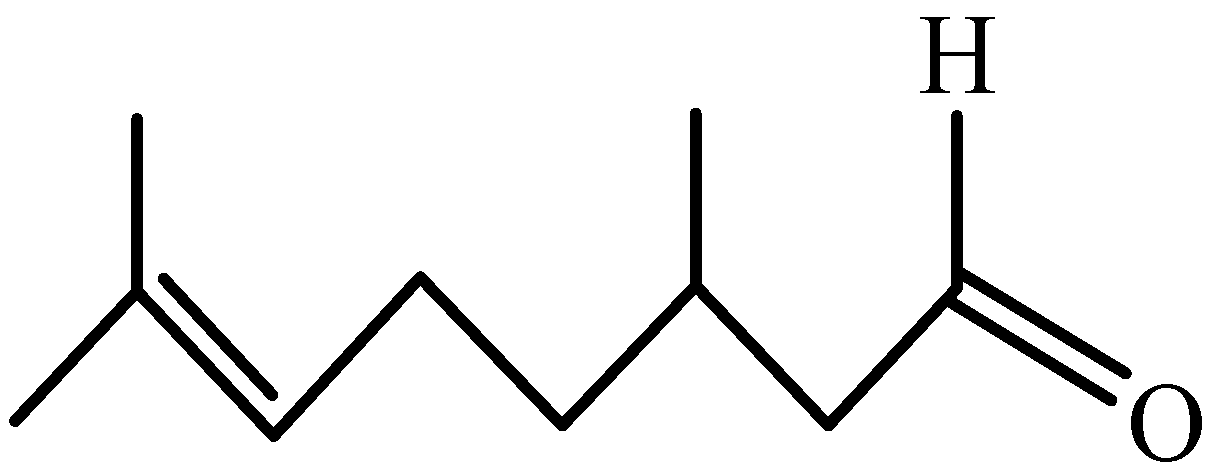
Natural Product Citronellal can Significantly Disturb Chitin Synthesis and Cell Wall Integrity in Magnaporthe oryzae
Abstract
1. Introduction
2. Materials and Methods
2.1. Magnaporthe oryzae, Medium, Rice Leaves and Citronellal
2.2. Antifungal Activity of Citronellal on Mycelial Growth
2.3. Determination of the Mycelial Quantity of M. oryzae
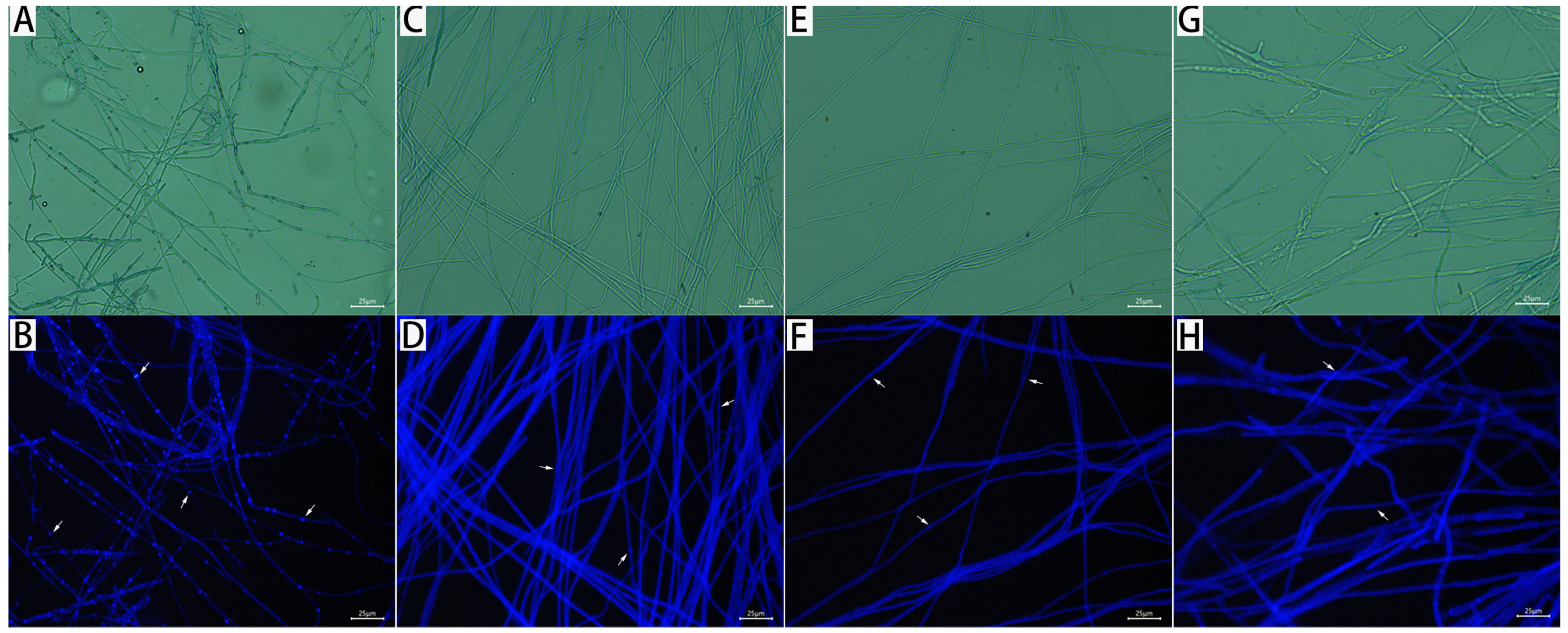
2.4. Observation of Mycelial Morphology
2.5. Mycelia Morphology and Ultrastructure of Mycelial Cell
2.6. Scanning Cell Membrane and Cell Wall Integrity
2.7. Determination of Mycelial Cell Membrane Permeability
2.8. Determination of Mycelial Chitinase Activity
2.9. Determination of Mycelial Β-1,3-Glucanase Activity
2.10. Detection of Expression of Mycelial Chitinase Gene
2.11. Detection of Expression of Mycelial Β-1,3-Glucanase Gene
2.12. Evaluation of Indoor Effectiveness
3. Results
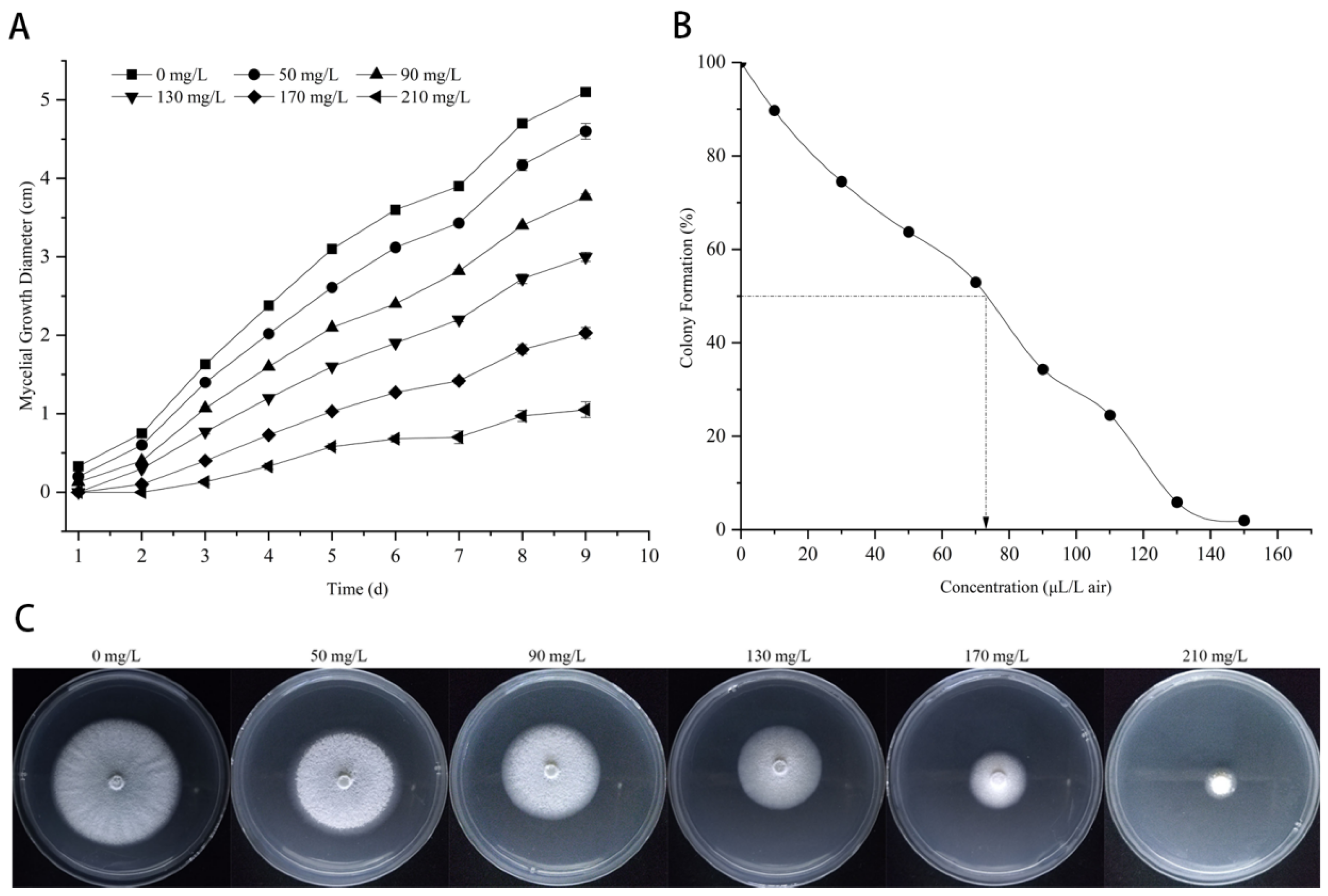
3.1. The Inhibition of Citronellal on Mycelial Growth of M. oryzae
3.2. Effect of Citronellal on Mycelial Quantity of M. oryzae
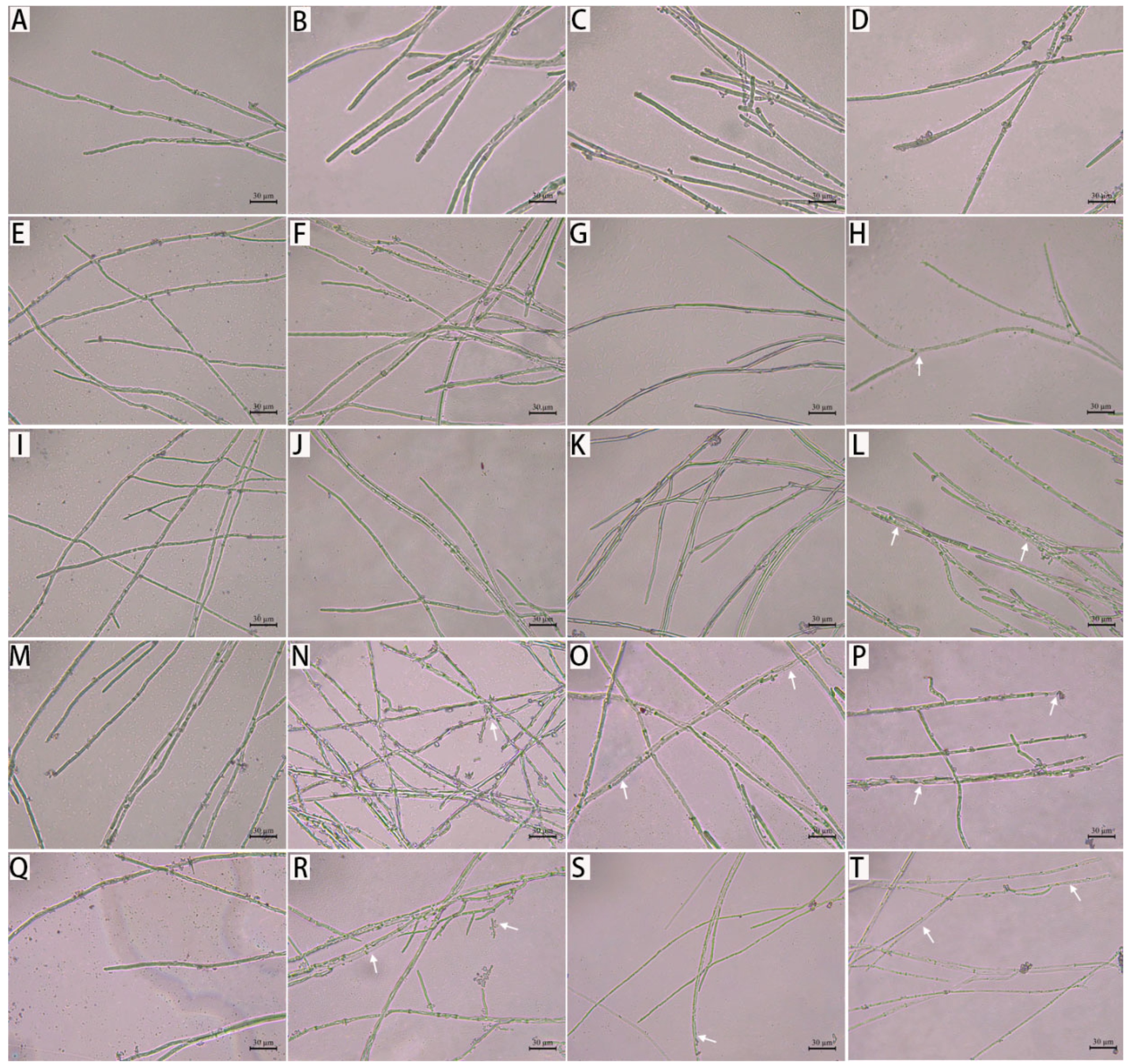
3.3. Effect of Citronellal on Mycelial Morphology of M. oryzae
3.4. Effect of Citronellal on Mycelial Morphology and Ultrastructure of M. oryzae
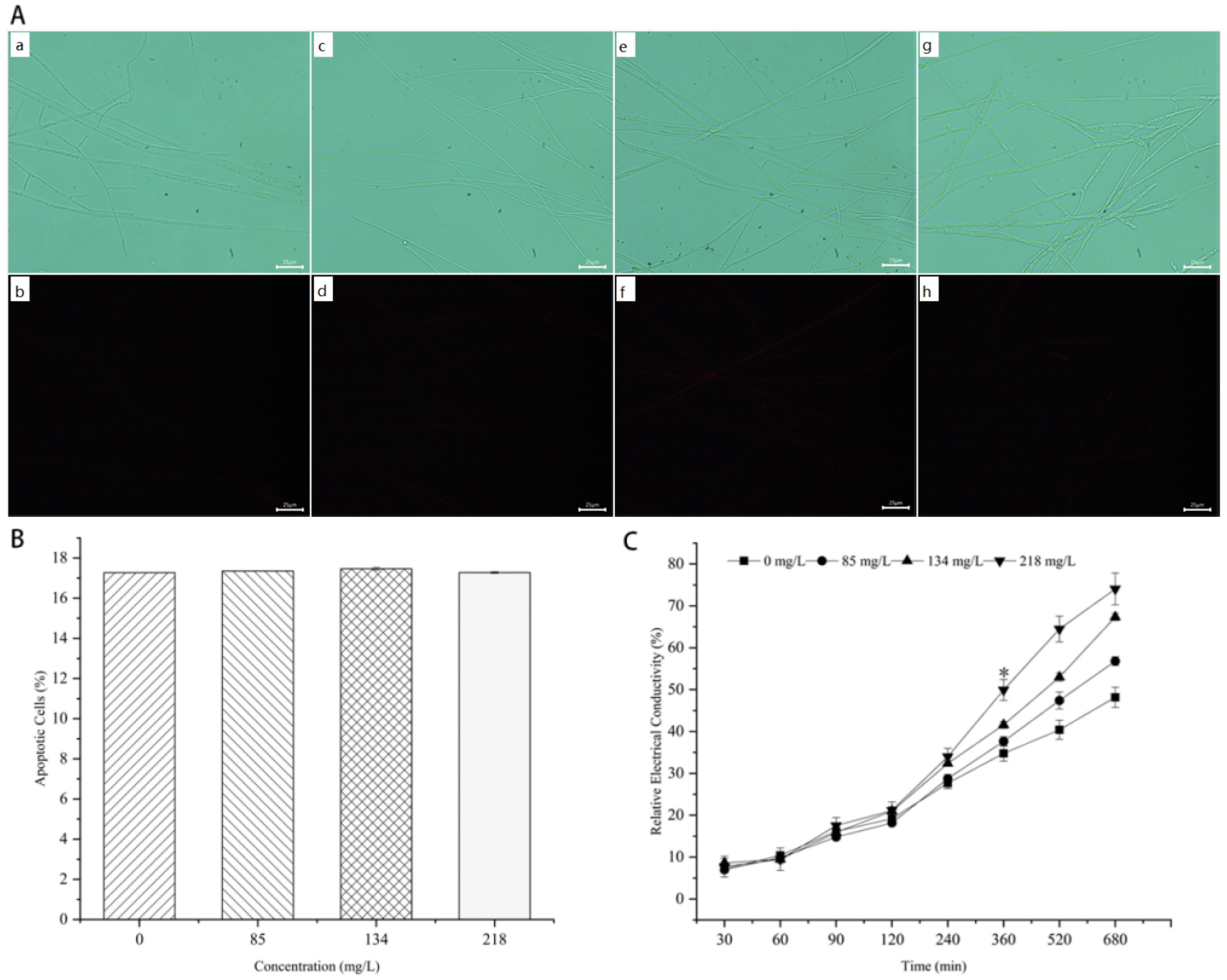
3.5. Mycelial PI and CFW Fluorescence Reaction of M. oryzae
3.6. Effect of Citronellal on Mycelial Cell Membrane Permeability of M. oryzae
3.7. Effect of Citronellal on Chitinase Activity in M. oryzae
3.8. Effect of Citronellal on Β-1,3-Glucanase Activity of M. oryzae
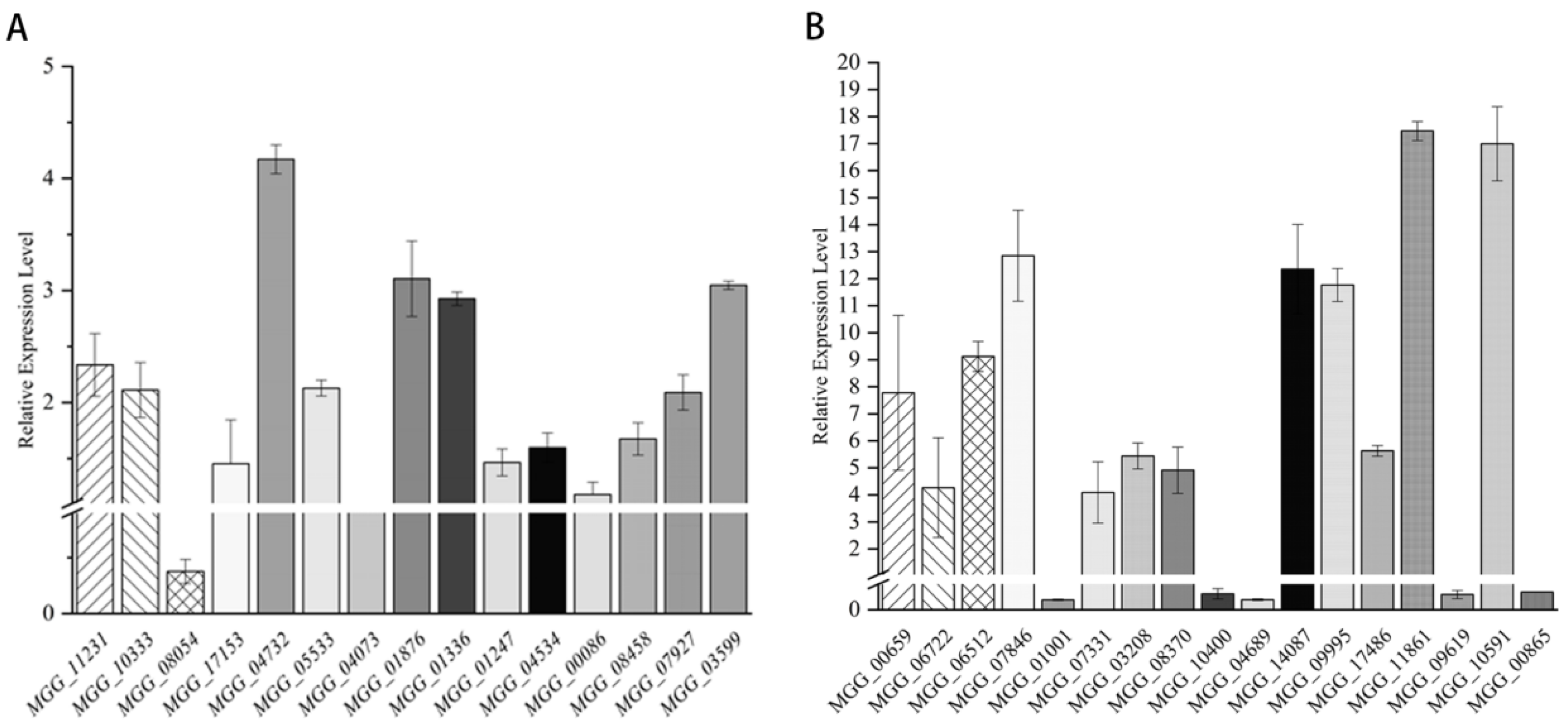
3.9. Effect of Citronellal on Chitinase Gene Expression in M. oryzae
3.10. Effect of Citronellal on Β-1,3-Glucanase Gene Expression of M. oryzae
3.11. Indoor Control Effect of Citronellal on Rice Blast
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ito, S. Contemporary Global Rice Economies: Structural Changes of Rice Production/Consumption and Trade. J. Nutr. Sci. Vitaminol. 2019, 65, S23–S25. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Baisakh, N.; Thet, K.M.; Tu, J.; Datta, S. Pyramiding transgenes for multiple resistance in rice against bacterial blight, yellow stem borer and sheath blight. Theor. Appl. Genet. 2002, 106, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-T.; Li, Y.; Li, T.-P.; Liang, Y.; Hu, L.; Zhang, D.; Zhou, C.-Y.; Yang, C.; Zhang, X.; Zha, S.-S.; et al. Stable Introduction of Plant-Virus-Inhibiting Wolbachia into Planthoppers for Rice Protection. Curr. Biol. 2020, 30, 4837–4845.e5. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Kim, K.-T.; Choi, J.; Cheong, K.; Ko, J.; Choi, G.; Lee, H.; Lee, G.-W.; Park, S.-Y.; Kim, S.; et al. Alternative splicing diversifies the transcriptome and proteome of the rice blast fungus during host infection. RNA Biol. 2022, 19, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-Q.; Meng, F.-Z.; Yin, L.-F.; Yin, W.-X.; Lv, L.; Yang, X.-L.; Chang, X.-Q.; Zhang, S.; Luo, C.-X. Transcriptomic Analysis of Resistant and Wild-Type Isolates Revealed Fludioxonil as a Candidate for Controlling the Emerging Isoprothiolane Resistant Populations of Magnaporthe oryzae. Front. Microbiol. 2022, 13, 874497. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, G.D.A.; Chaibub, A.A.; Oliveira, M.I.D.S.; Mizubuti, E.S.G.; de Filippi, M.C.C. Evidence of Pyricularia oryzae adaptability to tricyclazole. J. Environ. Sci. Health Part B 2021, 56, 869–876. [Google Scholar] [CrossRef]
- Ruan, H.; Tian, P.; Shi, N.; Du, Y.; Chen, F.; Chen, F. Characterization of pyraclostrobin-resistant Magnaporthe oryzae. J. Phytopathol. 2022, 170, 233–241. [Google Scholar] [CrossRef]
- Naranjo, S.E.; Ellsworth, P.C.; Frisvold, G.B. Economic Value of Biological Control in Integrated Pest Management of Managed Plant Systems. Annu. Rev. Èntomol. 2015, 60, 621–645. [Google Scholar] [CrossRef]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: An Updated Review on Antimicrobial Activities, Mechanism of Action and Their Potential Application in the Food Industry. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Chang, Y.; Harmon, P.F.; Treadwell, D.D.; Carrillo, D.; Sarkhosh, A.; Brecht, J.K. Biocontrol Potential of Essential Oils in Organic Horticulture Systems: From Farm to Fork. Front. Nutr. 2022, 8, 5138. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Cheng, F.; Li, S. Cytochrome P450 enzymes in fungal natural product biosynthesis. Nat. Prod. Rep. 2021, 38, 1072–1099. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Y.; Wu, X.-M.; Yin, X.-H.; Liang, J.-N.; Li, M. The Natural Product Citral Can Cause Significant Damage to the Hyphal Cell Walls of Magnaporthe grisea. Molecules 2014, 19, 10279–10290. [Google Scholar] [CrossRef] [PubMed]
