Tomato Xylem Sap Hydrophobins Vdh4 and Vdh5 Are Important for Late Stages of Verticillium dahliae Plant Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation, RNA Sequencing, and Initial Bioinformatics Analysis
2.2. Quantification of Gene Transcription
2.3. Additional Bioinformatics Methods
2.4. Plasmid and Strain Construction
2.4.1. VDH2 Deletion Plasmid and Strain Construction
2.4.2. VDH4 Deletion Plasmid and Strain Construction
2.4.3. VDH5 Deletion Plasmid and Strain Construction
2.4.4. Construction of VDH4/5 Double Deletion Strains
2.4.5. Construction of the VDH1 Deletion Plasmid and the VDH1/4/5 Triple Deletion Strains
2.4.6. Construction of the VDH2/5 Double Deletion and VDH2/4/5 Triple Deletion Strains
2.4.7. Construction of VDH4 and VDH5 Single Gene Complementation Strains in the VDH4/5 Double Deletion Background
2.5. Isolation of Genomic DNA and Southern Hybridization
2.6. Phenotypical Analyses
2.7. Quantification of Conidiospore Formation
2.8. Infection of Tomato Plants
3. Results
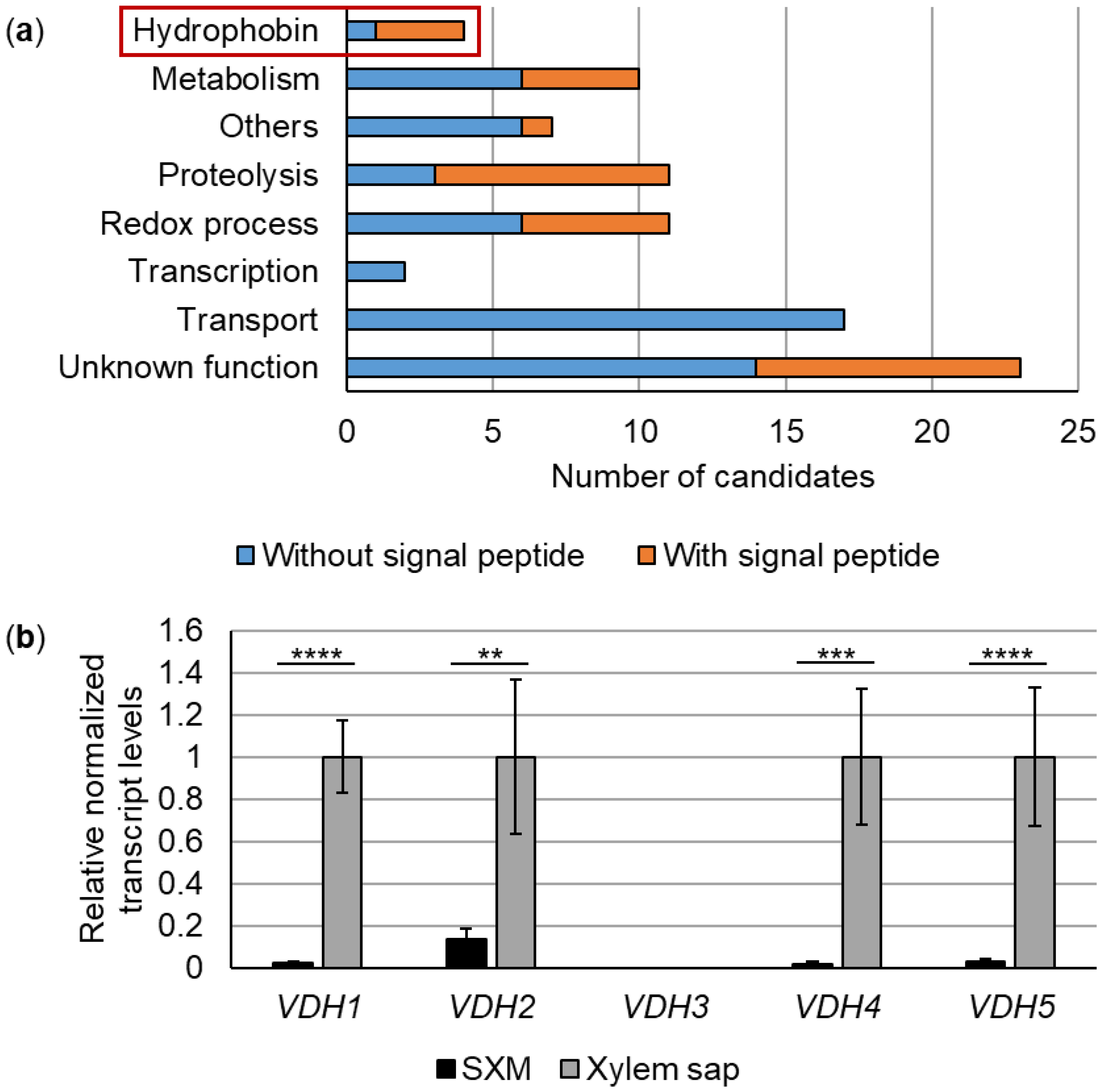
3.1. Expression of 85 V. dahliae Genes, Including Four Hydrophobin Genes, Is Strongly Induced in Tomato Xylem Sap
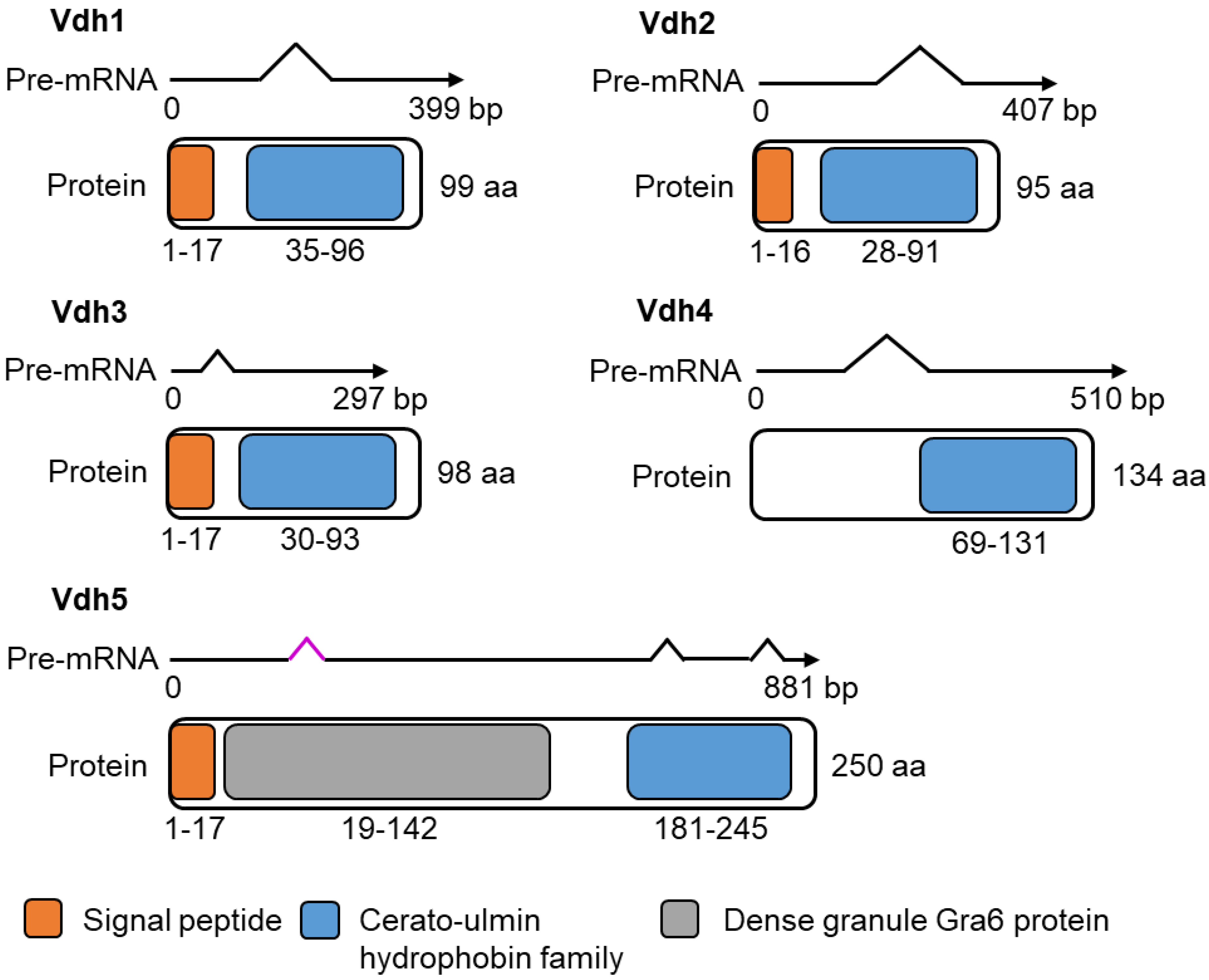
3.2. Vdh4 and Vdh5 Are Distinctive Members among the Five Hydrophobins Annotated in V. dahliae JR2
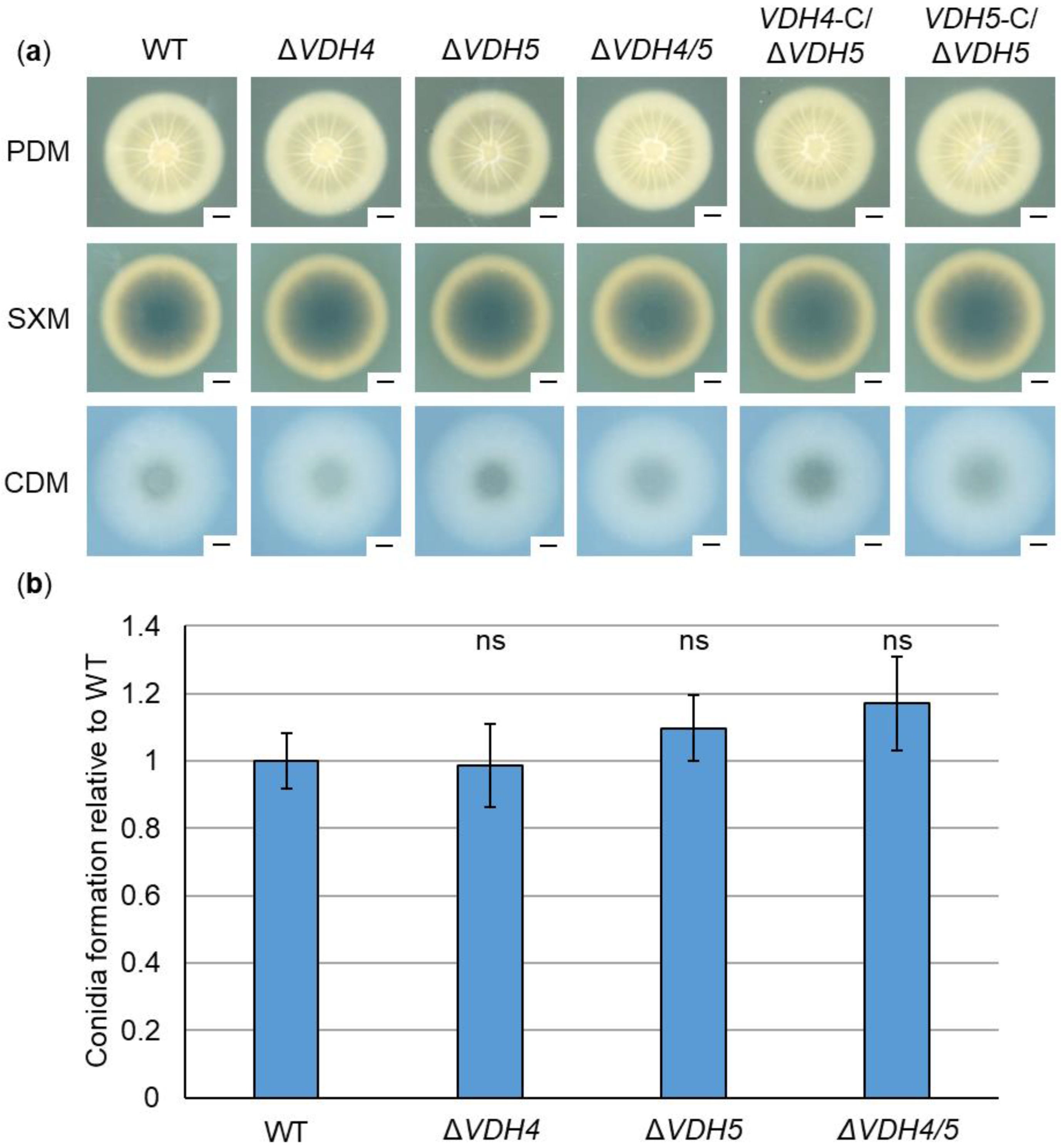
3.3. Hydrophobins Vdh2, Vdh4, and Vdh5 Are Dispensable for V. dahliae ex Planta Growth, Microsclerotia Formation, Conidiation, and Stress Response
3.4. Hydrophobins Vhd4 and Vdh5 Are Not Required for Initial Colonization of Tomato Plants
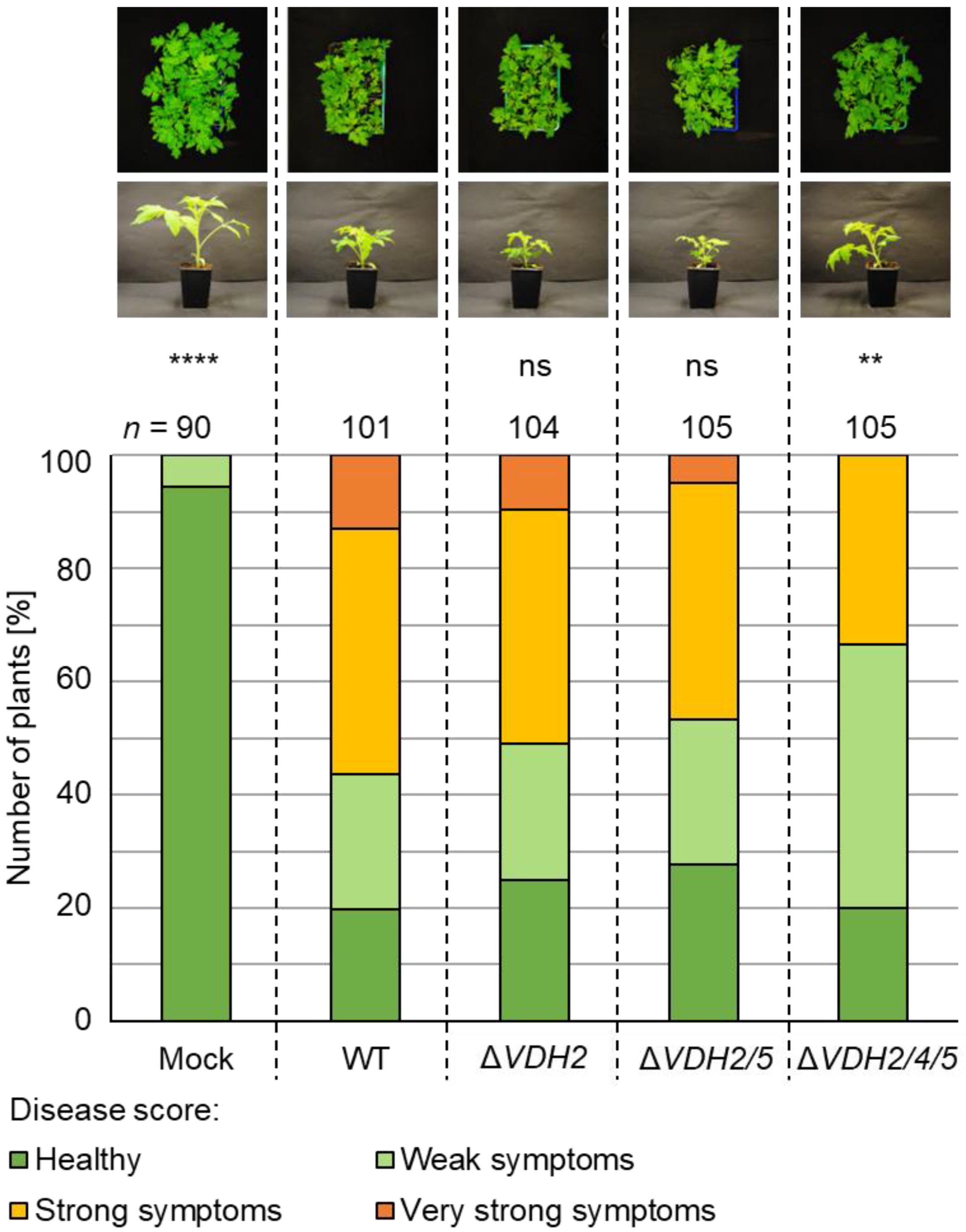
3.5. The Presence of Either VDH4 or VDH5 Is Required for Wild-Type-like Disease Development in Tomato Plants
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Vocht, M.L.; Reviakine, I.; Wösten, H.A.B.; Brisson, A.; Wessels, J.G.H.; Robillard, G.T. Structural and Functional Role of the Disulfide Bridges in the Hydrophobin SC3. J. Biol. Chem. 2000, 275, 28428–28432. [Google Scholar] [CrossRef] [PubMed]
- Sunde, M.; Kwan, A.H.Y.; Templeton, M.D.; Beever, R.E.; Mackay, J.P. Structural Analysis of Hydrophobins. Micron 2008, 39, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, L.; Liu, S.; Zhou, J.; Wu, Y.; Feng, Z.; Zhang, Y.; Zhu, H.; Wei, F.; Feng, H. Identification and Functional Analysis of a Novel Hydrophobic Protein VdHP1 from Verticillium dahliae. Microbiol. Spectr. 2022, 10, e0247821. [Google Scholar] [CrossRef] [PubMed]
- Wösten, H.A.B. Hydrophobins: Multipurpose Proteins. Annu. Rev. Microbiol. 2001, 55, 625–646. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, C.P.; Baker, S.; Gamauf, C.; Kenerley, C.M.; Druzhinina, I.S. Purifying Selection and Birth-and-Death Evolution in the Class II Hydrophobin Gene Families of the Ascomycete Trichoderma/Hypocrea. BMC Evol. Biol. 2008, 8, 4. [Google Scholar] [CrossRef]
- Stringer, M.A.; Dean, R.A.; Sewall, T.C.; Timberlake, W.E. Rodletless, a New Aspergillus Developmental Mutant Induced by Directed Gene Inactivation. Genes Dev. 1991, 5, 1161–1171. [Google Scholar] [CrossRef]
