In Vitro Activity of Amphotericin B in Combination with Colistin against Fungi Responsible for Invasive Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains
2.2. Medium Preparation
2.3. Drugs and Microplate Preparation
2.4. Inoculum Preparation and Inoculation of Microplates
2.5. Interpretation of the Results by Fractional Inhibition Concentration Index
2.6. Interpretation of the Results by Response Surface Analysis
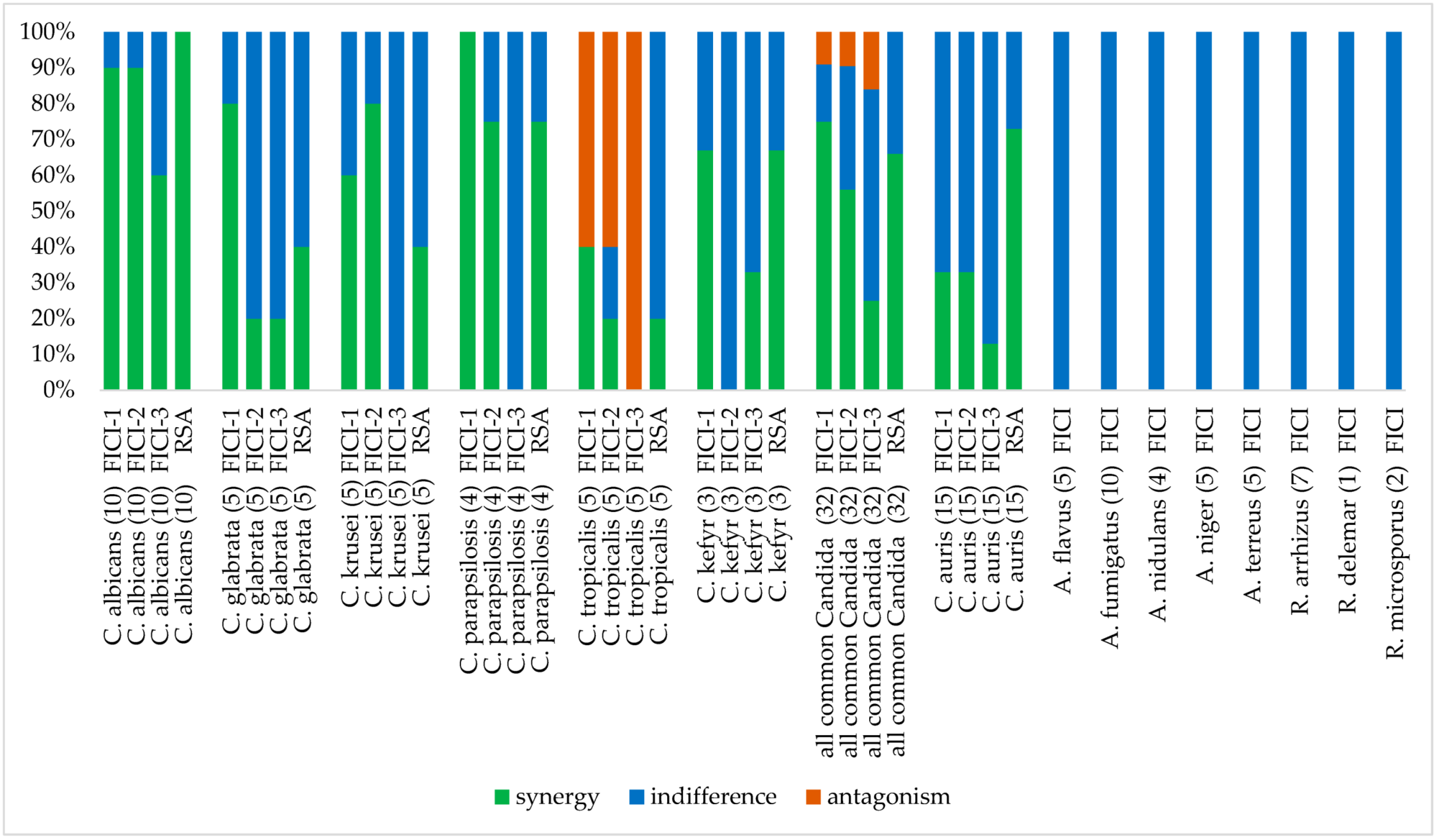
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cornet, M.; Fleury, L.; Maslo, C.; Bernard, J.-F.; Brücker, G.; Invasive Aspergillosis Surveillance Network of the Assistance Publique-Hôpitaux de Paris. Epidemiology of invasive aspergillosis in France: A six-year multicentric survey in the Greater Paris area. J. Hosp. Infect. 2002, 51, 288–296. [Google Scholar] [CrossRef]
- Skiada, A.; Pagano, L.; Groll, A.; Zimmerli, S.; Dupont, B.; Lagrou, K.; Lass-Florl, C.; Bouza, E.; Klimko, N.; Gaustad, P.; et al. Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin. Microbiol. Infect. 2011, 17, 1859–1867. [Google Scholar] [CrossRef]
- Cornillet, A.; Camus, C.; Nimubona, S.; Gandemer, V.; Tattevin, P.; Belleguic, C.; Chevrier, S.; Meunier, C.; Lebert, C.; Aupee, M.; et al. Comparison of Epidemiological, Clinical, and Biological Features of Invasive Aspergillosis in Neutropenic and Nonneutropenic Patients: A 6-Year Survey. Clin. Infect. Dis. 2006, 43, 577–584. [Google Scholar] [CrossRef]
- Gangneux, J.-P.; Dannaoui, E.; Fekkar, A.; Luyt, C.-E.; Botterel, F.; De Prost, N.; Tadié, J.-M.; Reizine, F.; Houzé, S.; Timsit, J.-F.; et al. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: The French multicentre MYCOVID study. Lancet Respir. Med. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Salmanton-García, J.; Sprute, R.; Stemler, J.; Bartoletti, M.; Dupont, D.; Valerio, M.; García-Vidal, C.; Falces-Romero, I.; Machado, M.; de la Villa, S.; et al. COVID-19–Associated Pulmonary Aspergillosis, March–August 2020. Emerg. Infect. Dis. 2021, 27, 1077–1086. [Google Scholar] [CrossRef]
- Prattes, J.; Wauters, J.; Giacobbe, D.R.; Salmanton-García, J.; Maertens, J.; Bourgeois, M.; Reynders, M.; Rutsaert, L.; Van Regenmortel, N.; Lormans, P.; et al. Risk factors and outcome of pulmonary aspergillosis in critically ill coronavirus disease 2019 patients—A multinational observational study by the European Confederation of Medical Mycology. Clin. Microbiol. Infect. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Ghosh, A.; Sarkar, A.; Paul, P.; Patel, P. The rise in cases of mucormycosis, candidiasis and aspergillosis amidst COVID-19. Fungal Biol. Rev. 2021, 38, 67–91. [Google Scholar] [CrossRef]
- Muthu, V.; Rudramurthy, S.M.; Chakrabarti, A.; Agarwal, R. Epidemiology and Pathophysiology of COVID-19-Associated Mucormycosis: India Versus the Rest of the World. Mycopathologia 2021, 186, 739–754. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.S.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2020, 64, 152–156. [Google Scholar] [CrossRef]
- Prestel, C.; Anderson, E.; Forsberg, K.; Lyman, M.; De Perio, M.A.; Kuhar, D.; Edwards, K.; Rivera, M.; Shugart, A.; Walters, M.; et al. Candida auris Outbreak in a COVID-19 Specialty Care Unit—Florida, July–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2021, 70, 56–57. [Google Scholar] [CrossRef]
- Bidaud, A.; Chowdhary, A.; Dannaoui, E. Candida auris: An emerging drug resistant yeast—A mini-review. J. Mycol. Med. 2018, 28, 568–573. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Arendrup, M.C. Invasive Candidiasis. N. Engl. J. Med. 2015, 373, 1445–1456. [Google Scholar] [CrossRef]
- Arastehfar, A.; Carvalho, A.; Nguyen, M.H.; Hedayati, M.T.; Netea, M.G.; Perlin, D.S.; Hoenigl, M. COVID-19-Associated Candidiasis (CAC): An Underestimated Complication in the Absence of Immunological Predispositions? J. Fungi 2020, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, A.; Tarai, B.; Singh, A.; Sharma, A. Multidrug-Resistant Candida auris Infections in Critically Ill Coronavirus Disease Patients, India, April–July 2020. Emerg. Infect. Dis. 2020, 26, 2694–2696. [Google Scholar] [CrossRef] [PubMed]
