–E30. [Google Scholar] [CrossRef]
- Lyengar, S.S.; Khan, J.A.; Brusco, M.; FitzSimmons, C.J. Cutaneous Sporothrix schenckii of the human eyelid. Ophthalmic Plast. Reconstr. Surg. 2010, 26, 305–306. [Google Scholar] [CrossRef]
- Milby, A.H.; Pappas, N.D.; O’Donnell, J.; Bozentka, D.J. Sporotrichosis of the upper extremity. Orthopedics 2010, 33, 1–3. [Google Scholar]
- Assi, M.; Lakkis, I.E.; Wheat, L.J. Cross-reactivity in the Histoplasma antigen enzyme immunoassay caused by sporotrichosis. Clin. Vaccine Immunol. 2011, 18, 1781–1782. [Google Scholar] [CrossRef] [PubMed]
- Parekh, P.K.; Butler, D.F. What is your diagnosis? Periorbital granulomatous plaque. Pediatr. Dermatol. 2011, 28, 457–458. [Google Scholar] [CrossRef] [PubMed]
- Rees, R.K.; Swartzberg, J.E. Feline-transmitted sporotrichosis: A case study from California. Dermatol. Online. J. 2011, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Sharon, V.R.; Kim, J.; Sudhakar, S.; Fung, M.A.; Maniar, A. Disseminated cutaneous sporotrichosis. Lancet Infect. Dis. 2013, 13, 95. [Google Scholar] [CrossRef]
- Adnan, M.M.; Fierro-Fine, A.; Zhao, L.; Khalil, M.O. Metastic melanoma masquerading as disseminated sporotrichosis. J. Community Support. Oncol. 2014, 12, 339–340. [Google Scholar] [CrossRef]
- Trotter, J.R.; Sriaroon, P.; Berman, D.; Petrovic, A.; Leiding, J.W. Sporothrix schenckii lymphadentitis in a male with X-linked chronic granulomatous disease. J. Clin. Immunol. 2014, 34, 49–52. [Google Scholar] [CrossRef]
- Bahr, N.C.; Janssen, K.; Billings, J.; Loor, G.; Green, J.S. Respiratory failure due to possible donor-derived Sporothrix schenckii infection in a lung transplant recipient. Case Rep. Infect. Dis. 2015, 2015, 925718. [Google Scholar]
- Hassan, K.; Turker, T.; Zangeneh, T. Disseminated sporotrichosis in an immunocompetent patient. Case Rep. Plast. Surg. Hand Surg. 2016, 3, 44–47. [Google Scholar] [CrossRef]
- Lederer, H.T.; Sullivan, E.; Crum-Cianflone, N.F. Sporotrichosis as an unusual case of osteomyelitis: A case report and review of the literature. Med. Mycol. Case Rep. 2016, 11, 31–35. [Google Scholar] [CrossRef]
- Aronowitz, P.B.; Gilroy, M.; Christiansen, K.N. Disseminated Sporotrichosis with Osteolytic Bone Involvement. J. Gen. Intern. Med. 2017, 32, 1063. [Google Scholar] [CrossRef][Green Version]
- Charles, K.; Lowe, L.; Shuman, E.; Cha, K.B. Painful linear ulcers: A case of cutaneous sporotrichosis mimicking pyoderma gangrenosum. JAAD Case Rep. 2017, 3, 519–552. [Google Scholar] [CrossRef] [PubMed]
- Hessler, C.; Kauffman, C.A.; Chow, F.C. The upside of bias: A case of chronic meningitis due to Sporothrix schenckii in an immunocompetent host. Neurohospitalist 2017, 7, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Barbaryan, A.; El Atrouni, W.; Bailuc, S.; Jones, M.W.; Bhakta, M.; Mahmoud, K.H.; Mirrakhimov, A.E. Isolated Sporothrix schenckii monoarthritis. Case Rep. Infect. Dis. 2018, 2018, 9037657. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, S.M.; Youness, H. The Infection Returns: A case of pulmonary sporotrichosis relapse after chemotherapy. Case Rep. Med. 2018, 2018, 1384029. [Google Scholar] [CrossRef]
- Patel, R.; Busby, L.P.; Motamedi, D. Delayed diagnosis in a case of smoldering sporotrichal monoarthropathy. J. Radiol. Case Rep. 2019, 13, 17–23. [Google Scholar] [CrossRef]
- Saeed, L.; Weber, R.J.; Puryear, S.B.; Bahrani, E.; Peluso, M.J.; Babik, J.M.; Haemel, A.; Coates, S.J. Disseminated cutaneous and osteoarticular sporotrichosis mimicking pyoderma gangrenosum. Open Forum Infect. Dis. 2019, 6, ofz395. [Google Scholar] [CrossRef]
- White, M.; Adams, L.; Phan, C.; Erdag, G.; Totten, M.; Lee, R.; Lu, X.; Mehta, S.; Miller, L.S.; Zhang, S.X. Disseminated sporotrichosis following iatrogenic immunosuppression for suspected pyoderma gangrenosum. Lancet Infect. Dis. 2019, 19, e385–e391. [Google Scholar] [CrossRef]
- Kaadan, M.I.; Dennis, M.; Desai, N.; Yadavalli, G.; Lederer, P. One health education for future physicians: A case report of cat-transmitted sporotrichosis. Open Forum Infect. Dis. 2020, 7, ofaa049. [Google Scholar] [CrossRef]
- Parker, N.; Strong, N.; Pichetsurnthorn, P.; Lalich, D.; Moore, T. Disseminated sporotrichosis with brain abscesses in an HIV-Infected patient. Cureus 2020, 12, e8016. [Google Scholar] [CrossRef]
- Shah, D.; Kim, A.E.; Elbadri, S.; Desai, B.; Ganti, L. An uncommon rash in the emergency department: Sporothrix Schenckii. Cureus 2021, 13, e16125. [Google Scholar] [CrossRef]
- Wellington, T.; Hauschild, J.; Krauland, K.J.; Verwiebe, E.G.; Markelz, A.E. Sporotrichosis in a U.S. Army basic trainee. Mil. Med. 2021, usab463. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, A.I.; Church, E.C.; McKay, K.M.; Carnes, S.K.; Morse, R.J.; Leveque, T.K.; Roxby, A.C. A disfiguring rash. Open Forum Infect. Dis. 2021, 8, ofab332. [Google Scholar] [CrossRef] [PubMed]
- Kenny, H.; Dougherty, M.; Churnin, I.; Early, S.; Gupta, A.; McGarey, P.O., Jr. Chronic laryngotracheal granulomatous disease secondary to Sporothrix schenckii in an immunocompromised patient. Ann. Otol. Rhinol. Laryngol. 2022, 34894211073002. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Morales, J.L.; Domínguez Romero, R.; Morales Esponda, M.; Rossiere Echazaleta, N.L.; Reyes Bonifant, G.; Santos Ramírez, A. Esporotricosis micematoide con invasión a médula espinal. Rev. Mex. Neuroci. 2011, 12, 50–54. [Google Scholar]
- Romero-Cabello, R.; Bonifaz, A.; Romero-Feregrino, R.; Sánchez, C.J.; Linares, Y.; Zavala, J.T.; Romero, L.C.; Romero-Feregrino, R.; Vega, J.T. Disseminated sporotrichosis. BMJ Case Rep. 2011, 2011, bcr1020103404. [Google Scholar] [CrossRef]
- Rojas-Padilla, R.; Pastrana, R.; Toledo, M.; Valencia, A.; Mena, C.; Bonifaz, A. Esporotricosis cutánea linfangítica por mordedura de araña. Dermatol. Rev. Mex. 2013, 57, 479–484. [Google Scholar]
- Espinoza-Hernández, C.J.; Jesús-Silva, A.; Toussaint-Caire, S.; Arenas, R. Disseminated sporotrichosis with cutaneous and testicular involvement. Actas Dermosifiliogr. 2014, 105, 204–206. [Google Scholar] [CrossRef]
- Chávez-López, G.; Estrada-Castañón, R.; Estrada-Chávez, G.; Vega-Memije, M.E.; Moreno-Coutiño, G. Esporotricosis cutánea diseminada: Un caso de la región de la montaña del estado de Guerrero, México. Dermatol. Rev. Mex. 2015, 59, 228–232. [Google Scholar]
- Cotino Sánchez, A.; Torres-Alvarez, B.; Gurrola Morales, T.; Méndez Martínez, S.; Saucedo Gárate, M.; Castanedo-Cazares, J.P. Mycosis fungoides-like lesions in a patient with diffuse cutaneous sporotrichosis. Rev. Iberoam. Micol. 2015, 32, 200–203. [Google Scholar] [CrossRef]
- Bonifaz, A.; Tirado-Sánchez, A.; Paredes-Solís, V.; Cepeda-Valdés, R.; González, G.M.; Treviño-Rangel, R.J.; Fierro-Arias, L. Cutaneous disseminated sporotrichosis: Clinical experience of 24 cases. J. Eur. Acad. Dermatol. Venereol. 2018, 32, e77–e79. [Google Scholar] [CrossRef]