- Li, R.-Y.; Wu, X.-M.; Yin, X.-H.; Long, Y.-H.; Li, M. Naturally produced citral can significantly inhibit normal physiology and induce cytotoxicity on Magnaporthe grisea. Pestic. Biochem. Physiol. 2015, 118, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Ding, Y.; Song, X.; Liu, S.; Li, M.; Li, R.; Ruan, H. Proteomic analysis reveals that naturally produced citral can significantly disturb physiological and metabolic processes in the rice blast fungus Magnaporthe oryzae. Pestic. Biochem. Physiol. 2021, 175, 104835. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, B.; Li, L.; Zhou, Q.; Tian, M.; Zhao, X.; Peng, J.; Liu, F.; Chen, Y.; Xu, Y.; et al. Effects of camptothecin on the rice blast fungus Magnaporthe oryzae. Pestic. Biochem. Physiol. 2019, 163, 108–116. [Google Scholar] [CrossRef]
- Mo, F.; Hu, X.; Ding, Y.; Li, R.; Long, Y.; Wu, X.; Li, M. Naturally produced magnolol can significantly damage the plasma membrane of Rhizoctonia solani. Pestic. Biochem. Physiol. 2021, 178, 104942. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Wu, X.; Jiang, M.; Jin, H.; Tao, K.; Hou, T. Unraveling the polypharmacology of a natural antifungal product, eugenol, against Rhizoctonia solani. Pest Manag. Sci. 2021, 77, 3469–3483. [Google Scholar] [CrossRef]
- Tibenda, J.J.; Yi, Q.; Wang, X.; Zhao, Q. Review of phytomedicine, phytochemistry, ethnopharmacology, toxicology, and pharmacological activities of Cymbopogon genus. Front. Pharmacol. 2022, 13, 997918. [Google Scholar] [CrossRef]
- Avoseh, O.N.; Ogunwande, I.A.; Lawal, O.A.; Atabo, J.; Ascrizzi, R.; Guido, F. Anti-inflammatory and anti-nociceptive activities of essential oil of Waltheria indica. Boletín Latinoam. Y Del Caribe Plantas Med. Y Aromáticas 2019, 18, 566–576. [Google Scholar] [CrossRef]
- Abbas, M.W.; Hussain, M.; Qamar, M.; Ali, S.; Shafiq, Z.; Wilairatana, P.; Mubarak, M.S. Antioxidant and Anti-Inflammatory Effects of Peganum harmala Extracts: An In Vitro and In Vivo Study. Molecules 2021, 26, 6084. [Google Scholar] [CrossRef]
- Wu, W.J.; Li, S.S.; Yang, M.; Lin, Y.W.; Zheng, K.B.; Akutse, K.S. Citronellal perception and transmission by Anopheles gambiae s.s. (Diptera: Culicidae) females. Sci. Rep. 2020, 10, 18615. [Google Scholar] [CrossRef] [PubMed]
- Tülek, Z.; Alaşalvar, H.; Başyiğit, B.; Berktas, S.; Salum, P.; Erbay, Z.; Telci, I.; Çam, M. Extraction optimization and microencapsulation of phenolic antioxidant compounds from lemon balm (Melissa officinalis L.): Instant soluble tea production. J. Food Process. Preserv. 2020, 45, 14995. [Google Scholar] [CrossRef]