- Ullmann, A.J.; Aguado, J.M.; Arikan-Akdagli, S.; Denning, D.W.; Groll, A.H.; Lagrou, K.; Lass-Flörl, C.; Lewis, R.E.; Munoz, P.; Verweij, P.E.; et al. Diagnosis and management of Aspergillus diseases: Executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin. Microbiol. Infect. 2018, 24 (Suppl. 1), e1–e38. [Google Scholar] [CrossRef] [PubMed]
- Verweij, P.E.; Ananda-Rajah, M.; Andes, D.; Arendrup, M.C.; Brüggemann, R.J.; Chowdhary, A.; Cornely, O.A.; Denning, D.W.; Groll, A.H.; Izumikawa, K.; et al. International expert opinion on the management of infection caused by azole-resistant Aspergillus fumigatus. Drug Resist. Updates 2015, 21–22, 30–40. [Google Scholar] [CrossRef]
- Cornely, O.A.; Alastruey-Izquierdo, A.; Arenz, D.; Chen, S.C.A.; Dannaoui, E.; Hochhegger, B.; Hoenigl, M.; Jensen, H.E.; Lagrou, K.; Lewis, R.E.; et al. Global guideline for the diagnosis and management of mucormycosis: An initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect. Dis. 2019, 19, e405–e421. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18 (Suppl. 7), 19–37. [Google Scholar] [CrossRef]
- Perlin, D.S. Echinocandin Resistance in Candida. Clin. Infect. Dis. 2015, 61 (Suppl. 6), S612–S617. [Google Scholar] [CrossRef]
- Chowdhary, A.; Prakash, A.; Sharma, C.; Kordalewska, M.; Kumar, A.; Sarma, S.; Tarai, B.; Singh, A.; Upadhyaya, G.; Upadhyay, S.; et al. A multicentre study of antifungal susceptibility patterns among 350 Candida auris isolates (2009–17) in India: Role of the ERG11 and FKS1 genes in azole and echinocandin resistance. J. Antimicrob. Chemother. 2018, 73, 891–899. [Google Scholar] [CrossRef]
- Mokhtari, R.B.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Lewis, R.E. Toward more effective antifungal therapy: The prospects of combination therapy. Br. J. Haematol. 2004, 126, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Marr, K.A.; Schlamm, H.T.; Herbrecht, R.; Rottinghaus, S.T.; Bow, E.J.; Cornely, O.A.; Heinz, W.J.; Jagannatha, S.; Koh, L.P.; Kontoyiannis, D.P.; et al. Combination Antifungal Therapy for Invasive Aspergillosis: A Randomized Trial. Ann. Intern. Med. 2015, 162, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Ibrahim, A.S.; Chin-Hong, P.V.; Kontoyiannis, D.P.; Morris, M.I.; Perfect, J.R.; Fredricks, D.; Brass, E.P. The Deferasirox–AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: A randomized, double-blinded, placebo-controlled trial. J. Antimicrob. Chemother. 2011, 67, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Pappas, P.G.; Karchmer, A.W.; Sobel, J.; Edwards, J.E.; Hadley, S.; Brass, C.; Vazquez, J.A.; Chapman, S.W.; Horowitz, H.W.; et al. A Randomized and Blinded Multicenter Trial of High-Dose Fluconazole plus Placebo versus Fluconazole plus Amphotericin B as Therapy for Candidemia and Its Consequences in Nonneutropenic Subjects. Clin. Infect. Dis. 2003, 36, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.; Cornely, O.A.; Dannaoui, E. Antifungal combinations in Mucorales: A microbiological perspective. Mycoses 2019, 62, 746–760. [Google Scholar] [CrossRef]
- Steinbach, W.J.; Stevens, D.A.; Denning, D.W. Combination and Sequential Antifungal Therapy for Invasive Aspergillosis: Review of Published In Vitro and In Vivo Interactions and 6281 Clinical Cases from 1966 to 2001. Clin. Infect. Dis. 2003, 37 (Suppl. 3), S188–S224. [Google Scholar] [CrossRef]
- Johnson, M.D.; MacDougall, C.; Ostrosky-Zeichner, L.; Perfect, J.R.; Rex, J. Combination Antifungal Therapy. Antimicrob. Agents Chemother. 2004, 48, 693–715. [Google Scholar] [CrossRef]
- Grégoire, N.; Aranzana-Climent, V.; Magréault, S.; Marchand, S.; Couet, W. Clinical Pharmacokinetics and Pharmacodynamics of Colistin. Clin. Pharmacokinet. 2017, 56, 1441–1460. [Google Scholar] [CrossRef]