- Bonifaz, A.; Toriello, C.; Araiza, J.; Ramírez-Soto, M.C.; Tirado-Sánchez, A. Sporotrichin skin test for the diagnosis of sporotrichosis. J. Fungi 2018, 4, 55. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Castañón, R.; Chávez-López, G.; Estrada-Chávez, G.; Bonifaz, A. Report of 73 cases of cutaneous sporotrichosis in Mexico. An. Bras. Dermatol. 2018, 93, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Reyes, J.; Ramos-Martínez, E.; Treviño-Rangel, R.; González, G.M.; Bonifaz, A. Auricular sporotrichosis. Atypical case report simulating bacterial cellulitis. Rev. Chil. Infectol. 2018, 35, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Puebla-Miranda, M.; Vásquez-Ramírez, M.; González-Ibarra, M.; Torres-López, I.H. Esporotricosis. Reporte de un caso ocupacional. Rev. Hosp. Jua. Mex. 2018, 85, 246–250. [Google Scholar]
- Rangel-Gamboa, L.; Martinez-Hernandez, F.; Maravilla, P.; Flisser, A. A population genetics analysis in clinical isolates of Sporothrix schenckii based on calmodulin and calcium/calmodulin-dependent kinase partial gene sequences. Mycoses 2018, 61, 383–392. [Google Scholar] [CrossRef]
- Rojas, O.C.; Bonifaz, A.; Campos, C.; Treviño-Rangel, R.J.; González-Álvarez, R.; González, G.M. Molecular identification, antifungal susceptibility, and geographic origin of clinical Strains of Sporothrix schenckii complex in Mexico. J. Fungi 2018, 4, 86. [Google Scholar] [CrossRef]
- Estrada-Castañón, R.; Estrada-Chávez, G.; Chávez-López, M.G. Diagnosis and management of fungal neglected tropical diseases in community settings-mycetoma and sporotrichosis. Trop. Med. Infect. Dis. 2019, 4, 81. [Google Scholar] [CrossRef]
- Mayorga-Rodríguez, J.; Mayorga-Garibaldi, J.L.; Muñoz-Estrada, V.F.; De León Ramírez, R.M. Esporotricosis: Serie de 1134 casos en una zona endémica de México. Med. Cut. Ibero Lat. Am. 2019, 47, 24–28. [Google Scholar]
- Alvarez-Rivero, V.; Hernandez-Castro, R.; Moreno-Coutiño, G.; Lozano-Platonoff, A. Disseminated sporotrichosis: An important differential diagnosis for venous ulcers. Adv. Skin. Wound Care 2020, 33, 1–3. [Google Scholar] [CrossRef]
- Bonifaz, A.; Morales-Peña, N.; Tirado-Sánchez, A.; Jiménez-Mendoza, D.R.; Treviño-Rangel, R.J.; González, G.M. Atypical sporotrichosis related to Sporothrix mexicana. Mycopathologia 2020, 185, 733–735. [Google Scholar] [CrossRef]
- Bonifaz, A.; Tirado-Sánchez, A.; Araiza, J.; Treviño-Rangel, R.; González, G.M. Deep mycoses and pseudomycoses of the foot: A single-center retrospective study of 160 cases, in a tertiary-care center in Mexico. Foot 2021, 46, 101770. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, E.; Arenas, R.; Hernández-Castro, R.; Frías-De-León, M.G.; Rodríguez-Cerdeira, C. Uncommon clinical presentations of sporotrichosis: A two-case report. Pathogens 2021, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Alvarado, S.; Salas-Campos, I.; Uribe-Lorío, L.; Gross, N.T. Molecular and Biochemical Identification and In Vitro Susceptibility to Itraconazole of Costa Rican Clinical Isolates of the Sporothrix schenckii Complex. Acta Sci. Microbiol. 2020, 3, 116–123. [Google Scholar] [CrossRef]
- Román-Carrillo, M.; Porres-Paredes, S.; Orozco, R.; Argueta, V. Cutaneous Sporotrichosis. Case report. Rev. Médica Gt Colmedegua 2018, 157, 90–92. [Google Scholar]
- Sánchez-Cárdenas, C.D.; Porras-López, C.; Morales-Ezquivel, O.; Frías-De-León, M.G.; Juárez-Durán, E.R.; Arenas, R.; Martínez-Herrera, E. Sporotrichosis: Epidemiological, clinical and mycological study of 53 cases in Guatemala. Life Sci. Press 2018, 2, 66–69. [Google Scholar] [CrossRef]
- Flórez-Muñoz, S.V.; Alzate, J.F.; Mesa-Arango, A.C. Molecular Identification and Antifungal Susceptibility of Clinical Isolates of Sporothrix schenckii Complex in Medellin, Colombia. Mycopathologia 2019, 184, 53–63. [Google Scholar] [CrossRef]
- Medina, R.; Flores, J.; Luque, M.T. Sporotrichosis in an Adolescent Patient. Honduras Pediátrica 2021, 34, 32–33. [Google Scholar] [CrossRef]
- Rios, M.E.; Suarez, M.D.; Moreno, J.; Vallee, J.; Moreno, J.P. Zoonotic Sporotrichosis Related to Cat Contact: First Case Report from Panama in Central America. Cureus 2018, 10, e2906. [Google Scholar] [CrossRef]
- Fernandez, M.C.; Reyes, N.; Gonzalez, J.C.; Montesino, M.; Apaulasa, K. Sporotrichosis. A propos of a case. Rev. Cubana Med. Trop. 2016, 68, 171–178. [Google Scholar]
- Pérez-Morales, L.; Iglesias-López, M.; Quiñones-Cherta, O.; Reyes-Rodríguez, I. Microbiological Isolation of Sporothrix Schenckii in an Immunocompromised Patient. A Case Report. Rev. Cienc. Médicas Cienfuegos 2014, 12, 662–669. [Google Scholar]
- Rojas, F.D.; Fernández, M.S.; Lucchelli, J.M.; Lombardi, D.; Malet, J.; Vetrisano, M.E.; Cattana, M.E.; de los Ángeles Sosa, M.; Giusiano, G. Cavitary Pulmonary Sporotrichosis: Case Report and Literature Review. Mycopathologia 2017, 182, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Etchecopaz, A.; Toscanini, M.A.; Gisbert, A.; Mas, J.; Scarpa, M.; Iovannitti, C.A.; Bendezú, K.; Nusblat, A.D.; Iachini, R.; Cuestas, M.L. Sporothrix brasiliensis: A review of an emerging south american fungal pathogen, its related disease, presentation and spread in Argentina. J. Fungi 2021, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, S.; Isla, G.; Szusz, W.; Vivot, W.; Hevia, A.; Davel, G.; Canteros, C.E. Molecular identification and susceptibility profile of Sporothrix schenckii sensu lato isolated in Argentina. Mycoses 2018, 61, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Picollo, M.; Epelbaum, C.; Bustos, A.C.; Carnovale, S.; Rosanova, M.T. Lymphocutaneous sporotrichosis in a pediatric patient, a case report. Rev. Chil. Infectol. 2021, 38, 811–815. [Google Scholar] [CrossRef] [PubMed]
- Alzuguir, C.L.C.; Pereira, S.A.; Magalhães, M.A.F.M.; Almeida-Paes, R.; Freitas, D.F.S.; Oliveira, L.F.A.; Pimentel, M.I.F. Geo-epidemiology and socioeconomic aspects of human sporotrichosis in the municipality of Duque de Caxias, Rio de Janeiro, Brazil, between 2007 and 2016. Trans. R. Soc. Trop. Med. Hyg. 2020, 114, 99–106. [Google Scholar] [CrossRef]
- Almeida-Paes, R.; de Oliveira, M.M.E.; Freitas, D.F.S.; do Valle, A.C.F.; Zancopé-Oliveira, R.M.; Gutierrez-Galhardo, M.C. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis Is Associated with Atypical Clinical Presentations. PLoS Negl. Trop. Dis. 2014, 8, e309. [Google Scholar] [CrossRef]
- Freitas, D.F.S.; do Valle, A.C.F.; da Silva, M.B.T.; Campos, D.P.; Lyra, M.R.; De Souza, R.V.; Veloso, V.G.; Zancopé-Oliveira, R.M.; Bastos, F.I.; Galhardo, M.C.G. Sporotrichosis: An Emerging Neglected Opportunistic Infection in HIV-Infected Patients in Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2014, 8, e3110. [Google Scholar] [CrossRef]
- Freitas, D.F.S.; de Siqueira Hoagland, B.; do Valle, A.C.F.; Fraga, B.B.; de Barros, M.B.; de Oliveira Schubach, A.; de Almeida-Paes, R.; Cuzzi, T.; Rosalino, C.M.V.; Zancopé-Oliveira, R.M.; et al. Sporotrichosis in HIV-infected patients: Report of 21 cases of endemic sporotrichosis in Rio de Janeiro, Brazil. Med. Mycol. 2012, 50, 170–178. [Google Scholar] [CrossRef]
- Arinelli, A.; Aleixo, A.L.Q.D.C.; Freitas, D.F.S.; Valle, A.C.F.D.; Almeida-Paes, R.; Gutierrez-Galhardo, M.C.; Curi, A.L.L. Ocular Sporotrichosis: 26 Cases with Bulbar Involvement in a Hyperendemic Area of Zoonotic Transmission. Ocul. Immunol. Inflamm. 2020, 28, 764–771. [Google Scholar] [CrossRef]
