Abstract
Aspergillus niger is an important industrial workhorse for the biomanufacturing of organic acids, proteins, etc. Well-controlled genetic regulatory elements, including promoters, are vital for strain engineering, but available strong promoters for A. niger are limited. Herein, to efficiently assess promoters, we developed an accurate and intuitive fluorescent-auxotrophic selection workflow based on mCherry, pyrG, CRISPR/Cas9 system, and flow cytometry. With this workflow, we characterized six endogenous constitutive promoters in A. niger. The endogenous glyceraldehyde-3-phosphate dehydrogenase promoter PgpdAg showed a 2.28-fold increase in promoter activity compared with the most frequently used strong promoter PgpdAd from A. nidulans. Six predicted conserved motifs, including the gpdA-box, were verified to be essential for the PgpdAg activity. To demonstrate its application, the promoter PgpdAg was used for enhancing the expression of citrate exporter cexA in a citric acid-producing isolate D353.8. Compared with the cexA controlled by PgpdAd, the transcription level of the cexA gene driven by PgpdAg increased by 2.19-fold, which is consistent with the promoter activity assessment. Moreover, following cexA overexpression, several genes involved in carbohydrate transport and metabolism were synergically upregulated, resulting in up to a 2.48-fold increase in citric acid titer compared with that of the parent strain. This study provides an intuitive workflow to speed up the quantitative evaluation of A. niger promoters and strong constitutive promoters for fungal cell factory construction and strain engineering.
1. Introduction
Aspergillus niger is an important industrial workhorse, owing to its good fitness for industrial fermentation and unique inherent physiological characters, including its high production capacity, robustness to an extreme acid environment, and powerful secretion efficiency to utilize a wide range of carbon sources [1,2,3]. A. niger has been widely exploited as cell factories in the industrial production of organic acids and enzymes, including citric acid, gluconic acid, amylase, and glucoamylase [2,3]. Over 80% of worldwide citric acid is produced by submerged fermentation using A. niger, with the global citric acid market of nearly 2.0 million tons [2]. With the rapid development of systems biology and synthetic biology, rational engineering has gradually become the dominant approach to reconstruct A. niger cell factories with higher titer, yield, and productivity [2,4,5]. Especially the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) system-mediated genome-editing techniques [6,7,8] have broken the bottleneck of highly efficient genetic manipulation.
In addition to genetic manipulation toolboxes, genetic regulation elements, especially well-controlled promoters, are crucial for strain engineering by fine-tuning the expression of target genes. For instance, some metabolite inducible promoters have been developed for Aspergillus, e.g., glucoamylase promoter (PglaA) in A. niger [9,10,11], but their regulation is dependent on carbon source availability and metabolism, possibly leading to unstable expression in complicated fermentation conditions. Although the metabolism-independent Tet-on system is induced by Doxycycline in A. niger [12,13], it is not economically suitable for industrial production. Hence, considering the requirement for strong and stable gene expression, low production cost, and simple fermentation control in industrial biomanufacturing, constitutive promoters are preferred. Compared to inducible promoters, only a few native constitutive promoters have been characterized in A. niger, including protein kinase promoter PpkiA [14], alcohol dehydrogenase promoter PadhA [11], glutamate dehydrogenase PgdhA [11], and multiprotein bridging factor promoter PmbfA [15]. However, owing to its relatively high activity, the glyceraldehyde-3-phosphate dehydrogenase promoter from Aspergillus nidulans (PgpdAd) [16] is by far the most frequently exploited promoter in A. niger and other Aspergilli spp. [17,18,19,20]. Therefore, alternative strong constitutive promoters are desired for engineering multiple genes for strain improvement.
Compared to well-established methods in bacteria and single-celled eukaryotes, such as Saccharomyces cerevisiae [21,22], accurate and efficient promoter evaluation approaches are rarely available for filamentous fungi due to the filamentous physiological features of irregular morphologies, which limits the discovery and evaluation of promoters. Traditionally, some enzymes are applied as reporters for promoter evaluation in filamentous fungi, including luciferase [12,13], β-galactosidase of E. coli [16,23,24], and β-glucuronidase of E. coli [15,25]. However, such enzyme-based approaches involve many labor-intensive and time-consuming steps for activity detection, for example, releasing target proteins from a mycelial culture and determining dry cell weight for accurate enzymatic activity data normalization. Recently, to avoid the influence of mycelial biomass, a fluorescence distribution for spore population is coming into use for the quantitative evaluation of promoter strength by fluorescence protein-coupled flow cytometry [26,27]. This approach provides a much more rapid screening platform for assessing promoters with desirable activity.
To accurately assess the promoter activity in filamentous fungi, it is worthy to note that the promoter–reporter cassette should be targeted to genomic locus-specific integration as a single copy. Huang et al. reported that two isolates of even the same promoter exhibited significantly different transcription levels of nearly two folds, because of random genetic insertion with various copy numbers and integration sites [28]. Apparently, the existence of a non-homologous end joining (NHEJ) system in filamentous fungi caused the indeterminacy of DNA repair. Multiple insertions frequently occur and have been found to improve gene expression [11]. Even with the same copy, integration at different genome loci also caused a variation in gene expression level, due to the impact of chromosomal structure and transcription complex accessibility [29]. Therefore, more genetic manipulation efforts are required to obtain positive isolates before promoter evaluation. Obviously, it is obligatory to have an efficient and easily detectable selection system for genetic manipulation. Otherwise, intensive efforts and much time have to be spent on confirming genotypes of isolates, as it often happens, including randomly picking colonies from selective transformation plates without any indicator, at least twice for spore purification and genotype verification. These conventional procedures impede the rapid promoter characterization in filamentous fungi.
To address these limitations, based on our previously developed 5S rRNA-CRISPR/Cas9 system for Aspergillus [6], we established an accurate and simplified promoter evaluation workflow with an intuitive fluorescent-auxotrophic selection in A. niger. In detail, the highly efficient CRISPR/Cas9 system ensures precise integration of a promoter-reporting cassette into a specific locus, to avoid inaccurate promoter activity determination. The fusion of a fluorescence protein with an auxotrophic selection marker was used as donor DNAs, which not only provides a reporter for rapidly and quantitatively reflecting the promoter activity via flow cytometry but also offers a double-checked indicator for conveniently distinguishing positive transformants with fluorescence grown on a selective transformation plate without uracil through fluorescence imaging. Using this workflow, we rapidly evaluated six endogenous constitutive promoters and identified the strongest promoter, a native glyceraldehyde-3-phosphate dehydrogenase promoter of A. niger (PgpdAg), which was a 2.28-fold increase in activity compared to the most frequently used strong promoter, PgpdAd from A. nidulans, and its application led to significant improvement of citric acid production.
2. Materials and Methods
2.1. Strains and Cultivation Conditions
The strains used in this study are listed in Table S1. Escherichia coli DH5α (Transgene, Beijing, China) was used for plasmid construction and cultured at 37 °C in Luria–Bertani broth containing ampicillin (100 μg/mL). The citric acid-producing strain A. niger D353.8 (kusA::hph, pyrG::hph, hygR) was stored in the lab [30]. A. niger strains were cultivated on defined minimal medium (MM), as reported previously [31], or on complete medium (CM) consisting of MM supplemented with 0.5% yeast extract and 0.1% casamino acids. Then, 1.5% agar was supplemented for plates. When necessary, 10 mM uracil was supplemented in the media for the pyrG mutants.