- Schwarz, P.; Djenontin, E.; Dannaoui, E. Colistin and Isavuconazole Interact Synergistically In Vitro against Aspergillus nidulans and Aspergillus niger. Microorganisms 2020, 8, 1447. [Google Scholar] [CrossRef]
- Schwarz, P.; Bidaud, A.-L.; Dannaoui, E. In vitro synergy of isavuconazole in combination with colistin against Candida auris. Sci. Rep. 2020, 10, 21448. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, P.; Bretagne, S.; Gantier, J.-C.; Garcia-Hermoso, D.; Lortholary, O.; Dromer, F.; Dannaoui, E. Molecular Identification of Zygomycetes from Culture and Experimentally Infected Tissues. J. Clin. Microbiol. 2006, 44, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Conidia Forming Moulds (EUCAST Definitive Document E.Def 9.3.2). 2020. Available online: http://www.eucast.org (accessed on 23 June 2013).
- Arendrup, M.C.; Meletiadis, J.; Mouton, J.W.; Lagrou, K.; Hamal, P.; Guinea, J. Method for the Determination of Broth Dilution Minimum Inhibitory Concentrations of Antifungal Agents for Yeasts (EUCAST Definitive Document E.Def 7.3.2). 2020. Available online: http://www.eucast.org (accessed on 23 June 2013).
- Meletiadis, J.; Meis, J.F.G.M.; Mouton, J.W.; Verweij, P.E. Analysis of Growth Characteristics of Filamentous Fungi in Different Nutrient Media. J. Clin. Microbiol. 2001, 39, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Di Veroli, G.Y.; Fornari, C.; Wang, D.; Mollard, S.; Bramhall, J.L.; Richards, F.M.; Jodrell, D.I. Combenefit: An interactive platform for the analysis and visualization of drug combinations. Bioinformatics 2016, 32, 2866–2868. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, A.-L.; Schwarz, P.; Herbreteau, G.; Dannaoui, E. Techniques for the Assessment of In Vitro and In Vivo Antifungal Combinations. J. Fungi 2021, 7, 113. [Google Scholar] [CrossRef]
- Lim, L.M.; Ly, N.; Anderson, D.; Yang, J.C.; Macander, L.; Jarkowski, A., 3rd; Forrest, A.; Bulitta, J.B.; Tsuji, B.T. Resurgence of Colistin: A Review of Resistance, Toxicity, Pharmacodynamics, and Dosing. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2010, 30, 1279–1291. [Google Scholar] [CrossRef]
- Brennan-Krohn, T.; Pironti, A.; Kirby, J.E. Synergistic Activity of Colistin-Containing Combinations against Colistin-Resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2018, 62, e00873-18. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Lewis, R.E.; Tarrand, J.; Leventakos, K.; Kontoyiannis, D.P. Antifungal Activity of Colistin against Mucorales Species In Vitro and in a Murine Model of Rhizopus oryzae Pulmonary Infection. Antimicrob. Agents Chemother. 2010, 54, 484–490. [Google Scholar] [CrossRef]
- Yousfi, H.; Ranque, S.; Rolain, J.-M.; Bittar, F. In vitro polymyxin activity against clinical multidrug-resistant fungi. Antimicrob. Resist. Infect. Control. 2019, 8, 66. [Google Scholar] [CrossRef]
- Chowdhary, A.; Singh, P.K.; Kathuria, S.; Hagen, F.; Meis, J.F. Comparison of the EUCAST and CLSI Broth Microdilution Methods for Testing Isavuconazole, Posaconazole, and Amphotericin B against Molecularly Identified Mucorales Species. Antimicrob. Agents Chemother. 2015, 59, 7882–7887. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Prakash, A.; Meletiadis, J.; Sharma, C.; Chowdhary, A. Comparison of EUCAST and CLSI Reference Microdilution MICs of Eight Antifungal Compounds for Candida auris and Associated Tentative Epidemiological Cutoff Values. Antimicrob. Agents Chemother. 2017, 61, e00485-17. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M.; Lee-Yang, W.; Ciblak, M.A.; Arthington-Skaggs, B.A.; Mellado, E.; Warnock, D.W.; Rodriguez-Tudela, J.L. Comparative Evaluation of NCCLS M27-A and EUCAST Broth Microdilution Procedures for Antifungal Susceptibility Testing of Candida Species. Antimicrob. Agents Chemother. 2002, 46, 3644–3647. [Google Scholar] [CrossRef] [PubMed][Green Version]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 23 June 2013).