- Pereira, M.A.; Freitas, R.J.; Nascimento, S.B.; Pantaleão, L.; Vilar, E.G. Sporotrichosis: A Clinicopathologic Study of 89 Consecutive Cases, Literature Review, and New Insights About Their Differential Diagnosis. Am. J. Dermatopathol. 2020, 42, 751–755. [Google Scholar] [CrossRef]
- Rocha, I.D.C.B.; Della Terra, P.P.; de Oliveira, R.C.; Zanotti, R.L.; Falqueto, A.; de Camargo, Z.P.; Rodrigues, A.M.; Goncalves, S.S. Molecular-based assessment of diversity and population structure of Sporothrix spp. clinical isolates from Espírito Santo-Brazil. Mycoses 2021, 64, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Caus, A.L.O.; Zanotti, R.L.; Faccini-Martínez, Á.A.; Paterlini, G.V.; Falqueto, A. Epidemiological and clinical aspects of sporotrichosis in Espírito Santo State, southeast Brazil: A study of three decades (1982–2012). Am. J. Trop. Med. Hyg. 2019, 100, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Poester, V.R.; Mattei, A.S.; Madrid, I.M.; Pereira, J.T.B.; Klafke, G.B.; Sanchotene, K.O.; Brandolt, T.M.; Xavier, M.O. Sporotrichosis in Southern Brazil, towards an epidemic? Zoonoses Public Health 2018, 65, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Benvegnú, A.M.; Dallazzem, L.N.D.; Chemello, R.M.L.; Beber, A.A.C.; Chemello, D. Case series of sporotrichosis at a teaching hospital in Brazil. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190509. [Google Scholar] [CrossRef] [PubMed]
- Grisolia, J.C.; Santos, L.A.; Coelho, L.M.L.; Silva, R.R.; de Camargo, Z.P.; Velloso, T.R.G.; Coelho, L.F.; Chavasco, J.K.; Malaquias, L.C.C. Seroepidemiological survey on sporotrichosis-infection in rural areas of the south of Minas Gerais State, Brazil. Braz. J. Microbiol. 2021, 52, 41–47. [Google Scholar] [CrossRef]
- Filho, J.E.; dos Santos, I.B.; Reis, C.M.S.; Patané, J.S.L.; Paredes, V.; Bernardes, J.P.R.A.; Poggiani, S.D.S.C.; Castro, T.D.C.B.; Gomez, O.M.; Pereira, S.A.; et al. A novel Sporothrix brasiliensis genomic variant in Midwestern Brazil: Evidence for an older and wider sporotrichosis epidemic. Emerg. Microbes Infect. 2020, 9, 2515–2525. [Google Scholar] [CrossRef]
- Marques, G.F.; Martins, A.L.G.P.; Sousa, J.M.P.; Brandão, L.S.G.; Wachholz, P.A.; Masuda, P.Y. Characterization of sporotrichosis cases treated in a dermatologic teaching unit in the State of São Paulo-Brazil, 2003–2013. Bras. Dermatol. 2015, 90, 273–275. [Google Scholar] [CrossRef]
- Veasey, J.; Neto, M.; Ruiz, L.; Zaitz, C. Clinical and laboratory profile of urban sporotrichosis in a tertiary hospital in the city of São Paulo. Bras. Dermatol. 2021, 96, 243–245. [Google Scholar] [CrossRef]
- Ferreira, L.C.; Barroso, P.F.; Tonomura, E.; Akiti, T.; Rodrigues, K.M. Osteomyelitis caused by Sporothrix schenckii in an immunocompetent patient. Rev. Soc. Bras. Med. Trop. 2016, 49, 527–529. [Google Scholar] [CrossRef][Green Version]
- Matos, A.M.F.; Moreira, L.M.; Barczewski, B.F.; De Matos, L.X.; De Oliveira, J.B.V.; Pimentel, M.I.F.; Almeida-Paes, R.; Oliveira, M.G.; Pinto, T.C.A.; Lima, N.; et al. Identification by MALDI-TOF MS of Sporothrix brasiliensis Isolated from a Subconjunctival Infiltrative Lesion in an Immunocompetent Patient. Microorganisms 2020, 8, 22. [Google Scholar] [CrossRef]
- do Monte Alves, M.; Pipolo Milan, E.; da Silva-Rocha, W.P.; Soares de Sena da Costa, A.; Araújo Maciel, B.; Cavalcante Vale, P.H.; de Albuquerque, P.R.; Lopes Lima, S.; Salles de Azevedo Melo, A.; Messias Rodrigues, A.; et al. Fatal pulmonary sporotrichosis caused by Sporothrix brasiliensis in Northeast Brazil. PLoS Negl. Trop. Dis. 2020, 14, e0008141. [Google Scholar] [CrossRef] [PubMed]
- Xavier, J.R.B.; Waller, S.B.; Osório, L.D.G.; Vives, P.S.; Albano, A.P.N.; de Aguiar, E.S.V.; Ferreira, M.R.A.; da Conceição, F.R.; de Faria, R.O.; Meireles, M.C.A.; et al. Human sporotrichosis outbreak caused by Sporothrix brasiliensis in a veterinary hospital in Southern Brazil. J. Mycol. Med. 2021, 31, 101163. [Google Scholar] [CrossRef] [PubMed]
- de Sá Menezes Carvalho, G.; Verrinder Veasey, J. Immunoreactive cutaneous sporotrichosis. Bras. Dermatol. 2020, 95, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Lacerda Filho, A.M.; Cavalcante, C.M.; Da Silva, A.B.; Inácio, C.P.; de Lima-Neto, R.G.; De Andrade, M.C.L.; Magalhães, O.M.C.; Dos Santos, F.D.A.G.; Neves, R.P. High-Virulence Cat-Transmitted Ocular Sporotrichosis. Mycopathologia 2019, 184, 547–549. [Google Scholar] [CrossRef] [PubMed]
- de Moura Barros, N.; de Souza Pessoa, A.; Martins Brotas, A. Systemic sporotrichosis in an alcoholic patient. Bras. Dermatol. 2020, 95, 376–378. [Google Scholar] [CrossRef]
- Lemes, L.R.; Veasey, J.V.; Soutto-Mayor, S.; Contin-Proenca, C. Ocular involvement in sporotrichosis: Report of two cases in children. Bras. Derm. 2021, 96, 349–351. [Google Scholar] [CrossRef]
- Henckens, N.F.T.; Rovers, J.F.J.; van Dommelen, L.; Bovenschen, H.J. Can cats cause colossal contagious cutaneous carbuncles? Derm. Online J. 2021, 27. [Google Scholar] [CrossRef]
- Lora Barraza, L.; Bissoli Tolomelli, J.; Graça Cunha, C.; Bernardes Filho, F.; Towersey, L.; Hay, R.; Casz Schechtman, R.; da Costa Nery, J.A. Facial Cutaneous Sporotrichosis in a Boy. J. Emerg. Med. 2019, 56, 222–223. [Google Scholar] [CrossRef]
- de Freitas Valente, M.; Bertazzoli Diogo, A.; Culau Merlo, V.F.; Pereira Pegas, J.R. Disseminated cutaneous sporotrichosis: Unusual presentation in an alcoholic patient. Rev. Inst. Med. Trop. São Paulo 2020, 62, e60. [Google Scholar] [CrossRef]
- Crestani, L.; de Castro e Souza, B.; Kakizaki, P.; Sakai-Valente, N.Y. Therapeutic failure with itraconazole in sporotrichosis due to bariatric surgery. Bras. Derm. 2020, 95, 241–243. [Google Scholar] [CrossRef]
- Arantes-Ferreira, G.S.; Watanabe, A.L.C.; Trevizoli, N.C.; Jorge, F.M.F.; Cajá, G.O.N.; Diaz, L.G.G.; Meireles, L.P.; Araújo, M.C.C.L. Disseminated Sporotrichosis in a Liver Transplant Patient: A Case Report. Transpl. Proc. 2019, 51, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Fichman, V.; Valle, A.C.F.D.; de Macedo, P.M.; Freitas, D.F.S.; Oliveira, M.M.E.; Almeida-Paes, R.; Gutierrez-Galhardo, M.C. Cryosurgery for the treatment of cutaneous sporotrichosis in four pregnant women. PLoS Negl. Trop. Dis. 2018, 12, e0006434. [Google Scholar] [CrossRef] [PubMed]
- Biancardi, A.L.; Freitas, D.F.; Vitor, R.D.; Andrade, H.B.; de Oliveira, M.M.; do Valle, A.C.; Zancope-Oliveira, R.M.; Galhardo, M.C.; Curi, A.L. Multifocal choroiditis in disseminated sporotrichosis in patients with HIV/AIDS. Retin. Cases Brief Rep. 2017, 11, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Fischman-Gompertz, O.; Rodrigues, A.M.; Fernandes, G.F.; Bentubo, H.D.L.; Pires de Camargo, Z.; Petri, V. Atypical Clinical Presentation of Sporotrichosis Caused by Sporothrix globosa Resistant to Itraconazole. Am. J. Trop. Med. Hyg. 2016, 94, 1218–1222. [Google Scholar] [CrossRef]
- Aparecida-Grazziotin, N.; Gonçalves, I.L.; Todeschini, D.; Grazziotin-Vedana, L.; Canello-Todeschini, C.M.; Grazziotin, C. Squamous cell carcinoma subsequent to scarring caused by sporotrichosis: A case report. Rev. Iberoam. Micol. 2019, 36, 83–85. [Google Scholar] [CrossRef]
