In Situ Corn Fiber Conversion for Ethanol Improvement by the Addition of a Novel Lignocellulolytic Enzyme Cocktail
Abstract
1. Introduction
2. Materials and Methods
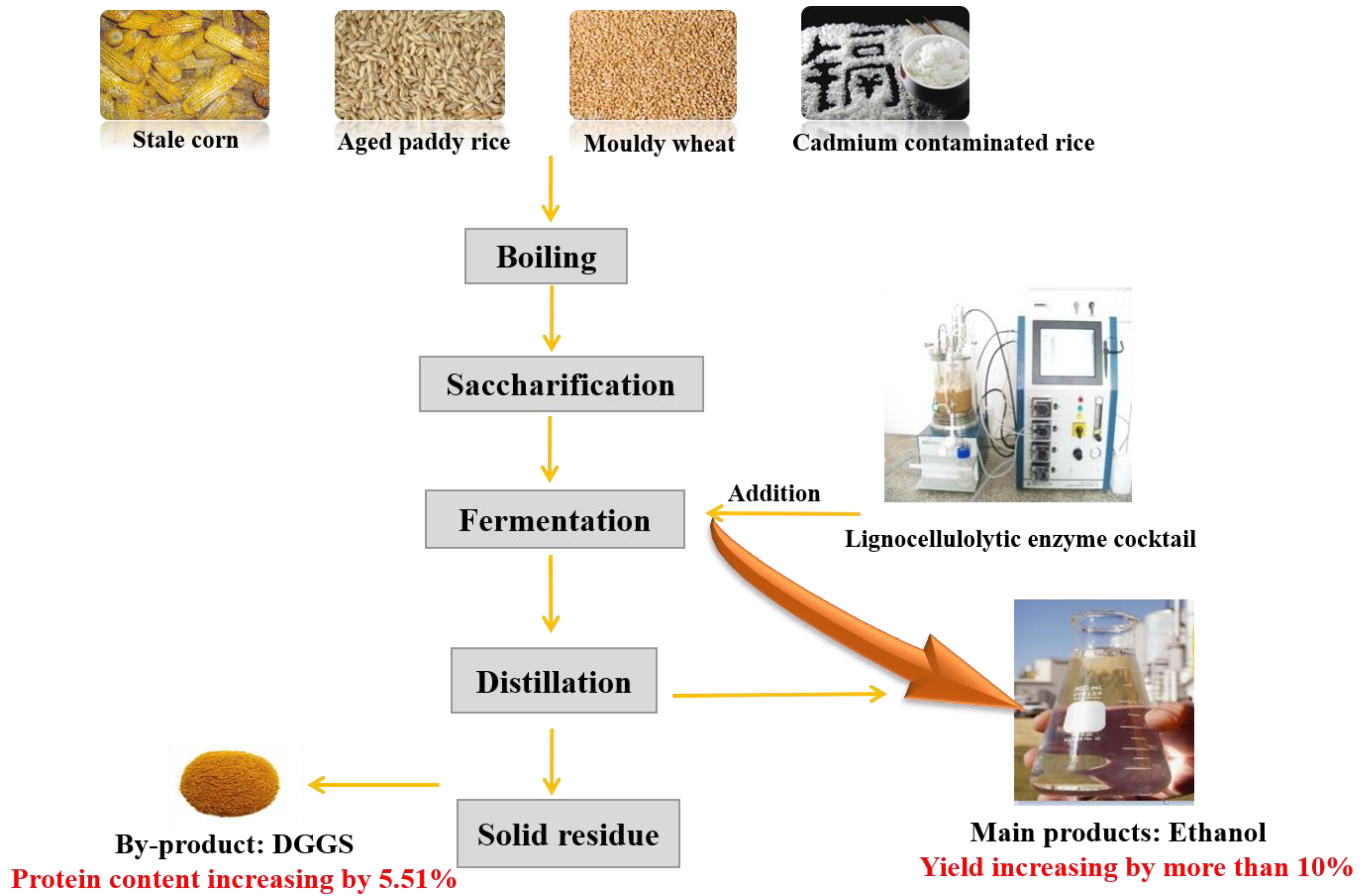
2.1. Mash Preparation
2.2. Culture Condition
2.3. Enzyme Preparations
2.3.1. Cellulase from T. reesei
2.3.2. Auxiliary Enzymes Expressed in A. niger
2.3.3. Lignocellulolytic Enzymatic Preparation Enzymes
2.4. Yeast Preparation
2.5. Simultaneous Saccharification and Fermentation (SSF) of Corn Mashes
2.6. Degradability of Residual Starch and Residual Cellulose
2.7. Analysis of Fermentation Samples
2.8. Statistical Analysis
3. Result and Discussion
3.1. Characteristics of Lignocellulolytic Enzymes
3.2. Effect of Lignocellulolytic Enzyme Loadings on Corn Mash Fermentation
3.3. Effect of Different Fermentation Tank Size on Ethanol Production
3.4. Further Degradation of Residual SSF Broth after Ethanol Evaporated by Lignocellulolytic Enzymes Cocktail
3.5. Comparison of DDGS Composition
3.6. Techno–Economic Analysis of the Strategy
4. Discussion
5. Patent
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Favaro, L.; Viktor, M.J.; Rose, S.H.; Viljoen-Bloom, M.; Zyl, W.H.V.; Basaglia, M.; Cagnin, L.; Casella, S. Consolidated bioprocessing of starchy substrates into ethanol by industrial Saccharomyces cerevisiae strains secreting fungal amylases. Biotechnol. Bioeng. 2015, 112, 1751–1760. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.S.; Yan, Y.S.; Feng, J.X. Efficient hydrolysis of raw starch and ethanol fermentation: A novel raw starch-digesting glucoamylase from Penicillium oxalicum. Biotechnol. Biofuels 2016, 9, 216. [Google Scholar] [CrossRef] [PubMed]
- Devantier, R.; Pedersen, S.; Olsson, L. Characterization of very high gravity ethanol fermentation of corn mash. Effect of glucoamylase dosage, pre-saccharification and yeast strain. Appl. Microbiol. Biotechnol. 2005, 68, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Ingledew, W.M. Yeasts for Production of Fuel Ethanol. In The Yeasts; Yeast Technology; Rose, A.H., Harrison, J.S., Eds.; Academic Press: Cambridge, MA, USA, 1993; Volume 5, pp. 245–291. [Google Scholar]
- Nghiem, N.; O’Connor, J.; Hums, M. Integrated Process for Extraction of Wax as a Value-Added Co-Product and Improved Ethanol Production by Converting Both Starch and Cellulosic Components in Sorghum Grains. Fermentation 2018, 4, 12. [Google Scholar] [CrossRef]