- Arendrup, M.; Friberg, N.; Mares, M.; Kahlmeter, G.; Meletiadis, J.; Guinea, J.; Andersen, C.; Arikan-Akdagli, S.; Barchiesi, F.; Chryssanthou, E.; et al. How to interpret MICs of antifungal compounds according to the revised clinical breakpoints v. 10.0 European committee on antimicrobial susceptibility testing (EUCAST). Clin. Microbiol. Infect. 2020, 26, 1464–1472. [Google Scholar] [CrossRef]
- Serrano-Lobo, J.; Gómez, A.; Sánchez-Yebra, W.; Fajardo, M.; Lorenzo, B.; Sánchez-Reus, F.; Vidal, I.; Fernández-Torres, M.; Sánchez-Romero, I.; de Alegría-Puig, C.R.; et al. Azole and Amphotericin B MIC Values against Aspergillus fumigatus: High Agreement between Spectrophotometric and Visual Readings Using the EUCAST EDef 9.3.2 Procedure. Antimicrob. Agents Chemother. 2020, 65, e01693-20. [Google Scholar] [CrossRef]
- Serrano-Lobo, J.; Gomez, A.; Munoz, P.; Escribano, P.; Guinea, J. Spectrophotometric azole and amphotericin B MIC readings against Aspergillus fumigatus sensu lato using the EUCAST 9.3.2 methodology. Are ≥90 and ≥95% fungal growth inhibition endpoints equally suitable? Med. Mycol. 2021, 60, myab072. [Google Scholar] [CrossRef]
- Bidaud, A.L.; Djenontin, E.; Botterel, F.; Chowdhary, A.; Dannaoui, E. Colistin interacts synergistically with echinocandins against Candida auris. Int. J. Antimicrob. Agents 2020, 55, 105901. [Google Scholar] [CrossRef]
- Reed, M.D.; Stern, R.C.; O’Riordan, M.A.; Blumer, J.L. The Pharmacokinetics of Colistin in Patients with Cystic Fibrosis. J. Clin. Pharmacol. 2001, 41, 645–654. [Google Scholar] [CrossRef]
- Garonzik, S.M.; Li, J.; Thamlikitkul, V.; Paterson, D.L.; Shoham, S.; Jacob, J.; Silveira, F.P.; Forrest, A.; Nation, R.L. Population Pharmacokinetics of Colistin Methanesulfonate and Formed Colistin in Critically Ill Patients from a Multicenter Study Provide Dosing Suggestions for Various Categories of Patients. Antimicrob. Agents Chemother. 2011, 55, 3284–3294. [Google Scholar] [CrossRef]
- Dannaoui, E.; Lortholary, O.; Dromer, F. Methods for antifungal combination studies in vitro and in vivo in animal models. J. Mycol. Med. 2003, 13, 73–85. [Google Scholar]
- Spitzer, M.; Robbins, N.; Wright, G.D. Combinatorial strategies for combating invasive fungal infections. Virulence 2017, 8, 169–185. [Google Scholar] [CrossRef]
- Schwarz, P.; Dromer, F.; Lortholary, O.; Dannaoui, E. In Vitro Interaction of Flucytosine with Conventional and New Antifungals against Cryptococcus neoformans Clinical Isolates. Antimicrob. Agents Chemother. 2003, 47, 3361–3364. [Google Scholar] [CrossRef][Green Version]
- Dannaoui, E.; Schwarz, P.; Lortholary, O. In Vitro Interactions between Antifungals and Immunosuppressive Drugs against Zygomycetes. Antimicrob. Agents Chemother. 2009, 53, 3549–3551. [Google Scholar] [CrossRef]
- Dannaoui, E.; Lortholary, O.; Dromer, F. In Vitro Evaluation of Double and Triple Combinations of Antifungal Drugs against Aspergillus fumigatus and Aspergillus terreus. Antimicrob. Agents Chemother. 2004, 48, 970–978. [Google Scholar] [CrossRef][Green Version]
- Bibi, M.; Murphy, S.; Benhamou, R.I.; Rosenberg, A.; Ulman, A.; Bicanic, T.; Fridman, M.; Berman, J. Combining Colistin and Fluconazole Synergistically Increases Fungal Membrane Permeability and Antifungal Cidality. ACS Infect. Dis. 2021, 7, 377–389. [Google Scholar] [CrossRef]