- Ribeiro, B.N.; Ribeiro, R.N.; Penna, C.R.; Frota, A.C. Bone involvement by Sporothrix schenckii in an immunocompetent child. Pediatr. Radiol. 2015, 45, 1427–3140. [Google Scholar] [CrossRef]
- Freitas, D.F.; Santos, S.S.; Almeida-Paes, R.; de Oliveira, M.M.; do Valle, A.C.; Gutierrez-Galhardo, M.C.; Zancopé-Oliveira, R.M.; Nosanchuk, J.D. Increase in virulence of Sporothrix brasiliensis over five years in a patient with chronic disseminated sporotrichosis. Virulence 2015, 6, 112–120. [Google Scholar] [CrossRef]
- Nassif, P.W.; Granado, I.R.; Ferraz, J.S.; Souza, R.; Nassif, A.E. Atypical presentation of cutaneous sporotrichosis in an alcoholic patient. Dermatol. Online J. 2012, 18, 12. [Google Scholar]
- Falqueto, A.; Bravim-Maifrede, S.; Araujo-Ribeiro, M. Unusual clinical presentation of sporotrichosis in three members of one family. Intern. J. Dermatol. 2012, 51, 434–438. [Google Scholar] [CrossRef]
- Marques-de-Macedo, P.; Sztajnbok, D.C.N.; Camargo, Z.P.; Rodrigues, A.M.; Lopes-Bezerra, L.M.; Bernardes-Engemann, A.R.; Orofino-Costa, R. Dacryocystitis due to Sporothrix brasiliensis: A case report of a successful clinical and serological outcome with low-dose potassium iodide treatment and oculoplastic surgery. Br. J. Dermatol. 2015, 172, 1116–1119. [Google Scholar] [CrossRef]
- Fichman, V.; Saraiva-Freitas, D.F.; Marques-de-Macedo, P.; Francesconi-do-Valle, A.C.; Almeida-Silva, F.; Zancopé-Oliveira, R.M.; Almeida-Paes, R.; Gutierrez-Galhardo, M.C. Sporotrichosis After Tattooing Caused by Sporothrix. Bras. Mycopathol. 2022, 187, 137–139. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno Araújo, M.J.C.L.; Nihei, C.H.; Rodrigues, A.M.; Higashino, H.; Ponzio, V.; Campos Pignatari, A.C.; Barcellos, M.A.; Braga, O.; Duayer, I.F. Case Report: Invasive Sinusitis due to Sporothrix Brasiliensis in a Renal Transplant Recipient. Am. J. Trop. Med. Hyg. 2021, 105, 1218–1221. [Google Scholar] [CrossRef]
- Lima, M.A.; Vallier, R.; Silva, M.M. Sporothrix brasiliensis meningitis in an immunocompetent patient. Pract. Neurol. 2021, 21, 241–242. [Google Scholar] [CrossRef]
- Veasey, J.V.; Carvalho, G.S.M.; Ruiz, L.R.B.; Neves-Neto, M.F.; Zaitz, C. Epidemiological and geographical distribution profile of urban sporotrichosis in the city of São Paulo. Bras. Derm. 2022, 97, 228–230. [Google Scholar] [CrossRef]
- Antonio, L.D.F.; Pimentel, M.I.F.; Lyra, M.R.; Madeira, M.D.F.; Miranda, L.D.F.C.; Paes, R.A.; Brito-Santos, F.; Carvalho, M.H.G.F.; Schubach, A.D.O. Sporothrix schenckii Sensu Lato identification in fragments of skin lesion cultured in NNN medium for differential diagnosis of cutaneous leishmaniasis. Diagn. Microbiol. Infect. Dis. 2017, 87, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; de Hoog, S.; de Camargo, Z.P. Emergence of pathogenicity in the Sporothrix schenckii complex. Med Mycol. 2013, 51, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, Z.; Pereira, C. Fungal diseases in children and adolescents in a referral centre in Bogota, Colombia. Mycoses 2018, 61, 543–548. [Google Scholar] [CrossRef]
- Macías, P.; Ordóñez, J.; Arenas, C.M.; Rodríguez, G. An eighteen-year-old man with tropical verrucous syndrome: Leishmaniasis vs, sporotrichosis. Biomedica 2021, 41, 240–246. [Google Scholar] [CrossRef]
- Arenas-Soto, C.M.; Téllez-Kling, A.M.; Alvarado-Álvarez, Z.L. A Lesion on the Ear Resulting From Infection Acquired in the Tropics. Actas Dermosifiliogr. 2016, 107, 599–600. [Google Scholar] [CrossRef]
- Niklitschek, S.; Porras, N.; González, S.; Romero, W. Sporotrichosis. Med. Clin. 2015, 145, 418. [Google Scholar] [CrossRef]
- Cruz, R.; Vieille, P.; Oschilewski, D. Sporothrix globosa isolation related to a case of lymphocutaneous sporotrichosis. Rev. Chil. Infectol. 2012, 4, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Choappa, R.M.; Vieille-Oyarzo, P.I.; Carvajal-Silva, L.C. Aislamiento de Sporothrix pallida complex en muestras clínicas y ambientales de Chile. Rev. Argent Microbiol. 2014, 46, 311–314. [Google Scholar] [CrossRef]
- García Duarte, J.M.; Wattiez Acosta, V.R.; Fornerón Viera, P.M.L.; Aldama Caballero, A.; Gorostiaga Matiauda1, G.A.; Rivelli de Oddone, V.B.; Pereira Brunelli, J.G. Sporotrichosis transmitted by domestic cat. A family case report. Rev. Del. Nacional. 2017, 9, 67–76. [Google Scholar] [CrossRef]
- Aguilar Fernández, G.; Araújo López, V. Mycosis and nocardiosis implantation: Sporotrichosis, Chromoblastomycosis, Mycetomas and Nocardiosis. Casuistics in the Central Laboratory of Public Health, Paraguay, period 1997–2019. Rev. Nac. 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Ramirez-Soto, M.; Lizarraga-Trujillo, J. Granulomatous sporotrichosis: Report of two unusual cases. Rev. Chil. Infectol. 2013, 5, 548–553. [Google Scholar]
- Ramírez-Soto, M.C. Sporotrichosis in the Ocular Adnexa: 21 Cases in an Endemic Area in Peru and Review of the Literature. Am. J. Ophthalmol. 2016, 162, 173–179.e3. [Google Scholar] [CrossRef]
- Ramírez-Soto, M.C.; Malaga, G. Subcutaneous mycoses in Peru: A systematic review and meta-analysis for the burden of disease. Int. J. Dermatol. 2017, 56, 1037–1045. [Google Scholar] [CrossRef]
- Ramírez-Soto, M.C.; Andagua-Castro, J.; Lizárraga-Trujillo, J. Palpebral sporotrichosis in a 6-year-old child. Int. J. Dermatol. 2016, 55, e625–e626. [Google Scholar] [CrossRef]
- Wolff, D.; Feldt, T.; Reifenberger, J.; Sebald, H.; Bogdana, C. The brief case: Cutaneous sporotrichosis in an immunocompetent patient after travel to Peru. J. Clin. Microbiol. 2018, 56, e01958-17. [Google Scholar] [CrossRef]
- Rueda, M.; Torres, N.; Bravo, F. Disseminated cutaneous sporotrichosis: An unusual case. Derm. Online J. 2018, 24. [Google Scholar] [CrossRef]
- Oyarce, J.A.; García, C.; Alave, J.; Bustamante, B. Epidemiological clinical and laboratory characterization of sporotrichosis in patients of a tertiary care hospital in Lima, Peru, from 1991 to 2014. Rev. Chil. Infectol. 2016, 3, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Schwalb, A.; Carcamo, P.M.; Seas, C. Lymphocutaneous Sporotrichosis. Am. J. Trop. Med. Hyg. 2022, 106, 758–759. [Google Scholar] [CrossRef] [PubMed]
- Solorzano, S.; Ramirez, R.; Cabada, M.M.; Montoya, M.; Cazorla, E. Disseminated cutaneous sporotrichosis with joint involvement in a woman with type 2 diabetes. Rev. Peru Med. Exp. Salud Publica 2015, 32, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, E.; Arrillaga, A.; Dalcín, L.; Carbia, M.; Arteta, Z.; Perera, P. Clinical and Epidemiological Characteristics of Sporotrichosis in Reference Center of Uruguay. J. Fungi 2022, 8, 322. [Google Scholar] [CrossRef] [PubMed]
- Barreto, L.; Velásquez, G.; Mendoza, M.; Camacho, E.; Goncalves, E.; Rodríguez, S.; Niño-Vega, G.A. Geographical distribution and ecological niche modeling of the etiological agents of human sporotrichosis in Venezuela. Braz. J. Microbiol. 2021, 52, 63–71. [Google Scholar] [CrossRef]
- Mata-Essayag, S.; Delgado, A.; Colella, M.T.; Landaeta-Nezer, M.E.; Rosello, A.; de Salazar, C.P.; Olaizola, C.; Hartung, C.; Magaldi, S.; Velasquez, E. Epidemiology of sporotrichosis in Venezuela. Int. J. Dermatol. 2013, 52, 974–980. [Google Scholar] [CrossRef]