- Bell-Pedersen, D.; Dunlap, J.C.; Loros, J.J. The Neurospora Circadian Clock-Controlled Gene, Ccg-2, Is Allelic to Eas and Encodes, a Fungal Hydrophobin Required for Formation of the Conidial Rodlet Layer. Genes Dev. 1992, 6, 2382–2394. [Google Scholar] [CrossRef]
- Temple, B.; Horgen, P.A.; Bernier, L.; Hintz, W.E. Cerato-Ulmin, a Hydrophobin Secreted by the Causal Agents of Dutch Elm Disease, Is a Parasitic Fitness Factor. Fungal Genet. Biol. 1997, 22, 39–53. [Google Scholar] [CrossRef]
- Talbot, N.J.; Ebbole, D.J.; Hamer, J.E. Identification and Characterization of MPG1, a Gene Involved in Pathogenicity from the Rice Blast Fungus Magnaporthe grisea. Plant Cell 1993, 5, 1575–1590. [Google Scholar] [CrossRef]
- van Wetter, M.-A.; Wösten, H.A.B.; Wessels, J.G.H. SC3 and SC4 Hydrophobins Have Distinct Roles in Formation of Aerial Structures in Dikaryons of Schizophyllum commune. Mol. Microbiol. 2000, 36, 201–210. [Google Scholar] [CrossRef]
- Kazmierczak, P.; Kim, D.-H.; Turina, M.; Van Alfen, N.K. A Hydrophobin of the Chestnut Blight Fungus, Cryphonectria parasitica, Is Required for Stromal Pustule Eruption. Eukaryot. Cell 2005, 4, 931–936. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Klimes, A.; Dobinson, K.F. A Hydrophobin Gene, VDH1, Is Involved in Microsclerotial Development and Spore Viability in the Plant Pathogen Verticillium dahliae. Fungal Genet. Biol. 2006, 43, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Segers, G.C.; Hamada, W.; Oliver, R.P.; Spanu, P.D. Isolation and Characterisation of Five Different Hydrophobin-Encoding CDNAs from the Fungal Tomato Pathogen Cladosporium fulvum. Mol. Gen. Genet. 1999, 261, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Schuren, F.H.J.; Wessels, J.G.H. Two Genes Specifically Expressed in Fruiting Dikaryons of Schizophyllum commune: Homologies with a Gene Not Regulated by Mating-Type Genes. Gene 1990, 90, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Klimes, A.; Amyotte, S.G.; Grant, S.; Kang, S.; Dobinson, K.F. Microsclerotia Development in Verticillium dahliae: Regulation and Differential Expression of the Hydrophobin Gene VDH1. Fungal Genet. Biol. 2008, 45, 1525–1532. [Google Scholar] [CrossRef]
- Fradin, E.F.; Thomma, B.P.H.J. Physiology and Molecular Aspects of Verticillium Wilt Diseases Caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Eynck, C.; Koopmann, B.; Grunewaldt-Stoecker, G.; Karlovsky, P.; von Tiedemann, A. Differential Interactions of Verticillium longisporum and V. dahliae with Brassica napus Detected with Molecular and Histological Techniques. Eur. J. Plant Pathol. 2007, 118, 259–274. [Google Scholar] [CrossRef]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K. Diversity, Pathogenicity, and Management of Verticillium Species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Kong, Z. Live-Cell Imaging Elaborating Epidermal Invasion and Vascular Proliferation/Colonization Strategy of Verticillium dahliae in Host Plants. Mol. Plant Pathol. 2022, 23, 895–900. [Google Scholar] [CrossRef]