- Macedo, D.; Leonardelli, F.; Dudiuk, C.; Vitale, R.G.; Del Valle, E.; Giusiano, G.; Gamarra, S.; Garcia-Effron, G. In Vitro and In Vivo Evaluation of Voriconazole-Containing Antifungal Combinations against Mucorales Using a Galleria mellonella Model of Mucormycosis. J. Fungi 2019, 5, 5. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Ricardo, E.; Branco, R.J.; Azevedo, M.M.; Rodrigues, A.G.; Pina-Vaz, C. Unveiling the Synergistic Interaction Between Liposomal Amphotericin B and Colistin. Front. Microbiol. 2016, 7, 1439. [Google Scholar] [CrossRef][Green Version]
- Zeidler, U.; Bougnoux, M.-E.; Lupan, A.; Helynck, O.; Doyen, A.; Garcia, Z.; Sertour, N.; Clavaud, C.; Munier-Lehmann, H.; Saveanu, C.; et al. Synergy of the antibiotic colistin with echinocandin antifungals in Candida species. J. Antimicrob. Chemother. 2013, 68, 1285–1296. [Google Scholar] [CrossRef]
- Schemuth, H.; Dittmer, S.; Lackner, M.; Sedlacek, L.; Hamprecht, A.; Steinmann, E.; Buer, J.; Rath, P.-M.; Steinmann, J. In vitroactivity of colistin as single agent and in combination with antifungals against filamentous fungi occurring in patients with cystic fibrosis. Mycoses 2012, 56, 297–303. [Google Scholar] [CrossRef]
- Lass-Flãrl, C.; Perkhofer, S.; Mayr, A.; Lass-Flörl, C. In vitro susceptibility testing in fungi: A global perspective on a variety of methods. Mycoses 2010, 53, 1–11. [Google Scholar] [CrossRef]
- MacCallum, D.M.; Desbois, A.P.; Coote, P.J. Enhanced efficacy of synergistic combinations of antimicrobial peptides with caspofungin versus Candida albicans in insect and murine models of systemic infection. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
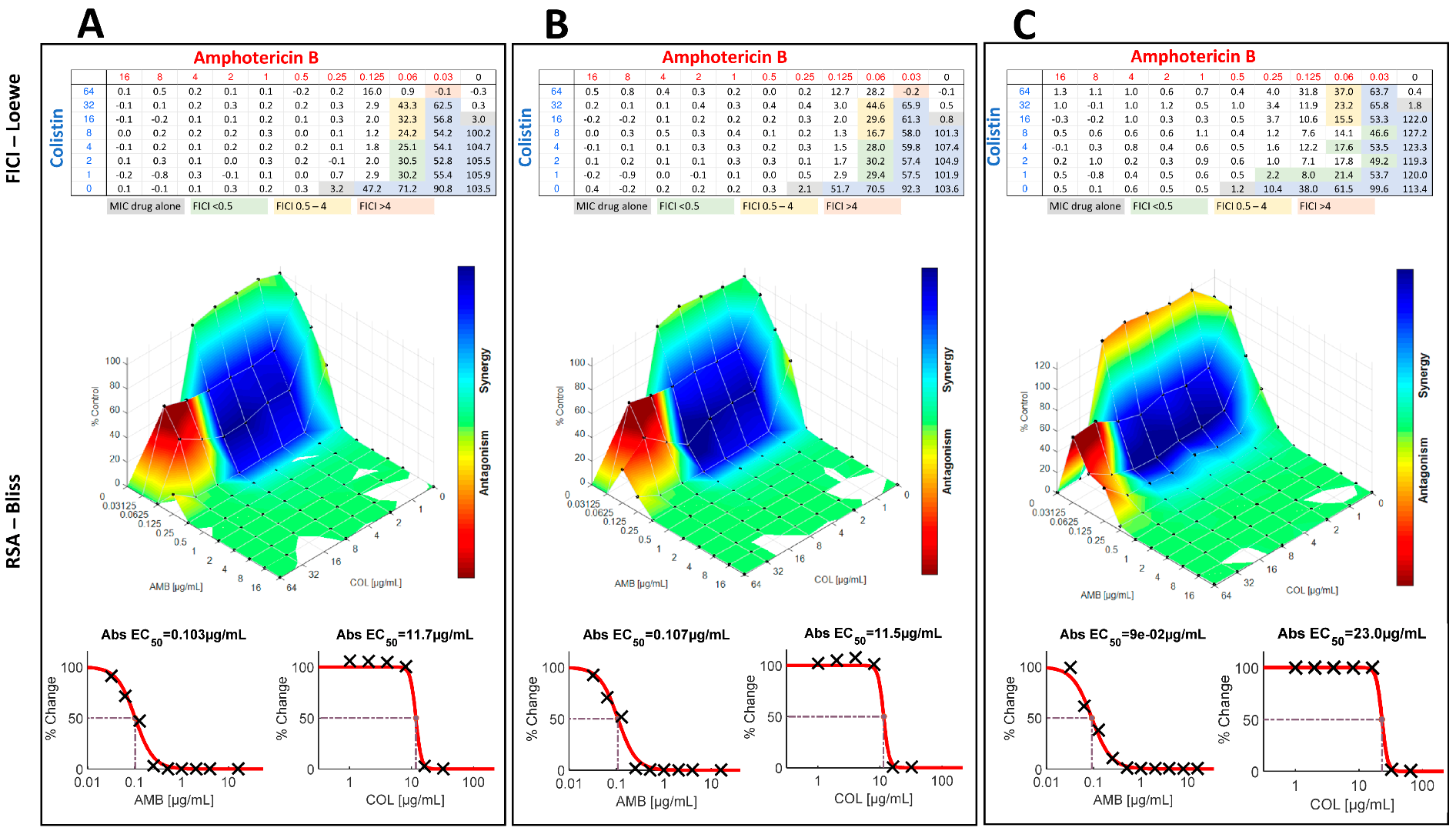
| Species | Collection Number | Checkerboard MICs (µg/mL) | Response Surface Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| AMB | COL | AMB/COL | FICI | INTPN | ΣSYN-ANT (ΣSYN; ΣANT) | INTPN | ||
| C. albicans | V2105126 | 0.25 | >68 | 0.06/1 | 0.2578 | SYN | 81.36 (81.48; −0.12) | SYN |
| C. albicans | N2101578 | 0.5 | >68 | 0.125/1 | 0.2578 | SYN | 79.68 (80.13; −0.45) | SYN |
| C. albicans | V2105568 | 0.25 | >68 | 0.06/2 | 0.2656 | SYN | 72.40 (72.73; −0.33) | SYN |
| C. albicans | N2101577 | 0.25 | >68 | 0.125/1 | 0.5078 | IND | 62.97 (63.75; −0.78) | SYN |
| C. albicans | V2105825iso3 | 0.25 | >68 | 0.03/1 | 0.1328 | SYN | 76.46 (76.73; −0.27) | SYN |
| C. albicans | ATCC 14053 | 0.25 | >68 | 0.06/1 | 0.2578 | SYN | 80.98 (82.44; −1.46) | SYN |
| C. albicans | V2105529 | 0.25 | >68 | 0.03/1 | 0.1328 | SYN | 87.84 (87.89; −0.05) | SYN |
| C. albicans | V2106139 | 0.25 | >68 | 0.06/1 | 0.2578 | SYN | 80.84 (81.29: −0.45) | SYN |
| C. albicans | V2106041 | 0.25 | 64 | 0.06/1 | 0.2656 | SYN | 79.58 (82.62; −3.04) | SYN |
| C. albicans | V2106305 | 0.25 | >68 | 0.03/2 | 0.1406 | SYN | 70.02 (70.43; −0.41) | SYN |
| C. glabrata | V2105272 | 0.5 | >68 | 0.25/1 | 0.5078 | IND | 52.39 (53.11; −0.72) | SYN |
| C. glabrata | V2105282 | 0.5 | >68 | 0.125/2 | 0.2656 | SYN | 10.67 (11.28; −0.61) | IND |
| C. glabrata | N2101711 | 0.5 | >68 | 0.125/2 | 0.2656 | SYN | 21.72 (23.48: −1.76) | IND |
| C. glabrata | V2105636 | 0.5 | >68 | 0.125/2 | 0.2656 | SYN | 32.39 (33.08; −0,69) | IND |