- Martínez-Méndez, D.; Hernández-Valles, R.; Alvarado, P.; Mendoza, M. Mycoses In Venezuela: Working Groups in Mycology Reported Cases (1984–2010). Rev. Iberoam. Micol. 2013, 30, 39–46. [Google Scholar]
- Camacho, E.; León-Navarro, I.; Rodríguez-Brito, S.; Mendoza, M.; Niño-Vega, G.A. Molecular epidemiology of human sporotrichosis in Venezuela reveals high frequency of Sporothrix globose. BMC Infect Dis. 2015, 15, 94. [Google Scholar] [CrossRef]
- Orofino-Costa, R.; Marques-de-Macedo, P.; Messias-Rodrigues, A.; Bernardes-Engemann, A.R. Sporotrichosis: An update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. Bras. Dermatol. 2017, 92, 606–620. [Google Scholar] [CrossRef]
- Rimma, Z.; Hernández Hernández, F. Sporotrichosis: The most frequent subcutaneous mycosis in Mexico. Rev. Fac. Med. 2019, 62, 48–55. [Google Scholar]
- Chakrabarti, A.; Bonifaz, A.; Gutierrez-Galhardo, M.C.; Mochizuki, T.; Li, S. Global epidemiology of sporotrichosis. Med. Mycol. 2015, 53, 3–14. [Google Scholar] [CrossRef] [PubMed]
- de Beer, Z.W.; Duong, T.A.; Wingfield, M.J. The divorce of Sporothrix and Ophiostoma: Solution to a problematic relationship. Stud. Mycol. 2016, 83, 165–191. [Google Scholar] [CrossRef] [PubMed]
- Toriello, C.; Brunner-Mendoza, C.; Ruiz-Baca, E.; Duarte-Escalante, E.; Pérez-Mejía, A.; Reyes-Montes, M.R. Sporotrichosis in Mexico. Braz. J. Microbiol. 2021, 52, 49–62. [Google Scholar] [CrossRef] [PubMed]

| Region | Country | City | Number of Reported Cases | Vulnerable Population | Diagnostic Method | Type of Sporotrichosis | Etiological Agents (%) | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (Years) | Taxonomy | ||||||||
| Before 2017 | After 2017 | |||||||||
| North America | Canada | Ontario | 1 | Male | 44 | PCR sequencing (ITS region) | Disseminated | S. schenckii | S. schenckii | [5] |
| Toronto | 1 | Male | 78 | Fungal culture, Biopsy (Histopathology) | Lymphocutaneous | S. schenckii complex | Sporothrix spp. | [6] | ||
| USA | California | 1 | Female | 7 | Fungal culture Biopsy (Histopathology) | Lymphocutaneous | S. schenckii | Sporothrix spp. | [7] | |
| Minnesota | 1 | Male | 61 | Fungal culture | Disseminated | S. schenckii | Sporothrix spp. | [8] | ||
| ND | 1 | Female | 87 | Fungal culture | Lymphocutaneous on the eyelid | S. schenckii | Sporothrix spp. | [9] | ||
| Pennsylvania | 1 | Male | 67 | Fungal culture Biopsy (Histopathology) | Lymphocutaneous | S. schenckii | Sporothrix spp. | [10] | ||
| Texas | 1 | Male | 34 | Fungal culture Biopsy (Histopathology) | Disseminated | Sporothrix spp. | Sporothrix spp. | [11] | ||
| Texas | 1 | Male | 9 | Fungal culture Biopsy (Histopathology) | Lymphocutaneous on the eyelid | S. schenckii | Sporothrix spp. | [12] | ||
| California | 1 | Female | 41 | Fungal culture | Lymphocutaneous | S. schenckii | Sporothrix spp. | [13] | ||
| Oregon | 1 | Male | 53 | Fungal culture | Disseminated | Sporothrix spp. | Sporothrix spp. | [14] | ||
| Oklahoma | 1 | Male | 66 | Latex agglutination test | Disseminated | S. schenckii | Sporothrix spp. | [15] | ||
| Florida | 1 | Male | 33 month-Old | Fungal culture Biopsy (Histopathology) | Atypical lymphadenitis | S. schenckii | Sporothrix spp. | [16] | ||
| Minnesota | 1 | Male | 49 | Fungal culture | Pulmonary sporotrichosis | Sporothrix spp. | Sporothrix spp. | [17] | ||
| Arizona | 1 | Male | 56 | Fungal culture | Lymphocutaneous and disseminated (10 months later) | S. schenckii | Sporothrix spp. | [18] | ||
| California | 1 | Male | 39 | Fungal culture | Sporothrical arthritis | S. schenckii | Sporothrix spp. | [19] | ||
| California | 1 | Male | 89 | Fungal culture Biopsy (Histopathology) | Disseminated | S. schenckii | Sporothrix spp. | [20] | ||
| Michigan | 1 | Female | 57 | Fungal culture Biopsy (Histopathology) | Lymphocutaneous | S. schenckii | Sporothrix spp. | [21] | ||
| California | 1 | Male | 34 | Latex agglutination test | Chronic meningitis | S. schenckii | Sporothrix spp. | [22] | ||
| Kansas | 1 | Male | 33 | Fungal culture MALDI-TOF | Sporothrical arthritis | S. schenckii | Sporothrix schenckii | [23] | ||
| Oklahoma | 1 | Male | 44 | Fungal culture Biopsy (Histopathology) | Pulmonary sporotrichosis | S. schenckii sensu lato | Sporothrix spp. | [24] | ||
| California | 1 | Male | 41 | Fungal culture | Sporothrical arthritis | S. schenckii | Sporothrix spp. | [25] | ||
| California | 1 | Female | 35 | Fungal culture | Disseminated | S. schenckii | Sporothrix spp. | [26] | ||
| Nebraska | 1 | Male | 62 | Fungal culture Biopsy (Histopathology) | Disseminated | S. schenckii | Sporothrix spp. | [27] | ||
| Boston | 1 | Female | 35 | MALDI-TOF | Fixed cutaneous | S. schenckii | S. schenckii | [28] | ||
| Kansas | 1 | Male | 30 | Fungal culture Biopsy (Histopathology) | Disseminated | S. schenckii | Sporothrix spp. | [29] | ||
| Florida | 1 | Male | 76 | History and physical examination | Lymphocutaneous | Sporothrix spp. | Sporothrix spp. | [30] | ||
| Oklahoma | 1 | Male | 23 | Fungal culture | Lymphocutaneous | S. schenckii complex | Sporothrix spp. | [31] | ||
| Washington | 1 | Female | 44 | Fungal culture PCR sequencing (ITS 1–2) | Disseminated | S. schenckii | S. schenckii | [32] | ||
| Arizona | 1 | Female | 72 | PCR DNA sequencing | Laryngotracheal granulomatous disease | S. schenckii | S. schenckii | [33] | ||
| Mexico | Veracruz | 1 | Male | 39 | Fungal culture Biopsy (Histopathology) | Atypical | S. schenckii | Sporothrix spp. | [34] | |
| Puebla | 1 | Male | 36 | Fungal culture Biopsy (Histopathology) | Disseminated | S. schenckii | Sporothrix spp. | [35] | ||
| Oaxaca | 1 | Male | 13 | Fungal culture | Lymphocutaneous on the left hand, forearm, and upper arm | Sporothrix spp. | Sporothrix spp. | [36] | ||
| Mexico City | 1 | Male | 54 | Fungal culture Biopsy (Histopathology) | Disseminated (Testicular involvement) | S. schenckii | Sporothrix spp. | [37] | ||
| Guerrero | 1 | Female | 36 | Fungal culture Biopsy (Histopathology) | Disseminated | Sporothrix spp. | Sporothrix spp. | [38] | ||
| Durango | 1 | Male | 68 | Fungal culture Biopsy (Histopathology) | Disseminated | Sporothrix spp. | Sporothrix spp. | [39] | ||
| ND | 24 | Male (16) Female (8) | Average: 35.5 | PCR sequencing (calmodulin gene) | Cutaneous disseminated 16 (66.7%) Cutaneous disseminated + Mucosal 3 (12.5%) Joint 1 (4.1%) Visceral 1 (4.1%) Fungaemia 1 (4.1%) Mucosal + Visceral + Fungemia: 1 (4.1%) Visceral + Fungaemia 1 (4.1%) | S. schenckii 23 (95.5%). S. globosa 1 (4.5%) | S. schenckii 23 (95.5%). S. globosa 1 (4.5%) | [40] | ||
| ND | 55 | Male (34) ND Female (18) | Sporotrichin Skin Test Fungal culture | Lymphocutaneous 32 (58.2%) Fixed cutaneous 19 (34.5%) Disseminated 4 (7.3%) | S. schenckii 54 (98%) S. globosa 1 (2%) | S. schenckii 54 (98%) S. globosa 1 (2%) | [41] | |||
| Guerrero | 73 | Male (33) Female (40) | Average: 25.8 | Fungal culture Biopsy (Histopathology) | Lymphocutaneous: 41 (56.16%) Fixed cutaneous 24 (32.87%) Disseminated 8 (10.95%) | S. schenckii | S. schenckii | [42] | ||
| Chihuahua | 1 | Female | 84 | Multiplex PCR (Calmodulin gene) | Fixed cutaneous (Auricular sporotrichosis) | S. schenckii (sensu stricto) | S. schenckii | [43] | ||