2.2. Plasmids Construction
The plasmids, protospacers, and primers used in this study are listed in Tables S1–S3, respectively. To establish a rapid promoter evaluation workflow using fluorescent protein–selection marker fusion, the red fluorescence protein-encoding gene mCherry was amplified with the primers mCherry-F and mCherry-R, then assembled into the backbone of pSM-AnpyrG [30] amplified with the primers of pSM-AnpyrG-Frev and pSM-AnpyrG-Rrev, using the ClonExpressTM one-step cloning kit (Vazyme, C113), resulting in pFPSM. To evaluate the promoter strength, seven promoter fragments, including PgpdAd, PgpdAg, PenoA, PpkiA, PcitA, PmdhA, PmbfA, were amplified with the corresponding primers, shown in Table S3. Intergenic regions located upstream of the coding sequences of the selected genes were used as promoter sequences, and their lengths are shown in Table S4. Then, these promoter fragments were cloned to the backbone of pFPSM digested with NheI, using the ClonExpressTM one-step cloning kit (Vazyme, C113), resulting in pYDD1–pYDD7, respectively. These plasmids were used as the templates for generating the donor DNAs containing the mCherry-pyrG fused gene expression cassettes driven by different promoters.
To apply the strong promoter for metabolic engineering, the cexA gene was amplified with the primers of cexA-F and cexA-R, then cloned into the backbone of pYDD1 and pYDD2, digested with NdeI and BglII, using the ClonExpressTM one-step cloning kit (Vazyme, C113), resulting in pXMD6 and pXMD7, respectively. The cexA expression plasmid harboring the truncated PgpdAg promoter was amplified using PgpdAg-775-F and PgpdAg-775-R as primers and pXMD7 as a template, resulting in pXMD8.
To construct the sgRNAs targeting adgA as the specific genomic locus, two protospacers were predicted by the sgRNAcas9 software [32] and designed with a minimal off-target possibility, as shown in Table S2. The targeting sgRNA constructs were built by the digestion of sgRNA expression plasmids psgRNA6.0 [6] with BbsI and ligation with synthetic double-stranded oligonucleotides, agdA-sgRNA1-F/agdA-sgRNA1-R and agdA-sgRNA2-F/agdA-sgRNA2-R, resulting in psgRNA6.18 and psgRNA6.19. The linear sgRNA targeting expression cassettes using the 5S rRNA promoter for A. niger transformation were amplified with the primers M13F and M13R, using psgRNA6.18 and psgRNA6.19 as the template, respectively, as previously described [6].
2.3. A. Niger Strains Construction
The standard protocol of A. niger genome editing using the CRISPR/Cas9 system-based 5S rRNA was performed as previously described [6]. For promoter strength evaluation, donor DNA constructs containing mCherry-pyrG fused gene expression cassettes driven by different constitutive promoters were integrated into the genome locus of α-glucosidase (agdA), to avoid the influence of the genomic context, resulting in the reported strains YDD1–YDD7, respectively. Briefly, 2 μg of the donor DNA constructs was co-transformed into the protoplasts of A. niger D353.8 together with the sgRNA targeting constructs AgdA-sgRNA1, AgdA-sgRNA1, and pCas9-AnpyrG. The transformants with fluorescence were selected after detecting under a fluorescence image system (Tanon, Tianjin, China). After subculturing in 24-well plates, genomic DNA of selected transformants was extracted and verified via diagnostic PCR and sequencing analysis with the primers of agdA-g-F and mCherry-R. Copy number analysis was conducted with quantitative PCR (qPCR) Lightcycler 96 (Roche) using ChamQ Universal SYBR qPCR Master Mix (Vazyme) according to manufacturer’s instructions. The qPCR signal of mcherry was normalized to the gpdA gene as reference. The qPCR primers are listed in Supplementary Table S3. The correct single-integration isolates were chosen for further fluorescence detection.
Similarly, citric acid-producing engineered strains XMD6–XMD8 (Table S1) were also constructed using the same tactics. The only differences were the donor DNAs containing the cexA expression cassette, which was amplified with the primers of MH-agdA-sgRNA1-F and MH-agdA-sgRNA1-R, using pXMD6–pXMD8 as the templates, respectively. After subculturing in 24-well plates, genomic DNA of selected transformants was extracted and verified via diagnostic PCR and sequencing analysis with the primers of agdA-g-F and cex-R.
2.4. Fluorescence Microscopic Analysis
To detect the fluorescence protein expression strength driven by different promoters, the conidia, hyphae, and mycelial pellets of each sample were prepared for the fluorescence microscopic analysis. Briefly, the conidia were collected with the 0.9% NaCl containing 0.05% Tween-80, after being sporulated on CM plates for 5 days. The hyphae were prepared according to the previous study [33]. Two disinfected coverslips were placed onto the bottom of a small petri dish containing 5 mL of liquid MM with 0.003% yeast extract. After inoculation with 106 spores for 8 h at 30 °C, coverslips with adherent hyphae were placed upside down on an object slide for the microscope analysis. The mycelial pellets were harvested for the analysis after being incubated in the CM liquid media for 48 h. Differential interference contrast (DIC) and red fluorescent images of the cells were captured with a 40× objective using a Leica DM5000B laser scanning confocal microscope (Leica, Wentzler, Germany) with excitation at 543 nm and detection at 586–670 nm. The results were assembled in Adobe Photoshop 7.0 (Adobe, San Jose, CA, USA).
2.5. Flow Cytometry Analysis
For quantitative determination of the fluorescence, the flow cytometry was conducted using conidia. A. niger strains were first sporulated on CM plates for 5 days. Then, conidia were collected, diluted in PBS, and filtered through a four-layer lens-cleaning paper prior to the flow cytometry analysis using a MoFlo™ XDP cell sorter (Beckman Coulter Inc., Brea, CA, USA). The green (561 nm) laser and 610 nm filter were used for the mCherry fluorescence measurements. The forward scatter (FSC) voltage and mCherry voltage were set as 43 and 610, respectively. Minor gating was performed on data to exclude obvious errors, such as dust particles, cell clusters, and conidia aggregates. A total of 100,000 cells of each sample were recorded and used for mCherry fluorescence and forward scatter (FSC). Flow cytometry results were analyzed with MoFlo XDP Summit 5.2 software (Beckman Coulter Inc., Brea, CA, USA). The t-test was used for the data statistical analysis.
2.6. Citric Acid Fermentation and Detection
To determine the application of the strongest promoter identified in this study, citric acid production was demonstrated by enhancing the citric acid synthetic flux and efflux. Citric acid fermentation was carried out using the liquefied corn media, as described in a previous study [34]. The final concentration of 1 × 105 spores/mL was inoculated in 20 mL of liquefied corn media in 100 mL shake flasks at 34 °C and 220 rpm for 120 h. The weight of the shake flasks was measured before and after the citric acid fermentation to eliminate measurement errors caused by evaporation. For the citric acid production in the 5 L bioreactor with a stirring paddle device, the same fermentation parameters [34] were utilized for 144 h, but the aeration rate was coupled to the dissolved oxygen concentration (>40%).