| C. glabrata | DSM 70614 | 0.25 | >68 | 0.06/1 | 0.2578 | SYN | 66.18 (67.17; −0.99) | SYN |
| C. krusei | V2105825iso4 | 0.5 | 64 | 0.125/2 | 0.2813 | SYN | 82.38 (83.33; −0.95) | SYN |
| C. krusei | V2105866 | 0.5 | 64 | 0.125/1 | 0.2656 | SYN | 49.18 (53.96; −4.78) | SYN |
| C. krusei | V2106177 | 0.5 | 64 | 0.25/1 | 0.5156 | IND | 43.68 (47.79; −4.11) | IND |
| C. krusei | V2105920 | 0.5 | 64 | 0.25/1 | 0.5156 | IND | 42.66 (44.51; −1.85) | IND |
| C. krusei | ATCC 6258 | 0.5 | 32 | 0.125/4 | 0.375 | SYN | 45.14 (47.64; −2.50) | SYN |
| C. parapsilosis | V2105056 | 0.25 | >68 | 0.06/1 | 0.2578 | SYN | 45.94 (45.96; −0.02 | SYN |
| C. parapsilosis | V2105223 | 0.25 | >68 | 0.06/1 | 0.2578 | SYN | 48.52 (49.02; −0.50) | SYN |
| C. parapsilosis | B2107379 | 0.5 | >68 | 0.06/2 | 0.1406 | SYN | 39.86 (40.36; −0.50) | IND |
| C. parapsilosis | ATCC 22019 | 0.5 | 64 | 0.06/1 | 0.1406 | SYN | 69.92 (70.41; −0.49) | SYN |
| C. tropicalis | V2105128 | 0.25 | 16 | 0.03/64 | 4.125 | ANT | 28.11 (43.14, −15.03) | IND |
| C. tropicalis | V2105245 | 0.25 | 16 | 0.03/64 | 4.125 | ANT | 21.56 (38.40; −16.84) | IND |
| C. tropicalis | V2105598 | 0.25 | 16 | 0.03/1 | 0.1875 | SYN | 17.29 (25.85; −8.56) | IND |
| C. tropicalis | B1907975 | 0.25 | 32 | 0.06/1 | 0.2813 | SYN | 36.13 (44.84; −8.71) | IND |
| C. tropicalis | V2106298 | 0.5 | 32 | 0.03/>68 | 4.125 | ANT | 55.85 (66.76; −10.91) | SYN |
| C. kefyr | V2105566 | 0.25 | 64 | 0.125/1 | 0.5156 | IND | 54.03 (63.85; −9,82) | SYN |
| C. kefyr | V2106126 | 0.5 | 64 | 0.125/1 | 0.2656 | SYN | 52.41 (54.37; −1.96) | SYN |
| C. kefyr | N2101899 | 0.5 | 32 | 0.125/1 | 0.2813 | SYN | 33.27 (36.56; −3.29) | IND |
| Species | Collection Number | Checkerboard MICs (µg/mL) | Response Surface Analysis | |||||
|---|---|---|---|---|---|---|---|---|
| AMB | COL | AMB/COL | FICI | INTPN | ΣSYN-ANT (ΣSYN; ΣANT) | INTPN | ||
| C. auris | CBS 10913 | 0.5 | >68 | 0.06/4 | 0.1563 | SYN | 84.31 (84.55; −0.24) | SYN |
| C. auris | CBS 12372 | 0.5 | >68 | 0.125/8 | 0.3125 | SYN | 32.54 (34.01; −1.47) | IND |
| C. auris | CBS 12373 | 0.5 | >68 | 0.125/2 | 0.2656 | SYN | 69.35 (70.74; −1.39) | SYN |
| C. auris | CBS 12766 | 1 | >68 | 0.5/1 | 0.5078 | IND | 26.82 (28.55; −1.73) | IND |
| C. auris | CBS 12767 | 1 | >68 | 0.5/1 | 0.5078 | IND | 40.81 (45.53; −4.72) | IND |
| C. auris | CBS 12768 | 1 | >68 | 0.5/1 | 0.5078 | IND | 47.90 (48.96; −1.06) | SYN |
| C. auris | CBS 12769 | 1 | >68 | 0.5/1 | 0.5078 | IND | 21.41 (21.63; −0.22) | IND |
| C. auris | CBS 12770 | 1 | >68 | 0.25/16 | 0.375 | SYN | 56.41(56.67; −0.26) | SYN |
| C. auris | CBS 12771 | 1 | >68 | 0.5/1 | 0.5078 | IND | 51.08 (51.37; −0.29) | SYN |
| C. auris | CBS 12772 | 1 | >68 | 0.5/1 | 0.5078 | IND | 54.38 (54.50; −0.12) | SYN |
| C. auris | CBS 12773 | 1 | >68 | 0.5/1 | 0.5078 | IND | 56.26 (56.28; −0.02) | SYN |
| C. auris | CBS 12774 | 1 | >68 | 0.5/1 | 0.5078 | IND | 53.74 (53.76; −0.02) | SYN |
| C. auris | CBS 12775 | 1 | >68 | 0.5/1 | 0.5078 | IND | 51.09 (51.14; −0.05) | SYN |
| C. auris | CBS 12776 | 1 | >68 | 0.5/1 | 0.5078 | IND | 44.93 (46.24; −1.31) | SYN |
| C. auris | CBS 12777 | 0.5 | >68 | 0.06/4 | 0.1563 | SYN | 60.03 (60.27; −0.24) | SYN |
| Species | Collection Number | Inhibition Endpoint | |||
|---|---|---|---|---|---|