- Singh, S.; Fatima, Z.; Hameed, S. Citronellal-induced disruption of membrane homeostasis in Candida albicans and attenuation of its virulence attributes. Rev. Soc. Bras. Med. Trop. 2016, 49, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Saibabu, V.; Singh, S.; Ansari, M.; Fatima, Z.; Hameed, S. Insights into the intracellular mechanisms of citronellal in Candida albicans: Implications for reactive oxygen species-mediated necrosis, mitochondrial dysfunction, and DNA damage. Rev. Soc. Bras. Med. Trop. 2017, 50, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.; Armstrong, V.; Navarro, F.; Castro, P.; Mendoza, L.; Cotoras, M. Characterization of the Fungitoxic Activity on Botrytis cinerea of N-phenyl-driman-9-carboxamides. J. Fungi 2021, 7, 902. [Google Scholar] [CrossRef] [PubMed]
- Soylu, E.M.; Soylu, S.; Kurt, S. Antimicrobial Activities of the Essential Oils of Various Plants against Tomato Late Blight Disease Agent Phytophthora infestans. Mycopathologia 2006, 161, 119–128. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wang, M.; Wang, H.; Zhao, T.; Cui, K.; Zhou, L. iTRAQ proteomic analysis of the inhibitory effect of 1,6-O,O-diacetylbritannilactone on the plant pathogenic oomycete Phytophthora capsici. Pestic. Biochem. Physiol. 2022, 184, 105125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, B.; Zhu, F.; Fu, Y. Baseline sensitivity and fungicidal action of propiconazole against Penicillium digitatum. Pestic. Biochem. Physiol. 2020, 172, 104752. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X.; Ling, L.; Qiu, H.; Zhen, Z.; Wang, Y.; Sun, G. Application of the Fluorescent Dye BODIPY in the Study of Lipid Dynamics of the Rice Blast Fungus Magnaporthe oryzae. Molecules 2018, 23, 1594. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Dawood, D.H.; Safwat, N.A. Antifungal action and induction of resistance by β-aminobutyric acid against Penicillium digitatum to control green mold in orange fruit. Pestic. Biochem. Physiol. 2020, 171, 104721. [Google Scholar] [CrossRef]
- Jiménez-Ortega, E.; Kidibule, P.E.; Fernández-Lobato, M.; Sanz-Aparicio, J. Structural inspection and protein motions modelling of a fungal glycoside hydrolase family 18 chitinase by crystallography depicts a dynamic enzymatic mechanism. Comput. Struct. Biotechnol. J. 2021, 19, 5466–5478. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Y.; Xie, Y. Paeonol Disrupts the Integrity of Aspergillus flavus Cell Walls via Releasing Surface Proteins, Inhibiting the Biosynthesis of β-1,3-Glucan and Promoting the Degradation of Chitin, and an Identification of Cell Surface Proteins. Foods 2021, 10, 2951. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhao, Q.; Zhou, A.; Wen, X.; Li, M.; Li, R.; Liao, X.; Xu, T. The Antifungal Effects of Citral on Magnaporthe oryzae Occur via Modulation of Chitin Content as Revealed by RNA-Seq Analysis. J. Fungi 2021, 7, 1023. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.; Mao, Y.; Lu, F.; Li, T.; Wang, J.; Duan, Y.; Zhou, M. In vitro fungicidal activity and in planta control efficacy of coumoxystrobin against Magnaporthe oryzae. Pestic. Biochem. Physiol. 2019, 162, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jiang, Y.; Ning, P.; Zheng, L.; Huang, J.; Li, G.; Jiang, D.; Hsiang, T. Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biol. Control 2011, 58, 139–148. [Google Scholar] [CrossRef]
- Chung, H.; Jeong, D.G.; Lee, J.-H.; Kang, I.J.; Shim, H.-K.; An, C.J.; Kim, J.Y.; Yang, J.-W. Outbreak of Rice Blast Disease at Yeoju of Korea in 2020. Plant Pathol. J. 2022, 38, 46–51. [Google Scholar] [CrossRef]
- Fernandez, J.; Orth, K. Rise of a Cereal Killer: The Biology of Magnaporthe oryzae Biotrophic Growth. Trends Microbiol. 2018, 26, 582–597. [Google Scholar] [CrossRef]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Yang, G.-Z.; Zhu, J.-K.; Yin, X.-D.; Yan, Y.-F.; Wang, Y.-L.; Shang, X.-F.; Liu, Y.-Q.; Zhao, Z.-M.; Peng, J.-W.; Liu, H. Design, Synthesis, and Antifungal Evaluation of Novel Quinoline Derivatives Inspired from Natural Quinine Alkaloids. J. Agric. Food Chem. 2019, 67, 11340–11353. [Google Scholar] [CrossRef]
- Kaur, H.; Bhardwaj, U.; Kaur, R.; Kaur, H. Chemical Composition and Antifungal Potential of Citronella (Cymbopogon nardus) Leaves Essential Oil and its Major Compounds. J. Essent. Oil Bear. Plants 2021, 24, 571–581. [Google Scholar] [CrossRef]
- Kaur, H.; Bhardwaj, U.; Kaur, R. Cymbopogon nardus essential oil: A comprehensive review on its chemistry and bioactivity. J. Essent. Oil Res. 2021, 33, 205–220. [Google Scholar] [CrossRef]
- Pontes, E.K.U.; Melo, H.M.; Nogueira, J.W.A.; Firmino, N.C.S.; de Carvalho, M.G.; Júnior, F.E.A.C.; Cavalcante, T.T.A. Antibiofilm activity of the essential oil of citronella (Cymbopogon nardus) and its major component, geraniol, on the bacterial biofilms of Staphylococcus aureus. Food Sci. Biotechnol. 2018, 28, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zong, Y.; Qin, G.; Li, B.; Tian, S. Plasma Membrane Damage Contributes to Antifungal Activity of Silicon Against Penicillium digitatum. Curr. Microbiol. 2010, 61, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; OuYang, Q.; Tao, N. Plasma membrane damage contributes to antifungal activity of citronellal against Penicillium digitatum. J. Food Sci. Technol. 2016, 53, 3853–3858. [Google Scholar] [CrossRef] [PubMed]