- Sapińska, E.; Balcerek, M.; Pielech-Przybylska, K. Alcoholic fermentation of high-gravity corn mashes with the addition of supportive enzymes. J. Chem. Technol. Biotechnol. 2013, 88, 2152–2158. [Google Scholar] [CrossRef]
- Li, X.J.; Chen, S.T.; Huang, H.; Jin, M.J. In situ corn fiber conversion improves ethanol yield in corn dry-mill process. Ind. Crop. Prod. 2018, 113, 217–224. [Google Scholar] [CrossRef]
- Gáspár, M.; Kálmán, G.; Réczey, K. Corn fiber as a raw material for hemicellulose and ethanol production. Process Biochem. 2007, 42, 1135–1139. [Google Scholar] [CrossRef]
- Eylen, D.V.; Dongen, F.V.; Kabel, M.; Bont, J.D. Corn fiber, cobs and stover: Enzyme-aided saccharification and co-fermentation after dilute acid pretreatment. Bioresour. Technol. 2011, 102, 5995–6004. [Google Scholar] [CrossRef]
- Ma, L.J.; Li, C.; Yang, Z.H.; Jia, W.D.; Zhang, D.Y.; Chen, S.L. Kinetic studies on batch cultivation of Trichoderma reesei and application to enhance cellulase production by fed-batch fermentation. J. Biotechnol. 2013, 166, 192–197. [Google Scholar] [CrossRef]
- Chen, L.; Yang, Z.H.; He, R.L.; Zhang, C.; Zhang, D.Y.; Chen, S.L.; Ma, L.J. Effect of pH on cellulase production and morphology of Trichoderma reesei and the application in cellulosic material hydrolysis. J. Biotechnol. 2013, 168, 470–477. [Google Scholar]
- He, R.L.; Ding, R.H.; Heyman, J.A.; Zhang, D.Y.; Tu, R. Ultra high-throughput picoliter-droplet microfuidics screening of the industrial cellulase-producing flamentous fungus Trichoderma reesei. J. Ind. Microbiol. Biotechnol. 2019, 46, 1603–1610. [Google Scholar] [CrossRef]
- Wang, G.K.; Jia, W.D.; Chen, N.; Zhang, K.; Wang, L.X.; Lv, P.; He, R.L.; Wang, M.; K Zhang, D.Y. A GFP-fusion coupling FACS platform for advancing the metabolic engineering of filamentous fungi. Biotechnol. Biofuels 2018, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, B.H.A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Yazdani, P.; Keikhosro, K.; Taherzadeh, M.J. Improvement of enzymatic hydrolysis of a marine macro-alga by dilute acid hydrolysis pretreatment. In Proceedings of the World Renewable Energy Congress, Linköping, Sweden, 8–13 May 2011. [Google Scholar]