- Carroll, C.L.; Carter, C.A.; Goodhue, R.E.; Lin Lawell, C.-Y.C.; Subbarao, K. A Review of Control Options and Externalities for Verticillium Wilts. Phytopathology 2018, 108, 160–171. [Google Scholar] [CrossRef]
- Pegg, G.F.; Brady, B.L. Verticillium Wilts; CABI Publishing: Wallingford, UK, 2002; ISBN 9781845933227. [Google Scholar]
- Fradin, E.F.; Zhang, Z.; Juarez Ayala, J.C.; Castroverde, C.D.M.; Nazar, R.N.; Robb, J.; Liu, C.-M.; Thomma, B.P.H.J. Genetic Dissection of Verticillium Wilt Resistance Mediated by Tomato Ve1. Plant Physiol. 2009, 150, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.J.; Dobinson, K.F. Sequence Tag Analysis of Gene Expression during Pathogenic Growth and Microsclerotia Development in the Vascular Wilt Pathogen Verticillium dahliae. Fungal Genet. Biol. 2003, 38, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Hollensteiner, J.; Wemheuer, F.; Harting, R.; Kolarzyk, A.M.; Diaz Valerio, S.M.; Poehlein, A.; Brzuszkiewicz, E.B.; Nesemann, K.; Braus-Stromeyer, S.A.; Braus, G.H.; et al. Bacillus thuringiensis and Bacillus weihenstephanensis Inhibit the Growth of Phytopathogenic Verticillium Species. Front. Microbiol. 2017, 7, 2171. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.-T.; Braus-Stromeyer, S.A.; Kusch, H.; Reusche, M.; Kaever, A.; Kühn, A.; Valerius, O.; Landesfeind, M.; Aßhauer, K.; Tech, M.; et al. Verticillium Transcription Activator of Adhesion Vta2 Suppresses Microsclerotia Formation and Is Required for Systemic Infection of Plant Roots. New Phytol. 2014, 202, 565–581. [Google Scholar] [CrossRef]
- Höfer, A.M.; Harting, R.; Aßmann, N.F.; Gerke, J.; Schmitt, K.; Starke, J.; Bayram, Ö.; Tran, V.T.; Valerius, O.; Braus-Stromeyer, S.A.; et al. The Velvet Protein Vel1 Controls Initial Plant Root Colonization and Conidia Formation for Xylem Distribution in Verticillium Wilt. PLoS Genet. 2021, 17, e1009434. [Google Scholar] [CrossRef]
- Yates, A.D.; Allen, J.; Amode, R.M.; Azov, A.G.; Barba, M.; Becerra, A.; Bhai, J.; Campbell, L.I.; Carbajo Martinez, M.; Chakiachvili, M.; et al. Ensembl Genomes 2022: An Expanding Genome Resource for Non-Vertebrates. Nucleic Acids Res. 2022, 50, D996–D1003. [Google Scholar] [CrossRef]
- Priebe, S.; Kreisel, C.; Horn, F.; Guthke, R.; Linde, J. FungiFun2: A Comprehensive Online Resource for Systematic Analysis of Gene Lists from Fungal Species. Bioinformatics 2015, 31, 445–446. [Google Scholar] [CrossRef]
- Blum, M.; Chang, H.-Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro Protein Families and Domains Database: 20 Years On. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef]
- Thumuluri, V.; Almagro Armenteros, J.J.; Johansen, A.R.; Nielsen, H.; Winther, O. DeepLoc 2.0: Multi-Label Subcellular Localization Prediction Using Protein Language Models. Nucleic Acids Res. 2022, 50, W228–W234. [Google Scholar] [CrossRef]