| 50% | 90% | ||||
| FICI | INTPN | FICI | INTPN | ||
| C. albicans | V2105126 | 0.2578 | SYN | 0.3125 | SYN |
| C. albicans | N2101578 | 0.5078 | IND | 0.3125 | SYN |
| C. albicans | V2105568 | 0.2656 | SYN | 0.5078 | IND |
| C. albicans | N2101577 | 0.3125 | SYN | 0.5078 | IND |
| C. albicans | V2105825iso3 | 0.2578 | SYN | 0.2578 | SYN |
| C. albicans | ATCC 14053 | 0.2578 | SYN | 0.5078 | IND |
| C. albicans | V2105529 | 0.2578 | SYN | 0.2578 | SYN |
| C. albicans | V2106139 | 0.2578 | SYN | 0.5078 | IND |
| C. albicans | V2106041 | 0.375 | SYN | 0.2656 | SYN |
| C. albicans | V2106305 | 0.2656 | SYN | 0.2813 | SYN |
| C. glabrata | V2105272 | 0.5078 | IND | 0.5078 | IND |
| C. glabrata | V2105282 | 0.5156 | IND | 0.5078 | IND |
| C. glabrata | N2101711 | 0.5156 | IND | 0.5078 | IND |
| C. glabrata | V2105636 | 0.5156 | IND | 0.5078 | IND |
| C. glabrata | DSM 70614 | 0.2578 | SYN | 0.3125 | SYN |
| C. krusei | V2105825iso4 | 0.2813 | SYN | 0.5313 | IND |
| C. krusei | V2105866 | 0.5156 | IND | 0.5156 | IND |
| C. krusei | V2106177 | 0.3125 | SYN | 0.5156 | IND |
| C. krusei | V2105920 | 0.2813 | SYN | 0.5313 | IND |
| C. krusei | ATCC 6258 | 0.375 | SYN | 0.5313 | IND |
| C. parapsilosis | V2105056 | 0.5078 | IND | 0.5156 | IND |
| C. parapsilosis | V2105223 | 0.2578 | SYN | 0.5313 | IND |
| C. parapsilosis | B2107379 | 0.2656 | SYN | 0.5078 | IND |
| C. parapsilosis | ATCC 22019 | 0.2656 | SYN | 0.5078 | IND |
| C. tropicalis | V2105128 | 4.25 | ANT | 8.5 | ANT |
| C. tropicalis | V2105245 | 4.125 | ANT | 8.5 | ANT |
| C. tropicalis | V2105598 | 0.3125 | SYN | 8.5 | ANT |
| C. tropicalis | B1907975 | 0.5313 | IND | 4.5 | ANT |
| C. tropicalis | V2106298 | 4.5 | ANT | 4.25 | ANT |
| C. kefyr | V2105566 | 0.5156 | IND | 0.5156 | IND |
| C. kefyr | V2106126 | 0.5156 | IND | 0.375 | SYN |
| C. kefyr | N2101899 | 0.5313 | IND | 0.5313 | IND |
| Species | Collection Number | Inhibition Endpoint | |||
|---|---|---|---|---|---|
| 50% | 90% | ||||
| FICI | INTPN | FICI | INTPN | ||
| C. auris | CBS 10913 | 0.2813 | SYN | 0.2656 | SYN |
| C. auris | CBS 12372 | 0.3125 | SYN | 0.5078 | IND |
| C. auris | CBS 12373 | 0.2656 | SYN | 0.5078 | IND |
| C. auris | CBS 12766 | 0.5078 | IND | 0.5313 | IND |
| C. auris | CBS 12767 | 0.5078 | IND | 0.5156 | IND |
| C. auris | CBS 12768 | 0.5078 | IND | 0.5078 | IND |
| C. auris | CBS 12769 | 0.5078 | IND | 0.5078 | IND |
| C. auris | CBS 12770 | 0.375 | SYN | 0.5078 | IND |
| C. auris | CBS 12771 | 0.5078 | IND | 0.5156 | IND |
| C. auris | CBS 12772 | 0.5078 | IND | 0.5078 | IND |
| C. auris | CBS 12773 | 0.5078 | IND | 0.5078 | IND |
| C. auris | CBS 12774 | 0.5078 | IND | 0.5156 | IND |
| C. auris | CBS 12775 | 0.5078 | IND | 0.5313 | IND |
| C. auris | CBS 12776 | 0.5078 | IND | 0.5156 | IND |
| C. auris | CBS 12777 | 0.2813 | SYN | 0.2656 | SYN |
| Species | Collection Number | MIC (µg/mL) | ||||
|---|---|---|---|---|---|---|
| AMB | COL | AMB/COL | FICI | INTPN | ||
| A. flavus | HEGP-6097 | 2 | >68 | 2/1 | 1.0078 | IND |
| A. flavus | HEGP-5899 | 2 | >68 | 2/1 | 1.0078 | IND |
| A. flavus | HEGP-4536 | 4 | >68 | 4/4 | 1.0313 | IND |
| A. flavus | HEGP-4251 | 2 | >68 | 2/2 | 1.0156 | IND |
| A. flavus | HEGP-4114 | 4 | >68 | 4/1 | 1.0078 | IND |
| A. fumigatus | HEGP-5780 | 2 | >68 | 1/8 | 0.5625 | IND |