- Lu, J.; Li, X.Z.; Yang, R.F.; Yang, L.; Zhao, J.; Liu, Y.; Qu, Y.B. Fed-Batch semi-simultaneous saccharification and fermentation of reed pretreated with liquid hot water for bio-ethanol production using Saccharomyces cerevisiae. Bioresour. Technol. 2013, 144, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Marcó, A.; Rubio, R.; Compañó, R.; Casals, I. Comparison of the Kjeldahl method and a combustion method for total nitrogen determination in animal feed. Talanta 2002, 57, 1019–1026. [Google Scholar] [CrossRef]
- Song, W.X.; Han, X.L.; Qian, Y.C.; Liu, G.D.; Yao, G.S.; Zhong, Y.H.; Qu, Y.B. Proteomic analysis of the biomass hydrolytic potentials of Penicillium oxalicum lignocellulolytic enzyme system. Biotechnol. Biofuels 2016, 9, 68. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chomvong, K.; Acosta-Sampson, L.; Estrela, R.; Gakazka, J.M.; Kim, S.R.; Jin, Y.S.; Cate, J.H.D. Leveraging transcription factors to speed cellobiose fermentation by Saccharomyces cerevisiae. Biotechnol. Biofuels 2014, 7, 126. [Google Scholar] [CrossRef][Green Version]
- Nie, D.; Yao, L.; Xu, X.; Zhang, Z.; Li, Y.L. Promoting corn stover degradation via sequential processing of steam explosion and cellulase/lactic acid bacteria-assisted ensilage. Bioresour. Technol. 2021, 337, 125392. [Google Scholar] [CrossRef]
- Liu, G.; Qin, Y.; Li, Z.H.; Qu, Y.B. Development of highly efficient, low-cost lignocellulolytic enzyme systems in the post-genomic era. Biotechnol. Adv. 2013, 31, 962–975. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Inhibition of cellulases by phenols. Enzyme Microb. Technol. 2010, 46, 170–176. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzyme Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef]
- Gao, L.; Gao, F.; Zhang, D.Y.; Zhang, C.; Wu, G.H.; Chen, S.L. Purification and characterization of a new β-glucosidase from Penicillium piceum and its application in enzymatic degradation of delignified corn stover. Bioresour. Technol. 2013, 147, 658–661. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, L.; Wang, M.; Chen, S.L.; Zhang, D.Y. Biochemical characterization of a novel feruloyl esterase from Penicillium piceum and its application in biomass bioconversion. J. Mol. Catal. B Enzym. 2016, 133, 384–394. [Google Scholar] [CrossRef]