- Agarwala, R.; Barrett, T.; Beck, J.; Benson, D.A.; Bollin, C.; Bolton, E.; Bourexis, D.; Brister, J.R.; Bryant, S.H.; Canese, K.; et al. Database Resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- Basenko, E.Y.; Pulman, J.A.; Shanmugasundram, A.; Harb, O.S.; Crouch, K.; Starns, D.; Warrenfeltz, S.; Aurrecoechea, C.; Stoeckert, C.J., Jr.; Kissinger, J.C.; et al. FungiDB: An Integrated Bioinformatic Resource for Fungi and Oomycetes. J. Fungi 2018, 4, 39. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Marx, A.; Backes, C.; Meese, E.; Lenhof, H.-P.; Keller, A. EDISON-WMW: Exact Dynamic Programing Solution of the Wilcoxon-Mann-Whitney Test. Genom. Proteom. Bioinforma. 2016, 14, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Nojima, H.; Okayama, H. High Efficiency Transformation of Escherichia coli with Plasmids. Gene 1990, 96, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Jessee, J.; Bloom, F.R. Plasmid Transformation of Escherichia coli and Other Bacteria. Methods Enzymol. 1991, 204, 63–113. [Google Scholar] [CrossRef] [PubMed]
- Lazo, G.R.; Stein, P.A.; Ludwig, R.A. A DNA Transformation-Competent Arabidopsis Genomic Library in Agrobacterium. Bio/Technol. Nat. Publ. Group 1991, 9, 963–967. [Google Scholar] [CrossRef]
- Jyothishwaran, G.; Kotresha, D.; Selvaraj, T.; Srideshikan, S.M.; Rajvanshi, P.K.; Jayabaskaran, C. A Modified Freeze-Thaw Method for Efficient Transformation of Agrobacterium tumefaciens. Curr. Sci. 2007, 93, 770–772. [Google Scholar]
- Bertani, G. Studies on Lysogenesis I. The Mode of Phage Liberation by Escherichia coli. J. Bacteriol. 1951, 62, 293–300. [Google Scholar] [CrossRef]
- Bui, T.-T.; Harting, R.; Braus-Stromeyer, S.A.; Tran, V.-T.; Leonard, M.; Höfer, A.; Abelmann, A.; Bakti, F.; Valerius, O.; Schlüter, R.; et al. Verticillium dahliae Transcription Factors Som1 and Vta3 Control Microsclerotia Formation and Sequential Steps of Plant Root Penetration and Colonisation to Induce Disease. New Phytol. 2019, 221, 2138–2159. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, J.; Guo, W.; Zhang, T. Functional Analysis of Autophagy Genes via Agrobacterium-Mediated Transformation in the Vascular Wilt Fungus Verticillium dahliae. J. Genet. Genom. 2013, 40, 421–431. [Google Scholar] [CrossRef]
- Leonard, M.; Kühn, A.; Harting, R.; Maurus, I.; Nagel, A.; Starke, J.; Kusch, H.; Valerius, O.; Feussner, K.; Feussner, I.; et al. Verticillium longisporum Elicits Media-Dependent Secretome Responses with Capacity to Distinguish between Plant-Related Environments. Front. Microbiol. 2020, 11, 1876. [Google Scholar] [CrossRef] [PubMed]
- Covert, S.F.; Kapoor, P.; Lee, M.; Briley, A.; Nairn, C.J. Agrobacterium tumefaciens-Mediated Transformation of Fusarium circinatum. Mycol. Res. 2001, 105, 259–264. [Google Scholar] [CrossRef]
- Smith, G. The Effect of Adding Trace Elements to Czapek-Dox Medium. Trans. Br. Mycol. Soc. 1949, 32, 280–283. [Google Scholar] [CrossRef]
- Harting, R.; Höfer, A.; Tran, V.-T.; Weinhold, L.-M.; Barghahn, S.; Schlüter, R.; Braus, G.H. The Vta1 Transcriptional Regulator Is Required for Microsclerotia Melanization in Verticillium dahliae. Fungal Biol. 2020, 124, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Starke, J.; Harting, R.; Maurus, I.; Leonard, M.; Bremenkamp, R.; Heimel, K.; Kronstad, J.W.; Braus, G.H. Unfolded Protein Response and Scaffold Independent Pheromone MAP Kinase Signaling Control Verticillium dahliae Growth, Development, and Plant Pathogenesis. J. Fungi 2021, 7, 305. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, J.-Y.; Song, J.; Li, J.-J.; Klosterman, S.J.; Li, R.; Kong, Z.-Q.; Subbarao, K.V.; Dai, X.-F.; Zhang, D.-D. Cytotoxic Function of Xylanase VdXyn4 in the Plant Vascular Wilt Pathogen Verticillium dahliae. Plant Physiol. 2021, 187, 409–429. [Google Scholar] [CrossRef]