| Baja California | 1 | Male | 23 | Fungal culture Biopsy (Histopathology) | Lymphocutaneous | S. schenckii | Sporothrix spp. | [44] | ||
| San Luis Potosi 8 Puebla 3 Mexico City 2 Queretaro 2 Guanajuato 2 Jalisco 1 Zacatecas 1 Michoacan 1 Morelos 1 State of Mexico 1 | 22 | ND | PCR sequencing (Calmodulin and calcium-calmodulin-dependent kinase genes) | Lymphocutaneous: 17 (77.3%) Fixed cutaneous 4 (18.2%) Disseminated 1 (4.5%) | S. schenckii: 18 (81.8%) S. globosa 4 (18.2%) | S. schenckii: 18 (81.8%) S. globosa 4 (18.2%) | [45] | |||
| Puebla 4 Nuevo Leon 2 Oaxaca 6 Mexico City 3 Jalisco 2 | 17 | ND | PCR sequencing (Calmodulin gene) | Lymphocutaneous: 16 (94.11%) Disseminated: 1 (5.88%) | S. schenckii: 16 (94.11%) S. globosa 1 (5.88%) | S. schenckii: 16 (94.11%) S. globosa 1 (5.88%) | [46] | |||
| Guerrero | 76 | Male (35) Female (41) | <18: 37 >18: 39 | Fungal culture Biopsy (Histopathology) | Lymphocutaneous 43 (56.8%) Fixed cutaneous 24 (32.3%) Disseminated 8 (11%) | Sporothrix spp. | Sporothrix spp. | [47] | ||
| Jalisco 1057 Nayarit 23 Zacatecas 20 Michoacan 19 Guanajuato 12 Veracruz 1 Chihuahua 1 | 1134 | Male (669) Female (465) | ND | Lymphocutaneous: 782 (69%) Fixed cutaneous: 308 (27.2%) Disseminated 44 (38.8%) | S. schenckii complex | Sporothrix spp. | [48] | |||
| ND | 1 | Male | 45 | PCR sequencing (Calmodulin gene) | Disseminated | S. schenckii complex | S. schenckii | [49] | ||
| ND | 1 | Male | 56 | Fungal Culture Biopsy (Histopathology) PCR sequencing (ITS and calmodulin gene) | Fixed cutaneous sporotrichosis | S. mexicana | S. mexicana | [50] | ||
| ND | 18 | Male (10) Female (8) | ND | PCR sequencing (ITS regions) | Lymphocutaneous 13 (72.2%) Fixed cutaneous 5 (27.8%) | S. schenckii 17 (94.4%) S. globosa: 1 (5.6%) | S. schenckii 17 (94.4%) S. globosa: 1 (5.6%) | [51] | ||
| Oaxaca | 2 | Male | 61 | Multiplex PCR (Calmodulin gene) | Fixed cutaneous 1 (50%) Disseminated 1 (50%) | S. schenckii sensu stricto | S. schenckii | [52] | ||
| Male | 21 | |||||||||
| Region | Country | City | Number of Reported Cases | Vulnerable Population | Diagnostic Method | Type of Sporotrichosis | Etiological Agents (%) | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Age (Years) | Taxonomy | ||||||||
| Before 2017 | After 2017 | |||||||||
| Central America | Costa Rica | San José | 57 (1994–2015) | No data | Direct microscopy, culture, PCR (enzymatic restriction and sequencing of the calmodulin gen) | ND | S. schenckii sensu stricto 53 (93%) S. brasiliensis 2 (3.5%) Sporothrix spp. 2 (3.5%) | S. schenckii 53 (93%) S. brasiliensis 2 (3.5%) Sporothrix spp. 2 (3.5%) | [53] | |
| Guatemala | Guatemala City | 11 | Male 7 Female 4 | Average 49 years | Fungal culture, Histopathology | Fixed cutaneous 9 (81.8%) Lymphocutaneous 2 (18.2%) | Sporothrix spp. (100%) | Sporothrix spp. (100%) | [54] | |
| Guatemala City | 53 (2007–2016) | Male 33 Female 20 | Average 44.1 years | Fungal culture, microscope with Lactophenol cotton blue | Lymphocutaneous 33 (62.2%) Fixed cutaneous 17 (32.1%) Disseminated 2 (3.8%) Chancre 1 (1.9%) | Sporothrix schenckii complex. (100%) | Sporothrix spp. (100%) | [55] | ||
| Guatemala City | 1 | ND | Fungal culture, PCR sequencing (ITS 1- 2 and β -tubulin) | ND | Sporothrix schenckii sensu stricto | Sporothrix schenckii | [56] | |||
| Honduras | Tegucigalpa | 1 | Male 1 | 14 years | Fungal culture | Lymphocutaneous 1 (100%) | S. schenckii | Sporothrix spp. | [57] | |
| Panamá | Chorrera District | 1 | Male 1 | 34 years | Clinical, Direct Microscopy, Fungal culture. | Lymphocutaneous 1 (100%) | ND | Sporothrix spp. | [58] | |
| Caribbean | Cuba | Pinar del Río | 1 | Female 1 | 57 years | Histopathology Fungal culture | Lymphocutaneous | Sporothrix schenckii
sensu lato (100%) | Sporothrix spp. (100%) | [59] |
| Cumanayagüa | 1 | Male | 67 | Histopathology, Fungal culture, Microscopy with lactophenol cotton blue | Lymphocutaneous | Sporothrix schenckii sensu lato (100%) | Sporothrix spp. (100%) | [60] | ||
| Region | Country | City | Number of Reported Cases | Vulnerable Population | Diagnostic Method | Type of Sporotrichosis | Etiological Agents (%) | References | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex (Number of Cases) | Age (Years) | Taxonomy | ||||||||
| Before 2017 | After 2017 | |||||||||
| South America | Argentina | Provincia de Chaco | 1 | Female | 65 | Bronchoalveolar lavage (BAL), Giemsa stain Fungal culture PCR sequencing (ITS 1–2) | Pulmonary | S. schenckii | S. schenckii | [61] |
| Buenos Aires | 16 | Male (4) Female (10) ND (7) | Average 32.5 | Fungal culture and PCR sequencing (Calmodulin gene) | Lymphocutaneous 33 (42.9%) Fixed cutaneous 17 (19.0%) ND (38.1%) | S. brasiliensis | S. brasiliensis | [62] | ||
| Misiones | 1 | Fungal culture and PCR sequencing (Calmodulin gene) | ||||||||
| El Calafate | 4 | Fungal culture, PCR sequencing (Calmodulin gene) and histopathology | ||||||||
| Buenos Aires | 15 | ND | Fungal culture (agar potato dextrose and brain heart infusion agar) PCR sequencing (Calmodulin gene) | ND | S. schenckii sensu stricto 9 (56.5%) S. brasiliensis 5 (34.7%) S. globosa 1 (8.7%) | S. schenckii 9 (56.5%) S. brasiliensis 5 (34.7%) S. globosa 1 (8.7%) | [63] | |||
| Buenos Aires | 1 | Female | 5 | Direct microscopy, Fungal culture (Sabouraud agar), Histopalology | Lymphocutaneous | S. schenckii complex | Sporothrix spp. | [64] | ||
| Brazil | Rio de Janeiro (Duque de Caxias) | 827 from 2007–2016 | Female (541) Male (286) | 42 | Fungal culture | ND | Sporothrix spp. | Sporothrix spp. | [65] | |
| Rio de Janeiro ND Teresópolis ND | 1563 (1999–2008 = 50 (3.20%)) | Male (16) Female (34) | Average 47 | Direct microscopy, Fungal culture, PCR sequencing (calmodulin gene) | Lymphocutaneous 24 (48%) Fixed cutaneous 15 (30%) Disseminated cutaneous 6 (12%) disseminated (involving internal tissues) 5 (10%) | S. brasiliensis 45 45 (90%) S. schenckii sensu stricto 5 (10%) | S. brasiliensis 45 (90%) S. schenckii 5 (10%) | [66] | ||
| Rio de Janeiro | Group 1 48 (1.33%) Group 2 3570 (98.67%) 1987–2013 | Group 1 HIV patients Male 33 Female 15 Group 2 Immunocompetent patients Male (1102) Female (2468) | Average: 38.4 Averag: 46.3 | Direct microscopy, Fungal culture. | ND | Sporothrix spp. | Sporothrix spp. | [67] | ||
| Rio de Janeiro | 21/1750 cases in HIV patients (1.2%) from 1999–2009 | Male (16) Female (5) | Average: 41.2 | Direct microscopy, Fungal culture, Histopathology | Lymphocutaneous 7 (33.3%) Disseminated 7 (33.3%) widespread cutaneous 5 (23.8%) fixed cutaneous 2 (9.5%) | S. schenckii sensu lato | Sporothrix spp. | [68] | ||
| Rio de Janeiro 16 Duque de Caxias 6 São João de Meriti 2 São Gonçalo 1 Maricá 1 | 26 from 2007–2017 | Female (19) Male (7) | Average: 25 | Direct microscopic, Fungal culture | Primary ocular 21 (80.8%) Associated cutaneous disease (3 lymphocutaneous, 1 the fixed cutaneous and 1 the disseminated 5 (19.2%) | Sporothrix spp. | Sporothrix spp. | [69] | ||
| Rio de Janeiro | 86 from 2009–2017 | Male (26) Female (60) | Average: 36.3 Average: 46 | Fungal culture Histopathology | ND | Sporothrix spp. | Sporothrix spp. | [70] | ||