For the citric acid detection, supernatants were filtered from cultures using filter paper. Total acids were first titrated using 0.1429 M NaOH with 20 μL 0.1% phenolphthalein as a pH indicator. Next, the supernatants were diluted in sterile distilled water depending on the estimated total acid. Samples were boiled for 15 min at 100 °C, after which supernatants were centrifuged at 12,000 rpm for 5 min and filtered through a 0.22 μm sterile filter membrane. Extracellular organic acids were detected by Prominence UFLC equipped with a UV detector (Shimadzu, Kyoto, Japan) and a Bio-Rad Aminex HPX-87H column (300 × 7.8 mm), according to the procedure described previously [34].
For the mycelial biomass after citric acid fermentation, the cultures were treated as described in a previous study [30]. Briefly, the mycelial cultures were vacuum filtered through filter paper, washed in 5-fold sterile water, and added to pre-weighed falcon tubes. The biomass was incubated at 50 °C until dry (minimum of 24 h), after which the dry weight was determined.
2.7. RNA Sequencing and Transcriptomics Analysis
The samples for transcriptomic profiling were prepared and detected according to a previous approach [35]. Briefly, the mycelial samples were collected by rapid vacuum filtration after cultivating for 72 h in a 5 L bioreactor, washed with 100 mL cold sterilized water, and then immediately placed into liquid nitrogen and stored at −80 °C. Total RNAs were extracted using RNAprep pure Plant Kit (DP432, Tiangen, Beijing, China) and assessed using the RNA Nano 6000 Assay Kit of the Agilent Bioanalyzer 2100 system (Agilent Technologies, CA, USA). RNA sequencing libraries were prepared using NEBNext UltraTM RNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA, USA) and then sequenced on an Illumina platform. Paired-end reads were generated at Beijing Biomarker technology Co., Ltd. (Beijing, China). Average clean reads of 52.22, 50.02, 51.43, 50.14, and 47.96 million were generated for assembly and further analysis after filtering and trimming of the raw data (Table S4). The percentage of bases with Phred scores at the Q30 level (an error probability of 0.1%) ranged from 94.30% to 94.65% and the GC content was 53.99–54.28%. These clean reads were then mapped to the reference genome sequence of the progenitor strain, which was annotated according to the reference genome of A. niger CBS 513.88 (Accession: PRJNA19263). Among all the samples, 90.06–95.71% of the clean reads were mapped to the reference genome (Table S5). These data are provided in the supplementary materials. RPKM (reads per kilobase of transcript per million fragments mapped) was applied to measure the expression level of each gene by StringTie using a maximum flow algorithm. Differential expression analysis was performed by edgeR [36], with the criteria for differentially expressed genes of fold change (FC) ≥ 1.5 and p value < 0.05. COG (cluster of orthologous groups of proteins) orthologous classification of DEGs was performed on the BMKCloud platform using a COG database (http://www.ncbi.nlm.nih.gov/COG accessed on1 March 2021) [37].
3. Results
3.1. Fluorescent-Auxotrophic Double Selection Coupling with Flow Cytometry Workflow
To increase the efficiency of promoter activity detection and improve the accuracy of clone selection in A. niger, we developed a fluorescent-auxotrophic double selection coupling with CRISPR/Cas9 system and flow cytometry (Figure 1A). The fluorescent protein mCherry was fused to the selection marker AnpyrG from A. nidulans. The promoter PgpdAd from A. nidulans was used as an example to establish this workflow (Figure 1B). Then, the CRISPR/Cas9 genome editing system was used to integrate this fluorescent-auxotrophic selection cassette at the agdA locus. With mCherry-PyrG as double indicators, a simple fluorescence image analysis was used to directly select potential positive transformants from the selective transformation plates without uracil (Figure S1); 30 of 38 primary transformants showed significant fluorescence after incubating for 4 days. Of these, 16 transformants with fluorescence were randomly picked up and reconfirmed by diagnostic PCR (Figure S1) and qPCR; all these isolates were confirmed with the expected genotype. Among them, the isolate YDD1.13 with a single copy of mCherry-pyrG fusion inserted at the agdA locus was selected for flow cytometry analysis. As shown in Figure 1B and Figure S1, all the conidia and mycelia pellets of A. niger YDD1.13 showed distinct red fluorescence, compared to the parent strain D353.8 without fluorescence (Figure 1C). To quantitively measure the promoter’s strength, a population of 100,000 conidia of YDD1.13 was analyzed by flow cytometry. It was observed that YDD1.13 displayed more conidia with high fluorescence intensity (55.16%), compared to that of the parent strain D353.8 (0.27%) (Figure 1D).
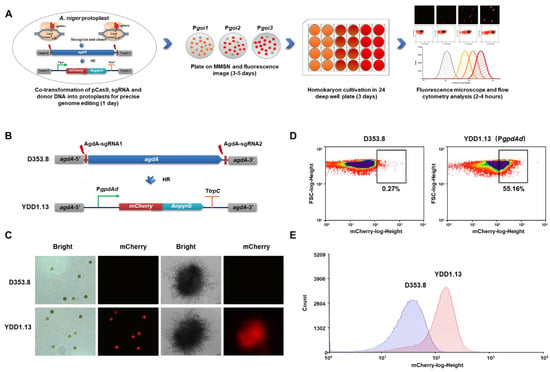
Figure 1.
Fluorescent-auxotrophic selection coupled with CRISPR/Cas9 system and flow cytometry. (A) Schematic overview of a promoter evaluation workflow based on an intuitive fluorescent-auxotrophic selection. This workflow combined the efficient CRISPR/Cas9 genetic manipulation system, the fluorescent protein-selection marker fused indicator, and flow cytometry-based analysis. CRISPR/Cas9 system was applied for integration of the reporter at the specific genome locus. Then, transformants with distinct fluorescence were picked in 24-deep-well plates based on fluorescence imaging. After genotype verification, fluorescence intensity was detected by laser scanning confocal microscope and flow cytometry. (B) Schematic diagram of mCherry-pyrG-expressing construct under the control of the PgpdAd promoter. The donor DNAs comprised the PgpdAd::mCherry-pyrG cassette, and 40-bp micro-homology arms targeted the flanking sequences of agdA gene. After being constructed, the donor DNAs were co-transformed with linear sgRNA constructs (agdA-sgRNA1 and agdA-sgRNA2) and the Cas9 expression cassette into protoplasts of the kusA and pyrG deficient chassis D353.8. Two DNA double-strand breaks (DSBs) at the flanking sequences of the agdA gene were generated by the Cas9 under the guide of two sgRNAs and then were repaired by homologous recombination (HR) with the integration of the donor DNAs, resulting in YDD1.13. (C) Representative fluorescence images in conidia and mycelial pellets of YDD1.13-expressing mCherry-pyrG with the PgpdAd promoter. The parent strain D353.8 was used as negative control. (D,E) Flow cytometry analysis of the conidia of YDD1.13. The 100,000 spores were analyzed by flow cytometry. Black boxes marking the same value of mCherry-log-height were used for direct comparison of YDD1.13 with the parent strain D353.8.