- Timper, P.; Wilson, J.P.; Johnson, A.W.; Hanna, W.W. Evaluation of Pearl Millet Grain Hybrids for Resistance to Meloidogyne spp. and Leaf Blight Caused by Pyricularia grisea. Plant Dis. 2002, 86, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Trindade, L.A.; Cordeiro, L.V.; Silva, D.D.F.; Figueiredo, P.T.R.; de Pontes, M.L.C.; Lima, E.D.O.; Carvalho, A.D.A.T. The antifungal and antibiofilm activity of Cymbopogon nardus essential oil and citronellal on clinical strains of Candida albicans. Braz. J. Microbiol. 2022, 53, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, C.; Li, Q.; Hu, A.; Peng, R.; Sun, F.; Zhang, W. Inhibition mechanism and antibacterial activity of natural antibacterial agent citral on bamboo mould and its anti-mildew effect on bamboo. R. Soc. Open Sci. 2021, 8, 202244. [Google Scholar] [CrossRef]
- Tan, X.; Long, C.; Meng, K.; Shen, X.; Wang, Z.; Li, L.; Tao, N. Transcriptome sequencing reveals an inhibitory mechanism of Penicillium digitatum by sodium dehydroacetate on citrus fruit. Postharvest Biol. Technol. 2022, 188, 111898. [Google Scholar] [CrossRef]
- Wang, Z.-Q.; Meng, F.-Z.; Zhang, M.-M.; Yin, L.-F.; Yin, W.-X.; Lin, Y.; Hsiang, T.; Peng, Y.-L.; Wang, Z.-H.; Luo, C.-X. A Putative Zn2Cys6 Transcription Factor Is Associated With Isoprothiolane Resistance in Magnaporthe oryzae. Front. Microbiol. 2018, 9, 2608. [Google Scholar] [CrossRef]
- OuYang, Q.; Okwong, R.O.; Chen, Y.; Tao, N. Synergistic activity of cinnamaldehyde and citronellal against green mold in citrus fruit. Postharvest Biol. Technol. 2019, 162, 111095. [Google Scholar] [CrossRef]
- Xu, Y.; Tong, Z.; Zhang, X.; Wang, Y.; Fang, W.; Li, L.; Luo, Z. Unveiling the Mechanisms for the Plant Volatile Organic Compound Linalool To Control Gray Mold on Strawberry Fruits. J. Agric. Food Chem. 2019, 67, 9265–9276. [Google Scholar] [CrossRef] [PubMed]
- OuYang, Q.; Liu, Y.; Oketch, O.; Zhang, M.; Shao, X.; Tao, N. Citronellal Exerts Its Antifungal Activity by Targeting Ergosterol Biosynthesis in Penicillium digitatum. J. Fungi 2021, 7, 432. [Google Scholar] [CrossRef] [PubMed]