| Espíritu Santo | 73 from 2016–2019 | Male Female | ND | Fungal culture, Microscopy with lactophenol cotton blue, PCR sequencing (Calmodulin gene and Mating type (MAT) gene) | ND | S. brasiliensis 55 (76%) S. schenckii sensu stricto 18 (24%) | S. brasiliensis 55 (76%) S. schenckii 18 (24%) | [71] | ||
| Espíritu Santo | 171 cases from 1982–2012 | Male (138) Female (33) | Average: 33.42 | Fungal culture | ND | Sporothrix spp. | Sporothrix spp. | [72] | ||
| Rio Grande do Sul | 83 from 2010–2016 | ND | Fungal culture | ND | Sporothrix spp. | Sporothrix spp. | [73] | |||
| Rio Grande do Sul | 43 from 2006–2015 | Male (31) Female (7) | Average: 43 | Fungal culture | Lymphocutaneous 22 (51%) Fixed cutaneous 14 (32.5%) Disseminated cutaneous 1 (2.5%) ND 6 (14%) | Sporothrix spp. | Sporothrix spp. | [74] | ||
| Minas Gerais | 282 | Male (153) Female (129) | Average: 42.52 | Fungal culture, Sporotrichin test, Histophatology, Production of S. schenckii antigens, Enzyme-linked immunosorbent assay | ND | S. schenckii | Sporothrix spp. | [75] | ||
| Brasilia | 91 from 1993–2018 | Male (64) Female (27) | ND | Direct microscopy, Fungal culture, PCR sequencing (Calmodulin gene) | Lymphocutaneous 34 (37.36%) Cutaneous fixed 6 (6.59%) Disseminated 5 (5.49%) ND 46 (50.55%) | S. globosa (ND) S. schenckii (ND) | S. globosa (ND) S. schenckii (ND) | [76] | ||
| São Paulo | 25 from 2003–2013. | Male (18) Female (7) | Average: 42.48 | Fungal culture Histopathology | Lymphocutaneous 20 (80%) Fixed cutaneous 5 (20%) | S. schencki sensu lato | Sporothrix spp. | [77] | ||
| São Paulo | 20 from 2012–2020 | Male (9) Female (11) | Average: 2.2 | Direct microscopy, Fungal culture, Histopathology | Lymphocutaneous 10 (50%) Multiple-inoculation 5 (25%) Fixed-cutaneous 3 (15%) Ocular-mucosal 2 (10%) | Sporothrix spp. | Sporothrix spp. | [78] | ||
| Rio de Janeiro | 1 | Male | 35 | Direct microscopy, fungal culture | Osteomyelitis | S. schenckii complex | Sporothrix spp. | [79] | ||
| Rio de Janeiro | 1 | Female | 68 | Direct microscopy (KOH), fungal culture (Sabouraud Dextrose Agar 2%, and Mycosel Agar, Brain Heart Infusion Agar, Potato Dextrose Agar), Lactophenol Cotton Blue and MALDI-TOF MS | Ocular | S. brasiliensis | S. brasiliensis | [80] | ||
| Rio Grande do Norte | 1 | Male | 50 | Direct microscopy (KOH), fungal culture (Mycosel Agar), PCR sequencing >(Calmodulin gene) | Pulmonary | S. brasiliensis | S. brasiliensis | [81] | ||
| Pelotas | 7 | ND | Gram-stain microscopy, fungal culture (Sabouraud-dextrose agar added with chloramphenicol and Mycosel), PCR sequencing (ITS1 and ITS4 and Calmodulin gene) | Lymphocutaneous 4 (57.1%) Ocular 3 (42.9%) | S. brasiliensis | S. brasiliensis | [82] | |||
| São Paulo | 1 | Female | 12 | Histopatology (Grocott stainin), fungal culture. | Immunoreactive cutaneous | Sporothrix spp. | Sporothrix spp. | [83] | ||
| Recife | 1 | Male | 25 | Histopatology (hematoxylin–eosin straining), fungal culture (Sabouraud dextrose agar with chloramphenicol), PCR sequencing (using the species-specific primers Sbra-F and Sbra-R and Calmodulin gene) | Ocular | S. brasilienis | S. brasilienis | [84] | ||
| Rio de Janeiro | 1 | Male | 44 | Fungal culture | Disseminated | Sporothrix spp. | Sporothrix spp. | [85] | ||
| São Paulo | 2 | Male | 3 and 12 | Fungal culture | Ocular | Sporothrix spp. | Sporothrix spp. | [86] | ||
| ND | 1 | Female | 45 | Histopathology, Fungal culture (Sabouraud dextrose agar), PCR sequencing (Whole genome sequencing) | Cutaneos carbuncle | S. brasiliensis | S. brasiliensis | [87] | ||
| Rio de Janeiro | 1 | Male | 11 | Fungal culture (Sabouraud’s dextrose agar), Culture microscopy with Lactofenol blue | Facial Cutaneous | Sporothrix spp. | Sporothrix spp. | [88] | ||
| Guarulhos, Sao Paulo | 1 | Male | 56 | Fungal culture, Histopathology (Peryodic Acid Schiff staining), | Disseminated | Sporothrix spp. | Sporothrix spp. | [89] | ||
| São Paulo | 1 | Female | 39 | Fungal culture (Sabouraud agar) | Lymphocutaneous | Sporothrix spp. | Sporothrix spp. | [90] | ||
| Brasilia | 1 | Male | 26 | Fungal culture | Disseminated | Sporothrix spp. | Sporothrix spp. | [91] | ||
| Rio de Janeiro | 4 from 2006–2016 | Female Age ranged from 18–34 | Average 25 | Fungal culture, PCR sequencing (Primer T3B fingerprintig assay) | Fixed cutaneous 2 (50%) Lymphocutaneous 2 (50%) | Sporothrix spp. 2 (50%) S. brasiliensis 2 (50%) | Sporothrix spp. 2 (50%) S. brasiliensis 2 (50%) | [92] | ||
| Rio de Janeiro | 3 from 2006 to 2013 | Male Age ranged from 25–43 | Average 32 | Fungal culture, PCR sequencing (primer T3B fingerprinting assay ) | Disseminated 3 | S. brasiliensis | S. brasiliensis | [93] | ||
| Rio de Janeiro | 1 | Male | 66 | Direct microscopy, fungal culture (Sabouraud dextrose agar, potato dextrose agar, corn meal agar and and brain heart infusion agar), Histopatology, PCR sequencing (Calmodulin gene) | Lymphocutaneous | S. globosa | S. globosa | [94] | ||
| Palmeira das Missões | 1 | Male | 73 | Fungal culture | ND | S. schenckii complex | Sporothrix spp. | [95] | ||
| Rio de Janeiro | 1 | Male | 5 | Fungal culture and Histopatology | Osteoarticular | S. schenckii | Sporothrix spp. | [96] | ||
| Rio de Janeiro | 1 | Male | 61 | Fungal culture, PCR sequencing (primer T3B fingerprinting assay) | Disseminated | S. brasiliensis | S. brasiliensis | [97] | ||
| ND | 1 | Male | 49 | Fungal culture | Disseminated | S. schenckii | Sporothrix spp. | [98] | ||
| Espírito Santo | 3 | Female | 30 and 10 | Direct microscopy (KOH), fungal culture (Sabouraud Dextrose agar and Mycosel agar®), assimilation of sugar test | Chancre 3 | S. brasiliensis | S. brasiliensis | [99] | ||
| Male | 14 | |||||||||
| Rio de Janeiro | 1 | Female | 9 | PCR sequencing (calmodulina gene) | Dacryocystitis | S. brasiliensis | S. brasiliensis | [100] | ||
| Rio de Janeiro | 2 | Female | 22 and 27 | Fungal culture, PCR sequencing (Calmodulina gene) | Fixed Cutaneous | S. brasiliensis | S. brasiliensis | [101] | ||
| Rio de Janeiro | 1 | Male | 6 | Fungal culture, PCR sequencing | Invasive Sinusitis | S. brasiliensis | S. brasiliensis | [102] | ||
| Rio de Janeiro | 1 | Male | 56 | Fungal culture, PCR sequencing | Meningitis, Lymphocutaneous | S. brasiliensis | S. brasiliensis | [103] | ||
| São Paulo | 20 from 2012–2020 | Male 9 (45%) | Age ranged from 2–81 mean 32.2 ± 25.10 | Fungal culture | ND | Sporothrix spp. | Sporothrix spp. | [104] | ||
| Females 11 (55%) | ||||||||||
| Rio de Janeiro | 64 from 2013–2015 | ND | Fungal culture (Sabouraud Dextrosa Agar, Mycosel ) | Lymphocutaneous 43 (67%) Fixed cutaneous 21 (33%) | S. schenckii sensu lato | Sporothrix spp. | [105] | |||
| Minas Gerais 1 Ceará 1 Goiás 1 Pernambuco2 São Paulo 1 | 6 | ND | Fungal culture (Potato Dextrose agar, Corn Meal agar), Carbohydrate assimilation tests, PCR sequiencing (calmodulin gene) | Lymphocutaneous 2 (33.3%) Disseminated 1 (16.7%) ND 3 (50%) | Sporothrix mexicana 3 (50%) Sporothrix globosa 3 (50%) | Sporothrix mexicana 3 (50%) Sporothrix globosa 3 (50%) | [106] | |||