- Becquer, A.; Guerrero-Galán, C.; Eibensteiner, J.L.; Houdinet, G.; Bücking, H.; Zimmermann, S.D.; Garcia, K. The Ectomycorrhizal Contribution to Tree Nutrition. Adv. Bot. Res. 2019, 89, 77–126. [Google Scholar] [CrossRef]
- Kaur, H.; Chowrasia, S.; Gaur, V.S.; Mondal, T.K. Allantoin: Emerging Role in Plant Abiotic Stress Tolerance. Plant Mol. Biol. Report. 2021, 39, 648–661. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Li, B.; Yang, X.; Dong, Y.; Qiu, D. A Verticillium dahliae Pectate Lyase Induces Plant Immune Responses and Contributes to Virulence. Front. Plant Sci. 2018, 9, 1271. [Google Scholar] [CrossRef]
- Braun, E.J.; Howard, R.J. Adhesion of Fungal Spores and Germlings to Host Plant Surfaces. Protoplasma 1994, 181, 202–212. [Google Scholar] [CrossRef]
- Lu, S.; Edwards, M.C. Genome-Wide Analysis of Small Secreted Cysteine-Rich Proteins Identifies Candidate Effector Proteins Potentially Involved in Fusarium graminearum-Wheat Interactions. Phytopathology 2016, 106, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.J.; Talbot, N.J. Hydrophobins and Repellents: Proteins with Fundamental Roles in Fungal Morphogenesis. Fungal Genet. Biol. 1998, 23, 18–33. [Google Scholar] [CrossRef] [PubMed]
- Lecordier, L.; Moleon-Borodowsky, I.; Dubremetz, J.F.; Tourvieille, B.; Mercier, C.; Deslée, D.; Capron, A.; Cesbron-Delauw, M.-F. Characterization of a Dense Granule Antigen of Toxoplasma Gondii (GRA6) Associated to the Network of the Parasitophorous Vacuole. Mol. Biochem. Parasitol. 1995, 70, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Labruyere, E.; Lingnau, M.; Mercier, C.; Sibley, L.D. Differential Membrane Targeting of the Secretory Proteins GRA4 and GRA6 within the Parasitophorous Vacuole Formed by Toxoplasma gondii. Mol. Biochem. Parasitol. 1999, 102, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Braus-Stromeyer, S.A.; Timpner, C.; Valerius, O.; von Tiedemann, A.; Karlovsky, P.; Druebert, C.; Polle, A.; Braus, G.H. The Plant Host Brassica napus Induces in the Pathogen Verticillium longisporum the Expression of Functional Catalase Peroxidase Which Is Required for the Late Phase of Disease. Mol. Plant-Microbe Interact. 2012, 25, 569–581. [Google Scholar] [CrossRef] [PubMed]
- López-Millán, A.F.; Morales, F.; Abadía, A.; Abadía, J. Effects of Iron Deficiency on the Composition of the Leaf Apoplastic Fluid and Xylem Sap in Sugar Beet. Implications for Iron and Carbon Transport. Plant Physiol. 2000, 124, 873–884. [Google Scholar] [CrossRef]