3.2. Evaluation of Endogenous Constitutive Promoters Using the Fluorescent-Auxotrophic Selection Workflow
To seek strong promoters, we selected six native promoters involved in the central metabolism in A. niger and assessed their strength with the above-established method. The native promoters included glyceraldehyde-3-phosphate dehydrogenase promoter (PgpdAg), enolase promoter (PenoA), pyruvate kinase promoter (PpkiA) [38], citrate synthase promoter (PcitA) [39], malate dehydrogenase promoter (PmdhA) [15], and constitutive transcription factor (PmbfA) [15]. To avoid the influence of an integration site, all the promoter-reporting cassettes were targeted to the adgA gene locus, owing to its good transcription complex accessibility [29]. For each construct, eight transformants with fluorescence were randomly picked up and reconfirmed by diagnostic PCR (Figure S2) and qPCR. It demonstrated that all these selected isolates were confirmed with a single copy of mCherry-pyrG fusion inserted at the agdA locus. Among them, four positive isolates were selected for flow cytometry analysis. As shown in Figure 2A and Figure S2C and Table S4, the fluorescence intensities of mCherry-pyrG fusion constructs driven by six promoters in conidia, hypha from spore germination, and mycelial pellets were significantly higher than that of the parent strain D353.8. It indicated that all the selected promoters could initiate the transcription of mCherry-pyrG in different developmental stages, suggesting these promoters were constitutive promoters. The strongest fluorescence of conidia-expressing mCherry-pyrG was found to be under the control of PgpdAg, whereas the five remaining endogenous constitutive promoters were still lower than PgpdAd from A. nidulans. The strength order of these promoters was PgpdAg > PgpdAd > PpkiA > PmdhA > PenoA ≈ PmbfA > PcitA (Figure 2B,C and Table S4). From the quantitative analysis, PgpdAg of A. niger showed a 2.28-fold increase in the median fluorescence value compared with PgpdAd of A. nidulans (Table S4).
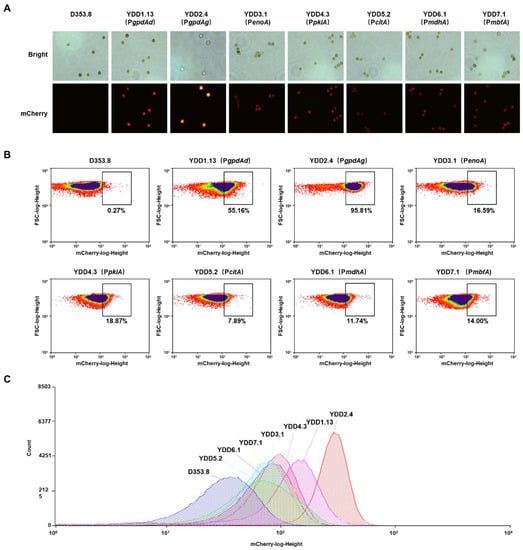
Figure 2.
Fluorescence analysis of constructs expressing mCherry-pyrG controlled by six constitutive promoters. (A) Representative fluorescence images in conidia of constructs expressing mCherry-pyrG controlled by six constitutive promoters. The parent strain D353.8 was used as negative control and YDD1.13 was used as positive control. (B,C) Representative flow cytometry analysis of the conidia of constructs YDD2 to YDD7 expressing mCherry-pyrG controlled by six selected promoters. The 100,000 spores were analyzed by flow cytometry. To reduce the interference of background fluorescence from the parent strain D353.8, the black box marking the same value of mCherry-log-height (higher than 102) was used for direct comparison of constructs expressing mCherry-pyrG controlled by different promoters. The number below the black box represents the percentage of spores with high fluorescence (mCherry-log-height higher than 102) of each construct. A. niger YDD1.13 with the promoter of PgpdAd was used as positive control, while the parent strain D353.8 was used as negative control.
3.3. Characterization of Essential Elements in the PgpdAg Promoter
To determine the essential elements of the PgpdAg promoter, we conducted the multiple sequence alignment of the PgpdAg promoters of six Aspergilli spp. and analyzed the truncation of PgpdAg in A. niger (Figure 3). As shown in Figure 3A and Table 1, six conserved motifs (Motifs I–VI) were predicted at the upstream of −631 to −272 of PgpdAg in A. niger. Among them, Motif III was identified as gpdA-box with two bases different from the gpdA-box of PgpdAd [16], which comprised a consensus sequence of 5′-CCARATATCGTGMSTCTCCTGCTTTGCCCGGTGTATGAAACCGGAAARG-3′ and located at −521 to −473 in PgpdAg from A. niger (Table 1). A G/C-rich conserved motif (Motif IV, GCGGCGCDMYCGGGAA) was found at the downstream of gpdA-box. Moreover, a conserved motif (Motif VI) was also predicted at the upstream of the transcription start. To unveil the effect of these motifs on promoter activity, the truncated PgpdAg strains were constructed with different donor DNAs by the CRISPR/Cas9 system. These donor DNAs were amplified with different forward primers targeting the PgpdAg promoter (such as MH-agdA-sgRNA1-F-PgpdAg1011) and the common reverse primer MH-agdA-sgRNA2-R (Table S3). For each construct, four correct isolates with a single copy of the reporter cassette were selected for flow cytometry analysis. As shown in Figure 3B, the impact of the truncated promoters on the transcription efficiency was investigated by the normalized mCherry fluorescence intensity of the verified transformants. Compared with the full-length promoter of 1889 bp, the deletion of −1889 to −1011 (PgpdAg-1011) and to −775 (PgpdAg-775) caused a slight decrease in fluorescence intensity. However, the removal of Motif I and Motif II (PgpdAg-531) led to a significant reduction in fluorescence intensity to 80.13%. Moreover, a further truncation to −319 (PgpdAg-319) drastically decreased the fluorescence intensity compared to PgpdAg-531, suggesting that key cis-elements including the gpdA-box exist. When all the conserved motifs were removed, it was hard to detect any fluorescence, namely, it showed no detectable differences between PgpdAg-157 and the negative control D353.8. These data indicated that the promoter region including motifs I–VI is important for full activity of the PgpdAg promoter in A. niger.
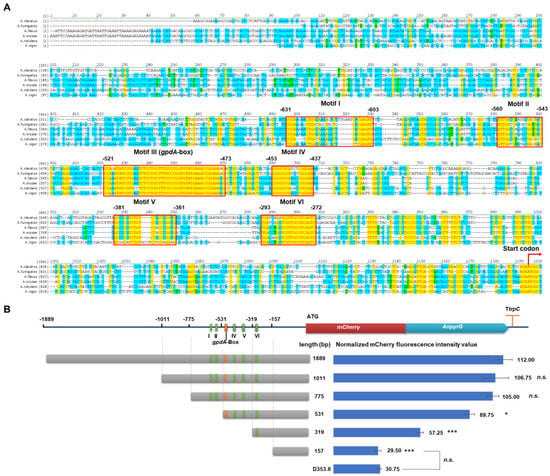
Figure 3.