| Colombia | Antioquia | 34 | ND | Fungal culture, PCR sequencing (ITS 1–2 and β-tubulin) | ND | S. schenckii sensu stricto 22 (65.7%) S. globosa 12 (34.2%) | S. schenckii 22 (65.7%) S. globosa 12 (34.3%) | [56] | ||
| Bogotá | 2.28% (14 cases/612 patients) | Male ND Female ND | Between: 0–18 | Fungal culture | ND | Sporothrix spp. | Sporothrix spp. | [107] | ||
| Casanare | 1 | Male | 18 | Fungal culture, Histopathology | Verrucose | Sporothrix spp. | Sporothrix spp. | [108] | ||
| Marandúa | 1 | Female | 48 | Fungal culture, Histopathology | Fixed cutaneous | S. schenckii sensu lato | Sporothrix spp. | [109] | ||
| Chile | Santiago | 1 | Male | 54 | Histopathology | Lymphocutaneous | Sporothrix spp. | Sporothrix spp. | [110] | |
| Valparaíso | 1 | Female | 75 | Fungal culture Direct microscopy, Sugar assimilation (sucrose) | Lymphocutaneous | Sporothrix globosa | Sporothrix globosa | [111] | ||
| Viña del Mar | 1 | Female | 64 | Direct microscopy, Fungal culture (Sabouraud with cycloheximide and potato dextrose agar) nitrogen-based agar, sequencing (D1/D2 region of the fungal 26S rRNA gene, it region; a partial fragment of the β-tubulin gene; ITS 1 and 2; and the 5.8S gene (SU)). | Onychomycosis | Sporothrix pallida | Sporothrix pallida | [112] | ||
| Paraguay | Itá | 2 | Male Male | 52 | Histopathology (Peryodic Acid Schiff), Fungal culture, direct microscopy with Giensa strein | Lymphocutaneous 1 (50%) Fixed cutaneous 1 (50%) | Sporothrix spp. | Sporothrix spp. | [113] | |
| Cordillera 2 Guairá, Central 2 Misiones 2 San Pedro 2 Caaguazú 1 | 11 from 1997–2019. | Male 10 Female 1 | Mean Age: 37,6 ± 20 Range: 24–69 | Direct microscopy (KOH 10%), fungal culture (Sabouraud agar with glucose 2%, potato dextrose agar with chloramphenicol), | Lymphocutaneous 11 (100%) | Sporothrix schenckii complex | Sporothrix spp. | [114] | ||
| Perú | Apurímac | 2 | Female | 65 | Direct microscopy, Giemsa stain Culture Microscopy with lactophenol cotton blue, Carbohydrate assimilation test (sucrose and raffinose) in nitrogen base | Fixed cutaneous | S. schenckii | S. schenckii | [115] | |
| Female | 67 | |||||||||
| Apurímac | 21 | Male (12) Female (9) | Average: 9 | Fungal culture | Lymphocutaneous 13 (62%) Fixed cutaneous 8 (38%) | Sporothrix spp. | Sporothrix spp. | [116] | ||
| Apurímac 2850 | 57 (15/100,000) | Male 1734 Female 1255 | ND | Fungal culture, Microscopy with lactophenol cotton blue and PCR sequencing | Lymphocutaneous 2942 (63%) Fixed cutaneous 1728 (37%) | S. schenckii 4651 (99.6%) S. schenckii sensu stricto 19 (0.4%) | Sporothrix spp. 4651 (99.6%) S. schenckii 19 (0.4%) | [117] | ||
| Cajamarca 1500 | 30 (3/100,000) | |||||||||
| La Libertad 100 | 4 (0.5/100,000) | |||||||||
| Cusco 200 | 2 (0.2/100,000) | |||||||||
| Otras regiones 20 | ≤1 (0.1/100,000) | |||||||||
| Abancay | 1 | Male | 6 | Fungal culture | Lymphocutaneous | Sporothrix spp. | Sporothrix spp. | [118] | ||
| Lima | 1 | Male | 23 | Fungal Culture Microscopy with lactophenol blue, MALDI-TOF MS, PCR sequencing (D1/D2 region of the fungal 26S rRNA gene) | Fixed cutaneous | S. schenckii | S. schenckii | [119] | ||
| Lima | 1 | Male | 42 | Histopathology, Microscopy, Fungal Culture | Disseminated cutaneous | S. schenkii sensu lato | Sporothrix spp. | [120] | ||
| Cajamarca | 94 from 1991 to 2014 | Males (67) Female (27) | Average: 36 | Direct microscopy, Gram and Giemsa stain, Fungal culture, Histopathology | Lymphocutaneous 44 (47%) Fixed cutaneous 37 (39%) Disseminated cutaneous 11 (12%) Extra-cutaneous 1 (1%) ND 1 (1%) | S. schenckii | Sporothrix spp. | [121] | ||
| Apurímac | ||||||||||
| Amazonas | ||||||||||
| Ancash | 1 | Male | 58 | Fungal culture | Lymphocutaneous | S. schenckii | Sporothrix spp. | [122] | ||
| Cusco | 1 | Female | 53 | Fungal culture (Sabouraud) | Disseminated | S. schenckii | Sporothrix spp. | [123] | ||
| Uruguay | Tacuarembó 10 Cerro Largo 9 Canelones 9 Montevideo 5 Rocha 4 Paysandú 3 Flores 3 Río Negro 2 Colonia 2 Artigas 1 Rivera 1 Maldonado 1 Soriano 1 Non-registered 20 | 157 from 1983 to 2020 | Male (152) | 13–79 age range | Gram staining and culture in Sabouraud | Nodular Lymphatic 120 (76.4%) Fixed cutaneous 30 (19.1%) ND 7 (4.5%) | Sporothrix spp. | Sporothrix spp. | [124] | |
| Female (5) | ||||||||||
| Venezuela | Caracas | 68 | ND | Fungal culture, PCR sequencing (Calmodulin locus and ITS regions) | ND | S. schenckii 42 (62%) S. globosa 26 (38%) | S. schenckii 42 (62%) S. globosa 26 (38%) | [125] | ||
| Aragua 55 Miranda 32 Other states 46 | 133 from 1963–2019 | Male (95) Female (38) | 0–15 15–30 >30 | Direct microscopy Fungal culture | Lymphocutaneous 84 (63.15%) Fixed cutaneous 48 (36.09%) Cornea 1 (0.7%) | S. schenckii sensu lato 130 (97.7%) ND 3 (2.3%) | Sporothrix spp. | [126] | ||
| Bolívar 14 | 0.55% (220 cases/39,806 patients) | ND | 25–45 years | Microscopy and fungal culture | ND | Sporothrix spp. | Sporothrix spp. | [127] | ||
| Caracas 160 | ||||||||||
| Carabobo 6 | ||||||||||
| Falcón 3 | ||||||||||
| Lara 5 | ||||||||||
| Mérida 1 | ||||||||||
| Monagas 24 | ||||||||||
| Sucre 1 | ||||||||||
| Táchira 2 | ||||||||||
| Zulia 4 | ||||||||||
| Costal Range 22 | 31 from 1973–2013 | Male 64% Female 36% | Microscopy, fungal culture, pruebas bioquímicas, PCR sequencing (Calmodulin gene and ITS 4–5) | Fixed cutaneous 18 (60%) Lymphocutaneous 11 (36.33%) Disseminated 1 (3.33)% | S. schenckii sensu stricto 17 S. globosa 13 and Ophiostoma stenoceras 1 | S. schenckii 17 (56.67%) S. globosa 13 (43.33%) | [128] | |||
| Andes 7 | ||||||||||
| Plains 2 | ||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Castro, R.; Pinto-Almazán, R.; Arenas, R.; Sánchez-Cárdenas, C.D.; Espinosa-Hernández, V.M.; Sierra-Maeda, K.Y.; Conde-Cuevas, E.; Juárez-Durán, E.R.; Xicohtencatl-Cortes, J.; Carrillo-Casas, E.M.; et al. Epidemiology of Clinical Sporotrichosis in the Americas in the Last Ten Years. J. Fungi 2022, 8, 588. https://doi.org/10.3390/jof8060588
Hernández-Castro R, Pinto-Almazán R, Arenas R, Sánchez-Cárdenas CD, Espinosa-Hernández VM, Sierra-Maeda KY, Conde-Cuevas E, Juárez-Durán ER, Xicohtencatl-Cortes J, Carrillo-Casas EM, et al. Epidemiology of Clinical Sporotrichosis in the Americas in the Last Ten Years. Journal of Fungi. 2022; 8(6):588. https://doi.org/10.3390/jof8060588
Chicago/Turabian StyleHernández-Castro, Rigoberto, Rodolfo Pinto-Almazán, Roberto Arenas, Carlos Daniel Sánchez-Cárdenas, Víctor Manuel Espinosa-Hernández, Karla Yaeko Sierra-Maeda, Esther Conde-Cuevas, Eder R. Juárez-Durán, Juan Xicohtencatl-Cortes, Erika Margarita Carrillo-Casas, and et al. 2022. "Epidemiology of Clinical Sporotrichosis in the Americas in the Last Ten Years" Journal of Fungi 8, no. 6: 588. https://doi.org/10.3390/jof8060588
APA StyleHernández-Castro, R., Pinto-Almazán, R., Arenas, R., Sánchez-Cárdenas, C. D., Espinosa-Hernández, V. M., Sierra-Maeda, K. Y., Conde-Cuevas, E., Juárez-Durán, E. R., Xicohtencatl-Cortes, J., Carrillo-Casas, E. M., Steven-Velásquez, J., Martínez-Herrera, E., & Rodríguez-Cerdeira, C. (2022). Epidemiology of Clinical Sporotrichosis in the Americas in the Last Ten Years. Journal of Fungi, 8(6), 588. https://doi.org/10.3390/jof8060588