- Singh, S.; Braus-Stromeyer, S.A.; Timpner, C.; Tran, V.T.; Lohaus, G.; Reusche, M.; Knüfer, J.; Teichmann, T.; von Tiedemann, A.; Braus, G.H. Silencing of Vlaro2 for Chorismate Synthase Revealed That the Phytopathogen Verticillium longisporum Induces the Cross-Pathway Control in the Xylem. Appl. Microbiol. Biotechnol. 2010, 85, 1961–1976. [Google Scholar] [CrossRef]
- Carella, P.; Wilson, D.C.; Kempthorne, C.J.; Cameron, R.K. Vascular Sap Proteomics: Providing Insight into Long-Distance Signaling during Stress. Front. Plant Sci. 2016, 7, 651. [Google Scholar] [CrossRef]
- Timpner, C.; Braus-Stromeyer, S.A.; Van Tuan, T.; Braus, G.H. The Cpc1 Regulator of the Cross-Pathway Control of Amino Acid Biosynthesis Is Required for Pathogenicity of the Vascular Pathogen Verticillium longisporum. Mol. Plant-Microbe Interact. 2013, 26, 1312–1324. [Google Scholar] [CrossRef][Green Version]
- Duressa, D.; Anchieta, A.; Chen, D.; Klimes, A.; Garcia-Pedrajas, M.D.; Dobinson, K.F.; Klosterman, S.J. RNA-Seq Analyses of Gene Expression in the Microsclerotia of Verticillium dahliae. BMC Genom. 2013, 14, 607. [Google Scholar] [CrossRef]
- Hu, D.; Wang, C.; Tao, F.; Cui, Q.; Xu, X.; Shang, W.; Hu, X. Whole Genome Wide Expression Profiles on Germination of Verticillium dahliae Microsclerotia. PLoS ONE 2014, 9, e100046. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Wang, Y.; Ma, J.; Klosterman, S.J.; Xiao, S.; Tian, C. Deep MRNA Sequencing Reveals Stage-Specific Transcriptome Alterations during Microsclerotia Development in the Smoke Tree Vascular Wilt Pathogen, Verticillium dahliae. BMC Genom. 2014, 15, 324. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Xie, C.; Dong, J.; Yang, X. Comparative Transcriptome Analysis Reveals Regulatory Networks and Key Genes of Microsclerotia Formation in the Cotton Vascular Wilt Pathogen. Fungal Genet. Biol. 2019, 126, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Wang, Y.; Tian, C. Transcriptomic Profiles of the Smoke Tree Wilt Fungus Verticillium dahliae under Nutrient Starvation Stresses. Mol. Genet. Genom. 2015, 290, 1963–1977. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Villamil, J.L.; García-Pedrajas, N.E.; Baeza-Montañez, L.; García-Pedrajas, M.D. The APSES Transcription Factor Vst1 Is a Key Regulator of Development in Microsclerotium- and Resting Mycelium-Producing Verticillium Species. Mol. Plant Pathol. 2018, 19, 59–76. [Google Scholar] [CrossRef]
- Sarmiento-Villamil, J.L.; Prieto, P.; Klosterman, S.J.; García-Pedrajas, M.D. Characterization of Two Homeodomain Transcription Factors with Critical but Distinct Roles in Virulence in the Vascular Pathogen Verticillium dahliae. Mol. Plant Pathol. 2018, 19, 986–1004. [Google Scholar] [CrossRef]
- van Esse, H.P.; Fradin, E.F.; de Groot, P.J.; de Wit, P.J.G.M.; Thomma, B.P.H.J. Tomato Transcriptional Responses to a Foliar and a Vascular Fungal Pathogen Are Distinct. Mol. Plant-Microbe Interact. 2009, 22, 245–258. [Google Scholar] [CrossRef][Green Version]
- Faino, L.; de Jonge, R.; Thomma, B.P.H.J. The Transcriptome of Verticillium dahliae-Infected Nicotiana benthamiana Determined by Deep RNA Sequencing. Plant Signal. Behav. 2012, 7, 1065–1069. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, H.; Zhu, X.; Wang, W.; He, X.; Shi, Y.; Yuan, Y.; Du, X.; Cai, Y. Analysis of Sea-Island Cotton and Upland Cotton in Response to Verticillium dahliae Infection by RNA Sequencing. BMC Genom. 2013, 14, 852. [Google Scholar] [CrossRef]