- Mouhoub, A.; Guendouz, A.; Belkamel, A.; Talibi, Z.E.A.; Koraichi, S.I.; El Modafar, C.; Delattre, C. Assessment of the antioxidant, antimicrobial and antibiofilm activities of essential oils for potential application of active chitosan films in food preservation. World J. Microbiol. Biotechnol. 2022, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.F.; Ghaderi, J.; Gómez-Guillén, M.C. Tailoring physico-mechanical and antimicrobial/antioxidant properties of biopolymeric films by cinnamaldehyde-loaded chitosan nanoparticles and their application in packaging of fresh rainbow trout fillets. Food Hydrocoll. 2021, 124, 107249. [Google Scholar] [CrossRef]
- Duarte, L.G.; Picone, C.S. Antimicrobial activity of lactoferrin-chitosan-gellan nanoparticles and their influence on strawberry preservation. Food Res. Int. 2022, 159, 111586. [Google Scholar] [CrossRef]





| Gene | Primer Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| actin | MGG_03982 | CTGGCACCGTCGTCGATGAAG | AAGGTCCGCTCTCGTCGTACTC |
| 42 kDa endochitinase | MGG_11231 | GGCAACTCCAGCGAGATATTTCCC | GGTATTCCCAGTCAACGTCAATCCC |
| class III chitinase | MGG_10333 | CCTCGCCTGTGCCTTCCATTTG | GTGACCAACAAAGAAGACGCCAAAC |
| chitinase 1 | MGG_08054 | CTCATCCCAACAAACTCG | GCGTCAAGCCACTCATAT |
| putative uncharacterized protein | MGG_17153 | ACCCGCTGGATGGTGTACTACG | CGACGAGTTGAGGAACAAGTCTGAC |
| acidic mammalian chitinase | MGG_04732 | AAGGGCAAGTACATCACCGACAAC | CATGGCATCGACGACAATGTTCTTG |
| chitinase 1 | MGG_05533 | CGTCGCCTATGTGACCAACTGG | GGCAAGAATATCCGCAAACGCATAC |
| class III chitinase | MGG_04073 | CATCACGCTCAACGACCACCAC | CCCTCCCAGCATCCCGAGAATC |
| chitinase 3 | MGG_01876 | CGTCGCTCTCCTAGCAACAACAG | GAGTGCGTCGTCTGGAAGTAGATG |
| bacteriodes thetaiotaomicron symbiotic chitinase | MGG_01336 | CGGAGGAAACGTGACCACAGAC | TTGACAGCCAGTGCCACAATGAG |
| chitinase 1 | MGG_01247 | AGAACCGTTACTGTCACCGTTGATG | GGACTTGTGCCAGAGGGTATGATG |
| chitinase | MGG_04534 | CCACCTGTCACTACAAGAGCGAATG | AAGCCAAACTCTGAGCAGCACAC |
| endochitinase | MGG_00086 | CACCCATCTGCTTTATGCGTTTGC | TCTGTGGGGTAGTGCTTGGAGAC |
| chitinase 1 precursor | MGG_08458 | CCCTCACCTCCCAGAAACA | TTGAGTAGGCTGCGATGGATA |
| endochitinase 1 | MGG_07927 | GTCGTTTCCTTCGCCCTGATAGTC | GTGGAGAAGATTGTGGTGGAGTGTC |
| acidic endochitinase SE2 | MGG_03599 | TTAGGCTGCTGACGGCTAGTCC | GTCAAACCTCACCGCCCGAATC |
| Gene | Primer Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|---|
| glucan 1,3-beta-glucosidase | MGG_00659 | CGACAAACATCCCACCTCAGACTC | AGAAACCGCCAGACCCAGACTC |
| 1,3-beta-glucanosyltransferase gel2 | MGG_06722 | ATTTCAAGAAGGTTCAGGGCGTCTC | CGGGCAGCGTAAAGTTGTTGTTG |
| glucan 1,3-beta-glucosidase 2 | MGG_06512 | CCAGGACCGTTACCGCAACATC | ACCATTCTTCCGCACCAAGTCATAC |
| endoglucanase family 5 glycoside hydrolase | MGG_07846 | CACCACTTCTTCACAGAGGCAGAC | GCTTCTTGATGACGGACGGGTTG |