Characterization of essential elements of the PgpdAg promoter. (A) Multiple sequence alignment of the PgpdA promoters of various Aspergilli spp. The GenBank accession numbers of the selected gpdA genes included A. niger CBS 513.88 (An16g01830), A. nidulans FGSC A4 (ANIA_08041), A. clavatus NRRL1(ACLA_003290), A. fumigatus Af293 (AFUA _5G01970), A. flavus NRRL 3357 (AFLA_025100), and A. oryzae RIB40 (AO090003001322). The conserved motifs are highlighted with red boxes. The transcription start site and start codon are shown as red arrows. (B) The conserved motifs’ prediction and truncation test of PgpdAg promoter in A. niger. GpdA-box and five predicted conserved motifs are represented as orange bars and green bars, respectively. The truncation design of the PgpdAg promoter is displayed as gray bars. The mean mCherry fluorescence intensity of each truncation is shown as blue bars. Results are the mean of three replicates, and error bars indicate standard deviations (n = 4). Pairwise Student’s t-test were conducted between PgpdAg truncated mutant relative to the full-length PgpdAg reported strain and between the mutant only containing UTR and the parent strain, respectively. The p values are indicated as (>0.05, n.s.; * < 0.05; *** < 0.001).

Table 1.
Predicted conserved motifs of PgpdAg and their location relative to start codon.
3.4. Application of PgpdAg Dramatically Improved Citric Acid Production in A. Niger
To assess industrial application of the endogenous PgpdAg promoter, we chose a citrate exporter cexA as an engineering target for citric acid production. The donor DNAs with cexA controlled by PgpdAd, PgpdAg and its truncated mutant PgpdAg-775 were integrated at the agdA gene locus, respectively (Figure 4A). After verified by the genomic PCR diagnosis and sequencing analysis of the four selected transformants (Figure S3), two isolates of each construction were selected for citric acid fermentation in shake flasks. As shown in Figure 4, all cexA overexpressed strains significantly increased citric acid titers. The citric acid production in XMD7.1 (74.82 ± 0.35 g/L) and XMD7.2 (75.26 ± 2.79 g/L) with PgpdAg was higher than those of XMD6.1 (52.18 ± 0.53 g/L) and XMD6.2 (54.17 ± 0.47 g/L) with PgpdAd (Figure 4B), which achieved an increase of 43.37% and 44.22%, respectively. Moreover, after the normalization of the citric acid titers to the biomass of these isolates, it also confirmed this superiority (Figure 4C), suggesting that the biomass of all isolates has no significant difference with the progenitor control strain D353.8. Together with the former promoter evaluation result, our study proved that the activity of the endogenous promoter PgpdAg was higher than PgpdAd in A. niger. Furthermore, cexA overexpressed strains under the control of the truncated promoter PgpdAg-775 XMD8.1 (75.66 ± 0.33 g/L) and XMD8.2 (74.93 ± 1.09 g/L) also showed a similar citric acid titer with the full-length promoter PgpdAg (Figure 4B). These data supported that PgpdAg and its truncated mutant PgpdAg-775 are able to give much stronger expression of the targets than the commonly used promoter PgpdAd.
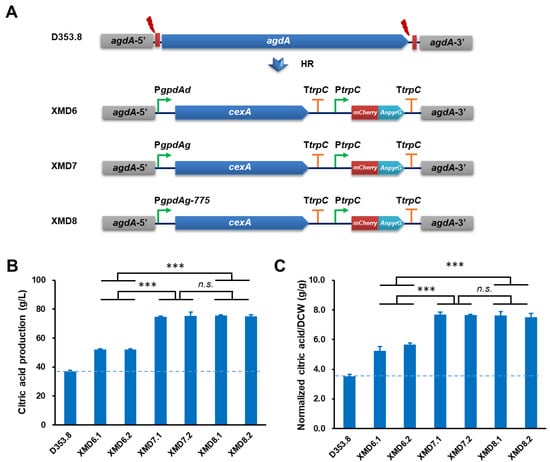
Figure 4.
Citric acid production of A. niger strains expressing cexA under the control of the PgpdA promoter. (A) Schematic diagram of constructs expressing cexA under the control donor DNAs containing the PgpdA promoter. The donor DNAs containing PgpdAd::CexA, PgpdAg::CexA, and PgpdAg-775::CexA expressing cassettes were co-transformed with linear sgRNA constructs (agdA-sgRNA1 and agdA-sgRNA2) and a Cas9 expression cassette into the protoplasts of A. niger D353.8. Two DNA double-strand breaks (DSBs) at the flanking sequences of agdA gene were generated by the Cas9 under the guide of two sgRNAs and then were repaired by HR with the integration of donor DNAs. (B,C) Citric acid production of the cexA overexpressed strains in A. niger. Citric acid titer (B) and normalized citric acid titer (g citric acid/g dry weight, C) were calculated for each strain. Then, 1 × 105 spores/mL were inoculated in 20 mL citrate fermentation media and incubated at 34 °C for 120 h. The extracellular citric acid was determined by the method of HPLC. A. niger XMD6.1 with cexA overexpression under the control of PgpdAd was used as positive control, while the parent strain D353.8 was used as negative control. Results are the mean of three replicates, and error bars indicate standard deviations (n = 3). Pairwise Student’s t-test was conducted between cexA overexpression mutants relative to the parent strains. The p values are indicated as (>0.05, n.s.; *** < 0.001).
Due to the high performance of the engineered A. niger strains in shake-flask fermentation, we conducted citric acid fermentations of the isolates XMD6.1 and XMD7.1 in 5 L bioreactors. As shown in Figure 5A, compared to XMD6.1, XMD7.1-expressing PgpdAg:cexA showed an increased citric acid accumulation. In detail, the citric acid titer of XMD7.1 reached up to 114 g/L, which was improved by 1.31-fold and 2.45-fold compared with those of XMD6.1 (87.0 g/L) and D353.8 (46.5 g/L), respectively. The average citric acid productivity of XMD7.1 was 0.79 g/L/h, while that of XMD6.1 was 0.61 g/L/h. These results further confirmed that, in comparison with PgpdAd, the endogenous promoter PgpdAg displayed a better effect on citric acid production in A. niger.
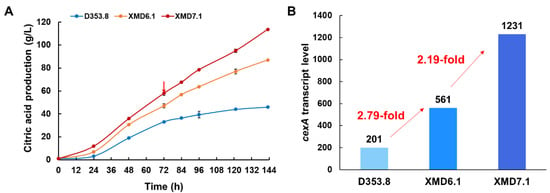
Figure 5.
Transcription analysis of cexA-expressing constructs in submerged citric acid fermentation. Citric acid production (A) and the cexA transcript level (B) of the cexA-expressing constructs in 5 L bioreactors. Then, 1 × 105 spores/mL were inoculated in submerged citric acid fermentation at 34 °C for 144 h. The extracellular citric acid was determined by the method of HPLC. Results are the mean of three replicates, and error bars indicate standard deviations (n = 3). The red arrow in (A) represents the time point of sampling for RNAseq analysis.