- Czarnecki, Z.; Nowak, J. Effects of rye pretreatment and enrichment with hemicellulolytic enzymes on ethanol fermentation efficiency. Electron. J. Pol. Agric. Univ. EJPAU Food Sci. Technol. 2001, 4, 12. [Google Scholar]
- Czarnecki, Z.; Nowak, J. Hemicellulase supplementation of regular and hybrid rye grain mashes for ethanol fermentation. Electron. J. Pol. Agric. Univ. EJPAU Food Sci. Technol. 2005, 8, 56. [Google Scholar]
- Joshi, B.; Bhatt, M.R.; Sharma, D.; Joshi, J.; Malla, R.; Sreerama, L. Lignocellulolytic ethanol production: Current practices and recent developments. Biotechnol. Mol. Biol. Rev. 2011, 6, 172–182. [Google Scholar]
- Abbas, C.; Bao, W.L. Cellulolytic Enzyme Enhancement of Dry Grind Corn Processing and Ethanol Production. International Application No. PCT/US2013/039496, 7 November 2013. [Google Scholar]
- Klosowski, G.; Mikulski, D.; Czuprynski, B.; Kotarska, K. Characterisation of fermentation of high-gravity maize mashes with the application of pullulanase, proteolytic enzymes and enzymes degrading nonstarch polysaccharides. J. Biosci. Bioeng. 2009, 109, 466–471. [Google Scholar] [CrossRef]
| 20 FPU/L | 30 FPU/L | 40 FPU/L | 45 FPU/L | 50 FPU/L | 60 FPU/L | 80 FPU/L | 100 FPU/L | 150 FPU/L | 200 FPU/L | CK | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethanol (g/L) | 105.1 ± 0.5 | 107.5 ± 0.7 | 112.7 ± 0.6 | 113.6 ± 0.6 | 114.2 ± 0.5 | 114.2 ± 0.7 | 114.3 ± 0.9 | 114.6 ± 0.7 | 113.8 ± 0.8 | 112.9 ± 0.7 | 100.7 ± 0.8 |
| Residual sugar (g/L) | 26.0 ± 1.4 | 21.1 ± 1.2 | 19.4 ± 1.0 | 18.1 ± 0.9 | 13.0 ± 0.7 | 13.3 ± 0.7 | 13.1± 0.8 | 13.2 ± 0.5 | 10.9 ± 0.4 | 11.1 ± 0.5 | 38.8 ± 1.7 |
| Viscosity (Pa·s) | 23.5 | 22.4 | 21.7 | 20.8 | 18.0 | 18.7 | 18.3 | 18.4 | 18.1 | 19.2 | 34.8 |
| Tank Size | 0.3 L | 1 L | 5 L | 70 L | Tank CK |
|---|---|---|---|---|---|
| Ethanol (g/L) | 114.2 ± 0.3 | 113.8 ± 0.8 | 115.8 ± 0.5 | 117.0 ± 0.1 | 103.6 ± 1.0 |
| Residual sugar (g/L) | 16.0 ± 0.7 | 16.2 ± 0.5 | 16.4 ± 0.4 | 16.1 ± 0.1 | 36.8 ± 0.9 |
| Viscosity (Pa·s) | 18.0 ± 1.0 | 22.9 ± 1.2 | 21.6 ± 1.4 | 19.8 ± 1.1 | 33.5 ± 1.5 |
| SSF | SHF | |||
|---|---|---|---|---|
| With Lignocellulolytic Enzymes | Without Lignocellulolytic Enzymes | With Lignocellulolytic Enzymes | Without Lignocellulolytic Enzymes | |
| Residual cellulose of corn mash (%) | 7.48 ± 0.10 | 12.64 ± 0.52 | 10.07 ± 0.10 | 13.2 ± 0.52 |
| Residual hemicellulose of corn mash (%) | 5.07 ± 0.10 | 5.35 ± 0.10 | 5.74 ± 0.13 | 5.35 ± 0.10 |