| A. fumigatus | HEGP-4020 | 2 | >68 | 1/4 | 0.5313 | IND |
| A. fumigatus | HEGP-4083 | 2 | >68 | 1/4 | 0.5313 | IND |
| A. fumigatus | HEGP-2659 | 2 | >68 | 1/16 | 0.625 | IND |
| A. fumigatus | HEGP-2664 | 2 | >68 | 2/1 | 1.0078 | IND |
| A. fumigatus | HEGP-R117 | 2 | >68 | 1/8 | 0.5625 | IND |
| A. fumigatus | HEGP-R279 | 2 | >68 | 1/2 | 0.5156 | IND |
| A. fumigatus | HEGP-R285 | 2 | >68 | 1/4 | 0.5313 | IND |
| A. fumigatus | HEGP-R290 | 2 | >68 | 1/16 | 0.625 | IND |
| A. fumigatus | HEGP-R291 | 2 | >68 | 1/4 | 0.5313 | IND |
| A. nidulans | HEGP-5711 | 4 | >68 | 0.5/64 | 0.625 | IND |
| A. nidulans | HEGP-6169 | 2 | >68 | 2/1 | 1.0078 | IND |
| A. nidulans | HEGP-5521 | 4 | >68 | 4/1 | 1.0078 | IND |
| A. nidulans | HEGP-5329 | 2 | >68 | 2/1 | 1.0078 | IND |
| A. niger | HEGP-6071 | 1 | >68 | 1/1 | 1.0078 | IND |
| A. niger | HEGP-6217 | 1 | >68 | 1/1 | 1.0078 | IND |
| A. niger | HEGP-6475 | 0.5 | >68 | 0.5/1 | 1.0078 | IND |
| A. niger | HEGP-6562 | 0.5 | >68 | 0.5/1 | 1.0078 | IND |
| A. niger | HEGP-6917 | 0.5 | >68 | 0.5/1 | 1.0078 | IND |
| A. terreus | HEGP-6625 | 4 | >68 | 4/1 | 1.0078 | IND |
| A. terreus | HEGP-6055 | 4 | >68 | 4/1 | 1.0078 | IND |
| A. terreus | HEGP-5599 | 4 | >68 | 4/1 | 1.0078 | IND |
| A. terreus | HEGP-5169 | 2 | >68 | 2/1 | 1.0078 | IND |
| A. terreus | HEGP-6398 | 4 | >68 | 4/1 | 1.0078 | IND |
| Species | Collection Number | Checkerboard MICs (µg/mL) | ||||
|---|---|---|---|---|---|---|
| AMB | COL | AMB/COL | FICI | INTPN | ||
| R. arrhizus | CBS 120809 | 0.5 | 16 | 0.25/8 | 1 | IND |
| R. arrhizus | IP 4.77 | 0.5 | 16 | 0.25/4 | 0.75 | IND |
| R. arrhizus | CBS 112.07 | 0.5 | 16 | 0.5/1 | 1.0625 | IND |
| R. arrhizus | CBS 120590 | 0.5 | 16 | 0.25/8 | 1 | IND |
| R. arrhizus | CBS 120591 | 0.5 | 16 | 0.25/4 | 0.75 | IND |
| R. arrhizus | CBS 120808 | 0.5 | 32 | 0.03/16 | 0.5625 | IND |
| R. arrhizus | IP 1443.75 | 0.5 | 16 | 0.25/4 | 0.75 | IND |
| R. delemar | CBS 120593 | 0.5 | 32 | 0.25/8 | 0.75 | IND |
| R. microsporus | CBS 120955 | 1 | 16 | 0.5/4 | 0.75 | IND |
| R. microsporus | IP 676.72 | 1 | 16 | 0.5/1 | 0.5625 | IND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarz, P.; Nikolskiy, I.; Bidaud, A.-L.; Sommer, F.; Bange, G.; Dannaoui, E. In Vitro Activity of Amphotericin B in Combination with Colistin against Fungi Responsible for Invasive Infections. J. Fungi 2022, 8, 115. https://doi.org/10.3390/jof8020115
Schwarz P, Nikolskiy I, Bidaud A-L, Sommer F, Bange G, Dannaoui E. In Vitro Activity of Amphotericin B in Combination with Colistin against Fungi Responsible for Invasive Infections. Journal of Fungi. 2022; 8(2):115. https://doi.org/10.3390/jof8020115
Chicago/Turabian StyleSchwarz, Patrick, Ilya Nikolskiy, Anne-Laure Bidaud, Frank Sommer, Gert Bange, and Eric Dannaoui. 2022. "In Vitro Activity of Amphotericin B in Combination with Colistin against Fungi Responsible for Invasive Infections" Journal of Fungi 8, no. 2: 115. https://doi.org/10.3390/jof8020115
APA StyleSchwarz, P., Nikolskiy, I., Bidaud, A.-L., Sommer, F., Bange, G., & Dannaoui, E. (2022). In Vitro Activity of Amphotericin B in Combination with Colistin against Fungi Responsible for Invasive Infections. Journal of Fungi, 8(2), 115. https://doi.org/10.3390/jof8020115