In order to further elucidate the transcription profile of the strains with cexA overexpressed by different promoters, comparative transcriptome analysis was conducted using the samples taken from D353.8, XMD6.1, and XMD7.1 in submerged citric acid fermentation for 72 h (Figure 5A). Compared to the parent strain D353.8, the cexA transcription level of XMD6.1 and XMD7.1 increased by 2.79-fold and 6.12-fold, respectively (Figure 5B). In comparison to XMD6.1, the cexA expression driven by PgpdAg in XMD7.1 was improved by 2.19-fold (Figure 5B), which was consistent with the promoter evaluation data using mCherry-pyrG as reporter. Moreover, it was observed that, compared to XMD6.1, 736 upregulated genes and 666 downregulated genes were detected in XMD7.1 (Figure S4). These differentially expressed genes were greatly enriched in carbohydrate transport and metabolism (G) (Figure S4), including several upregulated genes in starch hydrolysis, glucose uptake, and glycolysis, such as amyA (An12g06930), glaA (An03g06550), mstC (An02g03540), mstG (An05g01290), mstH (An15g03940), pfkB (An07g02100), and ppcA (An11g02550) (Table 2). As to intracellular citrate transportation, mitochondrial citrate/malate carrier protein CtpA (An11g11230) was also synergistically upregulated (Table 2). Meanwhile, with regards to citric acid degradation and by-product oxalate biosynthesis, cytoplasmic ATP-citrate lyase (AcsA, An11g00510 and AcsB, An11g00530), mitochondrial cis-aconitase AcoA (An08g10530), and cytoplasmic oxaloacetate acetylhydrolase OahA (An10g00820) were dramatically downregulated (Table 2).

Table 2.
Differentially expressed genes involved in central metabolism in PgpdA::cexA constructs.
4. Discussion
The filamentous fungus A. niger is an important workhorse in industrial biotechnology. Well-characterized promoters in regard to gene expression strength and regulation pattern are essential for strain engineering. However, because of the lack of fast and efficient promoter evaluation approaches, engineering of filamentous fungi is still short of available strong promoters. The filamentous physiological features of hypha polar growth and irregular morphologies bring several technical challenges for development of an accurate and simple approach for promoter characterization. Herein, we established a fast and easily handy fluorescent-auxotrophic selection coupled with flow cytometry workflow (Figure 1) in combination with a CRISPR/Cas9 genetic manipulation system.
In filamentous fungi, to ensure the accuracy of the promoter assessment, the reporter gene should be inserted into the identical, expected integration site as a single copy. However, the NHEJ DNA repair pathway dominates in filamentous fungi [40], and the entailing end processing of the breaks by NHEJ is typically error prone during genetic manipulation [41]. The NHEJ pathway inclines to generate unexpected multi-copy insertions at unspecific sites thus, leading to the inaccuracy of promoter evaluation. To address the integration indeterminacy of reporter genes, we utilized a highly efficient 5S rRNA promoter-driven CRISPR/Cas9 system [6] in an NHEJ-deficient strain [30], which enhanced the efficiency of the precise editing of the reporter genes [6] (Figure 1). To simplify the laborious screening procedure and avoid false-positive clones, we established a fluorescent-auxotrophic selection workflow. PyrG was used as the first selection marker to allow the growth of positive clones on the selective plates without uracil, whereas mCherry provided a second fluorescence checking for the intuitive selection of correct clones. All picked transformants with distinct fluorescence were verified to be correct isolates with the expected genotype (Figures S1 and S2). It should be noted that a few transformants did not show as distinct a fluorescence as others, possibly because of the inconsistent growth (Figure S1A). However, the transformants with distinct fluorescence were sufficient for screening the desirable isolates.
In contrast to bacteria and single-celled eukaryotes [21,22], the heterogeneous cultures with complex pelleted and dispersed morphologies made it impossible to measure biomass by cell turbidity. However, determining cell dry weight is usually tedious and time consuming [15,16,25]. To eliminate the influence of cell biomass variance and simplify this promoter strength detection procedure, the fluorescence value distribution of conidia populations determined by flow cytometry was applied in this study to reflect the promoter activity (Figure 1). Consistent with the results in T. reesei [27,42,43], the performance of the overall 100,000-conidia populations allowed us to quantitatively distinguish the difference of various promoters, overcome the tedious procedures of biomass measurement, and reduce the operating error (Figure 2). Recently, flow cytometry was applied to directly sort germinated conidia [44] or transformed protoplasts without plating [45], demonstrating the potential of high-throughput screening with mCherry-PyrG fusion by fluorescence-activated cell sorting (FACS). Moreover, coupled with droplet-based microfluidics [44], this fluorescent-auxotrophic selection workflow could be applied for screening inducible promoters under various conditions.
Using this workflow, we characterized six endogenous constitutive promoters of A. niger and identified a very strong endogenous PgpdAg promoter, which is even much stronger than the most frequently used strong promoter PgpdAd of A. nidulans [16]. In a case application, the cexA-expressing construct with PgpdAg promoter produced up to 114 g/L of citric acid (Figure 5), which was 2.48-fold higher than the parental strain, suggesting that our study provided an alternative, strong constitutive promoter PgpdAg, whereas a previously reported strong promoter PmbfA in A. niger ATCC1015 [15], whose transcription level was greater than gpdA in ATCC1015, showed only 18.01% and 41.01% lower strength than that of PgpdAg and PgpdAd in our strain D353.8, respectively (Figure 2 and Table S4). Moreover, the transcription profile of D353.8 suggested a 9.50-fold higher expression of gpdA than mbfA (Figure S5). Additionally, MbfA was not detected in the intracellular proteome of A. niger AB1.13 growing on a defined medium with xylose or maltose as a carbon substrate [46]. This difference of PmbfA activity might result from the discrepancy of gene regulation patterns in a different genetic background.
In addition, it is interesting to mention the broad effect of cexA overexpression on the transcription profiling (Figure S4 and Table 2). Generally, exporters such as CexA are usually focused on their efflux function of transporting metabolites. We observed that CexA overexpression dramatically influenced the transcriptional pattern of genes involved in carbohydrate transport and metabolism (Figure S4), suggesting its impacts on carbon metabolic flux redistribution. Three glucose transporters MstC, MstG, and MstH displayed the similar expression pattern as the cexA gene, which was consistent with the previous transcriptional analysis [47]. Additionally, we also discovered that several genes beneficial for intracellular citric acid accumulation were significantly regulated, including starch hydrolysis, precursor supply, mitochondrial citrate/malate shuttle, alternative oxidase respiratory chain, citric acid degradation, and by-product biosynthesis. It is, so far, not clear that such effect is directly caused by the cexA gene or by indirect regulation of the intracellular citric acid level.
5. Conclusions
In summary, we established an accurate and intuitive fluorescent-auxotrophic selection workflow to efficiently assess promoter activity in A. niger, which could pave the way for high-throughput promoter evaluation in filamentous fungi. With this workflow, we showed that PgpdAg is the strongest promoter out of the six tested promoters, providing an alternative competent promoter for metabolic engineering in A. niger.