- Tan, G.; Liu, K.; Kang, J.; Xu, K.; Zhang, Y.; Hu, L.; Zhang, J.; Li, C. Transcriptome Analysis of the Compatible Interaction of Tomato with Verticillium dahliae Using RNA-Sequencing. Front. Plant Sci. 2015, 6, 428. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Liu, K.; Jian, G.; Qi, F.; Si, N. Large-Scale Identification of Gossypium hirsutum Genes Associated with Verticillium dahliae by Comparative Transcriptomic and Reverse Genetics Analysis. PLoS ONE 2017, 12, e0181609. [Google Scholar] [CrossRef]
- Scholz, S.S.; Schmidt-Heck, W.; Guthke, R.; Furch, A.C.U.; Reichelt, M.; Gershenzon, J.; Oelmüller, R. Verticillium dahliae-arabidopsis Interaction Causes Changes in Gene Expression Profiles and Jasmonate Levels on Different Time Scales. Front. Microbiol. 2018, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Ruiz, J.; Leyva-Pérez, M.O.; Gómez-Lama Cabanás, C.; Barroso, J.B.; Luque, F.; Mercado-Blanco, J. The Transcriptome of Verticillium dahliae Responds Differentially Depending on the Disease Susceptibility Level of the Olive (Olea europaea L.) Cultivar. Genes 2019, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Chen, D.; Liao, S.; Zhang, Y.; Yu, F.; Wan, P.; Yu, D.; Wu, Q.; Zhang, Y. Transcriptome Analysis Reveals Downregulation of Virulence-Associated Genes Expression in a Low Virulence Verticillium dahliae Strain. Arch. Microbiol. 2019, 201, 927–941. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.G.H. Developmental Regulation of Fungal Cell Wall Formation. Annu. Rev. Phytopathol. 1994, 32, 413–437. [Google Scholar] [CrossRef]
- Whiteford, J.R.; Spanu, P.D. Hydrophobins and the Interactions between Fungi and Plants. Mol. Plant Pathol. 2002, 3, 391–400. [Google Scholar] [CrossRef]
- Zhou, B.-J.; Jia, P.-S.; Gao, F.; Guo, H.-S. Molecular Characterization and Functional Analysis of a Necrosis- and Ethylene-Inducing, Protein-Encoding Gene Family from Verticillium dahliae. Mol. Plant-Microbe Interact. 2012, 25, 964–975. [Google Scholar] [CrossRef]
- Santhanam, P.; van Esse, H.P.; Albert, I.; Faino, L.; Nürnberger, T.; Thomma, B.P.H.J. Evidence for Functional Diversification within a Fungal NEP1-like Protein Family. Mol. Plant-Microbe Interact. 2013, 26, 278–286. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Liang, Y.; Dong, Y.; Yang, X.; Yuan, J.; Qiu, D. The Verticillium dahliae SnodProt1-like Protein VdCP1 Contributes to Virulence and Triggers the Plant Immune System. Front. Plant Sci. 2017, 8, 1880. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, B.; Hua, C.; Meng, P.; Wang, S.; Chen, Z.; Du, Y.; Gao, F.; Huang, J. VdPKS1 Is Required for Melanin Formation and Virulence in a Cotton Wilt Pathogen Verticillium dahliae. Sci. China Life Sci. 2017, 60, 868–879. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Fang, Y.; Anchieta, A.; Goldman, P.H.; Hernandez, G.; Klosterman, S.J. Transcription Factor VdCmr1 Is Required for Pigment Production, Protection from UV Irradiation, and Regulates Expression of Melanin Biosynthetic Genes in Verticillium dahliae. Microbiology 2018, 164, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Temple, B.; Horgen, P.A. Biological Roles for Cerato-Ulmin, a Hydrophobin Secreted by the Elm Pathogens, Ophiostoma ulmi and O. novo-ulmi. Mycologia 2000, 92, 1–9. [Google Scholar] [CrossRef]