| Residual starch of corn mash (%) | 5.34 ± 0.26 | 6.58 ± 0.86 | 5.06 ± 0.34 | 6.02 ± 0.86 |
| Residual soluble sugar (g/L) | 3.68 ± 0.86 | 6.42 ± 0.34 | 4.68 ± 0.56 | 6.11 ± 0.14 |
| Composition | With Lignocellulolytic Enzymes | Without Lignocellulolytic Enzymes | p-Value |
|---|---|---|---|
| Crude protein | 29.63 ± 0.89 | 24.12 ± 0.73 | <0.01 |
| Cellulose | 21.20 ± 0.42 | 33.12 ± 0.59 | <0.01 |
| Starch | 11.26 ± 0.24 | 14.07 ± 0.33 | <0.01 |
| Ash | 5.01 ± 0.11 | 5.48 ± 0.18 | <0.01 |
| Xylan | 5.25 ± 0.13 | 8.21 ± 0.32 | <0.01 |
| Arabinan | 1.89 ± 0.10 | 3.19 ± 0.17 | <0.01 |
| Water extractives | 14.26 ± 0.52 | 6.01 ± 0.21 | <0.01 |
| Ether extractives | 11.50 ± 1.02 | 5.80 ± 0.72 | <0.01 |
| Enzymes/Microorganisms | Raw Sources | Solid Concentration (%) | Fermentation Conditions | Ethanol (g/L) | Reference |
|---|---|---|---|---|---|
| Lignocellulolytic enzymes from T. reesei and A. niger/S. cerevisiae | Corn | 27.0 | 32 °C, SSF, 36 h | 117.0 | This study |
| Cellulolytic enzyme from T. reesei | Corn | 28.0 | 30 °C, SSF, 45 h | 95.7 | [29] |
| Multi-enzyme complex from Aspergillus sp enzymes/S. cerevisiae | Corn | 28.0 | 32 °C, SSF, 72 h | 85.7 | [30] |
| Multi-enzyme complex CeluStar XL/S. cerevisiae | Corn | 28.5 | 30 °C, SSF, 72 h | 104.9 | [6] |
| Genencor cellulase/S. cerevisiae | Corn | 27.0 | 30 °C, SSF, 48 h | 111.3 | [7] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, L.; Zhang, D.; Wu, X. In Situ Corn Fiber Conversion for Ethanol Improvement by the Addition of a Novel Lignocellulolytic Enzyme Cocktail. J. Fungi 2022, 8, 221. https://doi.org/10.3390/jof8030221
Gao L, Zhang D, Wu X. In Situ Corn Fiber Conversion for Ethanol Improvement by the Addition of a Novel Lignocellulolytic Enzyme Cocktail. Journal of Fungi. 2022; 8(3):221. https://doi.org/10.3390/jof8030221
Chicago/Turabian StyleGao, Le, Dongyuan Zhang, and Xin Wu. 2022. "In Situ Corn Fiber Conversion for Ethanol Improvement by the Addition of a Novel Lignocellulolytic Enzyme Cocktail" Journal of Fungi 8, no. 3: 221. https://doi.org/10.3390/jof8030221
APA StyleGao, L., Zhang, D., & Wu, X. (2022). In Situ Corn Fiber Conversion for Ethanol Improvement by the Addition of a Novel Lignocellulolytic Enzyme Cocktail. Journal of Fungi, 8(3), 221. https://doi.org/10.3390/jof8030221