6. Patents
The engineered promoters described in this paper are covered by patents CN202110268572.8. P.Z., X.Z., J.S., Y.L., W.Z., L.Z., and Y.M. are listed as co-inventors of the patents.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/jof8060568/s1. Figure S1: Construction of A. niger strain expressing mCherry-pyrG under the control of the PgpdAd promoter Figure S2: Constructs expressing mCherry-pyrG controlled by six constitutive promoters. Figure S3: Construction of A. niger strains expressing cexA under the control of the PgpdA promoter. Figure S4: Comparative transcriptome analysis of cexA expressing constructs in submerged citric acid fermentation. Figure S5: Relative transcription level of genes of the analysed promoters in A. niger D353.8. Table S1: Strains and plasmids used in the study. Table S2: Protospacers used in this study. Table S3: Primers used in this study. Table S4: Promoter strength evaluation by flow cytometry analysis. Table S5: Summary of RNA sequencing and mapping in this study.
Author Contributions
X.Z., P.Z. and J.S. conceived the study. X.Z. designed the experiments. Y.L. (Yudan Lu) and L.Z. performed the experiments. L.W. took part in the flow cytometry analysis. Y.L. (Yu Lei) performed qPCR analysis. X.Z. drafted and revised the manuscript. Y.W., P.Z., T.Z. and J.S. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Key R&D Program of China (2018YFA0900500), National Natural Sciences Foundation of China (32070082 and 31961133021), and the Tianjin Synthetic Biotechnology Innovation Capacity Improvement Project (TSBICIP-PTJS-003 and TSBI-CIP-IJCP-003).
Institutional Review Board Statement
This study does not contain any studies with human or animal subjects.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this manuscript and Supplementary Material and can be requested from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meyer, V.; Wu, B.; Ram, A.F. Aspergillus as a multi-purpose cell factory: Current status and perspectives. Biotechnol. Lett. 2011, 33, 469–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Z.; Zheng, X.; Tong, Y.; Shi, Y.C.; Sun, J. Systems metabolic engineering for citric acid production by Aspergillus niger in the post-genomic era. Microb. Cell. Fact. 2019, 18, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, V.; Cairns, T.; Barthel, L.; King, R.; Kunz, P.; Schmideder, S.; Muller, H.; Briesen, H.; Dinius, A.; Krull, R. Understanding and controlling filamentous growth of fungal cell factories: Novel tools and opportunities for targeted morphology engineering. Fungal. Biol. Biotechnol. 2021, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, Y.; Cao, W.; Liu, H. Improved Production of malic acid in Aspergillus niger by abolishing citric acid accumulation and enhancing glycolytic flux. ACS Synth. Biol. 2020, 9, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Steiger, M.G.; Rassinger, A.; Mattanovich, D.; Sauer, M. Engineering of the citrate exporter protein enables high citric acid production in Aspergillus niger. Metab. Eng. 2019, 52, 224–231. [Google Scholar] [CrossRef]
- Zheng, X.; Zheng, P.; Zhang, K.; Cairns, T.C.; Meyer, V.; Sun, J.; Ma, Y. 5S rRNA promoter for guide rna expression enabled highly efficient CRISPR/Cas9 genome editing in Aspergillus niger. ACS Synth. Biol. 2019, 8, 1568–1574. [Google Scholar] [CrossRef]
- Rozhkova, A.M.; Kislitsin, V.Y. CRISPR/Cas Genome Editing in Filamentous Fungi. Biochemistry 2021, 86, S120–S139. [Google Scholar] [CrossRef]
- Zheng, X.; Zheng, P.; Sun, J. CRISPR/Cas-based genome editing in Aspergillus niger. Sheng Wu Gong Cheng Xue Bao 2021, 37, 980–990. [Google Scholar]
- Ganzlin, M.; Rinas, U. In-depth analysis of the Aspergillus niger glucoamylase (glaA) promoter performance using high-throughput screening and controlled bioreactor cultivation techniques. J. Biotechnol. 2008, 135, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Henriksen, A.L.S.; Even, S.; Muller, C.; Punt, P.J.; van den Hondel, C.; Nielsen, J. Study of the glucoamylase promoter in Aspergillus niger using green fluorescent protein. Microbiology 1999, 145 Pt 3, 729–734. [Google Scholar] [CrossRef] [Green Version]
- Fleissner, A.; Dersch, P. Expression and export: Recombinant protein production systems for Aspergillus. Appl. Microbiol. Biotechnol. 2010, 87, 1255–1270. [Google Scholar] [CrossRef] [PubMed]
- Meyer, V.; Wanka, F.; van Gent, J.; Arentshorst, M.; van den Hondel, C.A.; Ram, A.F. Fungal gene expression on demand: An inducible, tunable, and metabolism-independent expression system for Aspergillus niger. Appl. Environ. Microbiol. 2011, 77, 2975–2983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanka, F.; Cairns, T.; Boecker, S.; Berens, C.; Happel, A.; Zheng, X.; Sun, J.; Krappmann, S.; Meyer, V. Tet-on, or Tet-off, that is the question: Advanced conditional gene expression in Aspergillus. Fungal. Genet. Biol. 2016, 89, 72–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, A.H.; Dersch, P. A novel expression system for intracellular production and purification of recombinant affinity-tagged proteins in Aspergillus niger. Appl. Microbiol. Biotechnol. 2010, 86, 659–670. [Google Scholar] [CrossRef]
- Blumhoff, M.; Steiger, M.G.; Marx, H.; Mattanovich, D.; Sauer, M. Six novel constitutive promoters for metabolic engineering of Aspergillus niger. Appl. Microbiol. Biotechnol. 2013, 97, 259–267. [Google Scholar] [CrossRef]
- Punt, P.J.; Dingemanse, M.A.; Kuyvenhoven, A.; Soede, R.D.; Pouwels, P.H.; van den Hondel, C.A. Functional elements in the promoter region of the Aspergillus nidulans gpdA gene encoding glyceraldehyde-3-phosphate dehydrogenase. Gene 1990, 93, 101–109. [Google Scholar] [CrossRef]
- el-Enshasy, H.; Hellmuth, K.; Rinas, U. GpdA-promoter-controlled production of glucose oxidase by recombinant Aspergillus niger using nonglucose carbon sources. Appl. Biochem. Biotechnol. 2001, 90, 57–66. [Google Scholar] [CrossRef]
- Zhang, H.; Yan, J.N.; Zhang, H.; Liu, T.Q.; Xu, Y.; Zhang, Y.Y.; Li, J. Effect of gpd box copy numbers in the gpdA promoter of Aspergillus nidulans on its transcription efficiency in Aspergillus niger. FEMS Microbiol. Lett. 2018, 365, fny154. [Google Scholar] [CrossRef]
- Alazi, E.; Knetsch, T.; Di Falco, M.; Reid, I.D.; Arentshorst, M.; Visser, J.; Tsang, A.; Ram, A.F.J. Inducer-independent production of pectinases in Aspergillus niger by overexpression of the D-galacturonic acid-responsive transcription factor gaaR. Appl. Microbiol. Biotechnol. 2018, 102, 2723–2736. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, G.; Diano, A.; Nielsen, J. Recombinant bacterial hemoglobin alters metabolism of Aspergillus niger. Metab. Eng. 2009, 11, 8–12. [Google Scholar] [CrossRef]