- Kim, S.; Ahn, I.-P.; Rho, H.-S.; Lee, Y.-H. MHP1, a Magnaporthe grisea Hydrophobin Gene, Is Required for Fungal Development and Plant Colonization. Mol. Microbiol. 2005, 57, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Drakakaki, G.; Dandekar, A. Protein Secretion: How Many Secretory Routes Does a Plant Cell Have? Plant Sci. 2013, 203–204, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y.; Xiao, H.-L.; Gui, Y.-J.; Zhang, D.-D.; Li, L.; Bao, Y.-M.; Dai, X.-F. Characterization of the Verticillium dahliae Exoproteome Involves in Pathogenicity from Cotton-Containing Medium. Front. Microbiol. 2016, 7, 1709. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Zhang, D.-D.; Short, D.P.G.; Zhou, L.; Song, S.-S.; Liu, Y.; Wang, D.; Kong, Z.-Q.; Cui, W.Y.; et al. SNARE-Encoding Genes VdSec22 and VdSso1 Mediate Protein Secretion Required for Full Virulence in Verticillium dahliae. Mol. Plant-Microbe Interact. 2018, 31, 651–664. [Google Scholar] [CrossRef]
- Hakanpää, J.; Szilvay, G.R.; Kaljunen, H.; Maksimainen, M.; Linder, M.; Rouvinen, J. Two Crystal Structures of Trichoderma reesei Hydrophobin HFBI-The Structure of a Protein Amphiphile with and without Detergent Interaction. Protein Sci. 2006, 15, 2129–2140. [Google Scholar] [CrossRef]
- Kajava, A.V.; Baxa, U.; Wickner, R.B.; Steven, A.C. A Model for Ure2p Prion Filaments and Other Amyloids: The Parallel Superpleated β-Structure. Proc. Natl. Acad. Sci. USA 2004, 101, 7885–7890. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurus, I.; Leonard, M.; Nagel, A.; Starke, J.; Kronstad, J.W.; Harting, R.; Braus, G.H. Tomato Xylem Sap Hydrophobins Vdh4 and Vdh5 Are Important for Late Stages of Verticillium dahliae Plant Infection. J. Fungi 2022, 8, 1252. https://doi.org/10.3390/jof8121252
Maurus I, Leonard M, Nagel A, Starke J, Kronstad JW, Harting R, Braus GH. Tomato Xylem Sap Hydrophobins Vdh4 and Vdh5 Are Important for Late Stages of Verticillium dahliae Plant Infection. Journal of Fungi. 2022; 8(12):1252. https://doi.org/10.3390/jof8121252
Chicago/Turabian StyleMaurus, Isabel, Miriam Leonard, Alexandra Nagel, Jessica Starke, James W. Kronstad, Rebekka Harting, and Gerhard H. Braus. 2022. "Tomato Xylem Sap Hydrophobins Vdh4 and Vdh5 Are Important for Late Stages of Verticillium dahliae Plant Infection" Journal of Fungi 8, no. 12: 1252. https://doi.org/10.3390/jof8121252
APA StyleMaurus, I., Leonard, M., Nagel, A., Starke, J., Kronstad, J. W., Harting, R., & Braus, G. H. (2022). Tomato Xylem Sap Hydrophobins Vdh4 and Vdh5 Are Important for Late Stages of Verticillium dahliae Plant Infection. Journal of Fungi, 8(12), 1252. https://doi.org/10.3390/jof8121252