| endo-1,3(4)-beta-glucanase 1 | MGG_01001 | ACCGAGGACTTGCCATCAATATGC | GTGAGGATGCCGATGTTGGTGTC |
| 1,3-beta-glucanosyltransferase gel1 | MGG_07331 | CCGCCAAGATTGAGGATGCTGAG | TTGTTGCTGCCCTGGTTGCTAG |
| glycolipid-anchored surface protein 5 | MGG_03208 | TGGATGGGATGGAACTGGGATGG | TAAAGTGCCCGTGACCTCTCCTG |
| 1,3-beta-glucanosyltransferase gel3 | MGG_08370 | GGGTCGTTGGTTAGGTGTTGGATAC | CCGCACCACTCGTAGATGTTGTAG |
| GPI-anchored cell wall beta-1,3-endoglucanase EglC | MGG_10400 | GCAATCCAGGGCTTCAACTACGG | TACAGGCGGGCAGAGTTCCATC |
| glucan 1,3-beta-glucosidase | MGG_04689 | CATCACCAAGAACCTGTCCACCAAG | TGTTGCTACCACCAGTGCTGTTTC |
| beta 1,3 exoglucanase | MGG_14087 | GCAAGGCAGACGACACAGTAGC | AGGAGTGGTGGTGGTGGAGTTG |
| glucan 1,3-beta-glucosidase | MGG_09995 | TTACCTTGTCAGCGGCACGATTC | GATGACTCCCAGCCCAAGAAACG |
| glucan 1,3-beta-glucosidase | MGG_17486 | ACTTCTTCCGCTCAACTCGCAAC | GGTGTTCTCCTCAGTACGCATCTTG |
| 1,3-beta-glucanosyltransferase gel4 | MGG_11861 | CGCAGTACGACCAGCACATCAAG | AGCACCGCCAATACCGTCAATG |
| GPI-anchored cell wall beta-1,3-endoglucanase EglC | MGG_09619 | AGGCGAGCGGTCAGGATAATGG | ACCCAGTCTCCGTGACCCAAAC |
| endo-beta-1,3-glucanase | MGG_10591 | CACTACGACGACCGCAGTCAATAC | GTCCTCCTGTTGTTGGTGGTGATG |
| 1,3-beta-glucan synthase component FKS1 | MGG_00865 | CGACGCCAACCAGGACAACTATC | GACGGCATTCTTGACACCAGGAG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, A.-A.; Li, R.-Y.; Mo, F.-X.; Ding, Y.; Li, R.-T.; Guo, X.; Hu, K.; Li, M. Natural Product Citronellal can Significantly Disturb Chitin Synthesis and Cell Wall Integrity in Magnaporthe oryzae. J. Fungi 2022, 8, 1310. https://doi.org/10.3390/jof8121310
Zhou A-A, Li R-Y, Mo F-X, Ding Y, Li R-T, Guo X, Hu K, Li M. Natural Product Citronellal can Significantly Disturb Chitin Synthesis and Cell Wall Integrity in Magnaporthe oryzae. Journal of Fungi. 2022; 8(12):1310. https://doi.org/10.3390/jof8121310
Chicago/Turabian StyleZhou, Ai-Ai, Rong-Yu Li, Fei-Xu Mo, Yi Ding, Ruo-Tong Li, Xue Guo, Ke Hu, and Ming Li. 2022. "Natural Product Citronellal can Significantly Disturb Chitin Synthesis and Cell Wall Integrity in Magnaporthe oryzae" Journal of Fungi 8, no. 12: 1310. https://doi.org/10.3390/jof8121310
APA StyleZhou, A.-A., Li, R.-Y., Mo, F.-X., Ding, Y., Li, R.-T., Guo, X., Hu, K., & Li, M. (2022). Natural Product Citronellal can Significantly Disturb Chitin Synthesis and Cell Wall Integrity in Magnaporthe oryzae. Journal of Fungi, 8(12), 1310. https://doi.org/10.3390/jof8121310