- Dhillon, N.; Shelansky, R.; Townshend, B.; Jain, M.; Boeger, H.; Endy, D.; Kamakaka, R. Permutational analysis of Saccharomyces cerevisiae regulatory elements. Synth. Biol. 2020, 5, ysaa007. [Google Scholar] [CrossRef]
- Redden, H.; Alper, H.S. The development and characterization of synthetic minimal yeast promoters. Nat. Commun. 2015, 6, 7810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubertozzi, D.; Keasling, J.D. Marker and promoter effects on heterologous expression in Aspergillus nidulans. Appl. Microbiol. Biotechnol. 2006, 72, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Díaz, J.; Chávez, R.; Larrondo, L.F.; Eyzaguirre, J.; Bull, P. Functional analysis of the endoxylanase B (xynB) promoter from Penicillium purpurogenum. Curr. Genet. 2008, 54, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Ouyang, L.; Qi, J.; Chu, J. Exploration and characterization of hypoxia-inducible endogenous promoters in Aspergillus niger. Appl. Microbiol. Biotechnol. 2021, 105, 5529–5539. [Google Scholar] [CrossRef] [PubMed]
- Rantasalo, A.; Landowski, C.P.; Kuivanen, J.; Korppoo, A.; Reuter, L.; Koivistoinen, O.; Valkonen, M.; Penttila, M.; Jantti, J.; Mojzita, D. A universal gene expression system for fungi. Nucleic. Acids Res. 2018, 46, e111. [Google Scholar] [CrossRef]
- Wang, G.; Jia, W.; Chen, N.; Zhang, K.; Wang, L.; Lv, P.; He, R.; Wang, M.; Zhang, D. A GFP-fusion coupling FACS platform for advancing the metabolic engineering of filamentous fungi. Biotechnol. Biofuels 2018, 11, 232. [Google Scholar] [CrossRef]
- Huang, X.; Lu, X.; Li, J.J. Cloning, characterization and application of a glyceraldehyde-3-phosphate dehydrogenase promoter from Aspergillus terreus. J. Ind. Microbiol. Biotechnol. 2014, 41, 585–592. [Google Scholar] [CrossRef]
- Schape, P.; Kwon, M.J.; Baumann, B.; Gutschmann, B.; Jung, S.; Lenz, S.; Nitsche, B.; Paege, N.; Schutze, T.; Cairns, T.C.; et al. Updating genome annotation for the microbial cell factory Aspergillus niger using gene co-expression networks. Nucleic. Acids Res. 2019, 47, 559–569. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.H.; Zheng, X.M.; Cairns, T.C.; Zhang, Z.D.; Wang, D.P.; Zheng, P.; Sun, J.B. Disruption or reduced expression of the orotidine-5’-decarboxylase gene pyrG increases citric acid production: A new discovery during recyclable genome editing in Aspergillus niger. Microb. Cell Fact. 2020, 19, 76. [Google Scholar] [CrossRef]
- Carvalho, N.D.; Arentshorst, M.; Jin Kwon, M.; Meyer, V.; Ram, A.F. Expanding the ku70 toolbox for filamentous fungi: Establishment of complementation vectors and recipient strains for advanced gene analyses. Appl. Microbiol. Biotechnol. 2010, 87, 1463–1473. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.; Shen, B.; Zhang, C.; Huang, X.; Zhang, Y. sgRNAcas9: A software package for designing CRISPR sgRNA and evaluating potential off-target cleavage sites. PLoS ONE 2014, 9, e100448. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zheng, P.; Sun, J.; Kun, Z.; Ma, Y. Heterologous and endogenous U6 snRNA promoters enable CRISPR/Cas9 mediated genome editing in Aspergillus niger. Fungal. Biol. Biotechnol. 2018, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Yu, J.; Cairns, T.C.; Zhang, L.; Zhang, Z.; Zhang, Q.; Zheng, P.; Sun, J.; Ma, Y. Comprehensive improvement of sample preparation methodologies facilitates dynamic metabolomics of Aspergillus niger. Biotechnol. J. 2019, 14, e1800315. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Cairns, T.C.; Ni, X.; Zhang, L.; Zhai, H.; Meyer, V.; Zheng, P.; Sun, J. Comprehensively dissecting the hub regulation of PkaC on high-productivity and pellet macromorphology in citric acid producing Aspergillus niger. Microb. Biotechnol. 2022, e14020. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatusov, R.L.; Galperin, M.Y.; Natale, D.A.; Koonin, E.V. The COG database: A tool for genome-scale analysis of protein functions and evolution. Nucleic. Acids Res. 2000, 28, 33–36. [Google Scholar] [CrossRef] [Green Version]
- Storms, R.; Zheng, Y.; Li, H.; Sillaots, S.; Martinez-Perez, A.; Tsang, A. Plasmid vectors for protein production, gene expression and molecular manipulations in Aspergillus niger. Plasmid 2005, 53, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Dave, K.; Punekar, N.S. Utility of Aspergillus niger citrate synthase promoter for heterologous expression. J. Biotechnol. 2011, 155, 173–177. [Google Scholar] [CrossRef]
- Meyer, V.; Arentshorst, M.; El-Ghezal, A.; Drews, A.C.; Kooistra, R.; van den Hondel, C.A.; Ram, A.F. Highly efficient gene targeting in the Aspergillus niger kusA mutant. J. Biotechnol. 2007, 128, 770–775. [Google Scholar] [CrossRef]
- Aleksandrov, R.; Hristova, R.; Stoynov, S.; Gospodinov, A. The chromatin response to double-strand dna breaks and their repair. Cells 2020, 9, 1853. [Google Scholar] [CrossRef] [PubMed]
- Mathis, H.; Margeot, A.; Bouix, M. Optimization of flow cytometry parameters for high-throughput screening of spores of the filamentous fungus Trichoderma reesei. J. Biotechnol. 2020, 321, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Steiger, M.G. Flow Cytometry for Filamentous Fungi. Methods Mol. Biol 2021, 2234, 147–155. [Google Scholar]
- Yang, Y.J.; Liu, Y.; Liu, D.D.; Guo, W.Z.; Wang, L.X.; Wang, X.J.; Lv, H.X.; Yang, Y.; Liu, Q.; Tian, C.G. Development of a flow cytometry-based plating-free system for strain engineering in industrial fungi. Appl. Microbiol. Biotechnol. 2022, 106, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Beneyton, T.; Wijaya, I.P.; Postros, P.; Najah, M.; Leblond, P.; Couvent, A.; Mayot, E.; Griffiths, A.D.; Drevelle, A. High-throughput screening of filamentous fungi using nanoliter-range droplet-based microfluidics. Sci. Rep. 2016, 6, 27223. [Google Scholar] [CrossRef]
- Lu, X.; Sun, J.; Nimtz, M.; Wissing, J.; Zeng, A.P.; Rinas, U. The intra- and extracellular proteome of Aspergillus niger growing on defined medium with xylose or maltose as carbon substrate. Microb. Cell Fact. 2010, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Laothanachareon, T.; Bruinsma, L.; Nijsse, B.; Schonewille, T.; Suarez-Diez, M.; Tamayo-Ramos, J.A.; Martins Dos Santos, V.A.P.; Schaap, P.J. Global transcriptional response of Aspergillus niger to blocked active citrate export through deletion of the exporter gene. J. Fungi 2021, 7, 409. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).